Abstract
Background: Telomere length may be a marker of biological aging. Multivitamin supplements represent a major source of micronutrients, which may affect telomere length by modulating oxidative stress and chronic inflammation.
Objective: The objective was to examine whether multivitamin use is associated with longer telomeres in women.
Design: We performed a cross-sectional analysis of data from 586 early participants (age 35–74 y) in the Sister Study. Multivitamin use and nutrient intakes were assessed with a 146-item food-frequency questionnaire, and relative telomere length of leukocyte DNA was measured by quantitative polymerase chain reaction.
Results: After age and other potential confounders were adjusted for, multivitamin use was associated with longer telomeres. Compared with nonusers, the relative telomere length of leukocyte DNA was on average 5.1% longer among daily multivitamin users (P for trend = 0.002). In the analysis of micronutrients, higher intakes of vitamins C and E from foods were each associated with longer telomeres, even after adjustment for multivitamin use. Furthermore, intakes of both nutrients were associated with telomere length among women who did not take multivitamins.
Conclusion: This study provides the first epidemiologic evidence that multivitamin use is associated with longer telomere length among women.
See corresponding editorial on page 1721.
INTRODUCTION
Telomeres, the TTAGGG tandem repeat sequence, and their binding proteins at the ends of chromosomes prevent chromosomes from detrimental recombination and degradation (1). In somatic cells, the length of telomeres decreases with each cell division, which may eventually lead to cell senescence or apoptosis. Therefore, telomere length has been proposed as a marker of “biological ageing” (2). Consistent with this hypothesis, preliminary epidemiologic studies have related shorter telomeres to higher mortality (3) and higher risk of some age-related chronic diseases (4–10). Experimental evidence suggests that oxidative stress and chronic inflammation contribute to the attrition of telomeres (2, 11). Several micronutrients, such as antioxidant vitamins and minerals, can modulate the states of oxidative stress and chronic inflammation and therefore may affect telomere length (12–15). Multivitamin supplements contain large amounts of many vitamins and minerals and therefore represent a major source of micronutrient intake (16). We therefore examined whether multivitamin use was associated with longer telomeres among 586 women from the Sister Study.
SUBJECTS AND METHODS
Study population
The Sister Study (http://www.sisterstudy.org/) is an ongoing risk-enriched prospective cohort of healthy sisters (age 35–74 y) of breast cancer patients (17). Recruitment began in 2004 and is expected to be completed in 2009. The enrollment includes a home visit for blood and urine collection, a 90-min computer-assisted telephone interview, and several self-administered questionnaires, including a detailed food-frequency questionnaire (FFQ). Details of this telomere project and sampling procedures were described elsewhere (18). Briefly, a total of 740 women were selected for telomere measurement from the first 2086 Sister Study participants by oversampling smokers, nonwhite women, and women with high perceived stress and by randomly sampling the rest. Exclusion criteria included missing or ineligible biological specimens, missing race or smoking data, major dental procedure or surgery in the past week, working on rotating shifts, recent chemotherapy or radiation treatment of cancer, or a diagnosis of breast cancer before the first annual follow-up. The sample selection criteria and sample size reflect the requirements for a US Department of Defense–funded study on stress and telomere length. We limited our analyses to 586 women with valid dietary information and duplicate laboratory assays on telomere length. The Sister Study was approved by the Institutional Review Board of the National Institute of Environmental Health Science, National Institutes of Health.
Telomere length measurement
A whole blood sample was collected at enrollment and stored at −80°C until DNA extraction. Total leukocyte DNA was then used as a template for polymerase chain reaction (PCR)–based measurement of relative telomere length according to previously published protocols (19). The assay used 100–200 ng template DNA in 1-μL aliquots for triplicate PCR amplifications per sample per plate. Cycle threshold was transformed into nanograms DNA based on a standard curve. The quantitative assay determines the amount of telomeric DNA (T) relative to the amount of single-copy control gene (human β-globin) DNA (S) and then calculates a T/S ratio. This PCR-based telomere assay was found to be highly correlated with Southern blot analysis (19). We further estimated the relative length of telomeres in base pairs (bp) by multiplying the T/S ratio with a constant of 4270 (19). Whereas this constant was derived and validated in another study, the assays here were performed in the same laboratory by using the same genetic controls. Of the 740 specimens submitted for telomere assays, 647 were run on duplicate plates including 3 internal controls (one each at a high, medium, and low T/S ratio) to further account for variation over time and plates. The CV across averaged adjusted replicates was 8.5%. These average plate-adjusted values were used for the present analyses.
