Abstract
Inefficient muscle long-chain fatty acid (LCFA) combustion is associated with insulin resistance, but molecular links between mitochondrial fat catabolism and insulin action remain controversial. We hypothesized that plasma acylcarnitine profiling would identify distinct metabolite patterns reflective of muscle fat catabolism when comparing individuals bearing a missense G304A uncoupling protein 3 (UCP3 g/a) polymorphism to controls, because UCP3 is predominantly expressed in skeletal muscle and g/a individuals have reduced whole-body fat oxidation. MS analyses of 42 carnitine moieties in plasma samples from fasting type 2 diabetics (n = 44) and nondiabetics (n = 12) with or without the UCP3 g/a polymorphism (n = 28/genotype: 22 diabetic, 6 nondiabetic/genotype) were conducted. Contrary to our hypothesis, genotype had a negligible impact on plasma metabolite patterns. However, a comparison of nondiabetics vs. type 2 diabetics revealed a striking increase in the concentrations of fatty acylcarnitines reflective of incomplete LCFA β-oxidation in the latter (i.e. summed C10- to C14-carnitine concentrations were ∼300% of controls; P = 0.004). Across all volunteers (n = 56), acetylcarnitine rose and propionylcarnitine decreased with increasing hemoglobin A1c (r = 0.544, P < 0.0001; and r = −0.308, P < 0.05, respectively) and with increasing total plasma acylcarnitine concentration. In proof-of-concept studies, we made the novel observation that C12-C14 acylcarnitines significantly stimulated nuclear factor κ-B activity (up to 200% of controls) in RAW264.7 cells. These results are consistent with the working hypothesis that inefficient tissue LCFA β-oxidation, due in part to a relatively low tricarboxylic acid cycle capacity, increases tissue accumulation of acetyl-CoA and generates chain-shortened acylcarnitine molecules that activate proinflammatory pathways implicated in insulin resistance.
Introduction
Reduced skeletal muscle long-chain fatty acid (LCFA)8 combustion in the fasted state and a blunted increase of carbohydrate oxidation in response to increased insulinemia have been associated with insulin resistance and type 2 diabetes mellitus (T2DM) in humans (1). This conclusion is supported by cross-limb respiratory quotient (RQ) measurements that indicated higher RQ during fasting, an attenuated RQ response following insulin stimulation, reduced exercise-associated fat oxidation, and a more limited muscle uptake and combustion of isotopically-labeled LCFA in T2DM (2–7). LCFA utilization by skeletal muscle mitochondria relative to fatty acid (FA) delivery to the tissue may exacerbate intramyocellular lipid (IMCL) accumulation in nonexercise-trained individuals and IMCL content is strongly correlated with insulin resistance (8–15). The mechanisms that underlie dysfunctional tissue mitochondrial FA catabolism and the molecular factors that link this phenomenon to impaired insulin action remain controversial.
Most discussions of this problem have centered on perturbations of mitochondrial bioenergetics and build-up of lipid-derived metabolites. For example, limited mitochondrial number, volume, or reduced tricarboxylic acid (TCA) cycle and electron transport chain capacity in muscle appear to be commonplace in insulin-resistant, first-degree insulin-resistant relatives of T2DM patients (16–18) and in obese insulin-resistant or T2DM individuals (19–22). There is evidence that cytosolic lipid-derived moieties such as ceramide and diacylglyerol inhibit V-akt murine thymoma viral oncogene homolog/protein kinase B and activate protein kinase C enzymes, respectively, thereby attenuating insulin signaling, and these metabolites can increase under conditions of excess FA and lipid accumulation [for reviews, see (23,24)]. The importance of efficient mitochondrial FA β-oxidation in regulating muscle insulin sensitivity was highlighted by studies in which insulin-stimulated glucose uptake and glycogen synthesis improved following maneuvers that increased carnitine palmitoyltransferase-1 (CPT-1) activity in cultured muscle cells (25). This outcome was maintained under conditions of IMCL accumulation (25), reminiscent of what is observed in some well-trained athletes in whom muscle FA β-oxidation and insulin sensitivity are robust despite high levels of IMCL (14). In contrast, recent studies by Muoio et al. (26) have shown that in cultured muscle cells, a higher rate of LCFA carboxyl-carbon flux toward acid-soluble metabolites corresponds to an insulin resistance phenotype. These investigators postulated that incomplete mitochondrial catabolism of LCFA in muscle leads to chain-shortened FA moieties that are involved in, or reflect, events that trigger insulin resistance, an idea supported further by their observation of higher acylcarnitine levels in muscle cells and plasma of obese/insulin-resistant rodent models and improved insulin sensitivity in fat-fed malonyl-CoA decarboxylase knockout mice that have more limited LCFA β-oxidation (26). Thus, although most studies concur with the notion that muscle mitochondrial LCFA metabolism is a key regulator of insulin action, many questions remain regarding the mechanisms by which LCFA intermediary metabolism participates in this regulation.
Identification of circulating metabolites or metabolite signatures reflective of muscle LCFA combustion capacity or activity are needed to yield facile, minimally invasive tools for assessing diabetes risk, gauging efficacy of diabetes prevention and reversal interventions, and gaining insight into the connection between fat metabolism and insulin sensitivity. To date, most investigations of muscle metabolism have relied on invasive and expensive muscle biopsy enzyme and histology analyses, cross-muscle catheterization for RQ measurements, cross-limb metabolite arteriovenous level determinations, or NMR-based technologies for the assessment of muscle lipid accumulation (2,4,6,9,27). A major challenge to identify circulating selective biomarkers of muscle FA combustion in humans is the paucity of comparative experimental models in which β-oxidation is altered exclusively or predominantly in muscle cells. A set of interesting observations regarding humans harboring an uncoupling protein 3 (UCP3) missense polymorphism allele has prompted us to consider whether metabolomics studies in this unique population will overcome this challenge. Garvey et al. (28) discovered that a significant number of Gullah-speaking African-American persons (20%) were heterozygous for a g/a polymorphism in the exon 6 splice donor site, predicted to yield a truncated UCP3 protein missing the 6th transmembrane domain. Interestingly, in UCP3 heterozygotic (g/a) persons, the fasting RQ and nonprotein RQ values were significantly elevated relative to controls and estimated whole-body fat oxidation was reduced by ∼50% (28). Considering contemporary views of UCP3 function in supporting mitochondrial FA β-oxidation (29,30) and the muscle specificity of UCP3 expression in humans (31), it follows that the perturbations in FA combustion resulting from this polymorphism should primarily, if not exclusively, reflect muscle lipid metabolism.
Thus, we hypothesize that humans heterozygous for the UCP3 g/a missense polymorphism will display a distinctive plasma metabolite profile indicative of reduced muscle FA oxidation compared to those without the polymorphism (g/g genotype). Studies herein leveraged “targeted metabolomics” to assay plasma concentrations of 42 individual acylcarnitines, free carnitine, and total acylcarnitine to assess genotype-related differences in a cohort of overweight to obese females with or without the UCP3 g/a polymorphism. Additional analyses evaluated acylcarnitine profile shifts specific to T2DM by comparing T2DM volunteers to nondiabetics regardless of UCP3 genotype.
