Abstract
Ingestion of different nutrients, such as fats and sugars, normally produces different effects on physiology, the brain, and behavior. However, they do share certain neural pathways for reinforcement of behavior, including the mesolimbic dopamine (DA) system. When these nutrients are consumed in the form of binges, this can release excessive DA, which causes compensatory changes that are comparable to the effects of drugs of abuse. In this article, we review data obtained with animal models of fat and sugar bingeing. The concept of “food addiction” is described and reviewed from both clinical and laboratory animal perspectives. Behavioral manifestations of addictive-like behavior and concomitant alterations in DA and opioid systems are compared for sugar and fat bingeing. Finally, in relation to eating disorders and obesity, we discuss how fat may be the macronutrient that results in excess body weight, and sweet taste in the absence of fat may be largely responsible for producing addictive-like behaviors that include a withdrawal syndrome.
Introduction
Although binge-eating behavior is traditionally associated with eating disorders, it is becoming more prevalent in the United States through its emergence in a variety of clinical and nonclinical populations. Binge eating has been linked to obesity, which presently afflicts 33% of the adult U.S. population (1,2) and may also be a predictor of body-fat gain among children (3). Binge eating is also associated with increased frequency of body weight fluctuation, depression, anxiety, and substance abuse (4–6). The presence of bingeing behavior in several different eating disorders, as well as in nonclinical populations, has made it important to study from a public-health perspective.
The Diagnostic and Statistical Manual of Mental Disorders (ed. 4) defines binge eating as a series of recurrent binge episodes in which each episode is defined as eating a larger amount of food than normal during a short period of time (usually within any 2-h period) (7). Binge-eating episodes are associated with 3 or more of the following: 1) eating until feeling uncomfortably full, 2) eating large amounts of food when not physically hungry, 3) eating much more rapidly than normal, 4) eating alone because one is embarrassed by how much s/he is eating, 5) feeling disgusted, depressed, or guilty after overeating, or 6) marked distress or anxiety regarding binge eating.
Aside from diagnosed patients, there is also a far larger population of individuals who often binge on food, but perhaps not regularly enough to warrant a clinical diagnosis. It is not always clear where one draws the line between simply eating a large meal and a pathological binge. However, the physiological consequences of binge eating may be similar, whether engaged in naturally because of hunger, casually for social or hedonic reasons, or regularly enough to warrant a diagnosis.
What are the common binge foods?
To put it simply, people usually binge on highly palatable energy-rich food. These foods are typically high in fats, sugars, or often both (8,9). Binge episodes often involve consumption of bread or pasta, followed in frequency by sweets, fatty foods, or salty snacks (10). Individuals with a preference for bingeing on sweet foods tend to binge more frequently.
Why do people not binge on broccoli? There must be some property of palatable “dessert” and “snack” foods rich in sugar and/or fat that promotes binge eating. Sugars and fats are well known to have different effects on physiology and brain chemistry (11), which may be related to their different effects on behavior. To understand the behavioral and neurochemical basis of binge eating on specific macronutrients, we turn to laboratory animal models of binge eating.
Animal models of binge eating
Binge eating is a multifaceted behavior, with emotional and cultural components that are difficult to reproduce with animal models. Nonetheless, animal models of binge eating are fundamental to understanding the physiological and neurochemical basis of this behavior.
Models of sugar bingeing
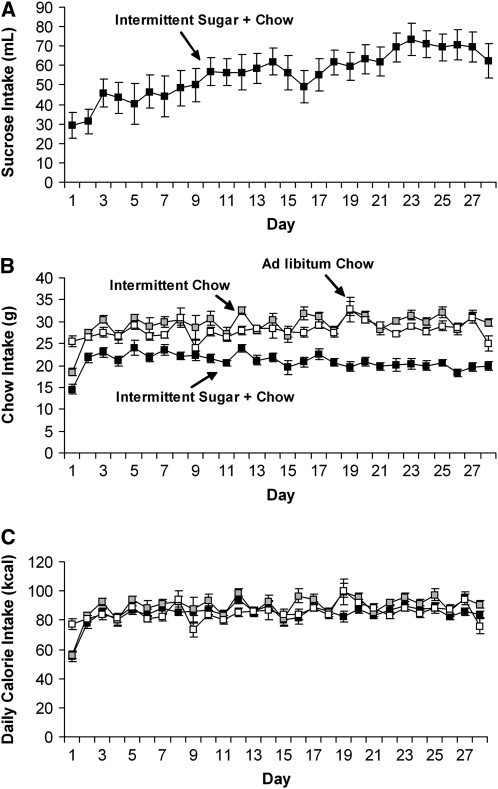
Several laboratories have used limited access to sugar solutions to model binge eating (12–15). The findings all suggest that animals will engage in binge-type eating on a sweet food when it is offered intermittently. Our laboratory has developed a model of sugar bingeing (16) in which rats are maintained on daily 12-h food restriction, followed by access to a 25% glucose or 10% sucrose solution (similar to the sugar concentration of a soft drink) and a nonpurified rodent diet. After a few days on this schedule, these rats escalate their daily intake of sugar (Fig. 1 A) and begin to binge, as indicated by an increase in their intake of the sugar solution during the first hour of access. Rats that have access to the sugar solution and nonpurified diet ad libitum consume a total daily amount similar to that consumed by the bingeing rats, but they seldom engage in discrete bingeing episodes. Body weight and total daily caloric intake do not differ from normal in rats that are bingeing on sugar (Fig. 1 C), indicating that the rats are able to regulate their energy intake and compensate for the excess energy by eating less rodent nonpurified diet (Fig. 1 B).
