Abstract
Food intake is regulated by 2 complementary drives: the homeostatic and hedonic pathways. The homeostatic pathway controls energy balance by increasing the motivation to eat following depletion of energy stores. In contrast, hedonic or reward-based regulation can override the homeostatic pathway during periods of relative energy abundance by increasing the desire to consume foods that are highly palatable. In contrast to the consumption of food, the motivation to use drugs of abuse is mediated only by the reward pathway. In this article we review the extensive research that has identified several mechanisms by which repeated exposure to drugs of abuse alters neuronal function and increases the motivational incentive to obtain and use these substances. We then compare our current understanding of drug-induced changes in neuronal reward circuitry with what is known about the consequences of repeated consumption of highly palatable foods such as high-fat and high-sugar diets. Next, we discuss the normal homeostatic regulation of food intake, which is a unique aspect of food addiction. Finally, we discuss the clinical implications of these neuronal adaptations in the context of obesity and neuropsychiatric syndromes such as bulimia nervosa and Prader-Willi syndrome.
Introduction
Within the field of medicine, the term addiction is applied only to drugs of abuse such as alcohol and cocaine. Although the concept of food addiction has received considerable attention from the popular media in recent years, there is not actually a diagnosis for food addiction in medical science. In contrast to addiction to drugs of abuse, much less is known about the behavioral and neurobiological consequences of repeated exposure to highly palatable foods. Given the requirement of food for life, much debate has centered on defining the term food addiction. For the purposes of this discussion, we use a simplified but useful definition of food addiction as “a loss of control over food intake.” [For a full discussion of the definition of food addiction, the reader is directed to an excellent review by Rogers and Smit (1).] Using drugs of abuse as a model, we compare the neuronal regulation of food intake to drug consumption and discuss the potential for food to be considered addictive.
Hedonic aspects of substance dependence and food intake
Considerable evidence in rodents and humans now supports the theory that both drugs of abuse and the consumption of highly palatable foods converge on a shared pathway within the limbic system to mediate motivated behaviors (2,3). Much of this work has focused on the mesolimbic dopamine pathway because all common drugs of abuse increase dopamine signaling from nerve terminals originating in the ventral tegmental area (VTA)5 onto neurons in the nucleus accumbens (also called the ventral striatum) (Fig. 1). Increased dopaminergic transmission is thought to occur either by direct action on dopaminergic neurons (stimulants, nicotine) or indirectly through inhibition of GABAergic interneurons in the VTA (alcohol, opiates) (2,3). Also implicated in mediating drug-induced activation of VTA dopamine neurons is the peptide neurotransmitter orexin, which is expressed by a population of lateral hypothalamic neurons that broadly innervate much of the brain including the VTA (4–6).
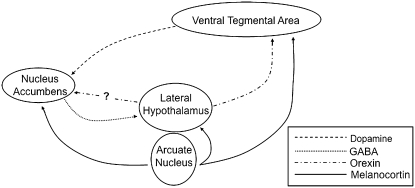
FIGURE 1 .
Schematic representation of neural circuits that regulate feeding. Dopaminergic neurons originating in the VTA project to neurons within the nucleus accumbens of the ventral striatum. The lateral hypothalamus receives input from GABAergic projections from the nucleus accumbens as well as melanocortinergic neurons from the Arc of the hypothalamus. In addition, melanocortin receptors are also found on neurons in the VTA and the nucleus accumbens.
Natural rewards, such as food, stimulate similar responses within the mesolimbic dopamine pathway. Presentation of highly palatable foods induces potent release of dopamine into the nucleus accumbens (3). This release of dopamine is believed to coordinate many aspects of an animal's attempts to obtain food rewards, including increased arousal, psychomotor activation, and conditioned learning (remembering food-associated stimuli). The mechanism by which food stimulates dopamine signaling is unclear; however, it does appear that taste receptors are not required, as mice lacking sweet receptors are still able to develop a strong preference for sucrose solutions (7). One possibility is that orexin neurons may be activated during feeding, with the consequent release of orexin directly stimulating VTA dopamine neurons (8).
