Abstract
Supplemental vitamin E alleviates age-related defects in interleukin (IL)-2 production, T cell proliferation, and immune synapse formation. Here, we evaluated the effect of in vitro supplementation with 46 μmol/L of vitamin E on T cell receptor-proximal signaling events of CD4+ T cells from young (4–6 mo) and old (22–26 mo) C57BL mice. Aged murine CD4+ T cells stimulated via CD3 and CD28, tyrosine 191 of the adaptor protein Linker for Activation of T cells (LAT), was hypo-phosphorylated. Supplementation with vitamin E eliminated this difference in the tyrosine phosphorylation of LAT. By using a flow cytometric assay, the age-related differences in the activation-induced phosphorylation of LAT were observed in both naïve and memory T cell subsets. In addition, supplementation with vitamin E eliminates the age-related differences in LAT phosphorylation in both T cell subsets. Neither age nor vitamin E supplementation altered the fraction of LAT entering the membrane compartment. Furthermore, neither age nor vitamin E influenced the phosphorylation of Lck and Zap70, indicating that associated changes in LAT phosphorylation were not caused by alterations in activation states of the upstream kinases Lck and Zap70.
Introduction
Aging is associated with reduced T cell function as exhibited by a decline in interleukin (IL)6-2 production and proliferative capacity. Age-associated defects in T cell receptor (TCR)-initiated signaling pathways reduce TCR-induced tyrosine phosphorylation (1–4), limit the elevation of intracellular calcium (5,6), and hinder the activation of mitogen-activated protein kinase pathways (7,8). Although recent findings have demonstrated that the redistribution of key signaling proteins into the immune synapse is disrupted with increased age (9,10), the underlying biochemical alterations responsible for these signaling defects remain controversial.
TCR engagement activates a cascade of intracellular signals, including protein kinases, protein phosphatases, and adaptor proteins (11,12). TCR activation is initiated when the tyrosine kinase Lck phosphorylates TCR-associated intracellular tyrosine-based activation motifs. Intracellular tyrosine-based activation motifs phosphorylation enables the recruitment and activation of the tyrosine kinase Zap70, which is responsible for the phosphorylation of downstream adaptor proteins, including Linker for Activation of T cells (LAT) and SLP76 (13). The phosphorylation of LAT, a membrane-anchored protein, is required for the recruitment of adaptor and effector proteins, including Grb2, Gads, SLP76, Vav1, phospholipase C-γ1, and phosphoinositide 3-kinase (14,15). Ultimately, these complexes drive the changes in cell morphology and gene expression that are associated with efficient T cell activation.
Current models of T cell activation emphasize the role of signaling microclusters in the initiation of antigen receptor-dependent signals (16). These structures exclude the tyrosine phosphatase CD45 and are highly enriched in tyrosine kinases and critical adaptor proteins. LAT plays a pivotal role in the assembly of these structures through its interactions with distinct membrane microdomains, known as lipid rafts, and through its ability to nucleate protein-protein scaffolds (17–19). Because mutations precluding LAT tyrosine phosphorylation impair both microcluster assembly and T cell activation (14,20–22), we postulated that defects in LAT phosphorylation could contribute to age-related defects in T cell function.
We previously showed that supplementation with vitamin E, either in vitro or in vivo, restores age-associated declines in T cell function both in elderly humans and in aged mice (23–27). We demonstrated that vitamin E improves the ability of T cells to produce IL-2 and advance through the cell cycle (28). In addition, we showed that vitamin E supplementation, either in vivo or ex vivo, enhances the redistribution of Zap70, LAT, and Vav to immune synapses (29). The objective of the present study was to clarify the biochemical defects associated with aging and the mechanisms by which vitamin E enhances immune synapse formation, IL-2 production, and T cell proliferation in the aged.
Materials and Methods
Mice.
Young (4–6 mo) and old (22–26 mo) pathogen-free C57BL/6 mice obtained from the National Institute on Aging colonies at Harlan Sprague Dawley were fed autoclaved commercial nonpurified (Harlan Teklad 7012) mouse diet containing the nutrient composition and balance necessary to support efficient growth and maintenance, in addition to 114.36 IU/kg (77μg/kg) of dl-α-tocopheryl acetate and water, ad libitum (30). Mice were housed in filtered cages and maintained at a constant temperature (23°C) with a 12-h-light/-dark cycle. Mice were killed via CO2 asphyxiation and exsanguination. All handling and animal conditions were approved by the Animal Care and Use Committee of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University. Mice exhibiting tumors, splenomegaly, grossly visible skin lesions, or considerable pathology were excluded from the study. All rodents were obtained and maintained as viral-antibody free for up to 19 viruses.