Materials and Methods
Human volunteers and plasma samples
Archived plasma samples derived from BMI- and age-matched overweight to obese type 2 diabetic (n = 44) and nondiabetic (n = 12) Gullah-speaking African-American women with or without a UCP3 g/a missense polymorphism were evaluated (Table 1). Volunteers were recruited as part of the Project SuGAR study described in detail elsewhere (32,33). Considering that the group studied herein is of a single sex, displays extraordinarily low genetic admixture, lives in a relatively small geographical space, and has a common dietary intake pattern, we anticipated that this group is well suited for metabolomics studies, because biological signal-to-noise should be low in terms of metabolite signatures. The Institutional Review Boards of the Medical University of South Carolina, University of Alabama at Birmingham, and the University of California, Davis approved the studies and all participants provided informed consent. Blood was obtained between ∼0800 and 0900 by arm venipuncture into EDTA-treated collection tubes after an overnight fast (no food or drink since 2000 the night before). A portion of whole blood was used for isolation of DNA and UCP3 genotyping as previously described (28). Plasma was frozen at −20°C for 1–7 d before transport to −80°C freezers for longer-term storage; acylcarnitine breakdown is prevented under these conditions [(34) and C.L. Hoppel, unpublished data]. Standard plasma clinical chemistry assays included glucose and lactate (YSI 2300 Glucose-Lactate Analyzer, Yellow Springs Instrumentation), triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol and VLDL (Vitos Autoanalyzer, Johnson and Johnson), and hemoglobin A1c (HbA1c; Bio-Rad Hemoglobin Analyzer). An oral 75-g glucose tolerance test was conducted for 180 min and fasting glucose and lactate values reported herein are the mean of 2 values obtained at −10 and 0 min before glucose ingestion. Volunteers were instructed to avoid any unusual activity and intentional exercise in the 3 d leading up to the study and to continue to eat their habitual diet without unusual deviations. Patients with diabetes did not take doses of oral agents on the evening before and on the morning of the study. Patients treated with insulin could take regular or rapid-acting insulin at dinner the night before the study but were instructed to withhold any intermediate- or long-acting insulin on the evening before and to avoid insulin injections on the morning of the study.
TABLE 1.
Characteristics of overweight diabetic and nondiabetic African American Gullah-speaking female study participants with or without a UCP3 missense g/a polymorphic allele1
| g/g Diabetic | g/g Nondiabetic | g/a Diabetic | g/a Nondiabetic | |
|---|---|---|---|---|
| n | 22 | 6 | 22 | 6 |
| Body mass, kg | 91.8 ± 3.8 | 92.5 ± 6.3 | 92.6 ± 3.2 | 86.0 ± 7.6 |
| BMI, kg/m2 (range) | 35.2 ± 1.2 (26–44) | 33.2 ± 1.6 (27–37) | 36.0 ± 1.4 (28–47) | 32.7 ± 3.2 (24–43) |
| Age, y (range) | 55.0 ± 3.2 (19–87) | 49.7 ± 6.4 (28–66) | 54.5 ± 2.9 (24–83) | 48.5 ± 8.2 (21–69) |
| Plasma metabolites2 | ||||
| Glucose,3mg/dL | 200.6 ± 14.1a | 90.6 ± 3.2b | 217.3 ± 19.1a | 92.9 ± 5.4b |
| HbA1c, % | 8.8 ± 0.4a | 5.4 ± 0.5b | 9.5 ± 0.6a | 5.4 ± 0.5b |
| Lactate, mmol/L | 1.31 ± 0.07a | 1.31 ± 0.10a | 1.13 ± 0.08a,b | 0.83 ± 0.06b |
| Triglycerides, mg/dL | 119.6 ± 14.9 | 126.2 ± 28.1 | 110.5 ± 17.1 | 74.5 ± 18.1 |
| Cholesterol, mg/dL | 205.9 ± 8.0 | 225.7 ± 23.6 | 217.6 ± 11.7 | 184.3 ± 16.1 |
| HDL cholesterol, mg/dL | 43.0 ± 2.7 | 47.8 ± 5.5 | 47.27 ± 3.0 | 42.5 ± 4.8 |
| LDL cholesterol, mg/dL | 138.7 ± 7.8 | 152.0 ± 20.2 | 148.0 ± 9.4 | 126.7 ± 13.4 |
| VLDL cholesterol, mg/dL | 23.9 ± 3.0 | 25.0 ± 5.7 | 21.8 ± 3.4 | 14.7 ± 3.7 |
Values are means ± SEM. Means in a row with superscripts without a common letter differ, P < 0.05.
Blood was drawn from fasting participants.
To convert to mmol/L, multiply by: glucose, 0.05551; triglycerides, 0.01129; cholesterol, 0.02586.
Acylcarnitine profiling
Plasma samples for metabolomics assays were thawed on ice, aliquoted, and refrozen on dry ice prior to delivery to Case Western Reserve for acylcarnitine analyses. Comprehensive analyses of acylcarnitines, free carnitine, and total acylcarnitine from 100 μL of plasma were conducted using HPLC-MS methods described in detail elsewhere (35,36). The limit of detection (LOD) for individual acylcarnitines using this method is 20 nmol/L with the exception of acetylcarnitine, propionylcarnitine, and valproylcarnitine at 100 nmol/L. Acylcarnitine values below the LOD were considered 0 nmol/L and were included in the calculations of mean values reported herein; thus, some reported mean values fell below the LOD.
Effect of acylcarnitines on nuclear factor κ-B activation
Reagents.
dl-Carnitine hydrochloride, dl-decanoylcarnitine chloride (C10-carn), dl-laurylcarnitine (C12-carn), and dl-myristoylcarnitine chloride (C14-carn) were purchased from Sigma-Aldrich, lipopolysaccharide (LPS) (Escherichia coli 0111:B4; catalog no. 421/lot no. 4214A1) was from List Biological Laboratory, and all agents were dissolved in endotoxin-free water (Sigma).
Cell culture.
RAW264.7 cells (a murine monocytic cell line; ATCC TIB-71) were cultured in high-glucose DMEM containing 10% (v:v) heat-inactivated fetal bovine serum (Intergen), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen). Cells were maintained at 37°C in a 5% CO2/air environment. Cells were used between the passages from 3 to 10.
Plasmid transfection and luciferase assays.