FIGURE 1 .
Sugar and nonpurified diet (chow) intake during the 28-d access period in a rat model of sugar bingeing. Rats with intermittent sugar + nonpurified diet escalated their total daily sugar intake over time (A). Rats with intermittent sugar + nonpurified diet ate less nonpurified diet than the intermittent nonpurified diet group and the nonpurified diet ad libitum control group (B); however, the groups did not differ in total daily energy intake (1 kcal = 4.184 kJ) (C). Values are means ± SEM, n = 9–10/group. Reproduced with permission from Avena et al. (23).
Models of fat bingeing
Animals will also binge on pure fat, which suggests that binge eating is not exclusive to sweet taste. Corwin et al. (17) have shown that sated rats with access to rodent nonpurified diet ad libitum will binge on a vegetable fat (shortening) when it is presented for 2 h each day, and this effect is enhanced when the fat is offered only 3 times per week. A similar finding has been reported with shortening that is trans-fat-free (18). Rats with restricted access to vegetable fat do not show alterations in body weight or body-fat accrual compared with nonpurified diet–fed controls (17,19); however, they do show elevated plasma leptin levels (19).
Models of bingeing on sweet-fat mixtures
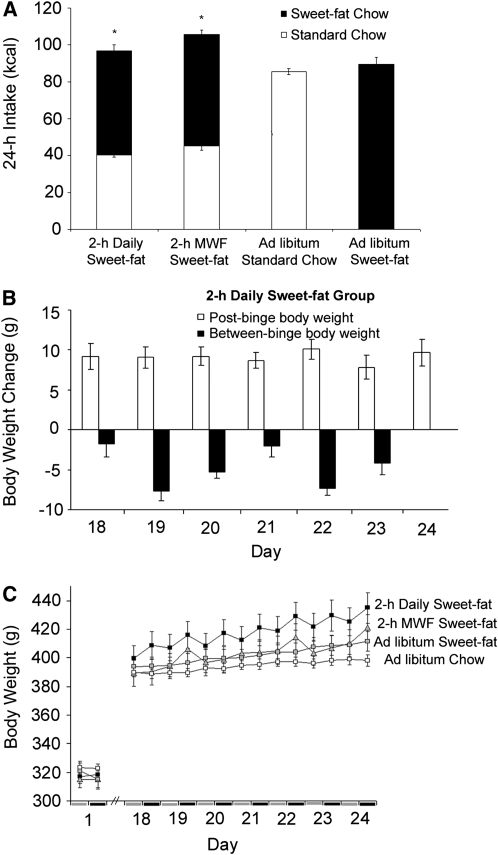
The combination of sweet and fat activates multiple taste receptors, postingestive signals, and neuropeptide systems. Sugar-fat combinations, in the form of cookies or sugar-fat mixtures, have been used by Boggiano and others to induce binge eating in laboratory models (20,21). We have developed a model of binge eating using a nutritionally complete sweet-fat diet in rats that are not food-restricted (22). Rats with 2-h daily access to a sweet-fat food [Research Diets #12451 pellets, 45% fat, 20% protein, 35% carbohydrate, 4.7 kcal/g (20 kJ/g)] binge on it, even though they have access to standard rodent nonpurified diet ad libitum for the other 22 h/d. By wk 3 of access, the bingeing behavior is most pronounced, and these rats consume, on average, 58% of their daily energy intake during the 2-h period of access to the sweet-fat food (Fig. 2 A). These rats self-restrict their intake of standard nonpurified diet, similar to the effects we have reported with sugar (23) and others have reported with fat (17,19) or sugar-rich diets (14). Cyclic bingeing and self-imposed food restriction result in fluctuations in daily body weight characterized by weight loss between binges (Fig. 2 B). However, even if we take into consideration the self-restriction of standard rodent nonpurified diet between binges, an overall increase in body weight occurs in rats bingeing on sweet-fat pellets when compared with control groups that are fed only standard rodent nonpurified diet or access to the same sweet-fat pellets ad libitum (Fig. 2 C). Thus, this model represents binge eating that can result in increased body weight.