Purification of CD4+ spleen cells.
Spleens were aseptically removed and placed in sterile, endotoxin-free RPMI 1640 (BioWhittaker) medium supplemented with 25 mmol/L HEPES, 2 mmol/L glutamine, 100 kU/L penicillin, and 100 mg/L streptomycin (all from Life Technologies; complete RPMI). Single-cell suspensions were acquired by gently disrupting spleens between 2 sterile frosted glass slides. RBC were lysed using a hemolytic ammonium chloride-based Gey's reagent. Untouched lymphocyte subpopulations were isolated from RBC-depleted splenocytes using depletion columns (Miltenyi Biotec). To assess purity, negatively selected cells were stained for PE-conjugated anti-CD4, anti-CD8, and anti-CD19 (BD PharMingen) and analyzed on a FACS Calibur flow cytometer (BD Biosciences). The results showed that the cell preparations were at least 93–98% CD4+ (data not shown).
Supplementation with α-tocopherol.
A 37-μg/L stock solution of natural vitamin E was made by dissolving RRR-α-tocopherol (Henkel) in ethanol. To enhance cellular uptake, the vitamin E stock solution was mixed in fetal bovine serum (FBS) at a final concentration of 1 μg/L and incubated for 1 h in a 37°C water bath protected from the light with periodic vortexing. Purified CD4+ T cells from young and old mice were adjusted to a final concentration of either 1 × 109 cells/L (for whole-cell protein extractions) or 3 × 109 cells/L (for membrane fractions). These cells were cultured with vitamin E [0.02 μg/L (46 μmol/L)] or 0.06% ethanol (vehicle control) as previously described (28). Under these conditions, comparable and significant increases were observed in the vitamin E content of both young and old T cells [data not shown; (29)]. This dose of vitamin E is equivalent to the average level of α-tocopherol measured in the plasma of humans taking a daily vitamin E supplement of 200 IU (134 mg). This dose is safe in humans and optimally enhances the immune responses of the elderly (25,31). Furthermore, this treatment mimics the effects of in vivo feeding with vitamin E (either 30 or 500 mg dl-α-tocopheryl acetate/(kg diet · d) with respect to antigen receptor-induced synapse formation (29), IL-2 production, and T cell proliferation (23,24,28).
Stimulation conditions and total whole-cell protein lysate preparation.
For each stimulation, 1 × 106 freshly isolated CD4+ T cells were treated with vitamin E and stimulated in RPMI using doses of anti-CD3 and anti-CD28 calibrated to reproduce the age-specific defects previously observed using plate-bound anti-CD3 [data not shown; (28)]. Briefly, T cells were preincubated with biotin-conjugated anti-CD3ɛ for 30 min at 4°C (0.001 μg/L; clone 145–2C11; BD PharMingen). Subsequently, the T cells were resuspended in fresh media containing anti-CD28 (0.002 μg/L; clone 37.51; Pharmingen). Stimulations were initiated by adding 0.01 μg/L streptavidin and placing the samples in a 37°C water bath. The reactions were stopped with ice-cold PBS containing 1 mmol/L sodium orthovanadate and 10 mmol/L sodium fluoride. Cells were washed and resuspended in lysis buffer containing 1% Triton X-100, 50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L sodium chloride, 10 mmol/L sodium fluoride, 1 mmol/L EDTA, 1 mmol/L sodium orthovanadate, 0.1 g/L 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), and a protease inhibitor cocktail tablet (Roche Diagnostics). After 30 min on ice, cellular debris was removed by centrifugation at 1000 × g; 1 min at 4°C. Protein lysates were stored at −80°C for Western blot analysis.
Plasma membrane isolation.