(2×)-Nuclear factor κ-B (NFκB)-dependent luciferase reporter construct (NFκB-Luc), which contains 2 copies of the NFκB consensus-binding site that drives luciferase expression, was provided by Frank Mercurio (Signal Pharmaceuticals). pRSV-β-galactosidase plasmid was from Dr. Jongdae Lee (University of California, San Diego, CA). RAW264.7 cells were seeded at ∼2.5 × 105/well in 24-well plates and after 24 h, cells were transiently transfected with NFκB-Luc and β-galactosidase plasmids using SuperFect transfection reagent (Qiagen) according to the manufacturer's instructions. Twenty-four hours later, groups of cells were stimulated for 15 h with vehicle control (endotoxin-free water), LPS, dl-carnitine, dl-laurylcarnitine, or dl-myristoylcarnitine as indicated in figure legends. Luciferase and β-galactosidase enzyme activities were determined using a luciferase assay system and β-galactosidase enzyme system (Promega) according to the manufacturer's instructions and luciferase activity was normalized by β-galactosidase activity to correct for transfection efficiency. Fold-differences (relative to control) in the normalized luciferase signals from treatment groups were calculated the day of each assay.
Statistical analyses
To identify differentially abundant carnitine metabolites across genotypes or in the presence or absence of T2DM, we evaluated individual plasma carnitine analyte concentrations using Mann-Whitney tests (Prism 4.03 software; GraphPad) considering the nonparametric nature of the data. Other metabolites (glucose, lactate, triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, VLDL cholesterol, and HbA1c) were compared across groups using 1-way ANOVA (Prism 4.03) and post hoc Newman-Keuls comparisons. We stratified our Mann-Whitney statistical comparisons of UCP3 genotypes into 3 parts: g/g vs. g/a comparisons regardless of diabetes status (n = 28 each genotype), g/g vs. g/a diabetics (n = 22 each genotype), and g/g vs. g/a nondiabetics (n = 6 each genotype). Diabetics were also compared to nondiabetics, regardless of genotype (n = 44 and n = 12, respectively). We used the Pearson correlation statistic to determine relationships between select metabolites, using all participant data unless otherwise noted. Treatment differences in NFκB-Luc induction were assessed by 1-way ANOVA followed by post hoc Dunnett's tests to compare treatment values to vehicle-treated control values. Results are means ± SEM and P < 0.05 was considered significant.
Results
Participant characteristics, plasma lipids, and indicators of diabetes status.
Mean body mass, BMI, and age did not differ among the diabetic or nondiabetic women with or without the g/a UCP3 missense allele (Table 1). Plasma triglyceride, total cholesterol, HDL and LDL cholesterol, and VLDL concentrations did not differ among the groups (Table 1). As expected, type 2 diabetics had significantly higher fasting plasma glucose and HbA1c (Table 1) and concentrations of these metabolites were significantly correlated with one another (Supplemental Fig. 1A). Diabetics also had grossly abnormal glucose tolerance during an oral glucose tolerance test (Supplemental Fig. 1B). UCP3 genotype did not influence diabetes outcome measures (fasting glucose, oral glucose tolerance test dynamics, or HbA1c).
Plasma metabolites differing between persons harboring g/g (wild-type) and g/a (missense) UCP3 alleles.
We hypothesized that relative to persons with the wild-type g/g UCP3 alleles, a dysfunctional g/a UCP3 allele would manifest in differences in fasting plasma acylcarnitine and metabolite profiles. However, just 2 genotype-related differences in circulating carnitine moieties were detected when we compared g/g and g/a volunteers. In diabetics only, plasma C12-carn was lower in g/a individuals (0.01 ± 0.01 μmol/L vs. 0.03 ± 0.01 μmol/L; P = 0.007) but was only detected in 3 of 22 g/a diabetics compared to 13 of 22 g/g diabetics. In nondiabetics, there was a significant genotype-related difference in plasma butyrylcarnitine (C4-carn; detected in 11 of the 12 nondiabetic volunteers), which was reduced by 57% in g/a participants (0.04 ± 0.008 μmol/L) relative to those with g/g wild-type alleles (0.08 ± 0.02 μmol/L).
In nondiabetics, plasma lactate concentrations were 36% lower in g/a compared to g/g volunteers (Table 1). The concentration in g/a diabetics tended to be lower (14%; P = 0.09) than in g/g diabetics (Table 1).
Differentially abundant plasma carnitine metabolites in T2DM.
Considering the minimal influence of genotype on acylcarnitine patterns, we turned our attention to the potential impact of T2DM on plasma acylcarnitine patterns by pooling data across genotype (n = 12 and n = 44 for nondiabetics and diabetics, respectively). Calculated total acylcarnitine values (defined as total minus free carnitine) and the total acylcarnitine:free carnitine ratio were significantly increased to 150–170% of nondiabetic levels in T2DM persons (Table 2). This magnitude of increase was also observed in the total summed acylcarnitine concentration (defined as the sum of all measured individual acylcarnitines) in diabetics (Table 2, bottom row).
TABLE 2.
Plasma concentrations of carnitine and acylcarnitines in fasting, obese nondiabetic and T2DM adult African American women1
| Nondiabetics, n = 12
|
Diabetics, n = 44
|
T2DM vs. nondiabetic P-value2
|
||||
|---|---|---|---|---|---|---|
| Analyte | Plasma concentration | Positive cases | Plasma concentration | Positive cases | T2DM | |
| μmol/L | n | μmol/L | n | % of nondiabetic | ||
