Abstract
Mesenchymal stem cells (MSCs) have been isolated from the pulp tissue of permanent teeth (dental pulp stem cells or DPSCs) and deciduous teeth (stem cells from human exfoliated deciduous teeth or SHED). We recently discovered another type of MSCs in the apical papilla of human immature permanent teeth termed stem cells from apical papilla (SCAP). Here we further characterized the apical papilla tissue and stem cell properties of SCAP using histological, immunohistochemical and immunocytofluorescent analyses. We found that apical papilla is distinctive to pulp in terms of containing less cellular and vascular components than those in pulp. Cells in apical papilla proliferated 2- to 3-fold greater than those in pulp in organ cultures. Both SCAP and DPSCs were as potent in osteo/dentinogenic differentiation as MSCs from bone marrows while weaker in adipogenic potential. The immunophenotype of SCAP is similar to that of DPSCs on the osteo/dentinogenic and growth factor receptor gene profiles. Double staining experiments showed that STRO-1 co-expressed with dentinogenic markers such as bone sialophosphoprotein (BSP), osteocalcin (OCN) and growth factors FGFR1 and TGFβRI in cultured SCAP. Additionally, SCAP express a wide variety of neurogenic markers such as nestin and neurofilament M upon stimulation with a neurogenic medium. We conclude that SCAP are similar to DPSCs but a distinct source of potent dental stem/progenitor cells. Their implications in root development and apexogenesis are discussed.
Keywords: Apical papilla, immature teeth, MSCs, SCAP, DPSCs, PDLSCs, BMMSCs, apexogenesis, immunohistochemistry, immunocytofluorescence
Introduction
Previously, we isolated and characterized human dental pulp stem cells (DPSCs) from the dental pulp of permanent teeth and stem cells from the pulp of human exfoliated deciduous teeth (SHED) (1, 2). DPSCs are capable of differentiating into odontoblast-like cells and produce ectopic dentin in the subcutaneous space of immunocompromised mice. SHED appear to be capable of forming odontoblast- and osteoblast-like cells and generating dentin and bone respectively when transplanted into immunocompromised mice. Very recently, we discovered another type of human MSCs from apical papilla (SCAP) which appear to be a different population of stem cells from DPSCs (3). We utilized SCAP to engineer bio-roots using minipigs as an animal model and demonstrated that these cells are a promising cell source to regenerate bio-roots for future clinical applications (3).
Little, if any, information on human apical papilla is available in the literature. In developing teeth, root formation starts as the epithelial cells from the cervical loop proliferate apically and influence the differentiation of odontoblasts from undifferentiated mesenchymal cells and cementoblasts from follicle mesenchyme (4). This apically extending two-layered epithelial wall (merging of the inner and outer enamel epithelium) forms Hertwig’s epithelial root sheath (HERS) which is responsible for determining the shape of the root(s) and forms cementum through epithelial-mesenchymal transition (EMT) (5). It has been known that dental papilla contributes to tooth formation and eventually converts to pulp tissue (4, 6, 7). As the root continues to develop after the bell stage, the location of the dental papilla becomes apical to the pulp tissue. The histological features at the junction of the pulp and apical papilla have not been well described. We report herein that apical papilla appears to be histologically distinct from pulp and contains unique potent MSCs.
Materials and methods
Sample collection
Normal human impacted third molars (n= 10) with immature roots were collected from healthy patients (6 donors aged 16–24 yrs) in the Dental Clinics at the University of Southern California and the University of Maryland. Bone marrow was obtained from the long bone of a healthy patient (aged ~20) undergoing orthopaedic surgery at Walter Reed Army Medical Center. Sample collection conformed to approved protocols by the respective Medical Institutional Review Boards and National Institute of Health. The informed consent of all human subjects who participated in the experimental investigation reported or described in this manuscript was obtained after the nature of the procedure and possible discomforts and risks had been fully explained. Tooth or bone samples were stored in medium and transported to labs for sample processing.