Freshly isolated CD4+ T cells from the spleen (3 × 106) were incubated with or without vitamin E and stimulated as above. However, cells were resuspended in a hypotonic lysis buffer containing 20 mmol/L HEPES, pH 7.6, 5 mmol/L EGTA, 1 mmol/L magnesium chloride, 10 mmol/L sodium fluoride, 5 mmol/L sodium pyrophosphate, 1 mmol/L sodium orthovanadate, 0.1 μg/L AEBSF, and a protease inhibitor cocktail tablet. After 30 min at 4°C, cells were disrupted using 2 15-s bursts from a Branson Sonifier 450 set to power level 2. Nuclei were removed by centrifuging at 1000 × g; 10 min at 4°C. Supernatants were transferred to Beckman polycarbonate centrifuge tubes and centrifuged at 200,000 × g; 1 h at 4°C in a Beckman Optima TLX tabletop ultracentrifuge. After the removal of the cytoplasmic fraction, the membrane-containing pellet was resuspended in solubilization buffer containing 20 mmol/L HEPES, pH 7.4, 150 mmol/L sodium chloride, 1 mmol/L magnesium chloride, 10 mmol/L sodium fluoride, 1% Triton X-100, 1 mmol/L sodium orthovanadate, 0.1 g/L AEBSF, and a protease inhibitor cocktail tablet (Roche Diagnostics). After 30 min at 4°C, solubilized membrane fractions were cleared by a second round of high-speed centrifugation.
Immunoblot analysis.
Total lysates and cellular fractions were separated by SDS-PAGE and Western blotted as described previously (32). All phosphorylation site-specific sera (Lck tyrosines 394 and 505, Zap70 tyrosine 319, and LAT tyrosine 191) were obtained from Cell Signaling Technology. Antibodies specific for total Lck and Zap70 were also from Cell Signaling. The LAT-specific antibody was from Upstate. The β-actin antibody was from Sigma. Densitometry was performed using the ChemiImager software package (Alpha Innotech).
Flow cytometry staining.
To analyze the phosphorylation of LAT by FACS, T cells were stimulated and fixed for 10 min in ice-cold PBS containing 1 mmol/L sodium orthovanadate, 10 mmol/L sodium fluoride, and 3.7% paraformaldehyde. T cells were surface-stained by incubating for 30 min at room temperature using PE-conjugated anti-CD44 and antigen presenting cells-conjugated anti-CD4 (BD Pharmingen) diluted in PBS and 2% FBS. For intracellular staining, the permeabilization and blocking steps were performed in permeabilization buffer (BD Pharmingen) containing 2% FBS and 2% goat serum. After 15 min at room temperature, the cells were stained with the LAT-specific primary antibody and then with a FITC-labeled goat anti-rabbit secondary antibody (Molecular Probes, Invitrogen).
Statistical analysis.
The effect of vitamin E for Western blot data were assessed by calculating the difference in response obtained when each animal's cells were treated with and without vitamin E (vitamin E-control). These differences were not normally distributed, so the differences for young and old animals were compared using the Kruskal-Wallace test. When the Kruskal-Wallace test showed significance, i.e. when the vitamin E effect was judged different for young and old animals, the vitamin E effect was assessed in young and old animals separately using the Wilcoxon's Signed Rank test. Flow cytometry data were analyzed using Student's paired t test. Systat 10 statistical software was used and results are presented as means ± SEM. Significance was set at P < 0.05.
Results
LAT phosphorylation is reversed by vitamin E.
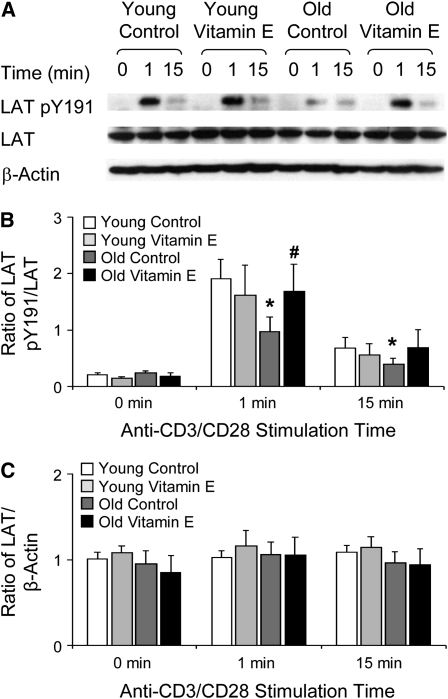
Previously, we demonstrated that aged T cells display vitamin E-reversible defects in the recruitment of signaling proteins to the immune synapse. To determine whether the age-related defect in LAT redistribution to the immune synapse was associated with defects in the tyrosine phosphorylation of LAT, we isolated CD4+ T cells from the spleens of young and old mice (28). The phosphorylation of LAT on tyrosine 191 occurred within 1 min of stimulation, remained high for 5 min, and declined within 15 min (data not shown). At 1 min, activated CD4+ T cells from young mice generated approximately twice the LAT pY191 in the aged cells (P = 0.04; Fig. 1A,B). At this time point, vitamin E supplementation increased the level of LAT pY191 in old CD4+ T cells (P = 0.05; Fig. 1A,B). This treatment eliminated the difference in LAT pY191 levels between old and young T cells without altering LAT phosphorylation in young T cells (Fig. 1B). Neither age nor vitamin E affected the expression of LAT (Fig. 1C), indicating that the age-associated and vitamin E-responsive defect must affect the efficiency of either LAT phosphorylation or dephosphorylation.