Exposure assessment
The Sister Study dietary survey was based on a modified Block 1998 FFQ with additional questions and changes (20, 21). The FFQ asked for the portion size and frequency of consumption of 146 food items in the past 12 mo. Intake of individual nutrients was then calculated with software from the Block Dietary Data Systems (Berkeley, CA). Participants were asked whether they had taken any vitamins or minerals regularly (at least once per month) during the past 12 mo. For those who answered “yes,” the FFQ further elicited details about the use of 3 types of multivitamins [regular once-a-day, Centrum (Wyeth Consumer Healthcare, Madison, NJ), or Thera type; stress-tabs or B-complex type; and antioxidant combination type] and 16 individual supplements of vitamins or minerals. For each supplement, participants were asked the frequency of use (ranging from “did not take” to “every day”) and, for users, the duration of use (ranging from “less than 1 year” to “10+ years”). We further calculated an overall frequency variable for multivitamin use by combining the use of all 3 types of multivitamins. The Sister Study also collected information on age, race, education, smoking status, perceived stress level, self-reported health status, adult-onset diabetes, and cardiovascular diseases (ie, heart attack, bypass surgery, angioplasty, congestive heart failure, cardiac arrhythmic, medicated angina, and stroke/transient ischemic attack). Body weight and height were measured during a home visit for blood collection, and body mass index (BMI) was calculated by dividing weight in kilograms by height squared in meters.
Statistical analyses
For multivitamins, the frequency and duration of use were defined categorically. The use of most individual vitamin and mineral supplements was infrequent; therefore, the participants were classified as users or nonusers. We compared the population characteristics by multivitamin use status using Student's t test for continuous variables and a chi-square test for categorical variables. Dietary intake of micronutrients was categorized into quartiles (≈25% of the participants in each quartile) after adjustment for energy intake with the residual method (22). The least-square means and SEM of relative telomere length for each exposure category were calculated with generalized linear regression models, adjusted for age (continuous), race (non-Hispanic white and others), BMI (continuous), education (≤high school, some college, associate degree/technical training, college graduate, and graduate degrees), cigarette smoking (never, former, and current smokers), presence of diabetes or cardiovascular diseases (yes, no), energy intake (continuous), perceived stress level (very low, low, moderate, high, and very high), self-reported health status (excellent, very good, good, and fair or poor), and physical activity (metabolic equivalent hours in quartiles). The statistical significance of a linear trend was tested by including the median of each category as a continuous variable in the regression model and interactions by including a multiplicative term between supplement use frequency and the stratifying variable.
To further explore the relation between multivitamin use and telomere length, we conducted stratified analyses according to median age (median: < and ≥53 y), smoking status (never and ever), BMI (< and ≥30), and the presence of diabetes or cardiovascular diseases (yes and no). In addition, we conducted a sensitivity analysis by excluding women who reported “fair or poor” health status.
Finally, we examined whether intakes of any important micronutrients that were commonly found in multivitamins were related to telomere length in the study population. Among multivitamin users, a substantial proportion of the micronutrient intake was from multivitamins. This was particularly true for vitamins C, D, and E and most B vitamins. We therefore fit the regression models with and without adjusting for multivitamin use and conducted an analysis among women who did not take multivitamins to evaluate the independent relation between dietary micronutrient intake and telomere length. All statistical analyses were conducted by using SAS software (version 9.1; SAS Institute, Cary, NC), and the significance tests were 2-tailed with α = 0.05.
RESULTS
Study sample characteristics are presented in Table 1. Compared with women who did not take multivitamins, regular users were older, more likely to be non-Hispanic white and never smokers, and had higher education level. However, multivitamin users and nonusers were not significantly based on other population characteristics.
TABLE 1.
