Abstract
Background: Few studies have allowed direct comparison of the association between body mass index (BMI; in kg/m2) and hypertension in different Asian ethnicities.
Objective: We compared the association of BMI with hypertension in Chinese, Indonesian, and Vietnamese adults and determined BMI cutoffs that best predicted hypertension in these populations.
Design: We included 7562 Chinese, 18,502 Indonesian, and 77,758 Vietnamese participants aged 18–65 y. Blood pressure, weight, and height were measured by trained health workers. To define an optimal BMI cutoff, we computed and searched for the shortest distance on receiver operating characteristic curves.
Results: Despite a low mean BMI, the prevalences of hypertension in Chinese, Indonesian, and Vietnamese men were 22.9%, 24.8%, and 14.4%, respectively, and in women were 16.6%, 26.9%, and 11.7%, respectively. At all BMI levels, the sex-specific prevalence of hypertension was higher in Indonesian adults than in Chinese and Vietnamese adults (P < 0.05 at almost all BMI levels). The overall and stratified analyses suggested optimal BMI cutoffs of 23–24, 21–22.5, and 20.5–21 for Chinese, Indonesian, and Vietnamese adults, respectively. The cutoffs were ≈0.5–1.0 units higher in women than in men and in the older (41–65 y) than in the younger (18–40 y) participants.
Conclusions: The study showed an ethnic difference in the BMI-hypertension association and in optimal BMI cutoffs between Chinese, Indonesian, and Vietnamese adults. Country-specific or even country-, sex-, and age-specific BMI cutoffs might be needed to identify persons at high risk of cardiovascular diseases.
INTRODUCTION
Asian populations tend to develop chronic diseases at a lower body mass index (BMI; in kg/m2) compared with other races and ethnicities (eg, whites, Hispanics, blacks, and Polynesians) (1). The main explanation for the ethnic difference is that Asians tend to have higher total body fat, abdominal fat, and visceral fat at a given BMI than do other races and ethnicities (2, 3). Asians, however, comprise many ethnic subgroups that differ in body composition, genotype, age structure, lifestyle, culture, religion, and socioeconomic status (2, 4). Thus, we would expect to find ethnic differences in the association between BMI and disease risk across Asian populations. The assumption of ethnic difference has not been verified in representative samples of East and Southeast Asians.
Although a BMI cutoff of 23 has been widely used to identify a moderate to high risk of cardiovascular disease in Asians (1), it is still unknown whether different Asian populations need different BMI cutoffs to define overweight. A BMI cutoff of 23–24 has been proposed by some authors who used sensitivity, specificity, and receiver operating characteristic (ROC) curve analyses in Chinese (5–8) and Indian (9) populations. However, these studies did not represent any Southeast Asian populations. The study by Huxley et al (10), which included a pooled sample of East, South, Southeast, and Middle East Asian populations, showed an optimal BMI cutoff for overweight of ≈24. The analysis, however, did not allow direct comparisons of the optimal BMI cutoffs in different Asian populations. Our study compared the association of BMI with hypertension in Chinese, Indonesian, and Vietnamese adults aged 18–65 y and determined optimal BMI cutoffs for predicting hypertension in these populations.
SUBJECTS AND METHODS
Study population
We used 3 data sets from recent representative surveys: the China Health and Nutrition Survey in 2004 (CHNS; n = 7562), the Indonesian Family Life Survey in 2000 (IFLS; n = 18,502), and the Vietnam National Health Survey in 2002 (VNHS; n = 77,758). The surveys were described elsewhere (11–14). To summarize, the CHNS in 2004 was part of an ongoing study established in the late 1980s in 9 China provinces that vary substantially in geography, economic development, public resources, and health indicators. The sample represented ≈50% of the Chinese population. The IFLS in 2000 was part of an ongoing longitudinal survey established in the early 1990s in 13 Indonesia provinces. The sample represented ≈83% of the Indonesian population. The VNHS in 2002 was the largest nationally representative health survey ever conducted in all 61 Vietnam provinces. Survey instructions, data sets, and questionnaires may be downloaded from the websites of the CHNS (http://www.cpc.unc.edu/china), the IFLS (http://www.rand.org/labor/FLS/IFLS), and the Vietnam Ministry of Health (http://www.moh.gov.vn).
We only included men and women aged 18–65 y (nonpregnant or nonlactating at the time of survey) (15), for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). The number of individuals with implausible values was 181, 228, and 548 for the CHNS, IFLS, and VNHS, respectivily, which represents 1–2% of the eligible participants with measured weight, height, and blood pressures. Removal of biologically implausible outliers did not affect point estimates, but did increase the precision of the estimate.
Study design
Blood pressure measurements were taken while the participants were in a seated position and on the right arm by trained health workers who followed a standardized procedure using regularly calibrated mercury sphygmomanometers (CHNS), Omron digital devices (IFLS), or aneroid manometers (VNHS) with appropriate-sized cuffs. In the CHNS and VNHS, systolic blood pressure was measured at the first appearance of a pulse sound (Korotkoff phase 1), and diastolic blood pressure was measured at the disappearance of the pulse sound (Korotkoff phase 5); 3 measurements of systolic or diastolic blood pressure were averaged to reduce the effect of measurement errors. In the IFLS, systolic and diastolic blood pressures were obtained with a digital device (11–13). Hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or diagnosis by a doctor (16). We did not include the use of an antihypertensive medication to define hypertension, because 1) in the VNHS and IFLS, information about the treatment was not available, and 2) in the CHNS, only a small proportion of Chinese adults was diagnosed (<7%) or treated (<5%) with any antihypertensive medications and none used the medications without being diagnosed by a doctor. Moreover, sensitivity analysis showed that incorporating these measures produced similar findings but with a smaller sample size.
