Abstract
Perinatal HIV infection is characterized by a sustained high-level viremia and a high risk of rapid progression to AIDS, indicating a failure of immunologic containment of the virus. We hypothesized that age-related differences in the specificity or function of HIV-specific T cells may influence HIV RNA levels and clinical outcome following perinatal infection. Here we define the HIV epitopes targeted by 76 pediatric subjects (47 HIV-infected and 29 HIV-exposed but uninfected), and assessed the ability of HIV-specific CD8 and CD4 T cells to degranulate and produce IFNγ, TNFα, and IL-2. No responses were detected among HIV-uninfected infants, while responses among infected subjects increased in magnitude and breadth with age. Gag-specific responses were uncommon during early infancy, and their frequency was significantly lower among children younger than 24 months old (p=0.014). Importantly, Gag responders exhibited significantly lower HIV RNA levels than nonresponders (logVL 5.8 vs. 5.0; p=0.005). Both the total and Gag-specific T cell frequency correlated inversely with viral load after correction for age, whereas no relationship with targeting of other viral proteins was observed. Functional assessment of HIV-specific T cells by multiparameter flow cytometry revealed that polyfunctional CD8 cells were less prevalent in children before 24 months of age, and that HIV-specific CD4 cell responses were of universally low frequency among antiretroviral-naïve children and absent in young infants. These cross-sectional data suggest that qualitative differences in the CD8 response, combined with a deficiency of HIV-specific CD4 cells, may contribute to the inability of young infants to limit replication of HIV.
Keywords: human, AIDS, T cells
INTRODUCTION
Compared to HIV-infected adults, infants with perinatally acquired HIV infection maintain much higher levels of plasma viremia during the initial years of infection (1, 2) and are at substantially higher risk for rapid progression to AIDS and death(3). These age-associated disparities in HIV viral kinetics and clinical outcome are likely attributable to developmental differences in the antiviral immune response of infants. However, the exact nature of these differences and their biological mechanisms remain poorly understood(4). Although T cell responses to HIV can be primed even during fetal life, as demonstrated by the presence of HIV-specific T cells in cord blood(5), early studies suggested that these responses are infrequent and remain narrowly directed against relatively few epitopes during infancy (5-8). However, recent studies using more sensitive assays have established that HIV-specific T cell responses are present in the majority of infants by one month of age (9, 10), and that older children exhibit HIV-1-specific T cell frequencies comparable to those of chronically HIV-1 infected adults (11).
Yet major gaps in our knowledge of the infant T cell response to HIV remain. The majority of pediatric studies performed to date have assessed responses to a limited panel of optimal epitopes (9, 12-14) or to vaccinia constructs expressing whole HIV-1 structural genes (5, 6, 15), and were therefore unable to evaluate the full breadth and epitope specificity of the response. Moreover, assessment of CD8 T cell effector functions beyond IFNγ production have been very limited, and even fewer data exist regarding the specificity and function of HIV-specific CD4 T cells in infants. The most comprehensive assessment of infant HIV-specific T cell responses performed to date suggested that the HIV proteins preferentially targeted by the T cell response may differ between early infancy and later childhood(10), but the impact of this difference upon containment of viral replication was not addressed. Such differences in T cell targeting during infancy could be of great consequence, as it has recently been demonstrated that CD8 T cells targeting different viral proteins have a divergent influence upon viral containment(16-18). Moreover, the “quality” of CD8 T cells, as assessed by their ability to exhibit multiple simultaneous effector functions, has been reported to correlate with control of viral replication(19).
We hypothesized that differences in the targets and/or functionality of HIV-specific T cells contribute to the efficacy of the antiviral immune response, and that age-related differences in these parameters may account for the inability of children to establish early containment of viremia. We examined CD8 and CD4 T cell responses to the full HIV-1 proteome in a cohort of infants and children born to HIV-positive mothers and assessed multiple effector functions by multiparameter flow cytometry. Our data indicate that HIV-specific T cell responses are present in the majority of HIV-infected infants, but that several qualitative features distinguish the responses observed in younger infants. Notably, Gag-specific T cell responses were less commonly detected in infants than in children older than 12 months of age, and targeting of Gag was associated with significantly lower plasma HIV-1 RNA levels. CD8 T cells exhibiting multiple effector functions (IFNγ, TNFα, and degranulation) were also detected less frequently in younger infants, and HIV-specific CD4 T cell responses were of very low magnitude in nearly all pediatric subjects, and absent in the youngest infants. These qualitative differences in the CD8 response, coupled with a lack of CD4 T cell help, may be important contributors to the generally poor ability of young infants to restrict viral replication.
MATERIALS AND METHODS
Study cohort
This study was performed in collaboration with the Kingston Perinatal AIDS Program (KPAIDS), a large-scale initiative for the prevention and treatment of perinatal HIV-1 infection in Jamaica(20). The KPAIDS Program provides antiretroviral therapy for the prevention of mother-to-child transmission of HIV-1, counsels HIV-positive mothers against breastfeeding, supplies free infant formula, and provides care and antiretroviral therapy for HIV-infected children in accordance with WHO guidelines. The study was approved by the Institutional Review Board of the Massachusetts General Hospital and the Ethics Committee of the University Hospital of the West Indies in Kingston, Jamaica. A parent or legal guardian of each subject provided written informed consent prior to participation.
Seventy-six infants and children (age 5 weeks to 10 years) born to HIV-positive mothers were enrolled. Because molecular diagnostic HIV testing of infants had not been instituted in Jamaica at the time this collaboration was initiated, some infants <18 months of age were of indeterminate HIV-1 infection status upon enrollment. Of these, 29 infants (age 5 weeks to 17 months) were later determined by PCR testing to be HIV-negative, and therefore comprise an “exposed/uninfected” cohort. Among the 47 HIV-infected subjects, 25 were antiretroviral therapy naïve at the time of enrollment. Plasma HIV-1 RNA levels were measured using the Roche Amplicor Monitor assay, with a detection range of 400 − 750,000 copies/ml. Values exceeding the upper limit of quantification were re-analyzed following dilution whenever sufficient plasma was available (3 subjects without sufficient plasma for repeat testing were assigned a viral load value of 750,001 copies/ml). Clinical and demographic information including age, sex, viral load, CD4 count and percentage, antiretroviral treatment status, and duration of therapy is provided in Table 1.
Table 1.
Clinical and demographic characteristics of the study cohort1
| Age (months) |
Sex (F) | Viral Load | CD42 | CD4%2 | Treatment Duration (months) |
|
|---|---|---|---|---|---|---|
|
Uninfected (n=29) |
7.6 (1.2−16.8) |
41% | <400 (all) | N/A | N/A | N/A |
|
HIV+ ARV3-naïve (n=25) |
13 (4−115) |
60% | 208,000 (2670−893,000) |
872 (42−2240) |
41 (7−52) |
N/A |
|
HIV+ on ARV3 (n=22) |
34.5 (13−96) |
59% | 29,050 (<50−750,001) |
1255 (226−2112) |
41 (16−54) |
8 (0.5−44) |
Sample processing and isolation of PBMCs
Blood samples were acquired from each child and from his or her biological parent whenever possible (69 mothers and 3 fathers). Blood from each pediatric subject was collected in both acid citrate dextrose tubes (for Elispot assays) and sodium heparin tubes (to permit whole blood stimulation for flow cytometry assays). Samples were shipped overnight to Boston and processed within 24 hours of collection. Upon arrival, peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Histopaque density gradient centrifugation (Sigma, St Louis, Mo).
Synthetic HIV-1 peptides
411 overlapping peptides (18mers with a 10-amino acid overlap) spanning the entire HIV-1 clade B consensus sequence were synthesized for use in screening assays on an automated peptide synthesizer by using Fmoc (9-fluorenylmethoxy carbonyl) chemistry. Previously described CD8-restricted HIV-1 optimal peptides (Los Alamos HIV Sequence Database www.hiv.lanl.gov) were synthesized by identical methods.