FIGURE 1 .
An age-related defect in LAT phosphorylation is reversed by vitamin E in CD4+ T cells isolated from the spleens of young and aged mice. (A) Lysates were serially Western blotted for LAT pY191, total LAT, and β-actin. One of 5 representative experiments is shown. (B) Densitometry was performed and the phosphorylated LAT signal was normalized to the total LAT signal. (C) Densitometry was performed and the total LAT signal was normalized to the β-actin signal. Ratios are the mean ± SEM, n = 5. *Different from young, P < 0.05 (Kruskal-Wallis nonparametric test); #different from vehicle control, P < 0.05 (Wilcoxon's Signed Rank t test).
LAT phosphorylation in old naïve and memory subsets is reversed by vitamin E.
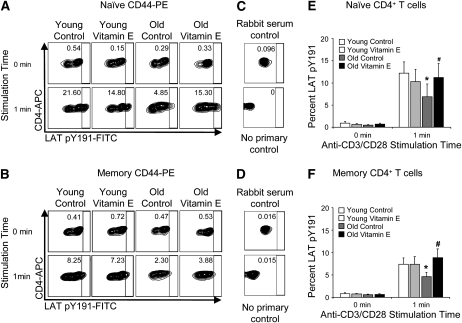
Before examining the mechanism of the age-related and vitamin E-responsive defect in LAT phosphorylation, we determined whether the observed defects in LAT phosphorylation were confined to specific subsets of T cells. By performing intracellular stains for LAT pY191, we were able to determine the efficiency of LAT phosphorylation in naïve and memory cells derived from young and old mice. As expected, we observed an age-related defect in the frequency of LAT phosphorylation (Fig. 2A–C). Notably, a defect was present in both the naïve (Fig. 2D; P = 0.003) and memory (Fig. 2E; P = 0.01) subpopulations of CD4+ T cells. These observations were consistent with the fact that LAT is less frequently recruited to the immune synapses formed by aged T cells (29). Although vitamin E supplementation did not alter the frequency of LAT pY191-positive young T cells following stimulation, vitamin E did increase the frequency of LAT pY191-positive T cells in both the naïve (Fig. 2D; P = 0.009) and memory (Fig. 2E; P = 0.018) subpopulations from old mice. These effects precisely parallel our observations of the recruitment of LAT into the immune synapses formed by vitamin E-treated cells from young and old mice (29).
FIGURE 2 .
An age-related defect in LAT phosphorylation in naïve and memory CD4+ T cells isolated from the spleens of young and aged mice is reversed by vitamin E. Fixed splenocytes were surface stained for CD4 and CD44, stained intracellular antigens, and analyzed by flow cytometry. (A–C) The percentages of pY191-LAT positive cells within populations gated for (A) naïve T cells (CD44lo, CD4+) or (B) memory T cells (CD44hi, CD4+) are shown. (C) Control intracellular stains were performed with normal rabbit serum or without primary antibody. All panels are representative of 7 independent experiments. (D,E) The frequencies of pY191-LAT positive cells within the naïve (D) and memory (E) CD4+ T cell subpopulations were determined in 7 replicate experiments examining the effects of age, vitamin E supplementation, and stimulation. The mean percentages ± SEM are shown, n = 7. *Different from young, P < 0.05 (Student's paired t test); #different from vehicle control, P ≤ 0.05 (Student's paired t test).