Cell culture
Root apical papilla was gently separated from the surface of the root, minced and digested in a solution of 3 mg/ml collagenase type I (Worthington Biochemicals Corp., Freehold, NJ) and 4 mg/ml dispase (Roche Diagnostic/Boehringer Mannheim Corp., Indianapolis, IN) for 30 minutes at 37°C. Single cell suspensions of SCAP were obtained by passing through a 70 μm strainer (Falcon, BD Labware, Franklin Lakes, NJ), seeded at 1×104 into 10-cm culture dishes (Costar, Cambridge, MA), and cultured with α-Modification of Eagle’s Medium (GIBCO/Invitrogen, Carlsbad, CA) supplemented with 15–20% fetal bovine serum (FBS) (Equitech-Bio Inc., Kerrville, TX), 100 mM L-ascorbic acid 2-phosphate (WAKO, Tokyo, Japan), 2 mM L-glutamine (Biosource/Invitrogen), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in 5% CO2. To assess colony-forming efficiency, day-10 cultures were fixed with 4% formalin, and then stained with 0.1% toluidine blue. Aggregates of ≥50 cells were scored as colonies. DPSCs and bone marrow mesenchymal stem cells (BMMSCs) were isolated and cultured as previously described (1, 8, 9). DPSCs were grown in the same medium for SCAP and BMMSCs were cultured in expansion medium containing Dulbecco’s Modified Eagle’s Medium (DMEM), 100 units/ml penicillin, 100 μg/ml streptomycin, 25 ng/ml Fungizone, and 10% preselected FBS. Cells were subcultured at 1:3 when they reached ~85% confluence. All primary cells used in this study were at 1–3 passages. For each experiment, same passage of cells were used.
In situ cell proliferation analysis
The root tips from extracted teeth containing pulp and apical papilla were cultured in a regular BMMSC culture medium and labeled with bromodeoxyuridine (BrdU) using a Zymed BrdU staining kit (Zymed/Invitrogen, Carlsbad, CA). The concentration of BrdU used was the same as for labeling cultured cells described previously (3, 10). After 3.5 and 18 hrs of BrdU incorporation, the tissues were fixed, demineralized and embedded. Samples were then sectioned and deparaffinized for immunohistochemical staining of BrdU positive cells using anti-BrdU antibodies. Positively stained cells were counted in randomly selected areas under a light microscope.
Differentiation stimulation
Conditions for the induction of calcium accumulation were as reported previously (1, 11). Osteo/dentinogenic medium contains the above basic elements plus 10 nM dexamethasone, 10 mM β-glycerophosphate, 50 μg/ml ascorbate phosphate and 10 nM 1, 25 dihydroxyvitamin D3 Calcium accumulation was detected by 2% Alizarin Red S (pH 4.2) staining. The induction of adipogenesis was as previously reported (2, 3, 12). Adipogenic medium contains basic elements plus 1 μM dexamethasone, 1 μg/ml insulin and 0.5 mM IBMX (3-isobutyl-1-methylxantine). Oil droplets accumulated within cells after adipogenic differentiation were stained by oil red O reagent. For neurogenic induction, cells were cultured in the presence of B27 supplement, bFGF (40 ng/ml) and EGF (20 ng/ml) (2).
Antibodies
Mouse antibodies included anti-DSP (LF-21) (Dr. Larry Fisher, NIDCR/NIH); anti- STRO-1 (Dr. Stan Gronthos, Institute of Medical and Veterinary Science, Australia), nestin, neurofilament M (NFM), neuronal nuclei (NeuN), and 2′, 3′-cyclic nucleotide-3′-phosphodiesterase (CNPase) from Chemicon; anti-βIII-tubulin from Promega and monoclonal anti-CD146/MUC18 (melanoma-associated glycoprotein) from Chemicon. Rabbit antibodies included anti-basic FGF (bFGF), TGFβRI, TGFβRII, NSE (neural-specific enolase), Flt-1 (VEGF receptor 1), Flg (FGFR1) and FGFR3 from Santa Cruz Biotechnology, Santa Cruz, CA; bone sialophosphoprotein (BSP, LF-120), alkaline phosphotase (ALP, LF-47), osteocalcin (OCN, LF-32), anti-alkaline phosphatase (ALP) (LF-47), and matrix extracellular phosphaglycoprotein (MEPE) (LF-155) from Dr. L. Fisher; and anti-endostatin, glutamic acid decarboxylase (GAD) from Chemicon. Rabbit and murine isotype-matched negative control antibodies were obtained from Caltag Laboratories (Caltag/Invitrogen).