IFN-γ ELISPOT assay
96-well polyvinylidene difluoride-backed plates (Millipore, Bedford, Mass.) were coated overnight with 2 μg/ml of anti-IFNγ MAb (Mabtech, Stockholm, Sweden). Fresh PBMC were added into these precoated plates at 50,000 − 100,000 cells/well in 100 μl of R10 medium. Peptides were added at a final concentration of 20 μg/ml, and plates were incubated for 16 hours at 37°C and 5% CO2, then processed by standard methods (21). Three negative control wells containing cells and media alone and a positive control well containing phytohemagglutinin were included in each assay. Individual IFNγ-secreting cells were counted using an AID enzyme-linked immunospot assay (Elispot) reader system (Cell Technology, Inc.). Results were calculated as the number of spot-forming cells per million input cells (SFC/million) after subtraction of the background response (mean SFC of all no-antigen wells; in all cases, ≤30 SFC/million). A response was considered positive if it was both ≥50 SFC/million PBMC and ≥3 times the average of the negative control wells.
Full genome ELISPOT screening for HIV-1-specific responses
Subjects were screened by ELISPOT for responses to all translated HIV-1 proteins using the overlapping peptide set described above. To minimize the cell number required for this comprehensive screening, we used a matrix approach that has been previously validated in both pediatric and adult cohorts studied in our laboratory (11, 22). The 411 peptides were pooled into five two-dimensional matrices such that each peptide was present in two different pools, and each pool contained between 7 and 11 peptides. The resulting 92 pools were plated at a final concentration of 20 μg/ml per peptide with 50,000 − 100,000 PBMC/well on a single 96-well plate; thus, only 5 to 10 × 106 cells were required to comprehensively screen all HIV-1 proteins. Each peptide within the “positive” pools was then tested individually in a separate ELISPOT assay the following day by using cells that had been incubated overnight at 106 PBMC/ml of R10.
Assessment of HIV-1-specific degranulation and cytokine production
Whole blood (300 μl, obtained in sodium heparin tubes) was aliquotted with the costimulatory antibodies anti-CD28 and CD49d (1μg/ml each, BD Biosciences) and FITC-conjugated anti-CD107a antibody (BD Biosciences) into 15ml polypropylene tubes. Overlapping HIV-1 peptides spanning the full viral genome were added at a final concentration of 2 μg/ml, in five separate pools: Gag (66 peptides), Nef (27 peptides), Pol (132 peptides), Env (113 peptides), and Rev, Tat, Vpr, Vif, and Vpu (72 peptides). Each assay included a positive control tube containing phytohemagglutinin (PHA; 1 μg/ml) and a negative control tube with no peptide. Samples were incubated at a 5 degree slant at 37°C for a total of six hours. The protein transport inhibitors brefeldin A (1 μg; GolgiPlug, BD Biosciences) and monensin (0.6 μg; GolgiStop, BD Biosciences) were added after the first hour. Following this stimulation step, 40 μl of 50mM EDTA solution was added to arrest cell activation and to remove adherent cells (15 minutes at room temperature). Simultaneous lysis of RBCs and fixation of nucleated cells was achieved with 3ml of 2X FACS Lysing solution (BD Biosciences). Cells were washed in PBS containing 1% heat inactivated fetal bovine serum (Sigma-Aldrich Corp.) and then permeabilized using 500 μl of 1X FACS Permeabilizing Solution 2 (BD Biosciences) for a 10-minute incubation. Finally, cells were stained with a panel of fluorescently labeled antibodies to CD3 (Pacific Blue), CD8 (APC-Cy7), CD4 (allophycocyanin; APC), IFNγ (PE-Cy7), TNFα (Alexa Fluor 700), and IL-2 phycoerythrin (PE) (all from BD Biosciences) for 30 minutes at room temperature in the dark.
Flow cytometry acquisition and analysis
Samples were acquired within 24 hours of staining on an LSR II flow cytometer using FACSDiva acquisition software (Becton Dickinson Immunocytometry Systems, v4.1.2), with bead compensation (CompBeads, Becton Dickinson). Only samples from which at least 20,000 CD4+ and 20,000 CD8+ T lymphocytes were acquired were included in the analysis (median 50,000 CD4+ events and 54,345 CD8+ events). All data were analyzed using FlowJo software (Tree Star, San Carlos, CA, USA). A lymphocyte gate was set based on forward- and side-scatter, and CD3+ events within this gate were analyzed. CD8+ T cells were identified by first gating on CD3+CD4− cells, and then gating on cells positive for CD8. Similarly, CD4+ T cells were identified by first gating on CD3+CD8− cells, and then gating on cells positive for CD4. The frequency of CD4+ and CD8+ T cells producing each cytokine (or degranulating, in the case of CD107a) was determined by subtracting the percentage of cytokine-positive cells in the unstimulated CD28/49d control tube from that of each peptide-stimulated tube. For this single parameter analysis, a response was considered positive if it was ≥3 times the negative control, and if it exceeded 0.01%, and if there were at least at 10 cytokine-positive events (corresponding to 0.02% if 50,000 CD4+ or CD8+ T cells were acquired). The highest background levels observed for any subject in the CD8+ subset were 0.03%, 0.098%, 0.028% and 0.18% for IFNγ, TNFα, IL-2 and CD017a respectively. The maximum background levels observed in the CD4+ subset were 0.012%, 0.067% and 0.041% for IFNγ, TNFα and IL-2 respectively. “Double-positive” (IFNγ+TNFα+) and “triple-positive” (IFNγ+TNFα+CD107a+) cells were identified by sequential gating on these parameters; a response was considered positive if it exceeded 0.005% and if it met criteria for positivity for each of the individual parameters.
Statistical analysis
Data analysis was performed using SAS (version 9.1.3, SAS Institute, Cary, NC). All statistical tests were two-tailed, with p<0.05 considered significant. Two-group comparisons of viral load and of HIV-specific T cell magnitude were performed by Wilcoxon rank-sum test, with Bonferroni correction for multiple comparisons where indicated. Categorical variables were compared using Fisher's Exact Test. The Chi-square test for trend was used to compare the frequency of responses to each viral protein across age strata. The correlation of age and viral load with immune response parameters (total magnitude of HIV-specific T cells response and the number of peptides targeted) was assessed using the Spearman rank correlation coefficient. Multiple regression controlling for log-age was used to analyze the correlation between log-viral load and the breadth (number of peptides targeted) and magnitude (total SFC/million) of immune response and their interaction. The combined effects of immune response were evaluated as a 3-degree of freedom contrast. Models with rank- and log-transformation (specifically, log(# peptides + 1), log(SFC/million + 1), and a separate indicator for SFC/million = 0 for all peptides) of immune response parameters were explored to reduce the influence of highly leveraged points, but our inference did not change. The influence of response to specific viral proteins was evaluated by including indicator variables for targeting of each viral protein in the model described above. A final model was selected by stepwise regression, retaining log-age and total immune response parameters at all steps. The relationship between viral load and magnitude of Gag vs. non-Gag targeting T cells was tested in a multiple regression controlling for log-age and total breadth of immune response. Pearson correlation coefficients are provided for all multiple regresssion analyses.