The importance of the mesolimbic dopamine pathway in human illness has recently been confirmed. Stoeckel et al. reported that in normal-weight women, pictures of energy-dense food stimulated a significant increase in the activity of the dorsal caudate, a region of the dorsal striatum. In contrast, obese women presented with pictures of high-energy foods demonstrated increased activation in several limbic regions including the orbitofrontal and prefrontal cortices, amygdala, dorsal and ventral striatum, insula, anterior cingulate cortex, and hippocampus (9). This difference in activation suggests that obese individuals may have altered evaluation of food reward, resulting in aberrant motivation to consume high-energy foods.
As might be expected, prolonged activation of the limbic system by drugs of abuse leads to cellular and molecular adaptations that serve in part to maintain homeostasis in dopamine signaling (2). Within the dopaminergic neurons of the VTA, chronic drug use is associated with decreased basal dopamine secretion, decreased neuronal size, and increased activity of tyrosine hydroxylase (the rate-limiting enzyme in dopamine biosynthesis) and of the transcription factor cyclic AMP response element binding protein (CREB) (2,10). Within target neurons in the striatum, chronic drug use increases levels of CREB as well as those of another transcription factor, deltaFosB, both of which alter neuronal responsiveness to dopamine signaling (2). These adaptations are thought to be important for the aberrant motivation to obtain drugs of abuse observed in addicted patients. For instance, increasing deltaFosB levels in the striatum increases sensitivity to the rewarding effects of drugs of abuse such as cocaine and morphine and increases incentive motivation to obtain them (2).
Similar cellular and molecular changes have been described in rodents exposed to highly palatable foods. Mice exposed to a high-fat diet for 4 wk and then abruptly withdrawn to a less palatable semipurified diet showed decreased levels of active CREB in the striatum up to 1 wk after the switch (11). These findings are consistent with the work of Barrot et al. (12) who reported that decreasing CREB activity in the ventral striatum increases the preference for both a sucrose solution (a natural reward) and for morphine, a well-characterized drug of abuse. In addition, mice exposed to 4 wk of high-fat diet exhibited a significant elevation in the level of deltaFosB in the nucleus accumbens (11), similar to changes observed following exposure to drugs of abuse (2). Furthermore, increased expression of deltaFosB in this brain region enhances food-reinforced operant responding, demonstrating a clear role for deltaFosB in increasing motivation to obtain food rewards (13). Taken together, these studies demonstrate that limbic regions experience similar neuroadaptations following exposure to both food and drug rewards and that these adaptations alter the motivation to obtain both types of rewards.
Homeostatic aspects of food intake
Unlike hedonic aspects of feeding, which focus on the reward associated with food intake, homeostatic control of feeding is concerned primarily with regulation of energy balance. Most of this work has focused on circulating hormones that relay information about peripheral energy levels to the brain. Two of the most important peripheral hormones are leptin and ghrelin. Leptin is synthesized by white adipose tissue, and its level increases in proportion to fat mass. Among its many actions, high levels of leptin potently suppress food intake and stimulate metabolic processes to dissipate excessive energy stores (14). In contrast, ghrelin is a stomach-derived peptide whose level increases in response to negative energy balance and stimulates food intake and energy storage (14).
Although receptors for leptin and ghrelin are expressed widely throughout the body and central nervous system, the arcuate nucleus (Arc) of the hypothalamus is a site of particular importance, given its well-known role in regulating feeding and metabolism (15). Within the Arc, leptin receptors are expressed on 2 distinct subsets of neurons (Fig. 1). The first expresses the peptide neurotransmitter pro-opiomelanocortin (POMC) and cocaine-amphetamine-regulated transcript (CART). Leptin receptor signaling stimulates the activity of POMC/CART neurons and suppresses feeding while increasing metabolic rate. Second, activation of the leptin receptor inhibits a second set of neurons, which express neuropeptide Y (NPY) and agouti-related peptide (AgRP); these neurons normally increase food intake. Thus, POMC/CART neurons and NPY/AgRP neurons exert opposite effects on food intake and energy consumption. In this manner, leptin is a potent suppressor of feeding by stimulating anorexigenic POMC/CART neurons while reciprocally inhibiting the action of proappetite NPY/AgRP neurons (15). In contrast, ghrelin receptors are expressed mainly on NPY/AgRP neurons within the Arc; activation of ghrelin signaling stimulates these neurons and promotes feeding behavior (14).