FIGURE 2 .
Energy intake and body weight alterations in a rat model of fat bingeing. Total daily energy intake during wk 3 of access expressed as energy derived from standard nonpurified diet (chow) (white) versus sweet-fat nonpurified diet (black) (A). The 2-h daily sweet-fat group and a group that received 2-h of sweet-fat diet only on Mondays, Wednesdays, and Fridays (2-h MWF Sweet-fat) both consume >50% of their daily energy from sweet-fat diet when it is available. A sawtooth pattern emerges for the 2-h daily sweet-fat group in which they decrease in weight prebinge and increase in weight postbinge each day (B). However, despite this fluctuation in body weight throughout the day, the rats with 2-h daily sweet-fat gained significantly more total body weight than rats fed standard nonpurified diet ad libitum (1 kcal = 4.184 kJ) (C). Values are means ± SEM, n = 10/group. *Different from the standard chow ad libitum group, P < 0.05. Adapted with permission from Berner et al. (22).
Food addiction
Many scientists have speculated that obesity and eating disorders, such as bulimia and anorexia, may have properties of an “addiction” (24–30). Moreover, several popular self-help books have been written on the topic of “sugar addiction” (31–34, to name just a few). Clinical and laboratory animal studies reveal similarities between overeating and drug addiction.
Clinical support for the theory of food addiction
A recent clinical study suggests that carbohydrates can have abuse potential for “carbohydrate cravers” (35). Likewise, craving-related changes in response to palatable foods have been identified using brain imaging techniques, and these changes are similar to those seen during drug craving (36,37). Dopamine (DA)4 has been suggested to have a common role in drug abuse and obesity (28). Positron emission tomography scans reveal that obese subjects show a reduction in striatal D2 receptor availability that is correlated with the body weight of the subject (38) and similar in magnitude to the reductions reported in drug-addicted subjects (39). Opioids have also been the focus of clinical studies (25). Appetite dysfunctions in the form of either binge eating or self-starvation can affect endogenous opioid activity (40). Collectively, these clinical studies support the view that overeating can affect behavior and brain systems in a manner that resembles aspects of an addiction.
Behavioral evidence of sugar dependence in laboratory animals
Many of the behaviors and neurochemical changes that are characteristic of drug abuse are also apparent in our animal model of sugar bingeing described above and summarized in Table 1. This model is reviewed and related to the substance abuse literature in greater detail elsewhere (16).
TABLE 1.
Signs of dependence observed in sugar-bingeing rats1
| Sign | Result | Reference |
|---|---|---|
| Behavioral signs | ||
| Tolerance | Escalation of sugar intake over time | 15, 55 |
| Sensitization | Large meals of sugar in the form of binges | 15, 16, 55 |
| Opiate-like withdrawal | Anxiety, somatic indications | 23, 41 |
| Decreased body temperature | 45 | |
| Deprivation effect | Increase intake of sugar following an abstinence period | 42 |
| Incubation of responding | Temporal increase in responding for cues associated with sugar | 47 |
| Cross-sensitization | Locomotor sensitization to amphetamine | 43 |
| Locomotor sensitization to cocaine | 48 | |
| Consummatory sensitization to alcohol | 44 | |
| Neurochemical signs | ||
| Neurotransmitter | ||
| Dopamine | Repeated release in the NAc | 55, 72 |
| Increased D1 receptor binding in the NAc | 15 | |
| Decreased D2 receptor binding in the striatum | 15 | |
| Decreased D2 receptor binding in the NAc | 59 | |
| Decreased D2 receptor mRNA in the NAc | 53 | |
| Decreased D3 receptor mRNA in the STR and NAc | 53 | |
| Increased DAT in the NAc and VTA | 12 | |
| Increased DAT mRNA in the VTA | 12 | |
| Increased DA turnover in the NAc | 60 | |
| Acetylcholine | Delay in rise in the NAc | 55 |
| No rise in the NAc when sham feeding | 72 | |
| Opioids | Respond to naloxone with signs of opiate-withdrawal | 41 |
| Increased μ-opioid receptor binding in the NAc, cingulate, hippocampus, and locus coeruleus | 15 | |
| Decreased enkephalin gene expression in the NAc | 53 |
Adapted with permission from Avena et al. (71). STR, striatum; DAT, dopamine transporter; VTA, ventral tegmental area.