RESULTS
Absence of HIV-specific T cell responses among uninfected infants exposed to HIV in utero
Several studies have described HIV-1 specific CTL responses among highly exposed but persistently seronegative adults (23-25) and among infants exposed to HIV in utero (26-29). However, other investigators have failed to detect such responses among uninfected infants (5, 8, 30, 31). We recruited 29 HIV-exposed but uninfected infants and young children (age 5 weeks to 17 months) from a non-breastfeeding population and comprehensively screened these subjects for T-cell responses to HIV. All investigators were blinded to the infection status of these subjects at the time the assays were performed and analyzed, as no diagnostic testing results were available prior to enrollment and processing of samples. All subjects were subsequently documented to be HIV-uninfected based on negative HIV DNA and RNA PCR assays. Comprehensive screening by Elispot for responses to a panel of overlapping peptides spanning the entire translated HIV proteome demonstrated that there was no detectable virus-specific IFNγ response in any infant (Fig.1). Six of these subjects were further screened for IFNγ Elispot responses to optimal HIV-1 epitopes corresponding to their class I HLA alleles (7−46 peptides tested; median 24), which is the most sensitive method for detection of antigen-specific T cell responses. Again, no response to any optimal peptide was observed. To investigate the possibility that virus-specific T cells were present but merely lacked the ability to produce IFNγ, 17 of these HIV-negative subjects were assessed by multiparameter intracellular cytokine staining for IFNγ, TNFα, and IL-2 production and for degranulation (assessed by CD107a staining) following stimulation with overlapping peptide pools spanning all HIV proteins. Again, none of the subjects exhibited a response that significantly exceeded background. These data support multiple prior studies suggesting that HIV-negative infants exposed to HIV in utero do not mount durable T cell responses to the virus(5, 8, 30, 31).
FIGURE 1. Absence of HIV-1 specific T cell responses among uninfected infants exposed to HIV-1 in utero.
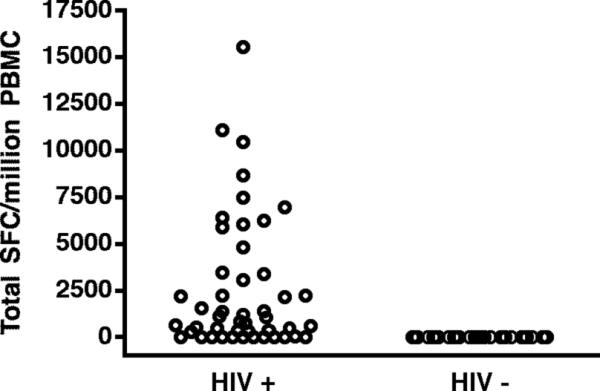
Twenty-nine HIV-exposed but uninfected infants and young children were screened by IFNγ ELISPOT for responses to overlapping peptides spanning all HIV-1 proteins. None had a detectable response, in contrast to HIV-infected subjects, 77% of whom had a detectable response (36 of 47).
The breadth and magnitude of the HIV-specific T cell response increases with age
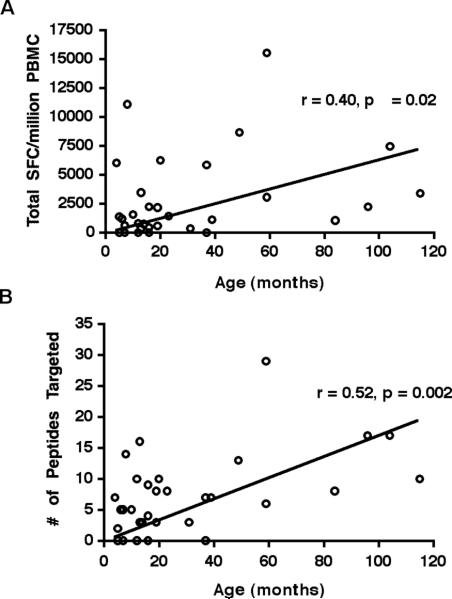
In order to quantify the breadth and magnitude of the HIV-specific T cell response in young children and determine how these parameters change with age, we comprehensively screened 47 HIV-1-infected infants and children for responses to a set of overlapping peptides spanning all HIV proteins by IFNγ Elispot. Thirty-six subjects (77%) exhibited an IFN-γ response to HIV, consistent with published pediatric studies (9, 10). Among those subjects in whom an HIV-specific response was detected, the total magnitude of response (determined by summation of all positive responses to individual screening peptides) varied widely from 50 to 15,540 SFC/million PBMC, with a median of 1855 SFC/million (Fig.1). The number of peptides targeted also varied widely, from 1 to 29 (median 8; data not shown). Infants who lacked any IFNγ response to HIV were generally <1 year of age (8 of 11 subjects) and exhibited a very high viral load (median 750,000 copies/ml). We analyzed the impact of age on the breadth and magnitude of the HIV-specific T cell response, limiting our analysis to subjects who were antiretroviral naïve (n=25) or who maintained a high level of HIV-1 viremia (≥ 50,000 RNA copies/ml) despite therapy (n=9), as drug-mediated viral suppression has been shown to diminish HIV-specific CTL responses (11, 32, 33). Among this subset of subjects, there was a positive correlation between age and both the total magnitude of HIV-specific T cell response (Spearman's r= 0.40, p= 0.02; Fig.2A) and the number of peptides targeted (Spearman's r= 0.52, p= 0.002; Fig. 2B). When the analysis was restricted to subjects who had never received antiretroviral therapy (n=25), the correlation between age and both the magnitude of response (Spearman's r= 0.40, p= 0.05) and the breadth of response (Spearman's r= 0.53, p= 0.007) was essentially unchanged. While these data were obtained cross-sectionally, they suggest that the antiviral T cell response increases throughout early childhood, recruiting more T cells and targeting more viral epitopes.
FIGURE 2. The magnitude and breadth of HIV-1-specific T cells increase with age.
IFNγ ELISPOT assays from 25 antiretroviral-naïve subjects and 9 subjects with VL≥ 50,000 copies/ml despite therapies were analyzed (n=34). There was a positive correlation between age and (A) the total magnitude (Spearman's r=0.40, p=0.02) and (B) the breadth (Spearman's r=0.52, p=0.002) of the HIV-1-specific T cell response.
Age-related differences in the HIV proteins preferentially targeted by virus-specific T cells
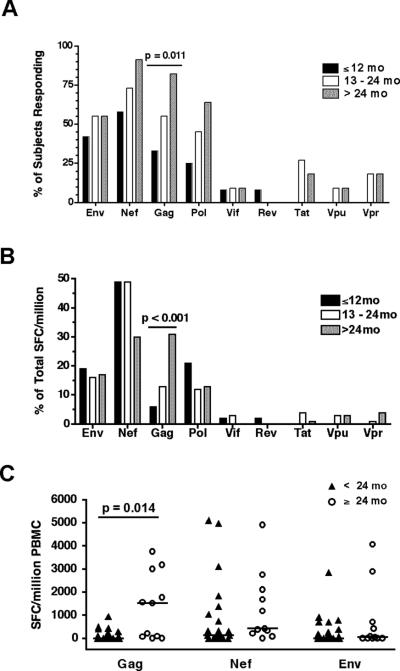
Recent population-based studies suggest that the efficacy of a CD8 T cell response may depend in part upon the HIV protein from which the targeted epitope is derived – in particular, CTL populations targeting Gag epitopes appear to be associated with superior viral containment (16-18). We therefore examined the distribution of T cell responses among all HIV proteins, and determined the relationship between age and the prevalence and magnitude of protein-specific responses. Thirty-four subjects who were antiretroviral-naïve or highly viremic despite therapy (≥50,000 HIV RNA copies/ml) were screened for responses to all HIV proteins by IFNγ Elispot. Each HIV protein was targeted by at least one subject. Overall, the most frequently recognized protein was Nef (recognized by 74% of subjects), followed by Gag (56%) and Env (50%). To determine whether age influenced the distribution of virus-specific responses, subjects were stratified into 3 age groups: ≤12 months (n=12), 13−24 months (n=12) and >24 months (n=11), and the prevalence of a response to each HIV-1 protein was determined separately for each age stratum (Fig. 3A). Responses to the HIV-1-Gag protein were particularly infrequent during early infancy (present in only 1 of the 6 infants younger than 7 months of age), but their frequency increased markedly with age from 33% to 58% to 82% across the three age strata (Chi-square test for trend, p=0.011; Fig 3A). No significant age-related increase in recognition was observed for the other viral proteins. When these data were analyzed as the relative proportion of the total HIV-specific response targeting each individual viral protein, an age-related increase in the contribution of Gag-specific responses was again noted (Chi-square test for trend, p<0.001; Fig 3B).