Emerging evidence now supports the idea that hormones known to regulate feeding, such as leptin and ghrelin, also exert effects on motivation to obtain food through regulation of mesolimbic dopamine signaling. Leptin can decrease the basal secretion of dopamine as well as feeding-stimulated dopamine release within the ventral striatum of rats (16). Furthermore, leptin receptor activation inhibits firing of VTA dopamine neurons (17), whereas long-term blockade of leptin signaling in the VTA increases locomotor activity and food intake (18). Imaging studies in human patients confirm the involvement of mesolimbic dopamine signaling in the action of leptin. Farooqi et al. (19) reported functional imaging results of 2 human patients with congenital deficiency in leptin. Both individuals displayed enhanced activation of striatal regions after seeing images of food. Importantly, this enhanced striatal activation could be normalized by 7 d of leptin replacement therapy. More recently, ghrelin has been shown to regulate mesolimbic dopamine signaling. Several investigators report that the ghrelin receptor is expressed by VTA neurons and that administration of ghrelin stimulates the release of dopamine into the striatum (20–22). Furthermore, Malik et al. (23) have confirmed a role for ghrelin in human patients. Healthy control subjects receiving infusions of ghrelin demonstrated increased activity in several limbic regions including the amygdala, orbitofrontal cortex, anterior insula, and striatum.
Effect of stress on feeding
Further complicating the picture is the impact of psychosocial stress on feeding and body weight homeostasis. Not only is a change in appetite 1 of the core diagnostic features of Major Depressive Disorder (24), but there is a ∼25% association rate between mood disorder and obesity (25). Therefore, it is very likely that stress may influence feeding and body weight independent of palatability of food or energy status of the individual. Recently, we have demonstrated an important role for ghrelin and orexin in the appetitive changes induced by chronic stress (26). Mice subjected to chronic social defeat stress responded with a significant elevation in levels of active ghrelin that correlate with an increase in both food intake and body weight. This effect on feeding and body weight was lost when mice lacking the ghrelin receptor were subjected to chronic social stress. Importantly, although stress regulation of food intake and body weight was blocked in ghrelin receptor-deficient mice, the animals displayed greater degrees of depressive symptoms. These findings indicate that stress-induced elevations in ghrelin not only may alter food intake but may also help compensate for the deleterious effect of stress on mood and motivation. These various actions of ghrelin appear to be mediated in part via activation of orexin neurons in the lateral hypothalamus (27). Other groups have demonstrated alterations in feeding systems after chronic stress as well. Lu reported that mice subjected to chronic mild stress have decreased levels of circulating leptin (28). Teegarden and Bale demonstrated, in a mouse line genetically vulnerable to the effects of stress, that chronic variable stress increases preference for a high-fat diet (29). These studies highlight the fact that mood disorders likely influence both hedonic and homeostatic aspects of food intake, making a clear definition of food addiction difficult (summarized in Table 1).
TABLE 1.
Neuronal factors that regulate food intake
| Factor | Pathways regulated | Site of action | Action on feeding | Effect of stress |
|---|---|---|---|---|
| Leptin | Both | Arcuate, VTA | Inhibits | Decreases |
| Ghrelin | Both | Arcuate, VTA | Stimulates | Increases |
| CREB | Hedonic | N. Accumbens, VTA | Inhibits | Increases |
| deltaFosB | Hedonic | N. Accumbens | Stimulates | Increases |
| α-MSH1 | Homeostatic | PVN1 | Inhibits | ? |
| AgRP | Homeostatic | PVN | Stimulates | ? |
| NPY | Homeostatic | Multiple sites | Stimulates | ? |
| Orexin | Hedonic | VTA | Stimulates | Decreases |
α-MSH, α-melanocyte stimulating hormone; PVN, paraventricular nucleus.