Briefly, rats given daily intermittent access to a sugar solution and nonpurified diet escalate their sugar intake and increase their intake during the first hour of daily access, which we define as a “binge” (15). Sugar-bingeing rats show signs of opiate-like withdrawal when administered a relatively high dose of the opioid antagonist naloxone (3 mg/kg, subcutaneous). Somatic signs of withdrawal, such as teeth chattering, forepaw tremor, and head shakes, as well as behavioral manifestations of anxiety, are observed (41). Similar signs of opiate-like withdrawal emerge spontaneously without the use of an opioid antagonist when all food is removed for 24 h (23,41). Sugar-bingeing rats lever press for 23% more sugar in a test after 2 wk without sugar than they ever did before (42), suggesting a change in the motivational impact of sugar that persists and increases throughout a period of abstinence. We have also shown that rats bingeing on sugar develop locomotor cross-sensitization to a low challenge dose of amphetamine (0.5 mg/kg, intraperitoneally) that has little or no effect on naive rats (43). When rats are bingeing on sugar and then are forced to abstain, they subsequently show enhanced intake of 9% alcohol (44), suggesting that intermittent access to sugar can be a gateway to alcohol use.
Other researchers have obtained supportive behavioral findings using similar models of sugar bingeing. Signs of anxiety have been reported in rats with limited access to a high-sucrose diet (14). The mere removal of sugar has been reported to decrease body temperature (45). Also, aggressive behavior has been observed during removal of a diet that involves intermittent sugar access (46). Using operant conditioning, Grimm et al. (47) find that sucrose seeking increases during a month of sugar abstinence in rats that had intermittent sugar access. Intermittent sucrose access cross-sensitizes not only with amphetamine (43) but also with cocaine (48) and facilitates sensitization to the DA agonist quinpirole (49). These results support the theory that the DA system is sensitized by intermittent sugar access; this is important because enhanced mesolimbic dopaminergic neurotransmission plays a role in the behavioral effects of sensitization as well as cross-sensitization (50) and may contribute to addiction (51,52).
Neurochemical evidence of sugar dependence
The evidence described above suggests that sugar bingeing can produce behaviors that are similar to those observed in drug-dependent rats. Concomitant neurochemical changes may result in, or perpetuate, these behaviors. These signs are also summarized in Table 1 and are explained in greater detail in an earlier article (16).
We have found changes in DA, acetylcholine (ACh), and opioid systems in sugar-bingeing rats that are similar to those observed with some drugs of abuse. Autoradiography reveals increased D1 receptor binding in the nucleus accumbens (NAc) and decreased D2 receptor binding in the striatum relative to nonpurified diet-fed rats (15). Rats with intermittent sugar and nonpurified diet access also have decreased D2 receptor mRNA in the NAc, and increased D3 receptor mRNA in the NAc and dorsal striatum compared with nonpurified diet-fed controls (53). Sugar-bingeing rats have a significant decrease in enkephalin mRNA (53), whereas μ-opioid receptor binding is significantly enhanced in the NAc shell, cingulate, hippocampus, and locus coeruleus (15).
One of the strongest neurochemical commonalities between sugar bingeing and drugs of abuse is their effect on extracellular DA. A hallmark of drugs that are abused is repeated increase in extracellular DA, whereas during normal feeding, the DA response fades out after repeated exposure to a food (54). When rats are bingeing on sugar, the release of DA is recurrent, which may make the brain adapt as it does to a drug of abuse. Rats that are bingeing on sugar apparently release DA every day, as measured on d 1, 2, and 21 of access (55). Control rats fed sugar or nonpurified diet ad libitum, rats with intermittent access to just nonpurified diet, or rats that taste sugar only 2 times, develop a blunted DA response that is typical of a food that loses its novelty.