Characteristics of the study participants1
| Multivitamin supplement2 |
||||
| All (n = 586) | Nonusers (n = 203) | Users (n = 378) | P value3 | |
| Age (y) | 53.6 ± 9.64 | 51.6 ± 9.1 | 54.6 ± 9.7 | 0.0005 |
| Non-Hispanic whites [n(%)] | 493 (84.1) | 162 (79.8) | 327 (86.5) | 0.03 |
| Education [n(%)] | 0.005 | |||
| ≤High school | 90 (15.4) | 40 (19.7) | 49 (13.0) | |
| Some college | 152 (25.9) | 63 (31.0) | 88 (23.3) | |
| Associate degrees/technical training | 84 (14.3) | 31 (15.3) | 53 (14.0) | |
| College graduate | 148 (25.3) | 37 (18.2) | 110 (29.1) | |
| Graduate degree | 112 (19.1) | 32 (15.8) | 78 (20.6) | |
| Smoking [n(%)] | 0.009 | |||
| Former | 173 (29.5) | 54 (26.6) | 116 (30.7) | |
| Current | 136 (23.2) | 62 (30.5) | 73 (19.3) | |
| BMI (kg/m2) | 27.5 ± 6.2 | 28.2 ± 6.6 | 27.2 ± 5.9 | 0.09 |
| Physical activity (MET hours) | 47.2 ± 30.0 | 44.2 ± 27.4 | 48.6 ± 31.0 | 0.07 |
| Self-reported health [n(%)] | 0.5 | |||
| Excellent | 185 (31.6) | 62 (30.5) | 121 (32.0) | |
| Very good | 211 (36.0) | 67 (33.0) | 142 (37.6) | |
| Good | 145 (24.7) | 56 (27.6) | 88 (23.3) | |
| Fair or poor | 45 (7.7) | 18 (8.9) | 27 (7.1) | |
| Perceived stress level [n(%)] | 0.08 | |||
| Very low | 109 (18.6) | 35 (17.2) | 74 (19.6) | |
| Low | 144 (24.6) | 43 (21.2) | 99 (26.2) | |
| Moderate | 108 (18.4) | 44 (21.7) | 63 (16.7) | |
| High | 128 (21.8) | 39 (19.2) | 89 (23.5) | |
| Very high | 97 (16.6) | 42 (20.7) | 53 (14.0) | |
| Self-reported diabetes or cardiovascular diseases [n(%)] | 112 (19.1) | 37 (18.2) | 74 (19.6) | 0.7 |
| Total energy (kcal) | 1590 ± 535 | 1590 ± 597 | 1594 ± 501 | 0.9 |
MET, metabolic equivalent task.
Five women were missing data on multivitamin use.
A Student's t test was used for continuous variables, and a chi-square test was used for categorical variables.
Mean ± SD (all such values).
Sixty-five percent of the women used multivitamins at least once per month, and most users (74%) took multivitamins on a daily basis. About 89% of the users took once-a-day type multivitamins, 21% took antioxidant combination, and 17% took stress-tabs or B-complex vitamins. Among users, multivitamins represented a major source of total vitamin and mineral intakes, contributing >50% of the total intake for vitamins C, E, D, B-6, B-12, folate, iron, and zinc and 30–50% for vitamin A, β-carotene, and calcium.
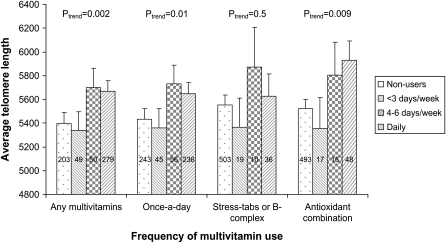
In general, the use of multivitamin supplements was associated with longer telomere length (Figure 1). Compared with nonusers, daily users had on average 5.1% longer telomeres (P for trend = 0.002). This difference (273 bp) corresponds to ≈9.8 y of age-related telomere loss since each year of age was associated with a 28-bp shorter telomere in our sample. Significant associations were also obtained for the once-a-day or the antioxidant combination type, but not for the stress-tab or B-complex type. Excluding women who reported fair or poor health did not change the results: the relative telomere length was 5398 bp for nonusers and 5645 bp for daily users (4.6% difference; P for trend = 0.009). Analysis of the duration of individual multivitamin use showed similar results. Compared with nonusers, the adjusted telomere length of those who took multivitamins for >5 y was ≈3% longer for once-a-day type multivitamins (P for trend = 0.09) and 8% for antioxidant combination type (P for trend = 0.02). The duration of stress-tabs or B complex use was not related to telomere length. Multivitamin use was also associated with longer telomere length in most of the subgroup analyses by age, sex, and smoking status (Table 2), although not all associations were significant. Use of individual micronutrient supplements was less common in this study sample, and, in general, they were not associated with telomere length after multivitamin use was accounted for (data not shown). The only exceptions were vitamin B-12 and iron: vitamin B-12 supplement users (n = 52) had a longer telomere length than did nonusers (n = 518): 5850 ± 159 compared with 5505 ± 89 bp (5.9% difference; P = 0.03), and iron users (n = 41) had a shorter telomere length than nonusers (n = 527): 5121 ± 183 compared with 5583 ± 87 bp (−9.0% difference; P = 0.007).
FIGURE 1.