BMI was calculated based on weight and height, which were measured by trained health workers who followed standardized procedures and used regularly calibrated equipment. The CHNS used SECA 880 scales and SECA 206 wall-mounted metal tapes; the IFLS and VNHS used SECA 890 scales and Shorr measuring boards (11–13). Covariates such as age, sex, smoking habits, alcohol consumption, and place of residence were collected by direct interviews. The covariates were measured in the same manner in all surveys.
Statistical methods
We used Poisson regression models to examine the association between BMI and hypertension. Potential confounding factors, such as age (centered at 40 y), sex, smoking habits (dichotomized to never smoker or ever smoker), alcohol consumption (dichotomized to current drinker or nondrinker), and place of residence (urban or rural) were taken into account in regression models. A covariate was considered as an effect-measure modifier if its interaction term with BMI in regression models had a P value <0.15 (chi-square test) or as a confounder if it caused a change in prevalence ratios of >10% compared with those of the fully adjusted models. On the basis of these criteria, the most reduced model had age as an effect-measure modifier (the association between BMI and hypertension was stronger in the younger participants) and sex as confounding factor. We purposely stratified our analyses by sex to make them comparable with other studies. To estimate the ethnic differences in the BMI and hypertension association, we compared the prevalences and prevalence ratios (PRs), which were obtained from crude, age-adjusted, or age-specific models.
To evaluate an optimal BMI cutoff, we computed and searched for the shortest distance on the sex-specific ROC curve, estimated at each 0.5 unit of BMI. A distance on the ROC curve is equal to 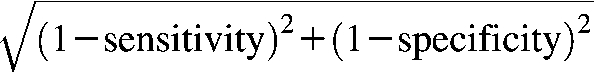 (8). On the basis of the ROC curves, we estimated the ROC area under the curve (AUC) that measured the predictability of hypertension by BMI. The AUC values ranged from 0.5 (lowest-no prediction) to 1.0 (highest-perfect prediction). Given the large sample sizes in each ethnicity, we performed stratified analyses by age and sex groups. We used 2-sided independent t tests to compare 2 means and chi-square tests to compare different proportions or test for trends of categorized variables. We conducted all analyses using Stata software version 9.2 (Stata Inc, College Station, TX).
(8). On the basis of the ROC curves, we estimated the ROC area under the curve (AUC) that measured the predictability of hypertension by BMI. The AUC values ranged from 0.5 (lowest-no prediction) to 1.0 (highest-perfect prediction). Given the large sample sizes in each ethnicity, we performed stratified analyses by age and sex groups. We used 2-sided independent t tests to compare 2 means and chi-square tests to compare different proportions or test for trends of categorized variables. We conducted all analyses using Stata software version 9.2 (Stata Inc, College Station, TX).
Role of the funding sources and ethical consideration
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The sponsors were not involved in the study design; the collection, analysis, or interpretation of data; and the writing or submission of the manuscript for publication. The Institutional Review Board of the School of Public Health, University of North Carolina at Chapel Hill, reviewed and approved the study.
RESULTS
The prevalence of hypertension in Chinese, Indonesian, and Vietnamese women was 16.6%, 26.9%, and 11.7%, respectively. Indonesian women had the highest mean diastolic and systolic blood pressures compared with the Chinese and Vietnamese women. The prevalence of hypertension in Chinese, Indonesian, and Vietnamese men was 22.9%, 24.8%, and 14.4%, respectively. Indonesian men had the highest mean diastolic and systolic blood pressures compared with Chinese and Vietnamese men (Table 1).
TABLE 1.