Immunohistochemistry and immunocytofluorescence
Deparaffinized sections were immersed in 3% H2O2/methanol for 15 minutes to quench the endogenous peroxidase activity, and incubated with primary antibodies (1:200 to 1:500 dilutions). Isotype-matched control antibodies were used under the same conditions for 1 hour. For enzymatic immunohistochemical staining, Zymed SuperPicTure polymer detection kit (Zymed/Invitrogen) was used according to the manufacturer’s protocol. Subsequently, sections were counterstained with hematoxylin. For immunocytofluorescent analysis, cells were subcultured into eight-chamber slides (2 ×104 cells per well) (Nunc). The cells were fixed in 4% formaldehyde for 15 min and then blocked and incubated with primary antibodies (1:200–1:500 dilutions) for 1 h, respectively. The samples were subsequently incubated with goat secondary antibodies of either IgG-rhodamine red or IgG-Cy2 (Jackson ImmunoResearch) for 45 min followed by counterstaining with DAPI (Chemicon) according to the manufacture’s instruction.
Results
Histological characteristics of apical papilla
A smooth-surfaced soft tissue was noted at the apex of extracted immature teeth and with a pair of pliers, this tissue (apical papilla) was easily detached from the apex exposing pulp tissue in the canal space. As shown in Fig. 1A, C, the apical papilla appears to be readily separated next to the pulp. Histological views of the longitudinal sections revealed that there is a cell rich zone between pulp and apical papilla (Fig. 1B). To distinguish the cell rich zone in mature pulp, here we term it “apical cell rich zone”. Apical papilla appears to contain less blood vessels and cellular components than do the dental pulp and the apical cell rich zone.
Fig. 1. Anatomy of apical papilla.
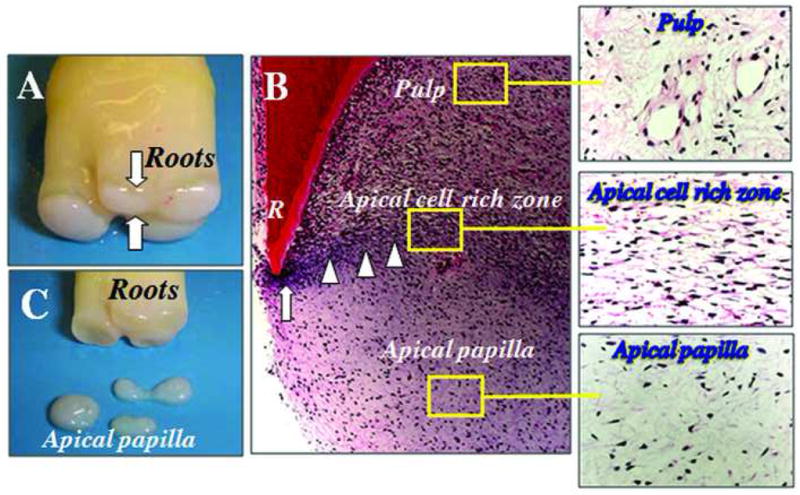
(A) An extracted human third molar depicting root attached to the root apical papilla (open arrows) at developmental stage. (B) Hematoxylin and eosin (H & E) staining of human developing root (R) depicting epithelial diaphragm (open arrows) and apical cell rich zone (open arrowheads). (C) Harvested root apical papilla for stem cell isolation.
Organ culture studies on cell proliferation in apical papilla
We asked whether cells in apical papilla proliferate at a different rate compared to cells in pulp when the extracted teeth are placed in cultures. BrdU was used to label the cells in the tissues in cultures. The results shown in Fig. 2 demonstrate that apical papilla contain 2- to 3-fold more BrdU positive cells than do pulp tissue in the same tooth 3.5 h or 18 h after the BrdU incorporation. Statistical analysis was examined by one-way factorial ANOVA followed by Fisher’s protected least significant difference (PLSD) post-hoc test. The data suggest that once the tissues are placed in cultures, cells in apical papilla start to enter the growth cycle more than those in pulp.