Least-squares mean (±SE) telomere length according to the frequency of multivitamin use. Generalized linear models were used in the analysis, adjusted for age, race, BMI, education, cigarette smoking, presence of diabetes or cardiovascular diseases, energy intake, perceived stress level, self-reported health status, and physical activity. Numbers within the bars represents the sample size for each exposure group.
TABLE 2.
Average telomere length according to frequency of multivitamin use in subgroups1
| Users |
||||||
| Nonusers | <3 d/wk | 4–6 d/wk | Daily | P for trend2 | P for interaction3 | |
| Age | 0.6 | |||||
| <53 y | ||||||
| No. of subjects | 122 | 27 | 35 | 99 | ||
| Lsmean ± SE | 5586 ± 141 | 5429 ± 233 | 5893 ± 213 | 5824 ± 146 | 0.05 | |
| ≥53 y | ||||||
| No. of subjects | 81 | 22 | 15 | 180 | ||
| Lsmean ± SE | 5168 ± 138 | 5291 ± 237 | 5373 ± 286 | 5494 ± 126 | 0.02 | |
| BMI | 0.5 | |||||
| <30 kg/m2 | ||||||
| No. of subjects | 137 | 34 | 37 | 190 | ||
| Lsmean ± SE | 5434 ± 126 | 5483 ± 194 | 5705 ± 197 | 5624 ± 122 | 0.08 | |
| ≥30 kg/m2 | ||||||
| No. of subjects | 66 | 15 | 13 | 89 | ||
| Lsmean ± SE | 5230 ± 162 | 5009 ± 314 | 5618 ± 336 | 5708 ± 158 | 0.008 | |
| Smoking | 0.6 | |||||
| Never | ||||||
| No. of subjects | 87 | 24 | 26 | 139 | ||
| Lsmean ± SE | 5483 ± 139 | 5307 ± 234 | 5717 ± 233 | 5628 ± 123 | 0.2 | |
| Ever | ||||||
| No. of subjects | 116 | 25 | 24 | 140 | ||
| Lsmean ± SE | 5368 ± 131 | 5450 ± 230 | 5765 ± 230 | 5788 ± 135 | 0.002 | |
| Diabetes or cardiovascular disease | 0.4 | |||||
| No | ||||||
| No. of subjects | 166 | 39 | 47 | 218 | ||
| Lsmean ± SE | 5587 ± 104 | 5508 ± 180 | 5817 ± 168 | 5796 ± 103 | 0.03 | |
| Yes | ||||||
| No. of subjects | 37 | 10 | 3 | 61 | ||
| Lsmean ± SE | 4908 ± 219 | 4966 ± 390 | 5813 ± 667 | 5590 ± 187 | 0.004 | |
Least-squares mean (Lsmean) ± SE values were derived from generalized linear regression models, adjusted for age, race, BMI, education level, cigarette smoking, presence of diabetes or cardiovascular diseases, energy intake, perceived stress level, self-reported health status, and physical activity. Stratified variables were also adjusted for in the subgroup analysis when possible.
Tested by including the median of each category as a continuous variable in the regression model.
Tested by including a multiplicative term in the regression model.
The total intake of most micronutrients was positively associated with telomere length (Table 3); however, these associations became statistically nonsignificant after multivitamin use was adjusted for. Micronutrient intake from foods was generally not related to telomere length, except for vitamins C and E (Table 4). Higher dietary intake of these 2 antioxidants was associated with longer telomere length in a dose-response manner even after multivitamin use was adjusted for. Among women who did not use multivitamins (n = 203), higher dietary intakes of β-carotene, folate, magnesium, and vitamins C, E, and A were each associated with longer telomere length (Table 4).
TABLE 3.