Characteristics of 18- to 65-y-old participants by sex and ethnic group1
| Women |
Men |
|||||||||||
| Chinese (n = 3913) |
Indonesian (n = 8888) |
Vietnamese (n = 39,711) |
Chinese (n = 3649) |
Indonesian (n = 9614) |
Vietnamese (n = 38,047) |
|||||||
| Est | 95% CI | Est | 95% CI | Est | 95% CI | Est | 95% CI | Est | 95% CI | Est | 95% CI | |
| Age (y) | 44.0 | (43.6, 44.4) | 39.12 | (38.8, 39.4) | 38.623 | (38.5, 38.8) | 43.7 | (43.3, 44.1) | 37.62 | (37.3, 37.8) | 37.123 | (37.0, 37.2) |
| Hypertension (%)4 | 16.6 | (15.4, 17.8) | 26.92 | (25.9, 27.9) | 11.723 | (11.3, 12.1) | 22.9 | (21.5, 24.2) | 24.8 | (23.8, 25.8) | 14.423 | (13.9, 14.9) |
| Systolic BP (mm Hg) | 117.8 | (117.2, 118.3) | 124.12 | (123.6, 124.7) | 116.223 | (115.9, 116.4) | 122.1 | (121.6, 122.6) | 125.82 | (125.4, 126.2) | 120.023 | (119.8, 120.3) |
| Diastolic BP (mm Hg) | 76.7 | (76.4, 77.1) | 80.42 | (80.1, 80.6) | 73.423 | (73.3, 73.6) | 80.1 | (79.7, 80.4) | 80.5 | (80.3, 80.8) | 76.123 | (75.9, 76.3) |
| BMI (kg/m2) | 23.1 | (23.0, 23.2) | 22.42 | (22.3, 22.5) | 20.423 | (20.3, 20.4) | 23.1 | (23.0, 23.2) | 21.22 | (21.2, 21.3) | 20.223 | (20.2, 20.3) |
| BMI (%) | ||||||||||||
| 15 to <18.5 kg/m2 | 6.0 | (5.3, 6.8) | 14.32 | (13.5, 15.1) | 25.923 | (25.3, 26.5) | 4.9 | (4.2, 5.6) | 16.62 | (15.8, 17.5) | 22.723 | (22.2, 23.3) |
| 18.5–22.9 kg/m2 | 47.2 | (45.6, 48.8) | 46.6 | (45.5, 47.8) | 58.723 | (58.1, 59.3) | 48.2 | (46.6, 49.8) | 59.62 | (58.5, 60.7) | 65.723 | (65.1, 66.3) |
| 23–24.9 kg/m2 | 19.8 | (18.6, 21.1) | 15.52 | (14.7, 16.3) | 9.223 | (8.9, 9.5) | 21.7 | (20.3, 23.0) | 12.02 | (11.3, 12.8) | 7.423 | (7.1, 7.7) |
| 25–29.9 kg/m2 | 23.4 | (22.1, 24.8) | 19.12 | (18.2, 20.0) | 5.723 | (5.5, 6.0) | 22.4 | (21.1, 23.8) | 10.52 | (9.8, 11.2) | 3.923 | (3.7, 4.2) |
| 30–35 kg/m2 | 3.6 | (3.0, 4.1) | 4.5 | (4.0, 4.9) | 0.523 | (0.4, 0.5) | 2.8 | (2.3, 3.3) | 1.22 | (1.0, 1.4) | 0.223 | (0.1, 0.2) |
| Weight (kg) | 56.4 | (56.1, 56.7) | 50.72 | (50.4, 50.9) | 47.323 | (47.2, 47.5) | 64.5 | (64.2, 64.9) | 55.72 | (55.5, 55.9) | 53.723 | (53.5, 53.8) |
| Height (cm) | 156.2 | (156.0, 156.4) | 150.22 | (150.0, 150.3) | 152.423 | (152.3, 152.5) | 167.1 | (166.8, 167.3) | 161.82 | (161.6, 161.9) | 162.823 | (162.7, 162.9) |
| Smoking status (%) | ||||||||||||
| Former smoker | 0.1 | (0.0, 0.2) | 0.62 | (0.4, 0.8) | 0.52 | (0.4, 0.6) | 5.6 | (4.8, 6.3) | 5.0 | (4.5, 5.5) | 13.023 | (12.5, 13.5) |
| Current smoker | 3.1 | (2.5, 3.6) | 5.12 | (4.6, 5.6) | 1.923 | (1.7, 2.1) | 58.9 | (57.3, 60.5) | 69.02 | (68.0, 70.0) | 65.023 | (64.3, 65.7) |
| Alcohol drinker (%) | 8.9 | (8.0, 9.8) | — | 1.92 | (1.7, 2.1) | 62.3 | (60.7, 63.8) | — | 53.323 | (52.4, 54.1) | ||
| Urban residence (%) | 34.0 | (32.5, 35.4) | 45.52 | (44.4, 46.7) | 26.523 | (26.0, 27.1) | 34.1 | (32.6, 35.6) | 44.92 | (43.9, 46.0) | 25.823 | (25.2, 26.5) |
Point estimates (Est) and 95% CIs were estimated with the use of survey and weighted commands to represent ≈50%, 83%, and 100% of the Chinese, Indonesian, and Vietnamese populations, respectively. The participants were 18- to 65-y-old men and women (nonpregnant or nonlactating) for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). BP, blood pressure.
Significantly different from Chinese, P < 0.05 (2-sided independent t test or chi-square test).
Significantly different from Indonesian, P < 0.05 (2-sided independent t test or chi-square test).
Defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or a previous diagnosis by a doctor.
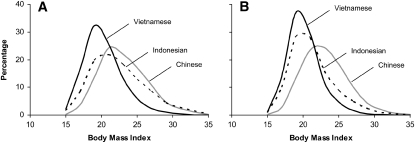
The mean BMIs of Chinese, Indonesian, and Vietnamese women were 23.1, 22.4, and 20.4, respectively. The BMI distributions were skewed to the right (skewness values: 0.5–1.0) (Figure 1A). The mean BMIs of Chinese, Indonesian, and Vietnamese men were 23.1, 21.2, and 20.2, respectively. The BMI distributions in men were also skewed to the right (skewness values: 0.5–1.0) (Figure 1B). Chinese women and men had a higher prevalence of overweight (BMI ≥ 25) than did Indonesian or Vietnamese women and men (Table 1).
FIGURE 1.
Distribution of BMI (in kg/m2) in women (A) and men (B). The samples included men from China (n = 3913), Indonesia (n = 8888), and Vietnam (n = 39,711) and women from China (n = 3649), Indonesia (n = 9614), and Vietnam (n = 38,047). The participants were 18- to 65-y-old men and women (nonpregnant or nonlactating) for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). Data were weighted to represent ≈50%, 83%, and 100% of the Chinese, Indonesian, and Vietnamese populations, respectively.