Fig. 2. Cell proliferation in pulp and apical papilla in cultures.
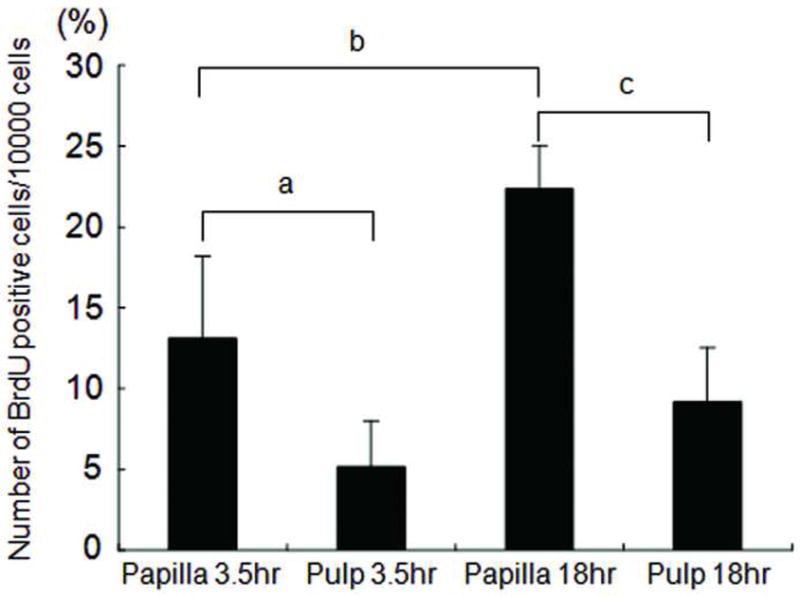
In vitro BrdU labeling after 3.5 and 18 hs. Representative data (out of three independence experiments) indicate the numbers of BrdU positive cells in the pulp compared with those in the apical papilla of the same tooth. Four randomly selected fields per sample were used to count the positively stained cells under the microscope. Error bars: SD; a, p=0.0091; b, p<0.0001; c, p=0.0002.
In situ immunohistochemical analysis of osteo/dentinogenic markers
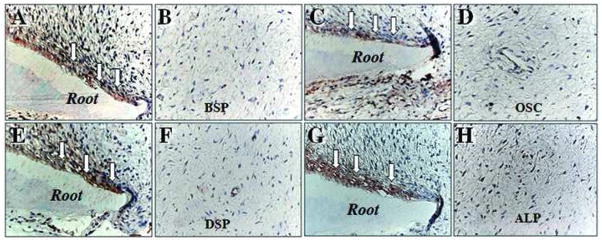
We next examined the in situ expression of osteo/dentinogenic markers in apical papilla. DSP, ALP, BSP, and OCN stained positively only in odontoblasts lined against the newly formed dentin (Fig. 3), whereas none of these markers were detected in the apical papilla.
Fig. 3. Immunocytofluorescent staining of osteo/dentinogenic markers in pulp and apical papilla.
Odontoblasts (open arrows) express osteo/dentinogenic markers including BSP (A), OCN (C), DSP (E), and ALP (G). Apical papilla showed a negative immunohistochemical staining for BSP (B), OSC (D), DSP (F), and ALP (H).