FIGURE 3. Proteins preferentially targeted by HIV-1-specific T cells differ by age.
(A) Prevalence of IFNγ ELISPOT responses to each of the 9 HIV-1 proteins among 34 antiretroviral-naïve or highly viremic subjects, stratified by age. Recognition of HIV-1-Gag increased with age (Chi-square test for trend, p=0.011). No statistically significant trend was observed for the other viral proteins. (B) The same data are displayed as the relative proportion of the total HIV-specific response targeting each individual viral protein (calculated as the protein-specific SFC/million divided by the total; Chi-square test for trend, p<0.001). (C) The total magnitude of the Gag-specific response in subjects older than 24 months was significantly higher than in those <24 months old (Wilcoxon rank-sum Test, p=0.014). Bars indicate median values.
We next compared the total magnitude of the T cell response to Gag, Nef, and Env between older and younger children (Fig 3C). Here again, the total magnitude of Gag-specific responses among older children (>24 months) significantly exceeded that observed among younger children (p=0.014; Fig. 3C). The magnitude of Nef-specific and Env-specific T cell responses did not differ between the older and younger children. When analysis was restricted to younger children with a detectable response to each protein, there was trend toward higher frequencies of Gag-specific cells among the older age group (median 351 vs. 1560 SFC/million) although this difference did not achieve statistical significance in the small sample of 19 responders (p=0.13). Finally, subjects were categorized according to their “dominant” response, defined as the HIV protein eliciting the highest frequency of responding T cells. A dominant response to Nef or Env was observed more frequently among children <24 months of age than among the older children (83% vs. 40%; p=0.07), 60% of whom mounted a dominant response to Gag. Together, these cross-sectional data suggest that the ability to mount responses to various viral proteins may differ by age, with preferential targeting of Nef and Env during infancy and a shift to targeting of Gag only during later childhood. Alternatively, there may be a survival bias favoring infants with Gag-specific T cell responses.
Total HIV- and Gag-specific T cell frequencies correlate inversely with viral load
The relationship between plasma viral load and the frequency of virus-specific T cells in HIV-infected individuals is complex. Although several studies have failed to detect a correlation between viral load and the total HIV-specific CD8 T cell response (11, 22, 34), some investigators have reported a negative correlation of viral load with responses to particular viral epitopes or proteins (16, 18, 35, 36), while others have reported a positive correlation (16, 34, 37). These conflicting findings have led to speculation that HIV-specific T cells of differing protein-specificity may vary in their antiviral efficacy. We performed a cross-sectional analysis of the relationship between viral load and virus-specific immune responses in antiretroviral-naïve pediatric subjects (n=25), looking first at the total HIV-1-specific T cell response and then at responses to individual viral proteins.
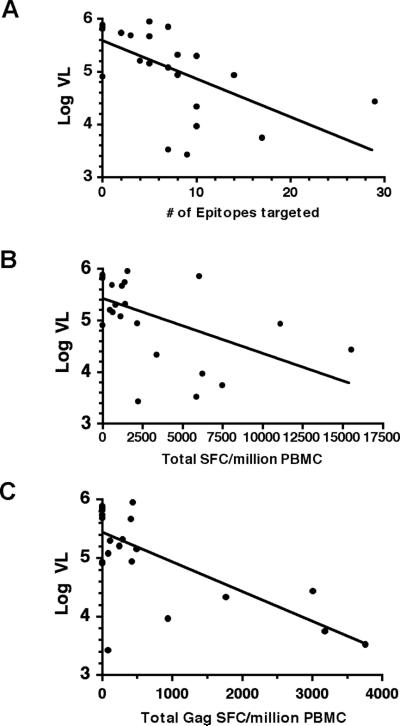
We found a highly significant inverse correlation between HIV viral load and the total breadth of the HIV-specific T cell response (Spearman's r= −0.71, p<0.001; Fig. 4A), as well as the total magnitude of this response (Spearman's r= −0.63, p<0.001; Fig. 4B). Because HIV RNA levels are known to decline over time in young children, we repeated this analysis controlling for age using multiple regression. The breadth and magnitude of the CD8+ T cell immune response remained inversely correlated with viral load after adjustment for age (p = 0.027).
FIGURE 4. Total and Gag-specific T cell responses correlate inversely with viral load.
Cross-sectional analysis of HIV-1-specific T cell responses among 25 antiretroviral-naïve subjects demonstrated a highly significant inverse correlation between HIV viral load and (A) the total breadth of the HIV-specific T cell response (Spearman's r=−0.71, p<0.001), as well as (B) the total magnitude of this response (Spearman's r=−0.63, p<0.001). The breadth and magnitude of the HIV-specific T cell immune response remained inversely correlated with viral load after controlling for age in a multivariate regression model (p = 0.027 for the aggregate effect of breadth and magnitude). (C) The total magnitude of the Gag-specific T cell response correlated inversely with viral load after adjusting for known covariates (p=0.037).
We next assessed whether targeting of particular HIV-1 proteins was associated with control of viremia by comparing plasma HIV-1 RNA levels among responders and nonresponders to each viral protein. Children who mounted a T cell response to Gag had significantly lower viral loads than to those who did not (p= 0.005; Fig. 5). No significant difference in viral load was observed between responders and non-responders to the other HIV-1 proteins. The association of viral load with a T cell response to Gag remained significant after Bonferroni correction for multiple comparisons (p=0.04). In light of this finding, we extended our multiple regression model to include indicator variables signifying the presence or absence of a response to each individual HIV-1 protein, in order to assess whether targeting of particular proteins was associated with differences in HIV-1 viral load. Stepwise regression yielded a model which indicates that targeting of Gag was associated with lower HIV-1 viral load (p= 0.009) whereas targeting of Nef was associated with higher viral load (p=0.02). Finally, we stratified all T cell responses into Gag and non-Gag responses and analyzed the relationship of the summed magnitude of these responses to viral load after adjustment for log-age and total breadth. This analysis revealed that the total magnitude of Gag-specific responses correlated inversely with viral load (p=0.037; Fig. 4C), whereas no significant correlation was observed between viral load and the total magnitude of non-Gag responses (data not shown). Together these findings provide additional support for an important role for Gag-specific T cell responses in the control of HIV-1 viremia. Alternatively, the ability to mount Gag-specific T cell responses may be impaired in the setting of high-level HIV-1 viremia.
FIGURE 5. Responders to HIV-1 Gag exhibit lower viral loads.
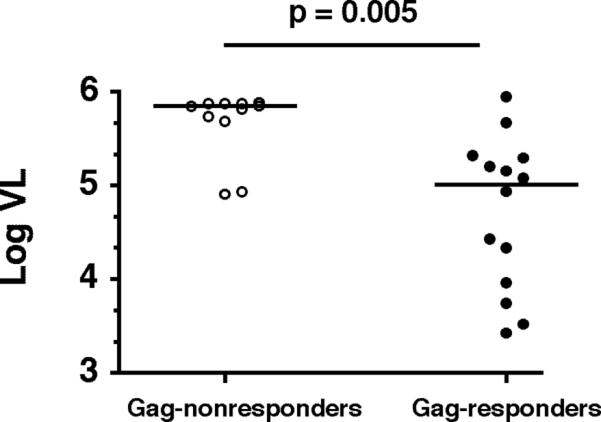
Subjects with Gag-specific T cell responses (n=14, median VL=703,000 copies/ml) had significantly lower viral loads than Gag-nonresponders (n=11, median VL=102,090 copies/ml; p=0.005). Bars indicate median values.