Average telomere length according to total intake of selected micronutrients with and without adjustment for multivitamin use1
| Model 12 (n = 586) |
Model 23 (n = 581) |
|||||
| Nutrient | Quartile 14 | Quartile 44 | P for trend5 | Quartile 14 | Quartile 44 | P for trend5 |
| Vitamin A (IU) | 49536 | 17,547 | 4927 | 17,547 | ||
| Lsmean ± SE | 5293 ± 106 | 5633 ± 108 | 0.02 | 5356 ± 124 | 5571 ± 124 | 0.3 |
| β-carotene (μg) | 1621 | 7226 | 1613 | 7206 | ||
| Lsmean ± SE | 5336 ± 107 | 5649 ± 106 | 0.03 | 5409 ± 120 | 5605 ± 120 | 0.2 |
| Vitamin C (mg) | 63 | 785 | 63 | 794 | ||
| Lsmean ± SE | 5289 ± 107 | 5670 ± 106 | 0.02 | 5318 ± 132 | 5620 ± 121 | 0.2 |
| Vitamin E (α-TE) | 7.6 | 328 | 7.6 | 328 | ||
| Lsmean ± SE | 5324 ± 104 | 5640 ± 111 | 0.1 | 5346 ± 158 | 5590 ± 127 | 0.5 |
| Vitamin B-6 (mg) | 1.2 | 4.9 | 1.2 | 4.9 | ||
| Lsmean ± SE | 5424 ± 107 | 5737 ± 107 | 0.005 | 5604 ± 149 | 5619 ± 140 | 0.5 |
| Vitamin B-12 (μg) | 2.3 | 17 | 2.3 | 17 | ||
| Lsmean ± SE | 5355 ± 104 | 5737 ± 106 | 0.002 | 5442 ± 142 | 5641 ± 127 | 0.1 |
| Folate (μg) | 236 | 867 | 236 | 867 | ||
| Lsmean ± SE | 5336 ± 108 | 5689 ± 107 | 0.01 | 5452 ± 146 | 5516 ± 146 | 0.8 |
| Vitamin D (IU) | 75 | 639 | 75 | 637 | ||
| Lsmean ± SE | 5393 ± 103 | 5564 ± 108 | 0.009 | 5430 ± 134 | 5500 ± 142 | 0.7 |
| Calcium (mg) | 444 | 1906 | 445 | 1906 | ||
| Lsmean ± SE | 5407 ± 106 | 5578 ± 111 | 0.04 | 5454 ± 120 | 5501 ± 126 | 0.4 |
| Selenium (μg) | 61 | 113 | 61 | 113 | ||
| Lsmean ± SE | 5369 ± 103 | 5663 ± 106 | 0.02 | 5446 ± 115 | 5602 ± 123 | 0.3 |
| Iron (mg) | 8.5 | 33 | 8.5 | 33 | ||
| Lsmean ± SE | 5391 ± 107 | 5568 ± 110 | 0.03 | 5459 ± 131 | 5459 ± 135 | 0.97 |
| Magnesium (mg) | 184 | 419 | 184 | 419 | ||
| Lsmean ± SE | 5314 ± 105 | 5659 ± 109 | 0.007 | 5364 ± 126 | 5589 ± 129 | 0.3 |
| Zinc (mg) | 7.1 | 31 | 7.1 | 30 | ||
| Lsmean ± SE | 5332 ± 102 | 5630 ± 106 | 0.008 | 5350 ± 134 | 5560 ± 132 | 0.4 |
Least-squares mean (Lsmean) ± SE values were derived from generalized linear regression models. TE, tocopherol equivalents.
Adjusted for age, race, BMI, education level, cigarette smoking, presence of diabetes or cardiovascular diseases, energy intake, perceived stress level, self-reported health status, and physical activity.
Adjusted as for model 1 and for multivitamin use. Five women with missing data on multivitamin use were not included in model 2.
Each quartile represents ≈25% of the study participants: quartile 1, the lowest 25%, and quartile 4, the highest 25%.
Tested by including the median of each category as a continuous variable in the regression model.
Median (all such values).
TABLE 4.
Average telomere length according to intake of selected micronutrients from foods1
| All women2 (n = 581) |
Multivitamin nonusers3 (n = 203) |
|||||
| Quartile 14 | Quartile 44 | P for trend5 | Quartile 14 | Quartile 44 | P for trend5 | |
| Vitamin A (IU) | 38456 | 13,160 | 3508 | 11,273 | ||
| Lsmean ± SE | 5399 ± 115 | 5527 ± 117 | 0.3 | 5230 ± 171 | 5674 ± 167 | 0.008 |
| β-carotene (μg) | 1168 | 5323 | 1052 | 4708 | ||
| Lsmean ± SE | 5463 ± 115 | 5549 ± 117 | 0.4 | 5120 ± 174 | 5484 ± 164 | 0.045 |
| Vitamin C (mg) | 42 | 153 | 38 | 134 | ||
| Lsmean ± SE | 5340 ± 115 | 5683 ± 113 | 0.03 | 4945 ± 173 | 5701 ± 153 | 0.002 |
| Vitamin E (α-TE) | 6.1 | 12 | 5.7 | 12 | ||
| Lsmean ± SE | 5311 ± 115 | 5672 ± 118 | 0.004 | 5134 ± 161 | 5533 ± 173 | 0.03 |
| Vitamin B-6 (mg) | 1.0 | 1.8 | 0.9 | 1.7 | ||
| Lsmean ± SE | 5434 ± 112 | 5625 ± 117 | 0.3 | 5308 ± 159 | 5300 ± 164 | 0.8 |
| Vitamin B-12 (μg) | 1.8 | 4.6 | 1.7 | 4.6 | ||
| Lsmean ± SE | 5574 ± 108 | 5611 ± 116 | 0.8 | 5317 ± 159 | 5414 ± 171 | 0.6 |
| Folate (μg) | 201 | 401 | 181 | 388 | ||
| Lsmean ± SE | 5371 ± 115 | 5530 ± 117 | 0.4 | 5091 ± 175 | 5586 ± 162 | 0.02 |
| Vitamin D (IU) | 50 | 250 | 42 | 215 | ||
| Lsmean ± SE | 5606 ± 110 | 5591 ± 114 | 0.8 | 5389 ± 157 | 5537 ± 164 | 0.3 |