Withdrawal from drugs such as morphine, nicotine, and alcohol is often accompanied by alterations in DA/ACh balance in the NAc: specifically, DA decreases while ACh increases (56–58). Rats bingeing on sugar also show this neurochemical imbalance in DA/ACh during withdrawal. This result occurs both when rats are given naloxone to precipitate opiate-like withdrawal (41) or after 36 h of food deprivation (23).
Others have reported supportive findings. There is a decrease in D2 receptor binding in the NAc of rats with intermittent access to sucrose and nonpurified diet compared with rats fed intermittent nonpurified diet only (59), and alterations occur in accumbens DA turnover and DA transporter binding in rats maintained on an intermittent sugar-feeding schedule (12,60).
Is there evidence of dependence on fat or sweet-fat combinations?
The literature suggests that, as with sugar, a similar addictive-like state may emerge with fat. Le Magnen (29) noted that naloxone could precipitate withdrawal in rats fed a cafeteria-style diet ad libitum that contains a variety of fat- and sugar-rich foods (e.g., cheese, cookies, chocolate chips). More recently, Teegarden and Bale (61) show that mice given access to diets high in fat or carbohydrate ad libitum for 4 wk and then forced to abstain endure an aversive environment to gain access to their preferred food. They conclude that withdrawal of such a diet elevates the stress state, contributing to dietary relapse. Also, Corwin and colleagues have shown an increase in progressive-ratio responding in rats that are bingeing on fat (62).
In terms of neurochemistry, it appears that binge eating of fat has effects on the accumbens DA and enkephalin systems that are similar to those observed with sugar bingeing. Limited exposure to fat (corn oil) will repeatedly release DA in the NAc, and this effect is caused by the taste of the oil (63). Rats with limited daily access to a sweet-fat diet show a significant decrease in enkephalin mRNA in the NAc (64), similar to the finding reported above with sugar (53). The role of opioids in the paraventricular nucleus of the hypothalamus has been studied using a binge model (65), and the findings suggest that d-Ala2, NMe-Phe4, Gly-ol5-enkephalin increases fat intake in fat-preferring rats but has no effect in sucrose-preferring rats. These results indicate a complex role for paraventricular nuclear opioids in food intake, with preference and nutrient type affecting the ability of these compounds to change behavior.
Based on this neurochemistry and the behaviors described above, it seems logical that fat bingeing might also produce addictive-like behaviors. However, the data are not clear. Although fat offered ad libitum has been reported to produce some addictive-like behaviors (29,61), bingeing might enhance these effects. We have investigated whether behavioral signs of dependence emerge when animals binge using a variety of different high-fat diets and sweet-fat combinations. We have tested rats with limited (12-h or 2-h) access to a sweet-fat diet (Research Diets #12451, 45% fat, 20% protein, 35% carbohydrate), 12-h access to a sweet-fat mixture (35.7% vegetable fat, 64.3% sucrose), or 12-h access to vegetable fat (100% Crisco vegetable shortening), all with nonpurified diet concurrently available. Control groups were fed these diets ad libitum or given standard nonpurified diet ad libitum. After 21–25 d on the diets, rats were administered 3 mg/kg subcutaneous naloxone and then observed for somatic signs of distress and anxiety in the elevated plus maze. No significant evidence of opiate-like withdrawal was found with any of these fat-rich dietary options, in either the bingeing groups or those given food ad libitum, even though these procedures gave positive results in our previous reports with sugar bingeing (41). In other studies, we attempted to elicit signs of spontaneous opiate-like withdrawal by food depriving the rats maintained on fat-rich diets for 24–36 h. Again, although we report signs of anxiety and somatic indications of distress following fasting in sugar-bingeing rats (23), this was not observed in rats that had been bingeing with a high-fat source in the diet.
Although we have not noted signs of opiate-like withdrawal in fat-bingeing rats, that does not mean that excessive fat intake cannot produce addictive-like behaviors. Withdrawal is not a necessary criterion for drug craving, just as food deprivation is not necessary for food craving (37). Moreover, different classes of drugs (e.g., DA agonists, opiates) result in specific behavioral and physiological withdrawal signs. Thus, it may be that different macronutrients may also produce different withdrawal signs. It has yet to be determined whether or not bingeing on fat can precipitate other addictive-like behaviors, including cross-sensitization and abnormal motivation caused by abstinence.
Why do signs of opiate-like withdrawal emerge with sugar but not fat bingeing?