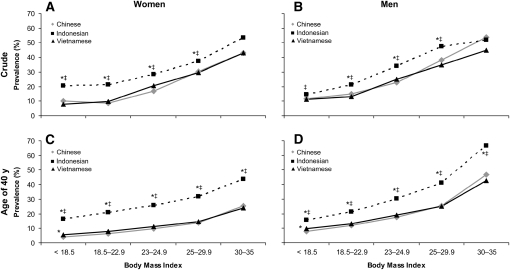
For each ethnicity, there was a significant trend of increased sex-specific prevalence of hypertension with an increase in BMI (P for trend <0.001). At each BMI level, the sex-specific prevalence of hypertension was higher in Indonesian adults than in Chinese and Vietnamese adults (P < 0.05 at almost all BMI levels) (Figure 2, A and B). The predicted prevalence of hypertension (in a hypothesized population in which all participants were aged 40 y) remained higher in Indonesian adults at all BMI levels than in Chinese and Vietnamese adults (P < 0.05 for all) (Figure 2, C and D). The prevalence estimates were precise (see Supplemental Table 1 under “Supplemental data” in the online issue).
FIGURE 2.
Prevalence of hypertension by BMI level in Chinese, Indonesian, and Vietnamese adults: crude prevalence in women (A), crude prevalence in men (B), at age of 40 y in women (C), and at age of 40 y in men (D). The samples included men from China (n = 3913), Indonesia (n = 8888), and Vietnam (n = 39,711) and women from China (n = 3649), Indonesia (n = 9614), and Vietnam (n = 38,047). The participants were 18- to 65-y-old men and women (nonpregnant or nonlactating) for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). Data were weighted to represent ≈50%, 83%, and 100% of the Chinese, Indonesian, and Vietnamese populations, respectively. The 95% CIs for the prevalence estimates are presented in Supplemental Table 1 under “Supplemental data” in the online issue. *Significantly different from the Chinese, P < 0.05 (chi-square test). ‡Significantly different from the Vietnamese, P < 0.05 (chi-square test). P for trend < 0.001 for all.
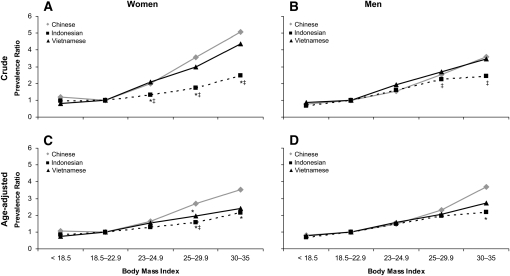
On average, each unit increase in BMI was associated with an increase of 18%, 8%, and 16% (women) and of 14%, 12%, and 14% (men) in the PR for hypertension in Chinese, Indonesian, and Vietnamese adults, respectively (crude models). In women, there was a significant trend of increased ethnic-specific PRs with an increase in BMI (P for trend < 0.001) in both crude and age-adjusted models. PR was highest in Chinese women and lowest in Indonesian women (Figure 3, A and B). There was also a significant trend of increased sex-specific PRs with an increase in BMI in Chinese, Indonesian, and Vietnamese men (P for trend < 0.001). PR was comparable in men, except among the obese (BMI ≥ 30), in an age-adjusted model (Figure 3, C and D). The PR estimates were precise (see Supplemental Table 2 under “Supplemental data” in the online issue).
FIGURE 3.
Prevalence ratios of hypertension by BMI level in Chinese, Indonesian, and Vietnamese: crude value in women (A), crude value in men (B), age-adjusted in women (C), and age-adjusted in men (D). The samples included men from China (n = 3913), Indonesia (n = 8888), and Vietnam (n = 39,711) and women from China (n = 3649), Indonesia (n = 9614), and Vietnam (n = 38,047). The participants were 18- to 65-y-old men and women (nonpregnant or nonlactating) for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). Data were weighted to represent ≈50%, 83%, and 100% of the Chinese, Indonesian, and Vietnamese populations, respectively. The 95% CIs for the prevalence estimates are presented in Supplemental Table 2 under “Supplemental data” in the online issue. *Significantly different from the Chinese, P < 0.05 (2-sided independent t test). ‡Significantly different from the Vietnamese, P < 0.05 (2-sided independent t test). P for trend < 0.001 for all.