Functionality of CD8+ T cells in perinatally HIV-infected subjects
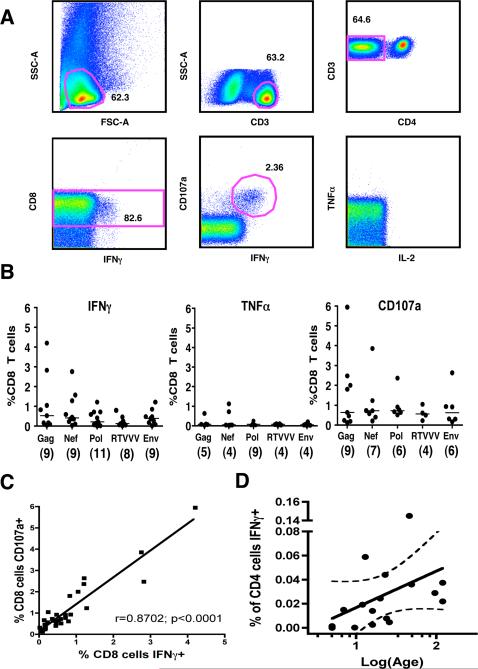
It has been reported previously that control of HIV-1 viremia in adults is associated with the presence of “polyfunctional” HIV-1-specific CD8+ T cells – that is, cells which exhibit the capacity to degranulate and to produce multiple cytokines simultaneously (19). However, no prior studies have assessed simultaneous production of multiple cytokines by virus-specific CD8 T cells in the pediatric population, and while infants have been shown to exhibit HIV-1-specific T cell responses even in cord blood (5, 8-10), it is not known whether such responses are functionally mature. We examined production of IFNγ, TNFα, and IL-2, and degranulation (assessed by staining for CD107a) following stimulation with 5 pools of overlapping HIV peptides using 9-parameter flow-cytometry (Fig. 6a). All 15 subjects were antiretroviral therapy-naïve, and they ranged in age from 4 to 120 months (median 23 months). HIV-specific CD8 T cell responses were detected in 93% of the subjects assessed (14 of 15), with individual subjects targeting a median of 3 of the 5 peptide pools. Production of IFNγ was the most commonly observed effector function, and it was very rare for a subject to exhibit other functions in the absence of IFNγ production. The highest frequencies of IFNγ-producing CD8 T cells were elicited by the Gag pool (Fig. 6b; 0.090 − 4.206%, median 0.521%, n=9), followed by Nef (0.103 − 2.756%, median 0.403%, n=9), Env (0.073 − 1.215%, median 0.388%, n=9), Pol (0.012 − 1.206%, median 0.214%, n=11), and Rev/Tat/Vif/Vpr/Vpu (0.014 − 0.791%, median 0.122%, n=8). Similar results were obtained for CD107a staining, as there was a very high degree of correlation between IFNγ production and degranulation (Fig. 6c; Spearman's r=0.87; p<0.0001). HIV-specific production of TNFα was observed in a subset of patients, generally at lower frequencies than IFNγ (Fig. 6b; median response ranging from 0.047% for Nef to 0.084% for Rev/Tat/Vif/Vpr/Vpu). IL-2 production was quite rare, observed in only 3 subjects, and at low frequencies (0.0102 − 0.0612%).
FIGURE 6. Multiple effector functions of HIV-specific T cells assessed by 9-parameter flow cytometry.
(A) Representative scheme for identification of Env-specific CD8 T cell responses is shown for a 12-month old antiretroviral-naïve subject. Gates for each of the 3 functions were established based on the negative control tube (i.e. cytokine vs. CD8 plot) for each individual subject. (B) The frequency of CD8 T cells producing IFNγ, producing TNFα, or degranulating in response to pooled overlapping peptides spanning Gag, Nef, Pol, RTVVV (Rev, Tat, Vif, Vpr, and Vpu), and Env. The number of responding subjects (among 15 antiretroviral-naïve subjects) is indicated in parentheses for each pool. Bars indicate median values. (C) A strong linear correlation was observed between CD8 T cell degranulation and production of IFNγ (Spearman's r=0.87; p<0.0001). (D) The frequency of IFNγ-producing CD4 cells specific for HIVGag increases linearly with log-age (Spearman's r=0.586; p=0.014; dotted line indicates 95% confidence band).
Polyfunctional CD8 T cells were present in the majority of pediatric subjects. HIV-specific CD8+ T cells producing both IFNγ and TNFα were observed in 64% of subjects overall. Triple-positive (IFNγ+TNFα+CD107a+) cells were present in 50% of subjects, and were observed in response to all 5 peptide pools. Quadruple-positive cells (IFNγ+TNFα+CD107a+IL2+) were not detected in any subject, given the rarity of IL-2-producing CD8 T cells. Age appeared to influence the ability to mount a polyfunctional response, as CD8+ T cells exhibiting three effector functions (IFNγ+ TNFα+ CD107a+) were detected less frequently in children <24 months of age (n=8) compared to older children, although this difference did not achieve statistical significance (p=0.11, Chi square test). Of note, such responses were not detected in any infant prior to 12 months of age, although degranulation and production of TNFα and IFNγ were observed as individual functions in much younger infants. These cross-sectional data suggest that, while young infants are capable of multiple CD8 effector functions, their capacity to mount a polyfunctional CD8 T cell response may be impaired. Alternatively, our findings are consistent with a survival bias favoring infants with polyfunctional HIV-specific CD8 cells.
HIV-1-specific CD4 T cell responses are of low frequency during infancy
Functionally preserved CD4+ T cells are important for the maintenance of effective virus-specific CD8+ T cells (38-40). However, prior studies suggest that infants and young children may have an age-related impairment in their ability to mount CD4+ T cell responses to HIV and other viruses such as CMV(10, 41-43). We assessed the frequency, functionality, and protein-specificity of HIV-specific CD4 T cells in 15 antiretroviral naïve children, all of whom had detectable HIV viremia, by using multicolor flow-cytometry to detect production of IFNγ, TNFα, and IL-2 following stimulation with 5 overlapping peptide pools spanning the entire HIV genome. Consistent with prior studies of HIV-infected adults, our analysis revealed that the majority of CD4 T cell responses targeted the HIV-Gag protein, with 8 of 15 subjects demonstrating an IFNγ response. However, most of these responses were of very low magnitude (0.0141 − 0.145% of CD4 T cells, median 0.033%) compared to Gag-specific CD4 T cell frequencies previously reported in untreated HIV+ adults cohorts(44, 45). Overall there was a statistically significant increase in the frequency of Gag-specific IFNγ-producing CD4 T cells with age (Fig. 6d; Spearman's r=0.586; p=0.014), with the 4 youngest infants failing to mount any significant IFNγ response to Gag or any other viral protein. IFNγ responses were also observed to the Env (3 subjects), Pol (2), Nef (2) and Rev/Tat/Vif/Vpr/Vpu pools (1), but these were of similarly low magnitude. Among the 8 children with Gag-specific IFNγ responses, simultaneous production of TNFα was detected in 5 (62.5%), and in each case double-positive IFNg+TNFα+ cells accounted for the majority of IFNγ-producing cells. No subject responded to Gag by producing TNFα alone. Production of TNFα was also observed in response to each of the other viral protein pools, at frequencies similar to that observed for IFNγ (0.0198% − 0.152%). Significant levels of IL-2 production by CD4 T cells were observed in only 2 subjects. Interestingly, both of these antiretroviral-naïve subjects demonstrated exceptionally good control of HIV-1 viremia compared to age-based norms; subject 00244C1, whose viral load was 2670 copies/ml at 16 months of age, demonstrated an IL-2 response to Pol, while subject 04035C1, whose viral load was 7930 copies/ml at 10 years of age, demonstrated IL-2 responses to both the Gag and Rev/Tat/Vif/Vpr/Vpu pools. Each of these IL-2-producing CD4 T cell populations was triple-positive (IFNγ+ TNFα+ IL-2+). Together these data, which provide the first comprehensive assessment of the CD4 response to all HIV proteins performed in children, indicate that virus-specific CD4 T cells are of strikingly low magnitude during early infancy.