| Total carnitine | 41.95 ± 3.38 | 12 | 42.96 ± 1.12 | 44 | ↔ | |
| Free carnitine | 36.58 ± 2.56 | 12 | 34.91 ± 0.94 | 44 | ↔ | |
| Calculated total acylcarnitines (total-free) | 5.38 ± 0.92 | 12 | 8.05 ± 0.49 | 44 | 0.01 | (↑) 150 |
| Acyl:free ratio | 0.14 ± 0.02 | 12 | 0.24 ± 0.02 | 44 | 0.002 | (↑) 170 |
| Acetylcarnitine (C2) | 3.41 ± 0.46 | 12 | 5.35 ± 0.29 | 44 | 0.002 | (↑) 157 |
| Propionylcarnitine (C3) | 0.38 ± 0.04 | 12 | 0.24 ± 0.02 | 41 | 0.0004 | (↓) 36 |
| Butyrylcarnitine (C4) | 0.06 ± 0.01 | 11 | 0.05 ± 0.01 | 40 | ↔ | |
| Isobutyrylcarnitine | 0.06 ± 0.01 | 12 | 0.07 ± 0.01 | 40 | ↔ | |
| Valerylcarnitine | <0.01 | 2 | <0.01 | 1 | ↔ | |
| Isovalerylcarnitine | 0.01 ± 0.004 | 6 | 0.02 ± 0.003 | 24 | ↔ | |
| 3-Hydroxy-isovalerylcarnitine | 0.01 ± 0.004 | 5 | 0.02 ± 0.002 | 25 | ↔ | |
| 2-Methyl-butyrylcarnitine | 0.01 ± 0.01 | 6 | 0.01 ± 0.003 | 15 | ↔ | |
| Tigloylcarnitine | 0.01 ± 0.004 | 3 | 0.01 ± 0.003 | 10 | ↔ | |
| 3-Methyl-crotonylcarnitine | n.d.3 | 0 | <0.01 | 2 | ↔ | |
| Hexanoylcarnitine (C6) | 0.01 ± 0.003 | 3 | 0.02 ± 0.003 | 20 | 0.09 | (↑) 323 |
| Phenylacetylcarnitine | 0.01 ± 0.006 | 4 | 0.02 ± 0.01 | 13 | ↔ | |
| Phenylpropionylcarnitine | n.d. | 0 | <0.01 | 2 | ↔ | |
| 4-Phenyl-butyrylcarnitine | 0.02 ± 0.01 | 4 | <0.01 | 3 | ↔ | |
| Benzoylcarnitine | 0.01 ± 0.004 | 6 | 0.01 ± 0.002 | 11 | ↔ | |
| 4-Methyl-hexanoylcarnitine | n.d. | 0 | <0.01 | 5 | ↔ | |
| Octanoylcarnitine (C8) | 0.03 ± 0.01 | 8 | 0.07 ± 0.01 | 40 | 0.008 | (↑) 223 |
| cis-3,4-Methylene-heptanoylcarnitine | 0.19 ± 0.03 | 12 | 0.23 ± 0.02 | 43 | ↔ | |
| Decanoylcarnitine (C10) | 0.05 ± 0.01 | 10 | 0.10 ± 0.03 | 37 | NS | (↑) 219 |
| cis-4-Decenoylcarnitine | n.d. | 0 | 0.01 ± 0.01 | 4 | ↔ | |
| cis-3,4-Methylene-nonanoylcarnitine | n.d. | 0 | 0.02 ± 0.01 | 17 | 0.01 | (↑) |
| 5-Decynoylcarnitine | n.d. | 0 | <0.01 | 5 | ↔ | |
| Lauroylcarnitine (C12) | 0.01 ± 0.004 | 3 | 0.02 ± 0.01 | 17 | ↔ | |
| Myristoylcarnitine (C14) | n.d. | 0 | 0.01 ± 0.003 | 14 | 0.03 | (↑) |
| Trans-2-tetradecenoylcarnitine | n.d. | 0 | <0.01 | 3 | ↔ | |
| Palmitoylcarnitine (C16) | 0.05 ± 0.01 | 12 | 0.05 ± 0.01 | 37 | ↔ | |
| Palmitoleoylcarnitine (C16:1) | n.d. | 0 | <0.01 | 4 | ||
| Stearoylcarnitine (C18) | n.d. | 0 | <0.01 | 4 | ↔ | |
| Oleoylcarnitine (C18:1) | 0.04 ± 0.01 | 10 | 0.09 ± 0.01 | 28 | 0.03 | (↑) 208 |
| Linoleoylcarnitine (C18:2) | 0.01 ± 0.01 | 3 | 0.03 ± 0.01 | 17 | ↔ | |
| Succinylcarnitine | 0.01 ± 0.004 | 3 | 0.01 ± 0.002 | 10 | ↔ | |
| Methyl-malonylcarnitine | n.d. | 0 | <0.01 | 2 | ↔ | |
| Adipoylcarnitine (C6-dicarb) | n.d. | 0 | 0.01 ± 0.002 | 7 | ↔ | |
| Suberoylcarnitine (C8-dicarb) | n.d. | 0 | 0.01 ± 0.002 | 13 | 0.03 | (↑) |
| Sebacoylcarnitine (C10-dicarb) | 0.01 ± 0.004 | 3 | 0.01 ± 0.002 | 9 | ↔ | |
| Summed C10-C14 acylcarnitines | 0.05 ± 0.01 | 10 | 0.14 ± 0.03 | 44 | 0.004 | (↑) 280 |
| Total acylcarnitines4 | 4.40 ± 0.55 | – | 6.50 ± 0.31 | – | 0.004 | (↑) 148 |
Values are means ± SEM. Not shown are valproyl-, 4-methyl-octanoyl-, trans-2-dodecenoyl-, trans-2-hexadecenoyl-, ethyl-malonyl-, glutaroyl-, and 3-methyl-glutaroyl- carnitines, which were undetected or observed in only a single volunteer.
Mann-Whitney test.
n.d., Not detected.
Total acylcarnitines = sum of acetylcarnitine through sebacoylcarnitine.
Acetylcarnitine represented the single most abundant acylcarnitine and this metabolite increased significantly in T2DM volunteers to 157% of nondiabetic levels (Table 2). T2DM was also characterized by increases in the levels of the medium-chain acylcarnitines C6-carn, C8-carn, and C10-carn to ∼200–300% of nondiabetic concentrations and a 36% reduction in plasma propionylcarnitine concentration (Table 2). However, the sum of the plasma C10-carn, C12-carn, and C14-carn concentrations in T2DM participants was ∼300% that of nondiabetic controls (see Table 2). Other diabetes-related plasma acylcarnitine concentration differences included: 1) a significant increase in LCFA oleoylcarnitine (to ∼200% of control levels; P = 0.03) and an upward trend in total summed LCFA-carnitines in T2DM (0.18 ± 0.02 μmol/L vs. 0.10 ± 0.02 μmol/L; P = 0.07), with the caveat that all LCFA metabolites were not detected in all volunteers; 2) the presence of suberoylcarnitine (dicarboxylic octanoylcarnitine) in 13 of 44 diabetics but in none of the nondiabetics; and 3) the detection of cis-3,4-methylene-nonanoylcarnitine in 17 of 44 diabetics but none of the nondiabetics. The latter metabolite and cis-3,4-methylene-heptanoylcarnitine have been detected previously in human blood and are considered to be end-products of gut bacteria-derived metabolites that are absorbed and further processed within the liver (37).
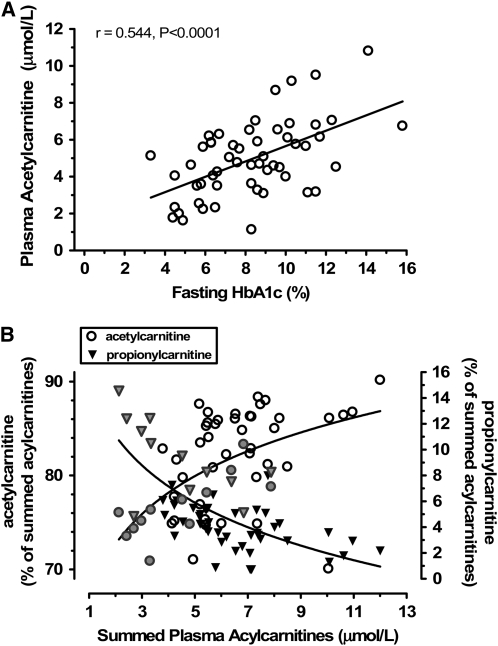
We next asked if acylcarnitines that differed in T2DM were associated with the degree of blood glucose control. Regression analyses revealed that acetylcarnitine levels were significantly correlated with plasma HbA1c (Fig. 1A), suggestive of higher acetylcarnitine generation with increasing severity of diabetes. On the other hand, propionylcarnitine levels were lower in T2DM (Table 2) and levels were inversely correlated with HbA1c (i.e. reduced with worsening diabetes severity; r = −0.308; P < 0.05). Additional variables that were correlated with HbA1c were the calculated total plasma acylcarnitine concentrations (r = 0.377; P = 0.005) and the acyl:free carnitine ratio (r = 0.509; P < 0.0001). Total summed acylcarnitine concentrations were also correlated with HbA1c (r = 0.502; P = 0.0001).