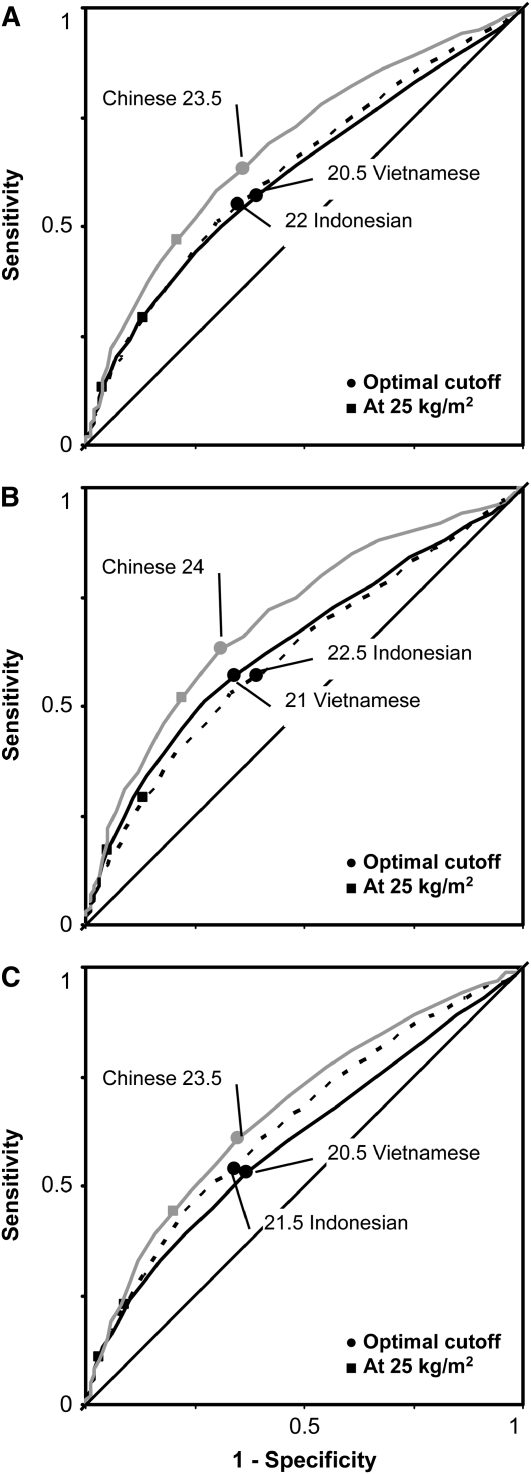
Based on the shortest distance on the ROC curve (corresponding to the largest sum sensitivity and specificity), optimal BMI cutoffs were 23.5, 22.0, and 20.5 in Chinese, Indonesian, and Vietnamese adults, respectively. A BMI of 25 provided lower sensitivities, especially in Vietnamese and Indonesian adults (Figure 4A). Similar trends were found in women (Figure 4B) and men (Figure 4C). Based on the ROC curve approach, optimal BMI cutoffs in Chinese adults were larger than those in Indonesian (≈1.5 units) and Vietnamese (≈3 units) adults. BMI cutoffs were ≈0.5–1.0 unit higher in women than in men and in the older (41–65 y) than in the younger (18–40 y) participants. Stratified and overall analyses showed optimal BMI cutoffs of 23–24, 21–22.5, and 20.5–21 for Chinese, Indonesian, and Vietnamese adults, respectively. AUC values for the prediction of hypertension by BMI, which ranged from 0.6 to 0.7, were highest in the Chinese in both the overall and stratified analyses (Table 2). The estimations of AUC values were precise (see Supplemental Table 3 under “Supplemental data” in the online issue).
FIGURE 4.
Receiver operating characteristic (ROC) curves for the prediction of hypertension by BMI in Chinese, Indonesian, and Vietnamese adults: in both sexes (A), in women (B), and in men (C). The samples included men from China (n = 3913), Indonesia (n = 8888), and Vietnam (n = 39,711) and women from China (n = 3649), Indonesia (n = 9614), and Vietnam (n = 38,047). The participants were 18- to 65-y-old men and women (nonpregnant or nonlactating) for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). The 95% CIs of the area under the ROC curve are presented in Supplemental Table 3 under “Supplemental data” in the online issue.
TABLE 2.
Optimal BMI cutoffs (in kg/m2) for overweight by sex and age groups1
| Chinese |
Indonesian |
Vietnamese |
|||||||||||||
| n | Cutoff | Sen | Spe | AUC | n | Cutoff | Sen | Spe | AUC | n | Cutoff | Sen | Spe | AUC | |
| Both sexes | |||||||||||||||
| All ages | 7562 | 23.5 | 0.63 | 0.64 | 0.68 | 18,502 | 22 | 0.55 | 0.65 | 0.632 | 77,758 | 20.5 | 0.57 | 0.61 | 0.622 |
| 18–40 y | 2843 | 23 | 0.63 | 0.66 | 0.68 | 11,373 | 21.5 | 0.57 | 0.61 | 0.632 | 44,646 | 20.5 | 0.56 | 0.65 | 0.64 |
| 41–65 y | 4719 | 24 | 0.59 | 0.65 | 0.66 | 7129 | 22.5 | 0.54 | 0.64 | 0.612 | 33,112 | 21 | 0.52 | 0.62 | 0.5923 |
| Women | |||||||||||||||
| All ages | 3913 | 24 | 0.63 | 0.69 | 0.70 | 8888 | 22.5 | 0.57 | 0.61 | 0.622 | 39,711 | 21 | 0.57 | 0.66 | 0.642 |
| 18–40 y | 1451 | 23 | 0.66 | 0.69 | 0.68 | 5200 | 22.5 | 0.56 | 0.64 | 0.63 | 21,766 | 20.5 | 0.57 | 0.64 | 0.64 |
| 41–65 y | 2462 | 24 | 0.64 | 0.63 | 0.68 | 3688 | 22.5 | 0.58 | 0.56 | 0.592 | 17,945 | 21 | 0.58 | 0.59 | 0.602 |
| Men | |||||||||||||||
| All ages | 3649 | 23.5 | 0.61 | 0.65 | 0.67 | 9614 | 21.5 | 0.54 | 0.66 | 0.64 | 38,047 | 20.5 | 0.53 | 0.63 | 0.6023 |
| 18–40 y | 1392 | 23.5 | 0.58 | 0.67 | 0.67 | 6173 | 21 | 0.57 | 0.61 | 0.64 | 22,880 | 20.5 | 0.55 | 0.65 | 0.63 |
| 41–65 y | 2257 | 23.5 | 0.61 | 0.63 | 0.66 | 3441 | 22 | 0.54 | 0.67 | 0.63 | 15,167 | 20.5 | 0.52 | 0.58 | 0.5823 |
The participants were 18- to 65-y-old men and women (nonpregnant or nonlactating) for whom measurements of weight, height, and blood pressure were complete and plausible (eg, BMI: 15–35; weight: 30–150 kg; height: 130–190 cm; and difference between systolic and diastolic blood pressures: ≥10 mm Hg). Area under the curve (AUC) values range from 0.5 (no prediction) to 1.0 (perfect prediction). The 95% CI values of the AUCs are presented in Supplemental Table 3 under “Supplemental data” in the online issue. Sen, sensitivity; Spe, specificity.