Osteo/dentinogenic and adipogenic potentials
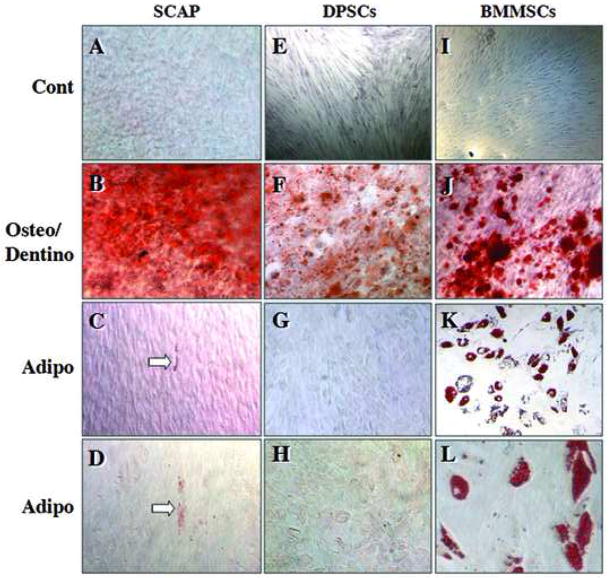
To compare osteo/dentinogenic and adipogenic potencies of SCAP and DPSCs to the most studied mesenchymal stem cells BMMSCs, cells were exposed to respective differentiation media. The results in Fig. 4 are representative data from several independent experiments of consistent results demonstrating that the accumulation of calcium in cultures of SCAP and DPSCs was comparable to that of BMMSCs (Fig. 4B, F, J, Alizarin red stain), whereas the adipogenic potential of SCAP and DPSCs was much weaker than BMMSCs. In Fig 4K, L, oil droplets accumulated in BMMSCs are clearly demonstrated by the oil red O stain and the positively stained adipocytes constituted up to 30–40% of cells in BMMSC cultures. In contrast, only a few cells in SCAP cultures showed the red stain (Fig. 4C, D, arrows) or very faint stain in DPSC cultures (Fig. 4G, H).
Fig. 4. Osteo-/dentino- and adipo-genesis of stem cells.
SCAP (A–D), DPSCs (E–H) and BMMSCs (I–L). Control unstimulated (A, E, I); osteo/dentinogenic stimulation of cells for 8 weeks (B, F, J); adipogenic stimulation for 3 weeks (C–L). Open arrows indicate oil red O stain. Data are representative from several independent experiments [original magnifications, E, I: 50x; B, F, G, J, K: 100x; A, C, D, H, L: 200x]
Immunophenotype of SCAP in cultures
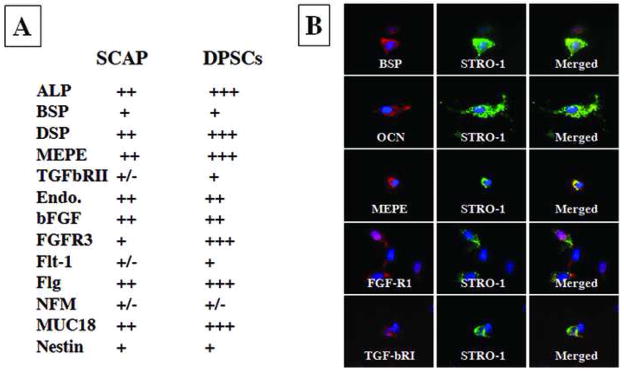
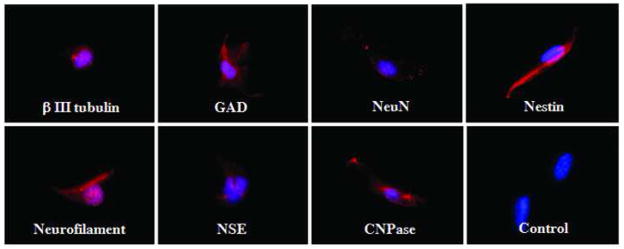
To further characterize gene expression profiles of SCAP, we selected a list of genes that are important for defining MSCs. Semi-quantitative analysis was performed after the immunocytofluorescent staining and the gene expression profiles of SCAP and DPSCs were compared as shown in Fig. 5A. SCAP and DPSCs express similar osteo/dentinogenic markers and growth factor receptors, however, SCAP express less amount of DSP, MEPE, TGFβRII, FGFR3, Flt-1, Flag, and MUC18. Double staining experiments revealed that STRO-1 co-expressed with osteo/dentinogenic markers such as BSP, OCN, MEPE and growth factors FGFR1 and TGFβRI (Fig. 5B). Approximately 20–30% of cells in cultures were positive for STRO-1 and ~70–80% positive for other markers. Additionally, we stimulated the SCAP with the neurogenic medium for 4 weeks followed by detection of neurogenic marker expression. The immunofluorescence images in Fig. 6 demonstrate the expression of β III tubulin, GAD, NeuN, nestin, GFAP, neurofilament M, NSE and CNPase.