DISCUSSION
Studies performed early in the HIV/AIDS epidemic indicated that HIV-specific T cells were infrequent and narrowly-targeted in infants(46, 47), but subsequent data suggest that these quantitative deficiencies may be largely attributable to the early stage of infection, as acute HIV infection is now known to induce a relatively narrow T cell response in adults as well as children(48). Indeed our data support other recent pediatric studies demonstrating that HIV-specific CD8 T cells can be detected in most infants during the first months of life(10, 49), and further indicate that this response progressively broadens throughout childhood, with HIV-specific T cell frequencies among older children approximating those of adults(11). Thus, there do not appear to be substantial quantitative differences in the CD8 response to HIV during infancy. However, in the present study, we describe several age-related qualitative differences in the HIV-specific T cell response that may contribute to the inability of infants to restrict viral replication. These differences include a propensity of infant CD8 T cells to target variable proteins such as Nef or Env rather than Gag, a lower degree of functionality among infant CD8 T cells, and a paucity of virus-specific CD4 T cell help during early infancy.
Our results in clade B -infected infants and young children support and extend those of another recent pediatric study which described age-related differences in the distribution of responses at the viral protein level among clade C-infected African infants (10). This prior study found that Env-specific responses predominated during the first six months of life, while responses to Gag were very uncommon. We observed a similar paucity of Gag-specific T cell responses, with only 17% of infants in this age group exhibiting a Gag-specific response. However in our cohort, responses to Nef were slightly more common than to Env during early infancy, perhaps reflecting differences in class I HLA allele frequencies or HIV-1 subtypes of the populations studied. Interestingly, both Nef- and Env-specific responses have previously been associated with higher levels of HIV viremia in clade C-infected Africans(16), and a similar association of Nef responses and poor viral control was observed in our multivariate model. Both Nef and Env are characterized by a relatively high degree of sequence variability, making them potentially elusive targets in a rapidly evolving virus, in contrast to Gag which is relatively conserved. Despite this sequence heterogeneity, preferential targeting of Nef was previously observed in a cohort of acutely infected adults, whose dominant responses shifted after prolonged antigen exposure to include Gag, Pol, and Env(50). The cross-sectional nature of our study did not permit us to differentiate whether the observed age-related differences reflect a temporal shift, in which acute-phase responses to Nef or Env are lost or eclipsed by late-emerging Gag-specific responses, or instead reflect a survival bias favoring infants who mount T cell response to Gag. The prospective longitudinal studies that would be required to distinguish these two possibilities may not be ethically feasible in the HAART era, as infants are at high risk for rapid progression to AIDS without early initiation of HAART(51).
The infrequent recognition of Gag by infant T cells may have important implications for viral control. In the present study, subjects who recognized at least one Gag epitope exhibited significantly lower HIV RNA levels than those who did not, and the frequency of Gag-specific T cells correlated inversely with viral load after adjustment for age. These findings are in agreement with other recent studies indicating that Gag-specific T cell responses are associated with better control of HIV viremia in adults (16-18). The potential mechanism underlying this association remains uncertain. The gag sequence is highly conserved relative to other HIV proteins, and the virus may be less able to escape from Gag-specific T cells due to functional constraints on the Gag protein, resulting in more durable antiviral T cell responses. Indeed, the inability of HIV to tolerate sequence changes within highly conserved Gag epitopes without great cost to viral replicative fitness has been postulated as an explanation for the association of HLA-B*27 and B*57 with spontaneous viral containment and long-term nonprogressive HIV disease(52-55). An alternative explanation for the superior efficacy of Gag-specific CTL is that HIV-infected cells have been demonstrated to present Gag-derived CD8 T cell epitopes within 2 hours of infection, prior to viral integration, transcription, and Nef-mediated downregulation of HLA(56). This phenomenon appears to be unique to Gag epitopes, owing to cross-presentation of the abundant Gag protein particles present in internalized virions, and could confer an ability to lyse infected target cells before the release of progeny virions, which would be highly advantageous for viral containment.
Our data add to mounting evidence that there is a generalized impairment of CD4 responses to HIV and other viruses such as CMV during early childhood(10, 41-43, 57). Previous studies have shown that virus-specific proliferative responses to both CMV(43) and HIV(42) are largely absent during the first five years of life. Perinatally CMV-infected infants exhibit a deficiency of virus-specific IFNγ production by CD4, but not CD8, T cells when compared to adults with acute CMV infection (41, 57). The results of the present study indicate that there is a similar temporal pattern in the emergence of CD4-mediated IFNγ responses to HIV among infants. We observed a virtual absence of HIV-specific CD4 cells during the first year of life, despite high-frequency CD8 responses in many of these infants, followed by a linear increase in the frequency of Gag-specific CD4 cells during early childhood. These results are in agreement with prior studies, mostly limited to measurement of Gag-specific IFNγ production, which found HIV-specific CD4 T cells to be absent during the first year of life (10), and detectable in older children but generally at lower frequencies than those reported in adults(44, 45, 58). Although our study is more comprehensive in that it assessed production of multiple cytokines in response to all HIV proteins, responses were nonetheless absent or of extremely low magnitude in the majority of infants. A generalized deficit in T cell help during infancy could have important consequences for the ability of infants to control viral infections, as it might be expected to compromise CD8 effector function and/or memory potential, similar to the “unhelped CD8 T cell” phenotype described following vaccination of CD4-depleted mice(39, 40, 59, 60). This impairment of T cell help during early infancy may help to explain the lower frequency of polyfunctional CD8 T cells observed among younger infants in the present study. It remains to be determined whether this age-related deficiency in virus-specific CD4 responses is due to developmental differences in antigen presenting cells, costimulatory pathways, or CD4 T cells themselves, or is instead due to active suppression by CD4 T regulatory cells(61), which are particularly abundant during infancy.
Mounting empiric evidence for the superior antiviral efficacy of Gag-specific T cells, combined with theoretical considerations such as the high degree of Gag sequence conservation and the kinetic advantage of early Gag epitope presentation, make HIV-Gag a particularly attractive vaccine target. The mechanism underlying the inefficient targeting of Gag by infants is unclear, and solving this puzzle will likely require a better understanding of the general determinants of immunodominance in the setting of viral infections – which may in turn lead to approaches to manipulate the hierarchy of epitopes targeted by vaccine-induced immune responses. Our data indicate that optimal immunization of neonates may require strategies to skew the immunodominance hierarchy away from that observed in natural pediatric infection, and toward induction of Gag-specific responses.
ACKNOWLEDGMENTS
The authors would like to thank all of the study subjects and their families for participating in this study. We would also like to thank the KPAIDS clinicians including Russell Pierre, Tracey Evans-Gilbert, and Paulette Palmer for their assistance with recruiting subjects and providing expert clinical care to the study population.