| Calcium (mg) | 366 | 931 | 331 | 824 | ||
| Lsmean ± SE | 5549 ± 110 | 5520 ± 116 | 0.9 | 5187 ± 165 | 5583 ± 163 | 0.06 |
| Selenium (μg) | 54 | 92 | 51 | 90 | ||
| Lsmean ± SE | 5518 ± 110 | 5574 ± 116 | 0.8 | 5264 ± 163 | 5563 ± 163 | 0.1 |
| Iron (mg) | 7.6 | 14 | 6.9 | 14 | ||
| Lsmean ± SE | 5511 ± 111 | 5605 ± 119 | 0.7 | 5365 ± 176 | 5367 ± 162 | 0.9 |
| Magnesium (mg) | 165 | 304 | 154 | 273 | ||
| Lsmean ± SE | 5382 ± 113 | 5572 ± 118 | 0.1 | 5183 ± 167 | 5603 ± 172 | 0.04 |
| Zinc (mg) | 6.4 | 12 | 5.7 | 12 | ||
| Lsmean ± SE | 5504 ± 114 | 5576 ± 116 | 0.6 | 5292 ± 162 | 5480 ± 168 | 0.2 |
Least-squares mean (Lsmean) ± SE values were derived from generalized linear regression models. TE, tocopherol equivalents.
The analysis among all women was adjusted for age, race, BMI, education level, cigarette smoking, presence of diabetes or cardiovascular diseases, energy intake, perceived stress level, self-reported health status, physical activity, and multivitamin use.
The analysis among multivitamin nonusers was adjusted as for all women, except for multivitamin use.
Each quartile represents ≈25% of the study participants: quartile 1, the lowest 25%, and quartile 4, the highest 25%.
Tested by including the median of each category as a continuous variable in the regression model.
Median (all such values).
DISCUSSION
In this cross-sectional analysis, multivitamin use was related to longer telomere length in women aged 35–74 y. Nutrient analysis suggests that one or more dietary antioxidant vitamins may contribute to this relation.
Telomeres typically shorten by a few dozen to a couple hundred bps per cell division (23); therefore, telomere length has been proposed as a marker of biological aging. Furthermore, because telomere attrition may eventually lead to chromosomal instability and cell death, excessive telomere shortening may play an important role in the development of some chronic diseases (1, 23). In recent epidemiologic studies, shorter leukocyte telomeres have been linked to higher mortality (3–5), accelerated aging (1), and higher risk of a variety of chronic diseases (4, 5, 24).
Telomere attrition in human somatic cells is likely the result of multiple forces, including the “end-replication” problem and low telomerase activity (2). Compared with these factors, oxidative stress is probably a more important contributor to telomere attrition (2). Telomeres are particularly vulnerable to oxidative damages, which often cannot be efficiently repaired (23). Furthermore, inflammatory reactions induce oxidative stress, and tumor necrosis factor-α significantly decreases telomerase activity and reduces telomere length in leukemic cells (25). Therefore, oxidative stress and chronic inflammation may be among the major mechanisms of telomere attrition. On the other hand, many micronutrients, such as dietary antioxidants, B-vitamins, and certain minerals, can modulate oxidative stress and inflammatory reactions (26–28) and therefore can contribute to the maintenance or attrition of telomeres. Few studies to date have investigated the role of these micronutrients in telomere maintenance. Earlier in vitro experiments showed that ascorbic acid or its derivatives (12, 29, 30) or α-tocopherol (13) slowed telomere shortening and increased the life span of certain somatic cells. In rats, iron overload significantly increased telomerase activity in liver cells but caused no change in telomere length (31). Recently, 2 population-based cross-sectional analyses examined dietary biomarkers in relation to telomere length (14, 15). In the first study, higher plasma vitamin D was associated with longer leukocyte telomere length among women, probably via antiinflammatory actions of vitamin D (14). In the other study, higher plasma homocysteine was associated with shorter telomere length, whereas higher folate was related to longer telomeres (15).