FIGURE 1 .
Evidence for alteration of acetylcarnitine and propionylcarnitine metabolism with increasing severity of diabetes and association with markers of incomplete fatty acid β-oxidation. (A) Significant positive correlation between fasting blood HbA1c and plasma acetylcarnitine concentration (Pearson correlation statistic, n = 56). (B) Association between the relative abundance of plasma acetylcarnitine (left axis, circles) and plasma propionylcarnitine (right axis, triangles) to increasing plasma acylcarnitine concentrations across all participants; diabetics (open circles, black triangles) and nondiabetics (gray symbols) are illustrated (2 diabetic outliers with relative levels of 40% and 60% of summed acylcarnitines are not shown). Best-fit lines were described by the equation: y = a × Ln(x) + b.
We observed a striking relationship between relative concentrations of plasma acetylcarnitine and propionylcarnitine (as a percent of total summed acylcarnitines) and the accumulation of acylcarnitine intermediates reflective of incomplete LCFA β-oxidation (Fig. 1B). Increasing concentrations of the latter were associated with progressively increasing levels of acetylcarnitine and decreasing levels of propionylcarnitine.
Activation of NFκB activity by medium-chain fatty acylcarnitine.
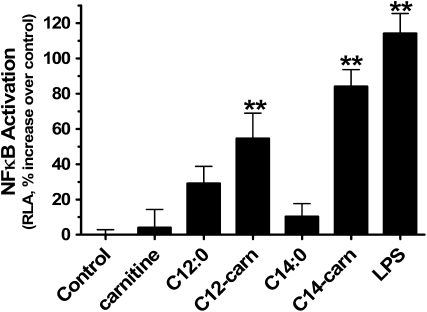
Hwang et al. (38–43) have shown that saturated medium-chain FA (MCFA) (C10:0, C12:0, C14:0, which can be components of the lipid A moiety of LPS) induce the proinflammatory state in cultured cells largely via increased NFκB-regulated transcriptional events and cytokine release downstream from activation of cell surface, intracellular toll-like receptors (TLR), and nucleotide-binding oligomerization domain-containing (Nod) proteins. NFκB-regulated pathways promote serine phosphorylation of insulin receptor substrate-1 and hence likely play a role in insulin resistance phenotypes [reviewed in (44,45)]. With this in mind, and considering the increased prevalence and overall plasma concentrations of C10- to C14-carnitines in T2DM (Table 2), we performed a proof-of-principle study to evaluate whether acylcarnitines can activate NFκB in the well-established RAW264.7 cell model. As expected, LPS treatment significantly induced NFκB activity consistent with its known interaction with the TLR4 system (Fig. 2). C12-carn and C14-carn, but not carnitine alone, also significantly activated NFκB as indicated by higher luciferase activity (Fig. 2). The activation of NFκB pathways by acylcarnitines was not an artifact of reagent LPS contamination, because, unlike LPS preparations, acylcarnitine stocks tested negative for LPS using a limulus amebocyte lysate assay (Lonza, no. 50–647U) and acylcarnitine-induced luciferase activity was not blocked by the LPS inhibitor polymixin B (Sigma) added to RAW264.7 cells at concentrations (5 and 10 mg/L) that maximally attenuate LPS-stimulated NFκB activation (data not shown).
FIGURE 2 .
Activity of NFκB is stimulated by MCFA-carn in RAW264.7 murine monocyte cells. Cells transfected with a (2×)-NF-κB-Luc containing 2 copies of the NFκB consensus-binding site were treated for 15 h with 20 μmol/L MCFA (C12:0 and C14:0), 20 μmol/L dl-carnitine alone, or 20 μmol/L dl-MCFA-carn (C12-carn and C14-carn). Relative luciferase activity (RLA) changes relative to vehicle control RLA are illustrated, with 5 μg/L LPS serving as a positive control. Results were derived from 3 (C12 studies; n = 9) to ≥3 (C14 studies; n = 21) independent cell culture experiments. Values are means ± SEM. **Different from vehicle-treated control, P < 0.01.
Discussion
Most studies support the idea that dysregulation in tissue lipid metabolism is involved in the pathogenesis of insulin resistance and T2DM, but the underlying mechanisms are not fully understood. Discovery of metabolite patterns specifically reflective of muscle LCFA catabolism should help clarify biochemical pathways linking myocellular lipid metabolism and insulin action and may provide clinically useful predictive and diagnostic biomarkers for insulin resistance and T2DM. In this study, we addressed this issue through comprehensive analysis of plasma acylcarnitines in samples derived from persons bearing a UCP3 G304A missense allele that results in abnormal UCP3 function in muscle and was previously reported to markedly reduce whole-body FA oxidation (28). Levels and patterns of specific acylcarnitine molecules reflect tissue-level accumulation of acyl-CoA and other CoA-bound carboxylic acids, a fraction of which are transferred to carnitine via carnitine acyltransferase enzymes and transported into the blood (46). Contrary to our expectations, there were few differences in acylcarnitine metabolites when we compared UCP3 g/g and g/a persons. Interestingly, plasma lactate concentrations were significantly reduced in g/a individuals, an outcome that might be explained by lower use of LCFA for muscle energy in g/a individuals and a greater reliance upon carbohydrate for energy. In this scenario, one would predict a decrease in the NADH:NAD+ ratio, a more limited LCFA-derived acetyl-CoA pool, and thus enhanced flow of glucose-derived pyruvate carbon into the TCA cycle via pyruvate dehydrogenase and reduced flux to lactate via lactate dehydrogenase. Further studies are required to test this model and define the tissue origin and uptake patterns of lactate and carnitine moieties in g/a compared to g/g individuals.
The reasons why more pronounced acylcarnitine shifts were not observed in g/a individuals with a dysfunctional UCP3 are unclear, because inborn errors of metabolism that disrupt FA catabolism globally manifest as markedly increased plasma and urinary concentrations of LCFA- and MCFA-carn (47). It is possible that distinct genotype-related differences in plasma acylcarnitines will only emerge following a challenge to engage muscle LCFA combustion, such as submaximal exercise. It is also plausible that despite a lower contribution of LCFA oxidation to energy production in g/a individuals (with concomitantly higher RQ and nonprotein RQ) (28), the relative degree of mismatch between intramitochondrial delivery of LCFA moieties to mitochondrial LCFA catabolism is similar across genotypes, preventing major genotype-related differences in acylcarnitine accumulation. Diet may also play a role, because it has been hypothesized that the UCP3 polymorphism interacts with a high-fat diet to increase risk of severe obesity (28). Clearly, further research is required to fully elucidate the metabolic ramifications of expression of the UCP3 g/a missense allele, using broader metabolomic profiling technologies and carefully controlled exercise/diet interventions to detect potential shifts in a greater number of pathways.