Fig. 5. Immunophenotype of SCAP.
(A) SCAP and DPSCs express similar osteo/dentinogenic markers and growth factor receptors. SCAP express less amounts of DSP, MEPE, TGFβRII, FGFR3, Flt-1, Flg, and MUC18. +++, strong; ++, moderate; +, weak; −, negative. (B) Double staining of SCAP showing STRO-1 co-expressed with osteo/dentinogenic markers such as BSP, OCN, MEPE and growth factor receptors FGFR1 and TGFβRI. Cell nuclei exhibit in blue fluorescence by DAPI counter stain. Expression of markers showing either in red of green fluorescence. Images are representative data of independent experiments with consistent results.
Fig. 6. Expression of neural markers in SCAP.
Immunocytofluorescent staining of cultured SCAP expressing a variety of neural markers (red fluorescence) including βIII tubulin, GAD, NeuN, nestin, GFAP, neurofilament M, NSE, and CNPase when cultured in neurogenic medium for 4 weeks. Blue fluorescence is the cell nucleus. Images are representative data of independent experiments with consistent results.
Discussion
Dental papilla has been considered to be the source of odontoblasts during tooth development. As the differentiated odontoblasts lay down the primary dentin, the dental papilla becomes encased within the dentin structure and evolves into pulp tissue. The apical end of the dental papilla, however, has not been discussed much in the literature. It is generally believed that the formation of root dentin is the result of signaling from HERS to the adjacent undifferentiated mesenchymal cells which then turn into odontoblasts that are responsible for the root dentin formation. The anatomical location of these undifferentiated mesenchymal cells has not been clearly elucidated. They may be residing either in pulp or apical papilla. Dental pulp also harbors undifferentiated mesenchymal cells that are known to be capable of differentiating into new odontoblasts to replace the lost original odontoblasts. To make a clear distinction of the odontoblasts from a different source, we herein term the odontoblasts derived from dental papilla during the developing stage the “primary odontoblasts” that make primary and secondary dentin, as opposed to the “replacement odontoblasts” derived from pulp to replace primary odontoblasts and make tertiary dentin or more specifically, the reparative dentin. Some authors described the replacement odontoblasts as odontoblast-like cells (13–16).
Earlier and recent evidence has shown that replacement odontoblasts derive from underlying mesenchymal cells are located in cell rich zone and cell proper particularly in the perivascular region (17–19). As mentioned above, isolated DPSCs from pulp have shown the capacity to differentiate into odontoblast-like cells and produce ectopic dentin in immunocompromised mice (1). By detecting the expression of STRO-1, one of the markers that defines MSCs, DPSCs were found and considered to reside in the perivascular and perineurosheath regions (20). This finding corresponds to that concerning the source of replacement odontoblasts, which is from the perivascular regions where stem/progenitor cells reside. Currently, however, it is unknown where the DPSCs in the pulp come from.
In the present study, we first discovered the loose physical connection between the pulp and the apical papilla. The latter tissue can be easily detached or peeled off from the apex. Under the histological view, we observed a layer of densely populated cell rich zone (apical cell rich zone) between the two tissues. The vascular density in apical papilla appears lower than that of pulp. More accurate measurement using labeled antibodies to stain vascular antigens such as the endothelial marker CD146 and/or CD34 is needed to confirm this observation. When we placed the apical papilla in cultures, cells within the tissue showed more BrdU incorporation than those in the pulp in cultures. Previously we studied the proliferation rates of SCAP and DPSCs and found that the former proliferate faster with greater population doublings than the latter (3), which finding corresponds to that in the present study. One property of adult MSCs is that they normally remain in a non-proliferative, quiescent state in vivo until stimulated by the signals triggered by tissue damage and remodeling (21). They enter the cell cycle when being isolated and exposed to cultural environments. It appears that similar activities in terms of proliferation are occurring when cells are still residing inside of the tissue which is being placed in cultures.