Containing most key vitamins and minerals 100% of the recommended daily intake, multivitamins are major sources of micronutrients in the US diet. According to the recent National Health and Nutrition Examination Survey, 35% of US adults took one or more types of multivitamins, the majority of whom were elderly white women (16). To our knowledge, this was the first epidemiologic study of multivitamin use and telomere length. Regular multivitamin users tend to follow a healthy lifestyle and have a higher intake of micronutrients, which sometimes makes it difficult to interpret epidemiologic observations on multivitamin use (16). In this study, we took extra caution in the data analyses by adjusting for and stratifying by important factors that may affect telomere length, including age, smoking status, and BMI (32, 33). Furthermore, we controlled for several indicators of socioeconomic status or lifestyle choice in all analyses. Women with less optimal health or chronic diseases may be more likely to use vitamin supplements; however, the exclusion of these women from the analysis did not alter the results. Previous epidemiologic results on multivitamin use and risk of chronic diseases vary, depending on the nutrient composition, the disease type, and the design of the study (34–36). Further investigations would be needed to understand the role of multivitamin use and telomere length and its implication in the etiology of chronic diseases.
Sorting out which micronutrients underlie our findings is difficult because multivitamins contain various vitamins and minerals and contribute in large amounts to daily micronutrient intakes. Nevertheless, higher intakes of the antioxidant vitamins C and E consistently showed associations with longer telomeres in different analyses. In multivitamin users, ≈63% of vitamin C and 84% of vitamin E were from supplemental sources. Whereas the evidence is not sufficient to conclude that these 2 dietary antioxidants mediated the observed relation, the results are consistent with experimental findings that vitamins C and E protect telomeres in vitro (12, 13, 29, 30).
This study had several limitations. The quantitative PCR-based assay measures the average telomere length across all leukocytes in the peripheral blood. We therefore could not exclude the possibility that multivitamin use might have shifted the composition of leukocyte subpopulations in a way that favored cells with longer telomeres as an alternative explanation for our finding. Furthermore, as the first study on this topic, our analysis was explanatory in nature; this was particularly true for subgroup analyses that included a smaller number of participants. Finally, although it is unlikely that telomere length affects multivitamin use, this analysis was cross-sectional and we were unable to make a direct causal inference. It is advisable to follow-up these findings in future large longitudinal studies.
Compared with national data, more women in our population took multivitamins (16). This may be a characteristic of women who volunteer for cohort studies and itself does not necessarily affect the validity of our study. Residual confounding is always a concern in epidemiologic research on health behaviors, such as multivitamin use. In this study, we adjusted for and stratified by a variety of potential confounders and conducted a sensitivity analysis by excluding women with fair or poor health. Finally, our dietary data relied on an FFQ, which was subject to measurement errors. However, the Block FFQ is widely used for dietary surveys and has been consistently updated and validated in various populations (20, 21, 37).
In summary, our study provides preliminary evidence linking multivitamin use to longer leukocyte telomeres. This finding should be further evaluated in future epidemiologic studies and its implications concerning aging and the etiology of chronic diseases should be carefully evaluated.
Supplementary Material
Acknowledgments
We thank Jack A Taylor for his helpful comments and Teresa Stepanek in RM Cawthon's laboratory for the telomere assay.