The relative lack of opiate-like withdrawal signs after fat bingeing underscores the importance of opioid systems in differentiating sugars and fats and their subsequent effects on behavior. The neuropeptide galanin (GAL) and its binding sites are expressed in brain areas important for both drug and food reward (11). GAL is considered a fat-stimulated peptide because its expression is increased in these brain regions in response to a high-fat meal (66). In addition, hypothalamic injection of GAL promotes the intake of fat in preference to carbohydrate in some situations (67,68). Interestingly, peripheral injection of galnon, a synthetic GAL agonist, decreases opiate withdrawal signs in morphine-dependent mice (69). A single systemic injection of galnon in GAL-knockout mice is sufficient to reverse some of the biochemical changes brought about by morphine administration (70). Thus, GAL may be an endogenous negative regulator of opiate reward by attenuating some of the behavioral and neurochemical effects of opiates. Based on these data, it is possible that that lack of opiate-like withdrawal signs in fat-bingeing rats may be caused by fat-induced endogenous GAL activation, which can inhibit the relevant opioid effects.
Implications for eating disorders and obesity
We began this article with a discussion relating binge eating to obesity. Indeed, the findings with animal models that have been presented suggest that binge eating of sugar, and possibly even fat, may have some addictive-like properties. However, sugar bingeing does not affect body weight, but a combination of sweet and fat does result in increased body weight (22). Thus, fat may be the macronutrient that results in excess body weight, and sweet taste may be largely responsible for producing addictive-like behaviors that include a withdrawal syndrome.
Other articles in this supplement include references (73–75).
Acknowledgments
We thank Miriam Bocarsly for her assistance in preparing the manuscript.
Published as a supplement to The Journal of Nutrition. Presented as part of the symposium “Food Addiction: Fact or Fiction” given at the 2008 Experimental Biology meeting, April 8, 2008 in San Diego, CA. The symposium was sponsored by the American Society for Nutrition and supported by an educational grant from The National Institute on Drug Abuse, The National Institute on Alcohol Abuse and Alcoholism, and The National Dairy Council. The symposium was chaired by Rebecca L. Corwin and Patricia S. Grigson.
Supported by USPHS grants DK-79793 (N.M.A.) and AA-12882 (B.G.H.).
Author disclosures: N. Avena, P. Rada, and B. Hoebel, no conflicts of interest.
Abbreviations used: ACh, acetylcholine; DA, dopamine; GAL, galanin; NAc, nucleus accumbens.
References
- 1.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. [DOI] [PubMed] [Google Scholar]
- 2.Stunkard AJ. Eating patterns and obesity. Psychiatr Q. 1959;33:284–95. [DOI] [PubMed] [Google Scholar]
- 3.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, Reynolds JC, Yanovski JA. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117:1203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramacciotti CE, Coli E, Paoli R, Gabriellini G, Schulte F, Castrogiovanni S, Dell'Osso L, Garfinkel PE. The relationship between binge eating disorder and non-purging bulimia nervosa. Eat Weight Disord. 2005;10:8–12. [DOI] [PubMed] [Google Scholar]
- 5.Grucza RA, Przybeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Compr Psychiatry. 2007;48:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord. 2007;40:727–32. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, ed. 4, Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000.
- 8.Guertin TL, Conger AJ. Mood and forbidden foods' influence on perceptions of binge eating. Addict Behav. 1999;24:175–93. [DOI] [PubMed] [Google Scholar]
- 9.Hadigan CM, Kissileff HR, Walsh BT. Patterns of food selection during meals in women with bulimia. Am J Clin Nutr. 1989;50:759–66. [DOI] [PubMed] [Google Scholar]
- 10.Allison S, Timmerman GM. Anatomy of a binge: food environment and characteristics of nonpurge binge episodes. Eat Behav. 2007;8:31–8. [DOI] [PubMed] [Google Scholar]
- 11.Leibowitz SF, Hoebel BG. Behavioral neuroscience and obesity. In: Bray G, Bouchard C, James P, editors. The Handbook of Obesity. New York: Marcel Dekker; 2004. p. 301–71.