BMMSCs are the gold standard MSCs in terms of multipotentiality. SCAP and DPSCs, on the other hand, appear to have a different profile of multipotency and are more committed to osteo/dentinogenicity (1, 3, 8). We confirmed the adipogenic aspect of the multipotentiality. Despite SCAP expressing many osteo/dentinogenic markers following ex vivo expansion, they express lower levels of DSP, MEPE, TGFβRII, FGFR3, Flt-1, Flg and MUC18 than do DPSCs. The in situ immunohistochemical staining of various osteo/dentiogenic markers revealed that only odontoblasts in the pulp express these genes, not in the apical papilla (Fig. 3).
Two criteria are normally used to define stem cells: i) capable of self-renewal and ii) give rise to progenitor cells that eventually differentiate into specialized cells. One approach to test the self-renewal property of stem cells is the population doubling (PD). The PD of SCAP was measured previously to be up to 80 (3), a number that is very high among identified postnatal stem cells. As a newly identified population of postnatal stem cells, SCAP exhibit heterogeneous nature by showing i) co-expression of STRO-1 with a variety of osteo/dentinogenic markers and ii) there are low percentage of STRO-1 positive cells while high percentage of cells positive with osteo/dentinogenic markers in cultures. In addition, SCAP show a positive staining for several neural markers including βIII tubulin, GAD, NeuN, nestin, GFAP, neurofilament M, NSE, and CNPase by immunocytochemical staining. It is possible that SCAP are derived from neural crest cells or at least associated with neural crest cells, analogous to dental stem cells such as DPSCs and SHED (2, 8).
The capacity of SCAP to differentiate into functional dentinogenic cells has been verified using animal models (3). Ex vivo expanded SCAP were transplanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate (HA/TCP) particles as a carrier. A typical dentin structure was regenerated in which a layer of dentin tissue was formed on the surface of the HA/TCP along with connective tissue (3). To verify the functional role of root apical papilla, one approach is to remove this tissue and determine how that would affect the root development. We performed a preliminary study using minipigs as a model in which the apical papilla of one root of a molar was surgically removed at early developing stage and this halted the development of that particular root, despite the pulp tissue being intact. In contrast, other roots of the tooth containing apical papilla showed normal growth and development (data not shown). Based on our current and previous findings, it is tempting to speculate that SCAP, not DPSCs, are a cell source for the primary odontoblasts that produce root dentin as depicted in Fig. 7.
Fig. 7. Diagram of hypothetical cell source for root dentin development.
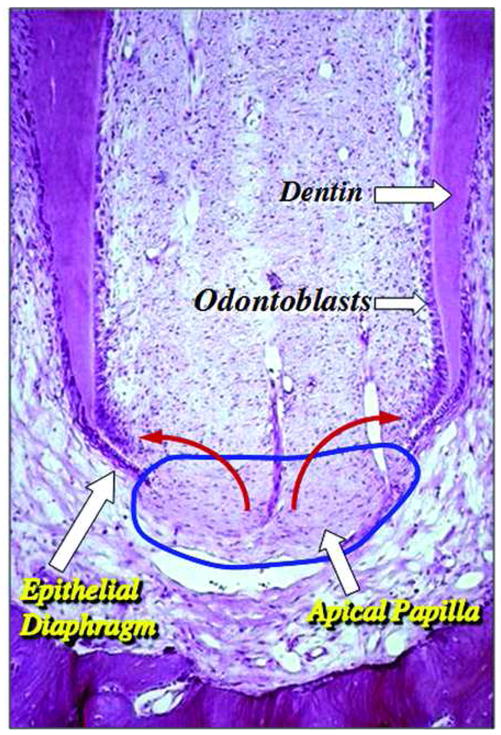
Apical papilla (blue circle) contains stem/progenitor cells which may be the source of the odontoblasts that produce root dentin (red arrows). [Original histological image of immature tooth from Macacca irus monkey, courtesy of Dr. C. Torneck; original image published in Principles and Practice of Endodontics, 3rd ed., chapter 2, page 6, Fig. 2--2; permission of image reuse obtained from Elsevier Ltd.]