Clinical implications
The term food addiction is generally applied to obesity by the popular media. Additionally 3 behavioral disorders, bulimia nervosa, binge eating disorder, and Prader-Willi syndrome, include compulsive food intake as a part of the clinical syndrome. Recent work has raised the possibility that aberrant mesolimbic dopamine signaling is involved in these disorders.
Although being overweight clearly contributes to the development of many disorders including diabetes and metabolic syndrome, by itself it is not considered a disease. Still, it is important to consider the effect of chronic exposure to highly palatable foods on the reward system in the development of obesity. Preliminary evidence from functional neuroimaging studies suggests that the limbic system may be hyperresponsive to food rewards in obese women, as stated earlier (9). Future research is needed to determine the functional differences between normal-weight and obese individuals, including the involvement of limbic activity in the rebound in weight gain that is observed in many individuals after successful weight loss. Several clinical methods are available for achieving weight loss, including diet and exercise, bariatric surgery, and medications such as rimonabant, a cannabinoid receptor antagonist. These treatment populations offer ideal subjects for functional neuroimaging techniques to identify mechanisms of weight loss and susceptibility to weight rebound.
Preclinical models also suggest the potential importance of neuronal adaptations in the development of obesity. The transcription factors CREB and deltaFosB, mentioned above, are of particular interest because of their well-established role in drug addiction. However, there is a clear lack of human postmortem studies on obese subjects. Human postmortem tissue needs to be analyzed for several neuronal adaptations that could potentially mediate, or be induced by, obesity, including size of dopaminergic neurons in the VTA and expression levels of CREB and deltaFosB in the ventral striatum. In addition, further testing of rodent models is indicated. Current data support a role for CREB and deltaFosB in mediating food reward but have not yet demonstrated the requirement for these transcription factors in the development of diet-induced or other rodent models of obesity. Experimental tools, including transgenic mouse lines and viral-mediated gene transfer, are already available to pursue this line of investigation.
Even less is known about the pathophysiology of compulsive food intake observed in bulimia nervosa, binge eating disorder, and Prader-Willi syndrome. Although clinical experience demonstrates a greatly enhanced motivation to obtain food in individuals with these disorders, suggesting a possible role for the mesolimbic dopamine system, little evidence exists to support this hypothesis. Two neuroimaging studies have demonstrated abnormal activation of the anterior cingulate cortex in patients with bulimia nervosa (30,31), whereas another study demonstrated dysfunction of the hypothalamus and orbitofrontal cortex in patients with Prader-Willi syndrome (32). The mechanism of abnormal limbic activation is not known but may involve altered levels of peripheral feeding hormones. For example, ghrelin levels are greatly elevated in Prader-Willi syndrome (33) and may account for the increase in motivation to obtain food seen in these patients. However, studies on the role of peripheral hormones such as ghrelin in the etiology of eating disorders such as bulimia nervosa and binge eating disorder have produced mixed results at best (34), emphasizing that the pathophysiology of these disorders is likely to involve complex interactions among many genetic, environmental, and psychological factors.
Creating a new diagnosis for food addiction requires careful analysis not only of the pertinent scientific information but also of social, legal, epidemiological, and economic considerations that are beyond the scope of this review. However, it is clear that chronic consumption of highly palatable foods can alter brain function in ways similar to drugs of abuse, particularly within the mesolimbic dopamine reward pathway. Determining the long-term consequences of diets high in sugar and fat on limbic function and motivated behaviors may yield important new insights into the cause and treatment of compulsive eating.
Other articles in this supplement include references (35–37).