The authors’ responsibilities were as follows—QX and LAD: study concept and design, statistical analysis, data interpretation, manuscript preparation and critical revision; CGP, DPS, and HC: study concept and design, data collection, statistical analysis, data interpretation, manuscript preparation, critical revision, and financial support; and RMC: study design, data collection and interpretation, and critical revision of the manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 2005;6:611–22 [DOI] [PubMed] [Google Scholar]
- 2.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 2005;5:197–203 [DOI] [PubMed] [Google Scholar]
- 3.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 2003;361:393–5 [DOI] [PubMed] [Google Scholar]
- 4.Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol 2006;60:174–80 [DOI] [PubMed] [Google Scholar]
- 5.Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon 4 and dementia. Ann Neurol 2006;60:181–7 [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol 2007;165:14–21 [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 2003;95:1211–8 [DOI] [PubMed] [Google Scholar]
- 8.Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol 2007;178:1492–6 [DOI] [PubMed] [Google Scholar]
- 9.Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, Santella RM. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res 2007;67:5538–44 [DOI] [PubMed] [Google Scholar]
- 10.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2007;16:815–9 [DOI] [PubMed] [Google Scholar]
- 11.Houben JMJ, Moonen HJJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 2008;44:235–46 [DOI] [PubMed] [Google Scholar]
- 12.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular vitamin C via suppression of oxidative stress. Life Sci 1998;63:935–48 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Moritoh Y, Miwa N. Age-dependent telomere-shortening is repressed by phosphorylated alpha-tocopherol together with cellular longevity and intracellular oxidative-stress reduction in human brain microvascular endotheliocytes. J Cell Biochem 2007;102:689–703 [DOI] [PubMed] [Google Scholar]
- 14.Richards JB, Valdes AM, Gardner JP, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr 2007;86:1420–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards JB, Valdes AM, Gardner JP, et al. Homocysteine levels and leukocyte telomere length. Atherosclerosis 2008;200:271–7 [DOI] [PubMed] [Google Scholar]
- 16.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999-2000. Am J Epidemiol 2004;160:339–49 [DOI] [PubMed] [Google Scholar]
- 17.Weinberg CR, Shore DL, Umbach DM, Sandler DP. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Epidemiology 2007;166:447–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks CG, Miller DB, McCanlies EC, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev 2009;18:551–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002;30:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeckner LS, Pullen CH, Walker SN, Abbott GW, Block T. Use and reliability of the World Wide Web version of the Block Health Habits and History Questionnaire with older rural women. J Nutr Educ Behav 2002;34(suppl 1):S20–4 [DOI] [PubMed] [Google Scholar]
- 21.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr 2006;9:84–93 [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S(discussion 1229S–31S) [DOI] [PubMed] [Google Scholar]
- 23.Monaghan P, Haussmann MF. Do telomere dynamics link lifestyle and lifespan? Trends Ecol Evol 2006;21:47–53 [DOI] [PubMed] [Google Scholar]
- 24.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006;29:283–9 [DOI] [PubMed] [Google Scholar]
- 25.Beyne-Rauzy O, Recher C, Dastugue N, et al. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene 2004;23:7507–16 [DOI] [PubMed] [Google Scholar]
- 26.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr 2005;25:151–74 [DOI] [PubMed] [Google Scholar]
- 27.Peyrin-Biroulet L, Rodriguez-Gueant R-M, Chamaillard M, et al. Vascular and cellular stress in inflammatory bowel disease. Revisiting the Role of Homocysteine The Am J Gastroenterol 2007;102:1108–15 [DOI] [PubMed] [Google Scholar]
- 28.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 2007;98(suppl 1):S29–35 [DOI] [PubMed] [Google Scholar]
- 29.Yokoo S, Furumoto K, Hiyama E, Miwa N. Slow-down of age-dependent telomere shortening is executed in human skin keratinocytes by hormesis-like-effects of trace hydrogen peroxide or by anti-oxidative effects of pro-vitamin C in common concurrently with reduction of intracellular oxidative stress. J Cell Biochem 2004;93:588–97 [DOI] [PubMed] [Google Scholar]
- 30.Kashino G, Kodama S, Nakayama Y, et al. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic Biol Med 2003;35:438–43 [DOI] [PubMed] [Google Scholar]
- 31.Brown KE, Meleah Mathahs M, Broadhurst KA, et al. Increased hepatic telomerase activity in a rat model of iron overload: A role for altered thiol redox state? Free Radic Biol Med 2007;42:228–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zannolli R, Mohn A, Buoni S, et al. Telomere length and obesity. Acta Paediatr 2008;97:952–4 [DOI] [PubMed] [Google Scholar]
- 33.Valdes AM, Andrew T, Gardner J, et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005;366:662–4 [DOI] [PubMed] [Google Scholar]
- 34.Huang H-Y, Caballero B, Chang S, et al. multivitamin/mineral supplements and prevention of chronic disease: executive summary. Am J Clin Nutr 2007;85(suppl):265S–8S [DOI] [PubMed] [Google Scholar]
- 35.Godfrey JR. Toward optimal health: Meir Stampfer, M.D., Dr.P.H., discusses multivitamin and mineral supplementation for women. J Womens Health 2007;16:959–62 [DOI] [PubMed] [Google Scholar]
- 36.Prentice RL. Clinical trials and observational studies to assess the chronic disease benefits and risks of multivitamin-multimineral supplements. Am J Clin Nutr 2007;85(suppl):308S–13S [DOI] [PubMed] [Google Scholar]
- 37.Block G, Wakimoto P, Jensen C, Mandel S, Green RR. Validation of a food frequency questionnaire for Hispanics. Prev Chronic Dis 2006;3:A77 (abstr). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.