The discovering of stem cells in apical papilla may also explain a clinical phenomenon that was presented in a number of recent clinical case reports showing that apexogenesis can occur in infected immature permanent teeth with periradicular periodontitis or abscess (22–24). It is likely that SCAP residing in the apical papilla survived the infection due to their proximity to the periapical tissues. Therefore, after endodontic disinfection, under the influence of the survived HERS, these cells give rise to primary odontoblasts to complete the root formation.
In view of our present studies along with what we recently reported, we conclude that apical papilla harbors multipotent MSCs that express various MSC markers. They are capable of forming odontoblast-like cells and produce dentin in vivo and are likely to be the cell source of primary odontoblasts for the root dentin formation. Further research is needed to elucidate the relationship between cells in apical papilla and those in the apical cell rich zone between pulp and apical papilla, and their potential roles in the odontoblast differentiation.
Acknowledgments
This study was supported in part by the University of Southern California School of Dentistry, grants from the National Natural Science Foundation of China #30428009 (S.W. and S.S.) and National Institutes of Health RO1 DE17449 (S.S.), by NIAMS/NIH Intramural Research Program (R.S.T); and by an Endodontic Research Grant from American Association of Endodontists Foundation (G.T.-J.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Souza R. Development of the pulpodentin complex. In: Goodis KMHaHE., editor. Seltzer and Bender’s Dental Pulp. Quintessence Publishing Co, Inc; Carol Stream, IL: 2002. [Google Scholar]
- 5.Sonoyama W, Seo BM, Yamaza T, Shi S. Human Hertwig’s Epithelial Root Sheath Cells Play Crucial Roles in Cementum Formation. J Dent Res. 2007;86:594–9. doi: 10.1177/154405910708600703. [DOI] [PubMed] [Google Scholar]
- 6.Linde A, Goldberg M. Dentinogenesis. Crit Rev Oral Biol Med. 1993;4:679–728. doi: 10.1177/10454411930040050301. [DOI] [PubMed] [Google Scholar]
- 7.Ruch JV, Lesot H, Begue-Kirn C. Odontoblast differentiation. Int J Dev Biol. 1995;39:51–68. [PubMed] [Google Scholar]
- 8.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 9.Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245–56. doi: 10.1385/MB:20:3:245. [DOI] [PubMed] [Google Scholar]
- 10.Huang GT, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324:225–36. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. Faseb J. 2004;18:980–2. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 13.Chiego DJ., Jr An ultrastructural and autoradiographic analysis of primary and replacement odontoblasts following cavity preparation and wound healing in the rat molar. Proc Finn Dent Soc. 1992;88(Suppl 1):243–56. [PubMed] [Google Scholar]
- 14.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 15.Smith AJ, Cassidy N, Perry H, Begue-Kirn C, Ruch JV, Lesot H. Reactionary dentinogenesis. Int J Dev Biol. 1995;39:273–80. [PubMed] [Google Scholar]
- 16.Tziafas D, Alvanou A, Panagiotakopoulos N, et al. Induction of odontoblast-like cell differentiation in dog dental pulps after in vivo implantation of dentine matrix components. Arch Oral Biol. 1995;40:883–93. doi: 10.1016/0003-9969(95)00069-2. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald M. Cellular mechanics of dentinal bridge repair using 3H-thymidine. J Dent Res. 1979;58:2198–206. doi: 10.1177/002203457905800411011. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald M, Chiego DJ, Jr, Heys DR. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 1990;35:707–15. doi: 10.1016/0003-9969(90)90093-p. [DOI] [PubMed] [Google Scholar]
- 19.Tecles O, Laurent P, Zygouritsas S, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005;50:103–8. doi: 10.1016/j.archoralbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 21.Song L, Webb NE, Song Y, Tuan RS. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24:1707–18. doi: 10.1634/stemcells.2005-0604. [DOI] [PubMed] [Google Scholar]
- 22.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–7. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 23.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Chueh LH, Huang GT. Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod. 2006;32:1205–13. doi: 10.1016/j.joen.2006.07.010. [DOI] [PubMed] [Google Scholar]