Published as a supplement to The Journal of Nutrition. Presented as part of the symposium “Food Addiction: Fact or Fiction?” given at the 2008 Experimental Biology meeting, April 8, 2008 in San Diego, CA. The symposium was sponsored by the American Society for Nutrition, and supported by an educational grant from The National Institute on Drug Abuse, The National Institute on Alcohol Abuse and Alcoholism, and The National Dairy Council. The symposium was chaired by Rebecca L. Corwin and Patricia S. Grigson.
Supported by the following grants: 1PL1DK081182-01, P01 MH66172, R01 MH51399, P50 MH066172-06, NARSAD Young Investigator Award, Astra-Zeneca, The Physician Scientist Training Program.
Author disclosures: M. Lutter and E. Nestler, no conflicts of interest.
Abbreviations used: AgRP, agouti-related peptide; Arc, arcuate nucleus; CART, cocaine-amphetamine-regulated transcript; CREB, cyclic AMP response element binding protein; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; VTA, ventral tegmental area.
References
- 1.Rogers PJ, Smit HJ. Food craving and food “addiction”: a critical review of the evidence from a biopsychosocial perspective. Pharmacol Biochem Behav. 2000;66:3–14. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–28. [DOI] [PubMed] [Google Scholar]
- 4.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. [DOI] [PubMed] [Google Scholar]
- 5.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. [DOI] [PubMed] [Google Scholar]
- 7.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–41. [DOI] [PubMed] [Google Scholar]
- 8.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47. [DOI] [PubMed] [Google Scholar]
- 10.Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–9. [DOI] [PubMed] [Google Scholar]
- 11.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–9. [DOI] [PubMed] [Google Scholar]
- 12.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR. DeltaFosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci. 2006;26:9196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity–the yin and yang of body weight control. Endocrinology. 2003;144:3749–56. [DOI] [PubMed] [Google Scholar]
- 15.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. [DOI] [PubMed] [Google Scholar]
- 16.Krugel U, Schraft T, Kittner H, Kiess W, Illes P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur J Pharmacol. 2003;482:185–7. [DOI] [PubMed] [Google Scholar]
- 17.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–22. [DOI] [PubMed] [Google Scholar]
- 18.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. [DOI] [PubMed] [Google Scholar]
- 22.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. 2005;26:2274–9. [DOI] [PubMed] [Google Scholar]
- 23.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994.
- 25.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008;11:752–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA. 2006;103:1593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav. 2008;93:713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank GK, Wagner A, Achenbach S, McConaha C, Skovira K, Aizenstein H, Carter CS, Kaye WH. Altered brain activity in women recovered from bulimic-type eating disorders after a glucose challenge: a pilot study. Int J Eat Disord. 2006;39:76–9. [DOI] [PubMed] [Google Scholar]
- 31.Penas-Lledo EM, Loeb KL, Martin L, Fan J. Anterior cingulate activity in bulimia nervosa: a fMRI case study. Eat Weight Disord. 2007;12:e78–82. [DOI] [PubMed] [Google Scholar]
- 32.Dimitropoulos A, Schultz RT. Food-related neural circuitry in Prader-Willi syndrome: response to high- versus low-calorie foods. J Autism Dev Disord. 2008;38:1642–53. [DOI] [PubMed] [Google Scholar]
- 33.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. [DOI] [PubMed] [Google Scholar]
- 34.Troisi A, Di Lorenzo G, Lega I, Tesauro M, Bertoli A, Leo R, Iantorno M, Pecchioli C, Rizza S, et al. Plasma ghrelin in anorexia, bulimia, and binge-eating disorder: relations with eating patterns and circulating concentrations of cortisol and thyroid hormones. Neuroendocrinology. 2005;81:259–66. [DOI] [PubMed] [Google Scholar]
- 35.Corwin RL, Grigson PS. Symposium overview. Food addiction: fact or fiction? J Nutr. 2009;139:617–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pelchat ML. Food addiction in humans. J Nutr. 2009;139:620–2. [DOI] [PubMed] [Google Scholar]
- 37.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]