- 12.Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1260–8. [DOI] [PubMed] [Google Scholar]
- 13.Wojnicki FH, Stine JG, Corwin RL. Liquid sucrose bingeing in rats depends on the access schedule, concentration and delivery system. Physiol Behav. 2007;92:566–74. [DOI] [PubMed] [Google Scholar]
- 14.Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–35. [DOI] [PubMed] [Google Scholar]
- 15.Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–52. [DOI] [PubMed] [Google Scholar]
- 16.Avena NM, Rada P, Hoebel BG. Evidence of sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–53. [DOI] [PubMed] [Google Scholar]
- 18.Wojnicki FH, Charny G, Corwin RL. Binge-type behavior in rats consuming trans-fat-free shortening. Physiol Behav. 2008;94:627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis JF, Melhorn SJ, Shurdak JD, Heiman JU, Tschop MH, Clegg DJ, Benoit SC. Comparison of hydrogenated vegetable shortening and nutritionally complete high-fat diet on limited access-binge behavior in rats. Physiol Behav. 2007;92:924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–14. [DOI] [PubMed] [Google Scholar]
- 21.Kinzig KP, Hargrave SL, Honors MA. Binge-type eating attenuates corticosterone and hypophagic responses to restraint stress. Physiol Behav. 2008;95:108–13. [DOI] [PubMed] [Google Scholar]
- 22.Berner LA, Avena NM, Hoebel BG. Bingeing, self-restriction, and increased body weight in rats with access to a sweet-fat diet. Obesity. 2008; Epub ahead of print. [DOI] [PubMed]
- 23.Avena NM, Bocarsly ME, Rada P, Kim A, Hoebel BG. After daily bingeing on a sucrose solution, food deprivation induces anxiety and accumbens dopamine/acetylcholine imbalance. Physiol Behav. 2008;94:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis C, Claridge G. The eating disorders as addiction: a psychobiological perspective. Addict Behav. 1998;23:463–75. [DOI] [PubMed] [Google Scholar]
- 25.Marrazzi MA, Luby ED. An auto-addiction opioid model of chronic anorexia nervosa. Int J Eat Disord. 1986;5:191–208. [Google Scholar]
- 26.Riva G, Bacchetta M, Cesa G, Conti S, Castelnuovo G, Mantovani F, Molinari E. Is severe obesity a form of addiction? Rationale, clinical approach, and controlled clinical trial. Cyberpsychol Behav. 2006;9:457–79. [DOI] [PubMed] [Google Scholar]
- 27.Trinko R, Sears RM, Guarnieri DJ, DiLeone RJ. Neural mechanisms underlying obesity and drug addiction. Physiol Behav. 2007;91:499–505. [DOI] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci. 2008;363:3191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Magnen J. A role for opiates in food reward and food addiction. In: Capaldi PT, editor. Taste, experience, and feeding. Washington, DC: American Psychological Association; 1990. p. 241–52.
- 30.Hoebel BG, Rada P, Mark GP, Pothos E. Neural systems for reinforcement and inhibition of behavior: Relevance to eating, addiction, and depression. In: Kahneman D, Diener E, Schwartz N, editors. Well-being: the foundations of hedonic psychology. New York: Russell Sage Foundation; 1999. p. 558–72.
- 31.Bennett C. Sugar shock!: How sweets and simple carbs can derail your life and how you can get back on track. New York: The Berkley Publishing Group; 2007.
- 32.DesMaisons K. Your last diet!: The sugar addict's weight-loss plan. Toronto: Random House; 2001.
- 33.Katherine A. Anatomy of a food addiction: an effective program to overcome compulsive eating. Carlsbad: Gurze Books; 1996.
- 34.Rufus E. Sugar addiction: a step-by-step guide to overcoming sugar addiction. Bloomington: Elizabeth Brown Rufus; 2004.