Contradictory reports regarding the influence of LCFA β-oxidation in regulating insulin action prompted us to examine plasma acylcarnitine differences between nondiabetics and T2DM samples. Some lines of research indicate that attenuated fasting muscle mitochondrial LCFA combustion (2–7) and accumulation of IMCL (8,9,11–15) are commonplace in T2DM and prediabetes. Increasing CPT-1 activity in rodent muscle cell lines improved insulin sensitivity even in the presence of LCFA and intracellular accumulation of IMCL, ceramide, and diacylglycerol (25), whereas pharmacologic CPT-1 blockade compromised muscle insulin sensitivity in rats (10). In contrast, other investigations have implicated inordinately high mitochondrial FA β-oxidation as a causative factor for poor insulin-stimulated glucose uptake in muscle (48). This has raised the question: “Is robust LCFA β-oxidation helpful or harmful with respect to glucose utilization in muscle?” The answer may be that it is not the rate of β-oxidation per se but rather the fate of its resulting metabolites that is important (i.e. how well does tissue and mitochondrial LCFA delivery match LCFA combustion to CO2?).
Recently, Koves et al. (26) have provided evidence in rodent obesity/insulin-resistance models and cultured rodent myotubes that insulin sensitivity is compromised when mitochondrial LCFA delivery, catabolism, and TCA cycle rates become increasingly mismatched [also see (24)]. This mismatch, or “metabolic overload,” was reflected in higher serum and muscle levels of acylcarnitines of short-, medium-, and long-chain length (26). These findings support the hypothesis that intracellular signals that are sensitive to incomplete oxidation of LCFA impact insulin signaling (24). Our results are consistent with this view of metabolic overload as follows. First, more indices of incomplete oxidation of LCFA carbon were evident in plasma of T2DM volunteers compared to nondiabetics, because the prevalence and concentrations of C6- to C14-carnitines were increased in T2DM (Table 2). Möder et al. (49) have reported an increase in urinary MCFA-carn, including C6- to C10-carnitines, in T2DM patients, also consistent with incomplete LCFA combustion in diabetic tissues. Second, excess availability of LCFA relative to their mitochondrial oxidation should give rise to LCFA-carnitine accumulation reflected in blood or urine. In the few studies that have examined this, blood LCFA-carnitine levels were up to 2-fold of control values (50,51) and even higher in urine (49) from T2DM/severely insulin-resistant individuals. Consistent with this, ∼100% or greater increases in the concentrations of several plasma LCFA-carnitines were observed in T2DM volunteers (Table 2). A limitation to the current study is that the tissue sources of plasma markers of incomplete LCFA oxidation are not known and additional studies are needed to pinpoint the specific generators of these metabolites.
What is the basis for incomplete LCFA β-oxidation in T2DM tissues? One possible contributing factor is a lower mitochondrial number, volume, and reduced mitochondrial substrate oxidation capacity in T2DM tissues such as muscle (16,17,20–22), which would limit total tissue capacity for TCA utilization of LCFA-derived carbon. Another contributor to this phenomenon might be diabetes-associated functional perturbations in the TCA cycle itself, independent of mitochondrial mass. This would be expected to expand tissue acetyl-CoA pools and increase conversion of the latter to acetylcarnitine via carnitine acetyltransferases and to acetate via thioesterases. Indeed, we and others (26,49–51) have observed significantly increased plasma acetylcarnitine concentrations in T2DM and circulating acetate of endogenous origin is also increased in diabetes (52,53). Further support for compromised TCA cycle/respiratory function in T2DM is the markedly reduced entry of 13C-labeled palmitate carbon into the TCA cycle of T2DM muscle (6), a 30–40% decrease in muscle TCA cycle activity in insulin-resistant offspring of T2DM patients (18), lower citrate synthase and succinate dehydrogenase enzyme activities in T2DM muscle (19,54), and evidence for increased blood pyruvate levels in T2DM (55,56). Notably, increased blood and urinary acetylcarnitine levels are a hallmark of inborn errors of metabolism in which propionyl-CoA anaplerosis (replenishment of TCA cycle intermediates) is disrupted vis a vis dysfunctional propionyl-CoA carboxylase or methylmalonyl-CoA mutase (57). We report a significant reduction in the plasma concentration of propionylcarnitine in T2DM and our novel results indicate that increasing plasma markers of incomplete LCFA β-oxidation are associated with rising plasma acetylcarnitine concentrations concurrent with reduced propionylcarnitine levels. This raises the intriguing possibility that a diabetes-associated decrease in tissue pools of anaplerotic propionyl-CoA limits the generation of TCA cycle intermediates and thus impacts oxidative disposal of acetyl-CoA and LCFA-CoA in mitochondria. Such a theory is consistent with a recent report of significantly lower TCA cycle intermediate levels in muscle of obese insulin-resistant rodents (26). Stimulation of anaplerosis has been proposed to explain the therapeutic benefit of propionylcarnitine in improving diabetic heart and muscle metabolism (58), and propionate and odd-chain fatty acids markedly reduced acetyl-CoA and acetylcarnitine levels in acetate-perfused rat hearts (59). Further studies will be required to evaluate if changes in tissue propionyl-CoA generation (i.e. from branched-chain amino acid catabolism) occur in the diabetic state and to understand if this modulates TCA cycle activity and accumulation of acylcarnitines.
Despite growing evidence in T2DM for dysregulated mitochondrial function and incomplete LCFA combustion, the molecular links between these processes and insulin signaling pathways remain unresolved. We provide novel evidence that acylcarnitine by-products of incomplete LCFA β-oxidation can stimulate proinflammatory NFκB-associated pathways. Our proof-of-principle studies were prompted by observations that NFκB is a downstream target of TLR2, TLR4, and Nod, and these factors can be powerfully activated by saturated MCFA (38–43). Because proinflammatory/NFκB-related events in immune cells and tissues are implicated in promoting insulin resistance [reviewed in (44,45); also see (60,61)], our finding that MCFA carnitines activate NFκB raises the intriguing possibility that excessive local tissue accumulation of such by-products can attenuate insulin signaling. More research is warranted to determine the mechanisms by which acylcarnitines activate NFκB (i.e. the roles of TLR and NOD), to assess if acylcarnitines inhibit insulin action, and to evaluate the acylcarnitine metabolite concentrations circa tissues for which NFκB plays a role in regulating insulin sensitivity.