Significantly different from Chinese, P < 0.05 (2-sided independent t test or chi-square test).
Significantly different from Indonesian, P < 0.05 (2-sided independent t test or chi-square test).
DISCUSSION
In this study, we found ethnic differences in the association between BMI and hypertension in Chinese, Indonesian, and Vietnamese adults. The differences could be explained by the ethnic variation in body fat. For example, at the same age, sex, and BMI, Indonesians have more body fat than do Chinese adults (17). On the assumption that total visceral fat is linearly associated with total body fat, Indonesian adults would have more visceral fat at a given BMI and thus have a higher cardiovascular disease risk than do Chinese adults (18, 19).
Differences in genes and gene-environment interactions would be another potential explanation for the differences in the BMI-specific prevalence of hypertension. Findings from gene-family environment, twin, and migration studies showed the roles of genetic, ethnic, and environmental factors in the development of chronic diseases (20–23). Chinese and Vietnamese populations are expected to share more genetic background than the Indonesian population because they evolved from a branch of Homo sapiens that differed from the branch that evolved into the Indonesians (4, 24). The different genetic backgrounds may interact differently with diverse environmental factors relating to hypertension, such as dietary intakes, physical activity levels, food habits, cultures, religions, demographic characteristics, and socioeconomic status (25, 26), which lead to different prevalences of hypertension. In-depth analyses that take into account genetic, individual, and environmental factors are still needed to explore the complex association between BMI and related chronic diseases.
The variation in blood pressure measurement protocols is unlikely to alter our overall conclusion. First, the surveys (the CHNS, IFLS, and VNHS) followed a standardized protocol for measuring blood pressures (11–13, 16). Second, the regularly calibrated sphygmomanometers used in these surveys are in the list of recommended equipment (27). Finally, rounding error in measuring blood pressure was not an issue in any of the surveys. However, because the first measurement of blood pressure is usually higher than the next measurements (16), blood pressures would be systematically elevated in the IFLS compared with the mean of 3 measurements (if they had been ideally collected). Compared with the mean of 3 measurements, the first measurement caused a negligible overestimation of systolic and diastolic blood pressures (<0.15 and <0.05 mm Hg in the CHNS and <1.25 and <0.75 mm Hg in the VNHS). A sensitivity analysis with a subtraction of 1.5 mm Hg from systolic and 1.0 mm Hg from diastolic blood pressures in the IFLS showed similar PR, AUC, and BMI cutoffs (see Supplemental Tables 4 and 5 under “Supplemental data” in the online issue). A similar conclusion was found in another sensitivity analysis in which the first measure of blood pressure in the CHNS and VNHS was used (see Supplemental Tables 6 and 7 under “Supplemental data” in the online issue).
Our study appears to provide stronger evidence of ethnic differences in the association between BMI and hypertension than does a study by Tesfaye et al (28), who found higher mean systolic and diastolic blood pressures and prevalences of hypertension in the Indonesians than in the Vietnamese adults. However, the BMI-hypertension association might be inflated in the Indonesian sample because it included older, heavier participants who lived in urban areas (10% in the Indonesian and 0% in Vietnamese samples were urban residents). Also, because the absolute values of corresponding BMI quintiles were larger in the Indonesian sample, the association would be systematically elevated in the Indonesians compared with the Vietnamese adults. To overcome these limitations, we 1) used large representative samples that ensured the generalizability of the finding; 2) used the same BMI categories in all populations, which ensured the comparability across countries; and 3) compared age-specific prevalence of hypertension in Chinese, Indonesian, and Vietnamese adults, which helped to eliminate the difference in age structures in different populations.
The ethnic difference in the association between BMI and hypertension is not the only explanation for the ethnic variation in optimal BMI cutoffs. The sensitivity and specificity of a screening test depend on distributions of exposure, outcome, and other covariates. For example, if we hold other variables unchanged and add a constant to BMI, the optimal BMI level will increase with the added value. This mathematic imputation shows that the BMI distribution affects optimal BMI level for predicting a disease outcome. Our finding that a higher BMI cutoff is found in a population with a higher mean BMI is consistent with previous studies (5, 7, 9).