- 35.Spring B, Schneider K, Smith M, Kendzor D, Appelhans B, Hedeker D, Pagoto S. Abuse potential of carbohydrates for overweight carbohydrate cravers. Psychopharmacology (Berl). 2008;197:637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–93. [DOI] [PubMed] [Google Scholar]
- 37.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–7. [DOI] [PubMed] [Google Scholar]
- 38.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. [DOI] [PubMed] [Google Scholar]
- 39.Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–7. [DOI] [PubMed] [Google Scholar]
- 40.Bencherif B, Guarda AS, Colantuoni C, Ravert HT, Dannals RF, Frost JJ. Regional mu-opioid binding in insular cortex is decreased in bulimia nervosa and correlated inversely with fasting behavior. J Nucl Med. 2005;46:1349–51. [PubMed] [Google Scholar]
- 41.Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–88. [DOI] [PubMed] [Google Scholar]
- 42.Avena NM, Long KA, Hoebel BG. Sugar-dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav. 2005;84:359–62. [DOI] [PubMed] [Google Scholar]
- 43.Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. [DOI] [PubMed] [Google Scholar]
- 44.Avena NM, Carrillo CA, Needham L, Leibowitz SF, Hoebel BG. Sugar-dependent rats show enhanced intake of unsweetened ethanol. Alcohol. 2004;34:203–9. [DOI] [PubMed] [Google Scholar]
- 45.Wideman CH, Nadzam GR, Murphy HM. Implications of an animal model of sugar addiction, withdrawal and relapse for human health. Nutr Neurosci. 2005;8:269–76. [DOI] [PubMed] [Google Scholar]
- 46.Galic MA, Persinger MA. Voluminous sucrose consumption in female rats: increased “nippiness” during periods of sucrose removal and possible oestrus periodicity. Psychol Rep. 2002;90:58–60. [DOI] [PubMed] [Google Scholar]
- 47.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: effects of reduced training and sucrose preloading. Physiol Behav. 2005;84:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. [DOI] [PubMed] [Google Scholar]
- 49.Foley KA, Fudge MA, Kavaliers M, Ossenkopp KP. Quinpirole-induced behavioral sensitization is enhanced by prior scheduled exposure to sucrose: A multi-variable examination of locomotor activity. Behav Brain Res. 2006;167:49–56. [DOI] [PubMed] [Google Scholar]
- 50.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. [DOI] [PubMed] [Google Scholar]
- 51.Vezina P. Sensitization, drug addiction and psychopathology in animals and humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1553–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–69. [DOI] [PubMed] [Google Scholar]
- 53.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–42. [DOI] [PubMed] [Google Scholar]
- 54.Bassareo V, Di Chiara G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J Neurosci. 1997;17:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–44. [DOI] [PubMed] [Google Scholar]
- 56.Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl). 2001;157:105–10. [DOI] [PubMed] [Google Scholar]
- 57.Rada P, Johnson DF, Lewis MJ, Hoebel BG. In alcohol-treated rats, naloxone decreases extracellular dopamine and increases acetylcholine in the nucleus accumbens: evidence of opioid withdrawal. Pharmacol Biochem Behav. 2004;79:599–605. [DOI] [PubMed] [Google Scholar]
- 58.Rada PV, Mark GP, Taylor KM, Hoebel BG. Morphine and naloxone, i.p. or locally, affect extracellular acetylcholine in the accumbens and prefrontal cortex. Pharmacol Biochem Behav. 1996;53:809–16. [DOI] [PubMed] [Google Scholar]
- 59.Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13:2213–6. [DOI] [PubMed] [Google Scholar]
- 61.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–9. [DOI] [PubMed] [Google Scholar]
- 62.Wojnicki FH, Roberts DC, Corwin RL. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharmacol Biochem Behav. 2006;84:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liang NC, Hajnal A, Norgren R. Sham feeding corn oil increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1236–9. [DOI] [PubMed] [Google Scholar]
- 64.Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure®) alters striatal enkephalin gene expression. Eur J Neurosci. 2003;18:2592–8. [DOI] [PubMed] [Google Scholar]
- 65.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am J Physiol Regul Integr Comp Physiol. 2007;293:R99–105. [DOI] [PubMed] [Google Scholar]
- 66.Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF. Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci USA. 1994;91:10375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–14. [DOI] [PubMed] [Google Scholar]
- 68.Smith BK, York DA, Bray GA. Effects of dietary preference and galanin administration in the paraventricular or amygdaloid nucleus on diet self-selection. Brain Res Bull. 1996;39:149–54. [DOI] [PubMed] [Google Scholar]
- 69.Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, Wynick D, Langel U, Picciotto MR. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci USA. 2003;100:9028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawes JJ, Brunzell DH, Narasimhaiah R, Langel U, Wynick D, Picciotto MR. Galanin protects against behavioral and neurochemical correlates of opiate reward. Neuropsychopharmacology. 2008;33:1864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avena NM, Rada P, Bocarsly M, Hoebel BG. Binge eating as a form of addiction: Evidence from an animal model of sugar addiction. In: Columbus F. editor. Binge eating: psychological factors, symptoms and treatment. New York: Nova Publishers. 2009; in press.
- 72.Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience. 2006;139:813–20. [DOI] [PubMed] [Google Scholar]
- 73.Corwin RL, Grigson PS. Symposium overview. Food addiction: fact or fiction? J Nutr. 2009;139:617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pelchat ML. Food addiction in humans. J Nutr. 2009;139:620–2. [DOI] [PubMed] [Google Scholar]
- 75.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–32. [DOI] [PMC free article] [PubMed] [Google Scholar]