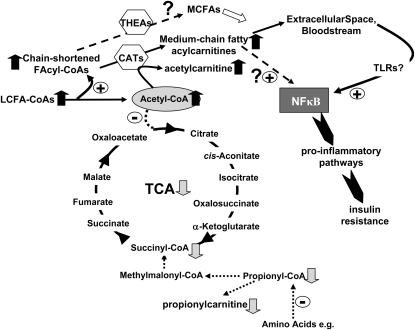
Comprehensive plasma acylcarnitine profiles in T2DM have revealed elevated circulating markers of incomplete LCFA catabolism and of acetylcarnitine, together with lower levels of propionylcarnitine. Because person-to-person differences in plasma acetylcarnitine and propionylcarnitine were correlated with HbA1c, these metabolites appear to be sensitive indicators of biochemical pathways that are responsive to, or underlie, the severity of diabetes and long-term blood sugar control. Our results support a working model for T2DM tissues (Fig. 3) in which limited TCA cycle activity relative to mitochondrial fuel delivery contributes to inefficient LCFA β-oxidation, in turn promoting accumulation of acylcarnitine by-products that activate NFκB-associated pathways to inhibit insulin action. This working model provides a basis for future hypothesis-testing regarding the roles of acylcarnitine accumulation and inefficient fat combustion in driving the diabetic phenotype.
FIGURE 3 .
Working model of tissue metabolism in T2DM and potential links between the TCA cycle, fatty acid metabolism, acylcarnitines, and insulin resistance. Considering results from the current experiments and literature reports, a unifying model was devised to illustrate metabolic and inflammatory events that may take place under diabetic conditions. This hypothetical paradigm proposes that in type 2 diabetic tissues, there is: 1) an inadequate capacity of the TCA relative to fuel delivery, due in part to reduced anaplerotic propionyl-CoA pools reflected in lower propionylcarnitine levels; 2) mismatched acetyl-CoA generation vs. entry into the TCA cycle, leading to accumulation of mitochondrial acetyl-CoA and hence increased acetylcarnitine generation via carnitine acyltransferase (CAT) activity (specifically, carnitine acetyltransferase); 3) incomplete LCFA catabolism and accumulation of chain-shortened acyl-CoA moieties that serve as substrates for CAT, with subsequent generation of medium-chain acylcarnitines. Thioesterases (THEA) might also act on acyl-CoA to increase free MCFA generation; 4) entry of a fraction of MCFA-carn into the bloodstream/extracellular space surrounding affected tissue; and 5) activation of proinflammatory NFκB through cell-surface TLR or other mechanisms, in turn promoting local or systemic insulin resistance.
Supplementary Material
Acknowledgments
We thank Maria Stoll and Pieter J. Oort for technical assistance and Thuan Nguyen for statistical help.
Supported by intramural USDA-Agricultural Research Service CRIS 5306-51530-016-00D (S. H. A.) and NIH-NIDDK R01-DK078328 (S. H. A.), USDA-ARS CRIS 5306-51530-15-00D (D. H. H.), NIH grants DK-38764 and P01-HL-55782 (W. T. G.), the Merit Review program of the Department of Veterans Affairs (W. T. G.), NIH grants DK064007, DK41868, and CA75613 (D. H. H.), USDA grant 2001-35200-10721 (D. H. H.), and the American Institutes for Cancer Research (01A095Rev to D. H. H.). We also acknowledge support from the UAB General Clinical Research Center M01-RR00032, University of Alabama, Birmingham, Clinical Nutrition Research Unit (P30-DK56336), and UAB Diabetes Research and Training Center (P60 DK079626).
Author disclosures: S. H. Adams, C. L. Hoppel, K. H. Lok, L. Zhao, S. W. Wong, P. E. Minkler, D. H. Hwang, J. W. Newman, and W. T. Garvey, no conflicts of interest.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: CPT-1, carnitine palmitoyltransferase; FA, fatty acid; HbA1c, hemoglobin A1c; IMCL, intramyocellular lipid; LCFA, long-chain fatty acid; LOD, limit of detection; LPS, lipopolysaccharide; MCFA, medium-chain fatty acid; MCFA-carn, MCFA-carnitine; NFκB, nuclear factor κ-B; NFκB-Luc, nuclear factor κ-B-dependent luciferase reporter construct; Nod, nucleotide-binding oligomerization domain-containing; RQ, respiratory quotient; TCA, tricarboxylic acid; T2DM, type 2 diabetes mellitus; TLR, toll-like receptor; UCP3, uncoupling protein 3.
References
- 1.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005;115:1699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandarino LJ, Consoli A, Jain A, Kelley DE. Interaction of carbohydrate and fat fuels in human skeletal muscle: impact of obesity and NIDDM. Am J Physiol. 1996;270:E463–70. [DOI] [PubMed] [Google Scholar]
- 4.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–41. [DOI] [PubMed] [Google Scholar]
- 5.Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49:2102–7. [DOI] [PubMed] [Google Scholar]
- 6.Blaak E.E., Wagenmakers A.J. The fate of [U- (13)C]palmitate extracted by skeletal muscle in subjects with type 2 diabetes and control subjects. Diabetes. 2002;51:784–9. [DOI] [PubMed] [Google Scholar]
- 7.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, et al. Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes. 1999;48:1113–9. [DOI] [PubMed] [Google Scholar]
- 9.Krssak M, Petersen KF, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6. [DOI] [PubMed] [Google Scholar]
- 10.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50:123–30. [DOI] [PubMed] [Google Scholar]
- 11.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. [DOI] [PubMed] [Google Scholar]
- 12.Mingrone G, Rosa G, Greco AV, Manco M, Vega N, Nanni G, Castagneto M, Vidal H. Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis. 2003;170:155–61. [DOI] [PubMed] [Google Scholar]
- 13.He J, Goodpaster BH, Kelley DE. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obes Res. 2004;12:761–9. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Brown NF. Skeletal muscle lipid and its association with insulin resistance: what is the role for exercise? Exerc Sport Sci Rev. 2005;33:150–4. [DOI] [PubMed] [Google Scholar]
- 15.Lara-Castro C, Newcomer BR, Rowell J, Wallace P, Shaughnessy SM, Munoz AJ, Shiflett AM, Rigsby DY, Lawrence JC, et al. Effects of short-term very low-calorie diet on intramyocellular lipid and insulin sensitivity in nondiabetic and type 2 diabetic subjects. Metabolism. 2008;57:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–23. [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50. [DOI] [PubMed] [Google Scholar]
- 21.Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. [DOI] [PubMed] [Google Scholar]
- 22.Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–9. [DOI] [PubMed] [Google Scholar]
- 23.Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda). 2004;19:183–90. [DOI] [PubMed] [Google Scholar]
- 24.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. [DOI] [PubMed] [Google Scholar]
- 25.Perdomo G, Commerford SR, Richard AM, Adams SH, Corkey BE, O'Doherty RM, Brown NF. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem. 2004;279:27177–86. [DOI] [PubMed] [Google Scholar]