However, the difference in mean BMI could not explain all of the variation in optimal BMI cutoffs. An increase in cardiovascular disease risk, which is associated with an increase in BMI, allows us to detect elevated cardiovascular disease risk at a lower BMI, and thus, leads to a decrease in optimal BMI cutoffs. In combination with other complex changes or differences in environment, although we know the difference in mean BMI of given populations, we are not able to predict the difference in optimal BMI cutoffs. For example, in our samples, the ethnic difference in the mean BMI was not proportionally related to the variation in the optimal BMI cutoffs. In this sample, Chinese men had a higher mean BMI, but a lower optimal BMI cutoff compared with another Chinese sample (5); Chinese women had the same mean BMI, but a higher optimal BMI cutoff compared with Indian women (9). Also, South Asian Canadians had a higher mean BMI, but a lower BMI cutoff based on lipid factors compared with Chinese Canadians (22). Because the variation in the mean BMI and prevalence of hypertension are probably clustered by groups of ethnicities, genetic factors, age, sex, lifestyles, and socioeconomic status, an ethnic- or country-specific BMI cutoff should be understood as the combination of all factors.
The recommendation to lower BMI cutoffs for Chinese, Indonesian, and Vietnamese adults is consistent with results from large-scale, cross-sectional studies in Asian populations (5, 7–10). In those studies, a BMI cutoff of 22–24 was associated with an increase in the prevalences of hypertension, diabetes mellitus, dyslipidemia, and cardiovascular diseases. The explanations for the ethnic differences were presented elsewhere (1, 29, 30). To summarize, first, Asian ethnicities tend to have more total body fat (2, 3) and more abdominal and visceral fat (31, 32) at a given BMI than do other races and ethnicities, which lead to increased cardiovascular disease risk (18, 19). Second, differences in gene, environmental, and gene-environment interactions (18, 20, 23–26, 33) might lead to ethnic variation in blood pressure phenotypes. Third, there is also speculation that any insults during fetal development and infancy might have also resulted in the elevated risks (34–38). In addition, Asian populations are younger and have a lower mean BMI than do Western populations, which both lead to a decrease in optimal cutoffs.
Our findings were based on large, recent, representative samples from China, Indonesia, and Vietnam, which ensured the generalizability of the findings to respective populations. However, similar to other cross-sectional studies, we cannot determine that the exposure to a higher BMI had preceded hypertension. Compared with optimal BMI cutoffs for overweight for Chinese adults obtained from the CHNS 2000–2004 cohort (30), these optimal BMI cutoffs in Chinese (from the CHNS cross-sectional sample in 2004) were 0.5–1.0 units larger. Given that almost all studies aimed at determining optimal BMI cutoffs for Asians were based on cross-sectional samples, a certain adjustment for the cross sectional cutoffs might be needed.
In conclusion, although ethnic differences exist in the association between BMI and hypertension and in optimal BMI cutoffs for overweight in Chinese, Indonesian, and Vietnamese adults, an optimal BMI cutoff for overweight of <25 may be more appropriate for the East and Southeast Asian populations. A lower BMI cutoff is important in developing countries because in these countries, overweight and related chronic diseases are becoming emerging problems (39) and are not adequately prevented, diagnosed, and managed (40). Country-specific or even country-, sex-, and age-specific BMI cutoffs for overweight would be needed to identify persons at a high risk of cardiovascular diseases and to reduce health and economic burdens of both overweight and chronic diseases.
Acknowledgments
We thank Frances Dancy for administrative assistance.
The authors' responsibilities were as follows—NTT: designed the study, acquired the data, analyzed and interpreted the data, and drafted the manuscript; LSA: assisted in the analysis and interpretation of the data and provided critical intellectual feedback on the manuscript; CMS: assisted in the data analysis and provided critical intellectual feedback on the manuscript; KH: assisted in the interpretation of the data and provided critical intellectual feedback on the manuscript; and BMP: assisted in the study design and in the interpretation of the data and provided critical intellectual feedback on the manuscript. All authors have read and approved the final version of the manuscripts. None of the authors had a conflict of interest related to any part of this study or manuscript.
REFERENCES
- 1.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63 [DOI] [PubMed] [Google Scholar]
- 2.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev 2002;3:141–6 [DOI] [PubMed] [Google Scholar]
- 3.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN., Jr Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 1994;60:23–8 [DOI] [PubMed] [Google Scholar]
- 4.Macaulay V, Hill C, Achilli A, et al. Single, rapid coastal settlement of Asia revealed by analysis of complete mitochondrial genomes. Science 2005;308:1034–6 [DOI] [PubMed] [Google Scholar]
- 5.Bei-Fan Z. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr 2002;11(Suppl 8)S685–93 [PubMed] [Google Scholar]
- 6.Ho SY, Lam TH, Janus ED. Waist to stature ratio is more strongly associated with cardiovascular risk factors than other simple anthropometric indices. Ann Epidemiol 2003;13:683–91 [DOI] [PubMed] [Google Scholar]