REFERENCES
- 1.Mofenson LM, Korelitz J, Meyer WA, 3rd, Bethel J, Rich K, Pahwa S, Moye J, Jr., Nugent R, Read J. The relationship between serum human immunodeficiency virus type 1 (HIV- 1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis. 1997;175:1029–1038. doi: 10.1086/516441. [DOI] [PubMed] [Google Scholar]
- 2.McIntosh K, Shevitz A, Zaknun D, Kornegay J, Chatis P, Karthas N, Burchett SK. Age- and time-related changes in extracellular viral load in children vertically infected by human immunodeficiency virus. Pediatr Infect Dis J. 1996;15:1087–1091. doi: 10.1097/00006454-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Rich KC, Fowler MG, Mofenson LM, Abboud R, Pitt J, Diaz C, Hanson IC, Cooper E, Mendez H. Maternal and infant factors predicting disease progression in human immunodeficiency virus type 1-infected infants. Women and Infants Transmission Study Group. Pediatrics. 2000;105:e8. doi: 10.1542/peds.105.1.e8. [DOI] [PubMed] [Google Scholar]
- 4.Feeney ME. HIV and children: the developing immune system fights back. West Indian Med J. 2004;53:359–362. [PubMed] [Google Scholar]
- 5.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali DL, Sullivan JL. HIV-1-Specific Cytotoxic T Lymphocyte Responses in the First Year of life. The Journal of Immunology. 1995;154:433–443. [PubMed] [Google Scholar]
- 6.Pikora CA, Sullivan JL, Panicali D, Luzuriaga K. Early HIV-1 Envelope-specific Cytotoxic T Lymphocyte Responses in Vertically Infected Infants. The Journal of Experimental Medicine. 1997;185:1153–1162. doi: 10.1084/jem.185.7.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buseyne F, Burgard M, Teglas J, Bui E, Rouzioux C, Mayaux M, Blanche S, Rivière Y. Early HIV-specific cytotoxic T lymphocytes and disease progression in children born to HIV-infected mothers. AIDS Research and Human Retroviruses. 1998;14:1435–1444. doi: 10.1089/aid.1998.14.1435. [DOI] [PubMed] [Google Scholar]
- 8.Luzuriaga K, Koupa RA, Pikoraa CA, Brettlera DB, Sulliva JL. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. The Journal of Pediatrics. 1991;119:230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 9.Lohman BL, Slyker JA, Richardson BA, Farquhar C, Mabuka JM, Crudder C, Dong T, Obimo E, Mbori-Ngacha D, Overbaugh J, Rowland-Jones S, John-Stewart G. Longitudinal Assessment of Human Immunodeficiency Virus Type 1 (HIV-1)-Specific Gramma Interferon Responses during the First Year of Life in HIV-1-Infected Infants. Journal of Virology. 2005;79:8121–8130. doi: 10.1128/JVI.79.13.8121-8130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thobakgale CF, Ramduth D, Reddy S, Mkhwanazi N, de Pierres C, Moodley E, Mphatswe W, Blanckenberg N, Cengimbo A, Prendergast A, Tudor-Williams G, Dong K, Jeena P, Kindra G, Bobat R, Coovadia H, Kiepiela P, Walker BD, Goulder PJ. Human immunodeficiency virus-specific CD8+ T-cell activity is detectable from birth in the majority of in utero-infected infants. J Virol. 2007;81:12775–12784. doi: 10.1128/JVI.00624-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney ME, Roosevelt KA, Tang Y, Pfafferott KJ, McIntosh K, Burchett SK, Mao C, Walker BD, Goulder PJ. Comprehensive screening reveals strong and broadly directed human immunodeficiency virus type 1-specific CD8 responses in perinatally infected children. J Virol. 2003;77:7492–7501. doi: 10.1128/JVI.77.13.7492-7501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luzuriaga K, McManus M, Catalina M, Mayack S, Sharkey M, Stevenson M, Sullivan JL. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: control of viral replication and absence of persistent HIV-1- specific immune responses. J Virol. 2000;74:6984–6991. doi: 10.1128/jvi.74.15.6984-6991.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiegel HM, DeFalcon E, Ogg GS, Larsson M, Beadle TJ, Tao P, McMichael AJ, Bhardwaj N, O'Callaghan C, Cox WI, Krasinski K, Pollack H, Borkowsky W, Nixon DF. Changes in frequency of HIV-1-specific cytotoxic T cell precursors and circulating effectors after combination antiretroviral therapy in children. J Infect Dis. 1999;180:359–368. doi: 10.1086/314867. [DOI] [PubMed] [Google Scholar]
- 14.Scott-Algara D, Buseyne F, Blanche S, Rouzioux C, Jouanne C, Romagne F, Riviere Y. Frequency and phenotyping of human immunodeficiency virus (HIV)-specific CD8+ T cells in HIV-infected children, using major histocompatibility complex class I peptide tetramers. J Infect Dis. 2001;183:1565–1573. doi: 10.1086/320708. [DOI] [PubMed] [Google Scholar]
- 15.Scott ZA, Chadwick EG, Gibson LL, Catalina MD, McManus MM, Yogev R, Palumbo P, Sullivan JL, Britto P, Gay H, Luzuriaga K. Infrequent detection of HIV-1-specific, but not cytomegalovirus-specific, CD8(+) T cell responses in young HIV-1-infected infants. J Immunol. 2001;167:7134–7140. doi: 10.4049/jimmunol.167.12.7134. [DOI] [PubMed] [Google Scholar]
- 16.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 17.Zuniga R, Lucchetti A, Galvan P, Sanchez S, Sanchez C, Hernandez A, Sanchez H, Frahm N, Linde CH, Hewitt HS, Hildebrand W, Altfeld M, Allen TM, Walker BD, Korber BT, Leitner T, Sanchez J, Brander C. Relative dominance of Gag p24-specific cytotoxic T lymphocytes is associated with human immunodeficiency virus control. J Virol. 2006;80:3122–3125. doi: 10.1128/JVI.80.6.3122-3125.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–2305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T-cells. Blood. 2006 doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christie CD. A paediatric and perinatal HIV/AIDS leadership initiative in Kingston, Jamaica. West Indian Med J. 2004;53:283–292. [PubMed] [Google Scholar]
- 21.Goulder PJ, Addo MM, Altfeld MA, Rosenberg ES, Tang Y, Govender U, Mngqundaniso N, Annamalai K, Vogel TU, Hammond M, Bunce M, Coovadia HM, Walker BD. Rapid definition of five novel HLA-A*3002-restricted human immunodeficiency virus-specific cytotoxic T-lymphocyte epitopes by elispot and intracellular cytokine staining assays. J Virol. 2001;75:1339–1347. doi: 10.1128/JVI.75.3.1339-1347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, Feeney ME, Rodriguez WR, Basgoz N, Draenert R, Stone DR, Brander C, Goulder PJ, Rosenberg ES, Altfeld M, Walker BD. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaul R, Dong T, Plummer FA, Kimani J, Rostron T, Kiama P, Njagi E, Irungu E, Farah B, Oyugi J, Chakraborty R, MacDonald KS, Bwayo JJ, McMichael A, Rowland-Jones SL. CD8+ lymphocytes respond to different HIV epitopes in seronegative and infected subjects. The Journal of Clinical Investigation. 2001;107:1303–1310. doi: 10.1172/JCI12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowland-Jones SL, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nature Medicine. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 25.Rowland-Jones SL, Dong T, Fowke KR, Kimani J, Krausa P, Newell H, Blanchard T, Ariyoshi K, Oyugi J, Ngugi E, Bwayo J, MacDonald KS, McMichael AJ, Plummer FA. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. The Journal of Clinical Investigation. 1998;102:1758–1765. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Maria A, Cirillo C, Morett L. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. The Journal of Infectious Diseases. 1994;170:1296–1299. doi: 10.1093/infdis/170.5.1296. [DOI] [PubMed] [Google Scholar]