- 26.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Stenger VA, Boada F, McKolanis T, Davis D, Ross R, Kelley DE. Skeletal muscle lipid concentration quantified by magnetic resonance imaging. Am J Clin Nutr. 2004;79:748–54. [DOI] [PubMed] [Google Scholar]
- 28.Argyropoulos G, Brown AM, Willi SM, Zhu J, He Y, Reitman M, Gevao SM, Spruill I, Garvey WT. Effects of mutations in the human uncoupling protein 3 gene on the respiratory quotient and fat oxidation in severe obesity and type 2 diabetes. J Clin Invest. 1998;102:1345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezaire V, Spriet LL, Campbell S, Sabet N, Gerrits M, Bonen A, Harper ME. Constitutive UCP3 overexpression at physiological levels increases mouse skeletal muscle capacity for fatty acid transport and oxidation. FASEB J. 2005;19:977–9. [DOI] [PubMed] [Google Scholar]
- 30.Costford SR, Seifert EL, Bezaire V, Gerrits MF, Bevilacqua L, Gowing A, Harper ME. The energetic implications of uncoupling protein-3 in skeletal muscle. Appl Physiol Nutr Metab. 2007;32:884–94. [DOI] [PubMed] [Google Scholar]
- 31.Pecqueur C, Couplan E, Bouillaud F, Ricquier D. Genetic and physiological analysis of the role of uncoupling proteins in human energy homeostasis. J Mol Med. 2001;79:48–56. [DOI] [PubMed] [Google Scholar]
- 32.McLean DC Jr, Spruill I, Argyropoulos G, Page GP, Shriver MD, Garvey WT. Mitochondrial DNA (mtDNA) haplotypes reveal maternal population genetic affinities of Sea Island Gullah-speaking African Americans. Am J Phys Anthropol. 2005;127:427–38. [DOI] [PubMed] [Google Scholar]
- 33.Sale MM, Lu L, Spruill IJ, Fernandez JK, Lok KM, Divers J, Langefeld CD, Garvey WT. Genome-wide linkage scan in Gullah-speaking African American Families with type 2 diabetes: the Sea Islands Genetic African American Registry (Project SuGAR). Diabetes. 2009;58:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancinelli A, Iannoni E, Calvani M, Duran M. Effect of temperature on the stability of long-chain acylcarnitines in human blood prior to plasma separation. Clin Chim Acta. 2007;375:169–70. [DOI] [PubMed] [Google Scholar]
- 35.Minkler PE, Ingalls ST, Hoppel CL. Strategy for the isolation, derivatization, chromatographic separation, and detection of carnitine and acylcarnitines. Anal Chem. 2005;77:1448–57. [DOI] [PubMed] [Google Scholar]
- 36.Minkler PE, Stoll MS, Ingalls ST, Yang S, Kerner J, Hoppel CL. Quantification of carnitine and acylcarnitines in biological matrices by HPLC electrospray ionization mass spectrometry. Clin Chem. 2008;54:1451–62. [DOI] [PubMed] [Google Scholar]
- 37.Yang S, Minkler P, Hoppel C. cis-3,4-Methylene-heptanoylcarnitine: characterization and verification of the C8:1 acylcarnitine in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857:251–8. [DOI] [PubMed] [Google Scholar]
- 38.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–9. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, Plakidas A, Lee WH, Heikkinen A, Chanmugam P, Bray G, Hwang DH. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J Lipid Res. 2003;44:479–86. [DOI] [PubMed] [Google Scholar]
- 40.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–51. [DOI] [PubMed] [Google Scholar]
- 41.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–9. [DOI] [PubMed] [Google Scholar]
- 42.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–7. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Kwon MJ, Huang S, Lee JY, Fukase K, Inohara N, Hwang DH. Differential modulation of Nods signaling pathways by fatty acids in human colonic epithelial HCT116 cells. J Biol Chem. 2007;282:11618–28. [DOI] [PubMed] [Google Scholar]
- 44.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–95. [DOI] [PubMed] [Google Scholar]
- 45.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramsay RR, Zammit VA. Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Aspects Med. 2004;25:475–93. [DOI] [PubMed] [Google Scholar]
- 47.Roe CR. Inherited disorders of mitochondrial fatty acid oxidation: a new responsibility for the neonatologist. Semin Neonatol. 2002;7:37–47. [DOI] [PubMed] [Google Scholar]
- 48.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. [DOI] [PubMed] [Google Scholar]
- 49.Moder M, Kiessling A, Loster H, Bruggemann L. The pattern of urinary acylcarnitines determined by electrospray mass spectrometry: a new tool in the diagnosis of diabetes mellitus. Anal Bioanal Chem. 2003;375:200–10. [DOI] [PubMed] [Google Scholar]
- 50.Genuth SM, Hoppel CL. Acute hormonal effects on carnitine metabolism in thin and obese subjects: responses to somatostatin, glucagon, and insulin. Metabolism. 1981;30:393–401. [DOI] [PubMed] [Google Scholar]
- 51.Inokuchi T, Imamura K, Nomura K, Nomoto K, Isogai S. Changes in carnitine metabolism with ketone body production in obese glucose-intolerant patients. Diabetes Res Clin Pract. 1995;30:1–7. [DOI] [PubMed] [Google Scholar]
- 52.Smith RF, Humphreys S, Hockaday TD. The measurement of plasma acetate by a manual or automated technique in diabetic and non-diabetic subjects. Ann Clin Biochem. 1986;23:285–91. [DOI] [PubMed] [Google Scholar]
- 53.Akanji AO, Humphreys S, Thursfield V, Hockaday TD. The relationship of plasma acetate with glucose and other blood intermediary metabolites in non-diabetic and diabetic subjects. Clin Chim Acta. 1989;185:25–34. [DOI] [PubMed] [Google Scholar]
- 54.Colberg SR, Simoneau JA, Thaete FL, Kelley DE. Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest. 1995;95:1846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith MJ, Taylor KW. Blood pyruvate and alpha-ketoglutarate in normal and diabetic subjects. BMJ. 1956;2:1035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diraison F, Large V, Brunengraber H, Beylot M. Non-invasive tracing of liver intermediary metabolism in normal subjects and in moderately hyperglycaemic NIDDM subjects. Evidence against increased gluconeogenesis and hepatic fatty acid oxidation in NIDDM. Diabetologia. 1998;41:212–20. [DOI] [PubMed] [Google Scholar]
- 57.Wikoff WR, Gangoiti JA, Barshop BA, Siuzdak G. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin Chem. 2007;53:2169–76. [DOI] [PubMed] [Google Scholar]
- 58.Broderick TL. ATP production and TCA activity are stimulated by propionyl-L-carnitine in the diabetic rat heart. Drugs R D. 2008;9:83–91. [DOI] [PubMed] [Google Scholar]
- 59.Pearson DJ, Tubbs PK. Carnitine and derivatives in rat tissues. Biochem J. 1967;105:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinha S, Perdomo G, Brown NF, O'Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004;279:41294–301. [DOI] [PubMed] [Google Scholar]
- 61.Radin MS, Sinha S, Bhatt BA, Dedousis N, O'Doherty RM. Inhibition or deletion of the lipopolysaccharide receptor Toll-like receptor-4 confers partial protection against lipid-induced insulin resistance in rodent skeletal muscle. Diabetologia. 2008;51:336–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.