- 7.Lin WY, Lee LT, Chen CY, et al. Optimal cut-off values for obesity: using simple anthropometric indices to predict cardiovascular risk factors in Taiwan. Int J Obes Relat Metab Disord 2002;26:1232–8 [DOI] [PubMed] [Google Scholar]
- 8.Wildman RP, Gu D, Reynolds K, Duan X, He J. Appropriate body mass index and waist circumference cutoffs for categorization of overweight and central adiposity among Chinese adults. Am J Clin Nutr 2004;80:1129–36 [DOI] [PubMed] [Google Scholar]
- 9.Mohan V, Deepa M, Farooq S, Narayan KM, Datta M, Deepa R. Anthropometric cut points for identification of cardiometabolic risk factors in an urban Asian Indian population. Metabolism 2007;56:961–8 [DOI] [PubMed] [Google Scholar]
- 10.Huxley R, James WP, Barzi F, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev 2008;9:53–61 [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health, General Statistical Office The report: results of the Vietnam National Health Survey 2001-2002. Hanoi, Vietnam: Medical Publishing House, 2003 [Google Scholar]
- 12.Popkin BM, Paeratakul S, Zhai F, Ge K. Dietary and environmental correlates of obesity in a population study in China. Obes Res 1995;3(suppl 2)135s–43s [DOI] [PubMed] [Google Scholar]
- 13.Strauss J, Beegle K, Sikoki B, Dwiyanto A. Y. H, Witoelar F. The third wave of the Indonesia Family Life Survey (IFLS3). Overview and field report. RAND Labor and Population Program, 2004. Available from: http://www.rand.org/FLS/IFLS (cited 18 March 2007)
- 14.Tuan NT, Tuong PD, Popkin BM. Body mass index (BMI) dynamics in vietnam. Eur J Clin Nutr 2008;62:78–86 [DOI] [PubMed] [Google Scholar]
- 15.WHO Expert Committee Physical status: the use and interpretation of anthropometry. Geneva, Switzerland: WHO, 1995 [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72 [DOI] [PubMed] [Google Scholar]
- 17.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes 1998;22:1164–71 [DOI] [PubMed] [Google Scholar]
- 18.Kaplan N. Primary hypertension: pathogenesis. In: Kaplan N, ed Kaplan's clinical hypertension. 9th ed Philadelphia, PA: Lippincott Williams & Wilkins, 2006:50–121 [Google Scholar]
- 19.Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol 2007;36:220–5 [DOI] [PubMed] [Google Scholar]
- 20.Luft FC. Twins in cardiovascular genetic research. Hypertension 2001;37:350–6 [DOI] [PubMed] [Google Scholar]
- 21.Misra A, Ganda OP. Migration and its impact on adiposity and type 2 diabetes. Nutrition 2007;23:696–708 [DOI] [PubMed] [Google Scholar]
- 22.Razak F, Anand SS, Shannon H, et al. Defining obesity cut points in a multiethnic population. Circulation 2007;115:2111–8 [DOI] [PubMed] [Google Scholar]
- 23.Cui J, Hopper JL, Harrap SB. Genes and family environment explain correlations between blood pressure and body mass index. Hypertension 2002;40:7–12 [DOI] [PubMed] [Google Scholar]
- 24.Maca-Meyer N, Gonzalez AM, Larruga JM, Flores C, Cabrera VM. Major genomic mitochondrial lineages delineate early human expansions. BMC Genet 2001;2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell AC, Adair LS, Popkin BM. Understanding the role of mediating risk factors and proxy effects in the association between socio-economic status and untreated hypertension. Soc Sci Med 2004;59:275–83 [DOI] [PubMed] [Google Scholar]
- 26.Merlo J, Asplund K, Lynch J, Rastam L, Dobson A. Population effects on individual systolic blood pressure: a multilevel analysis of the World Health Organization MONICA Project. Am J Epidemiol 2004;159:1168–79 [DOI] [PubMed] [Google Scholar]
- 27.O'Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ 2001;322:531–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tesfaye F, Nawi NG, Van Minh H, et al. Association between body mass index and blood pressure across three populations in Africa and Asia. J Hum Hypertens 2007;21:28–37 [DOI] [PubMed] [Google Scholar]
- 29.Misra A. Revisions of cutoffs of body mass index to define overweight and obesity are needed for the Asian-ethnic groups. Int J Obes Relat Metab Disord 2003;27:1294–6 [DOI] [PubMed] [Google Scholar]
- 30.Nguyen TT, Adair LS, He K, Popkin BM. Optimal cutoff values for overweight: using body mass index to predict incidence of hypertension in 18- to 65-year-old Chinese adults. J Nutr 2008;138:1377–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 2007;86:353–9 [DOI] [PubMed] [Google Scholar]
- 32.Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res 2001;9:381–7 [DOI] [PubMed] [Google Scholar]
- 33.Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation 2000;101:329–35 [DOI] [PubMed] [Google Scholar]
- 34.Adair LS, Cole TJ. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 2003;41:451–6 [DOI] [PubMed] [Google Scholar]
- 35.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab 2002;13:364–8 [DOI] [PubMed] [Google Scholar]
- 36.Demerath EW, Cameron N, Gillman MW, Towne B, Siervogel RM. Telomeres and telomerase in the fetal origins of cardiovascular disease: a review. Hum Biol 2004;76:127–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams S, Poulton R. Birth size, growth, and blood pressure between the ages of 7 and 26 years: failure to support the fetal origins hypothesis. Am J Epidemiol 2002;155:849–52 [DOI] [PubMed] [Google Scholar]
- 38.Singhal A, Lucas A. Early origins of cardiovascular disease: is there a unifying hypothesis? Lancet 2004;363:1642–5 [DOI] [PubMed] [Google Scholar]
- 39.Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr 2006;84:289–98 [DOI] [PubMed] [Google Scholar]
- 40.Mendis S, Abegunde D, Oladapo O, Celletti F, Nordet P. Barriers to management of cardiovascular risk in a low-resource setting using hypertension as an entry point. J Hypertens 2004;22:59–64 [DOI] [PubMed] [Google Scholar]