- 27.Legrand FA, Nixon DF, Loo CP, Ono E, Chapman JM, Miyamoto M, Diaz RS, Santos AMN, Succi RCM, Abadi J, Rosenberg MG, de Moraes-Pinto MI, Kallas EG. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:1–10. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheynier R, Langlade-Demoyen P, Marescot M, Blanche S, Blondin G, Wain-Hobson S, Griscelli C, Vilmer E, Plata F. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. European Journal of Immunology. 1992;22:2189–2473. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 29.Rowland-Jones SL, Nixon DF, Aldhous MC, Gotch F, Ariyoshi K, Hallam N, Kroll JS, Froebel K, McMichael A. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. The Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 30.McFarland EJ, Harding PA, Luckey D, Conway B, Young RK, Kuritzkes DR. High frequency of Gag- and envelope-specific cytotoxic T lymphocyte precursors in children with vertically acquired human immunodeficiency virus type 1 infection. The Journal of Infectious Diseases. 1994;170:766–774. doi: 10.1093/infdis/170.4.766. [DOI] [PubMed] [Google Scholar]
- 31.Buseyne F, Blanche S, Schmitt D, Griscelli C, RiviPre Y. Detection of HIV pecific Cell-Mediated Cytotoxicity in the Peripheral Blood from Infected Children. The Journal of Immunology. 1993;150:3569–3581. [PubMed] [Google Scholar]
- 32.Altfeld M, van Lunzen J, Frahm N, Yu XG, Schneider C, Eldridge RL, Feeney ME, Meyer-Olson D, Stellbrink HJ, Walker BD. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J Clin Invest. 2002;109:837–843. doi: 10.1172/JCI14789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg ES, Altfeld M, Poon SH, Phillips MN, Wilkes BM, Eldridge RL, Robbins GK, D'Aquila RT, Goulder PJ, Walker BD. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407:523–526. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 34.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J Virol. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath MJ. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 36.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 37.Buseyne F, Scott-Algara D, Porrot F, Corre B, Bellal N, Burgard M, Rouzioux C, Blanche S, Riviere Y. Frequencies of ex vivo-activated human immunodeficiency virus type 1-specific gamma-interferon-producing CD8+ T cells in infected children correlate positively with plasma viral load. J Virol. 2002;76:12414–12422. doi: 10.1128/JVI.76.24.12414-12422.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day CL, Walker BD. Progress in defining CD4 helper cell responses in chronic viral infections. J Exp Med. 2003;198:1773–1777. doi: 10.1084/jem.20031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 40.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu W, Chen S, Sharp M, Dekker C, Manganello AM, Tongson EC, Maecker HT, Holmes TH, Wang Z, Kemble G, Adler S, Arvin A, Lewis DB. Persistent and selective deficiency of CD4+ T cell immunity to cytomegalovirus in immunocompetent young children. J Immunol. 2004;172:3260–3267. doi: 10.4049/jimmunol.172.5.3260. [DOI] [PubMed] [Google Scholar]
- 42.Feeney ME, Draenert R, Roosevelt KA, Pelton SI, McIntosh K, Burchett SK, Mao C, Walker BD, Goulder PJ. Reconstitution of Virus-Specific CD4 Proliferative Responses in Pediatric HIV-1 Infection. J Immunol. 2003;171:6968–6975. doi: 10.4049/jimmunol.171.12.6968. [DOI] [PubMed] [Google Scholar]
- 43.Pass RF, Stagno S, Britt WJ, Alford CA. Specific cell-mediated immunity and the natural history of congenital infection with cytomegalovirus. J Infect Dis. 1983;148:953–961. doi: 10.1093/infdis/148.6.953. [DOI] [PubMed] [Google Scholar]
- 44.Harari A, Rizzardi GP, Ellefsen K, Ciuffreda D, Champagne P, Bart PA, Kaufmann D, Telenti A, Sahli R, Tambussi G, Kaiser L, Lazzarin A, Perrin L, Pantaleo G. Analysis of HIV-1- and CMV-specific memory CD4 T-cell responses during primary and chronic infection. Blood. 2002;100:1381–1387. doi: 10.1182/blood-2001-11-0080. [DOI] [PubMed] [Google Scholar]
- 45.Pitcher CJ, Quittner C, Peterson DM, Connors M, Koup RA, Maino VC, Picker LJ. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 46.Luzuriaga K, Holmes D, Hereema A, Wong J, Panicali DL, Sullivan JL. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. Journal of Immunology. 1995;154:433–443. [PubMed] [Google Scholar]
- 47.Luzuriaga K, Koup RA, Pikora CA, Brettler DB, Sullivan JL. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr. 1991;119:230–236. doi: 10.1016/s0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 48.Altfeld M, Rosenberg ES, Shankarappa R, Mukherjee JS, Hecht FM, Eldridge RL, Addo MM, Poon SH, Phillips MN, Robbins GK, Sax PE, Boswell S, Kahn JO, Brander C, Goulder PJ, Levy JA, Mullins JI, Walker BD. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169–180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohman BL, Slyker JA, Richardson BA, Farquhar C, Mabuka JM, Crudder C, Dong T, Obimbo E, Mbori-Ngacha D, Overbaugh J, Rowland-Jones S, John-Stewart G. Longitudinal assessment of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon responses during the first year of life in HIV-1-infected infants. J Virol. 2005;79:8121–8130. doi: 10.1128/JVI.79.13.8121-8130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichterfeld M, Yu XG, Cohen D, Addo MM, Malenfant J, Perkins B, Pae E, Johnston MN, Strick D, Allen TM, Rosenberg ES, Korber B, Walker BD, Altfeld M. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. Aids. 2004;18:1383–1392. doi: 10.1097/01.aids.0000131329.51633.a3. [DOI] [PubMed] [Google Scholar]
- 51.Violari A, Cotton M, Gibb D, Babiker A, Steyn J, Jean-Phillip P, McIntyre J. Antiretroviral therapy initiated before 12 weeks of age reduces early mortality in young HIV-infected infants: evidence from the Children with HIV Early Antiretroviral Therapy (CHER) Study.. 4th IAS Conference on HIV Pathogenesis, Treatment and Prevention..2007. [Google Scholar]
- 52.Martinez-Picado J, Prado JG, Fry EE, Pfafferott K, Leslie A, Chetty S, Thobakgale C, Honeyborne I, Crawford H, Matthews P, Pillay T, Rousseau C, Mullins JI, Brander C, Walker BD, Stuart DI, Kiepiela P, Goulder P. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J Virol. 2006;80:3617–3623. doi: 10.1128/JVI.80.7.3617-3623.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 54.Brockman MA, Schneidewind A, Lahaie M, Schmidt A, Miura T, Desouza I, Ryvkin F, Derdeyn CA, Allen S, Hunter E, Mulenga J, Goepfert PA, Walker BD, Allen TM. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J Virol. 2007;81:12608–12618. doi: 10.1128/JVI.01369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schneidewind A, Brockman MA, Yang R, Adam RI, Li B, Le Gall S, Rinaldo CR, Craggs SL, Allgaier RL, Power KA, Kuntzen T, Tung CS, LaBute MX, Mueller SM, Harrer T, McMichael AJ, Goulder PJ, Aiken C, Brander C, Kelleher AD, Allen TM. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J Virol. 2007;81:12382–12393. doi: 10.1128/JVI.01543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, Lee W, Burwitz BJ, Stephany JJ, Loffredo JT, Allison DB, Adnan S, Hoji A, Wilson NA, Friedrich TC, Lifson JD, Yang OO, Watkins DI. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J Immunol. 2007;178:2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen SF, Tu WW, Sharp MA, Tongson EC, He XS, Greenberg HB, Holmes TH, Wang Z, Kemble G, Manganello AM, Adler SP, Dekker CL, Lewis DB, Arvin AM. Antiviral CD8 T cells in the control of primary human cytomegalovirus infection in early childhood. J Infect Dis. 2004;189:1619–1627. doi: 10.1086/383249. [DOI] [PubMed] [Google Scholar]
- 58.Scott ZA, Beaumier CM, Sharkey M, Stevenson M, Luzuriaga K. HIV-1 Replication Increases HIV-Specific CD4(+) T Cell Frequencies but Limits Proliferative Capacity in Chronically Infected Children. J Immunol. 2003;170:5786–5792. doi: 10.4049/jimmunol.170.11.5786. [DOI] [PubMed] [Google Scholar]
- 59.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 60.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 61.Hartigan-O'Connor DJ, Abel K, McCune JM. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: implications for SIV disease progression. J Exp Med. 2007;204:2679–2692. doi: 10.1084/jem.20071068. [DOI] [PMC free article] [PubMed] [Google Scholar]