Age and vitamin E do not influence Lck or Zap70 phosphorylation.
To determine the mechanism responsible for the age-specific defect in LAT phosphorylation, we assessed the phosphorylation of upstream signaling intermediates, Lck and Zap70, activated by CD3 and CD28. We demonstrated that neither age nor vitamin E significantly influenced the phosphorylation of either site in Lck (Supplemental Fig. 1A,B). Similarly, we evaluated the phosphorylation of Zap70 at Tyrosine 319, a site whose phosphorylation is associated with the activation of this kinase. As observed with Lck, neither age nor vitamin E significantly affected the phosphorylation of Zap70 (Supplemental Fig. 1C). In addition, the levels of Lck and Zap70 are not affected by either age or vitamin E (data not shown). Thus, the age-related and vitamin E-responsive changes in LAT phosphorylation are not caused by gross defects in the expression or activation of TCR-proximal tyrosine kinases.
LAT phosphorylation in membrane fractions is reversed by vitamin E.
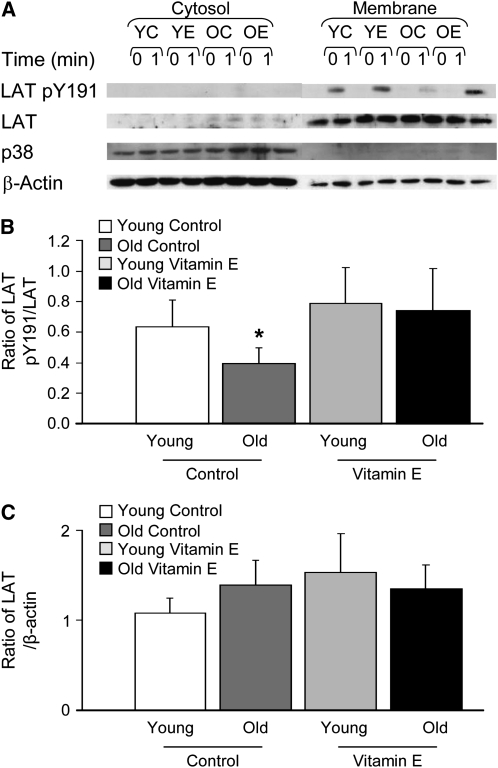
Vitamin E is a regulator of redox balance and is capable of acting as a membrane free radical scavenger. In addition, changes in the intracellular redox balance have been reported to alter the subcellular localization of LAT, resulting in its displacement from the plasma membran and in the perturbation of TCR signaling pathways (33). Therefore, vitamin E could, in principle, influence LAT phosphorylation by controlling the efficiency with which LAT partitions into the membrane. To determine whether this is the case, we generated membrane and cytosolic fractions from activated CD4+ T cells. However, the fraction of LAT retained in cellular membranes was comparable in the T cells of both young and old mice and was unaffected by vitamin E (Fig. 3A,C), disproving this hypothesis. In addition, the phosphorylation of membrane-associated LAT on Tyrosine 191 was reduced in T cells from old mice (Fig. 3A,B). Supplemental vitamin E selectively reversed this hypo-phosphorylation in aged T cells, eliminating the difference between vehicle-treated aged and young T cells. Thus, the association of LAT with the plasma membrane is unaffected by either age or vitamin E, and the biochemical properties of membrane-associated LAT are indistinguishable from those of total LAT.
FIGURE 3 .
Effect of age and vitamin E on membrane-associated phosphorylated LAT in CD4+ T cells isolated from the spleens of young and aged mice. (A) Cytoplasmic and membrane fractions were isolated and blotted for pY191 LAT, total LAT, total p38, and β-actin. The cytosolic mitogen-activated protein kinase p38 is absent from the membrane fractions and the lipid-raft associated adaptor LAT is absent from the cytoplasmic fractions, validating the fractionation procedure. One of 5 representative experiments is shown. (B) Densitometry was performed and the pY191 LAT signal was normalized to the total LAT signal. (C) Densitometry was performed and the LAT signal was normalized to the β-actin signal. Ratios are the mean ± SEM, n = 5. *Different from young, P < 0.05 (Kruskal-Wallis nonparametric test).
Discussion
Our goal in this study was to determine how TCR-proximal signals are affected by aging and by supplementation with vitamin E. Our previous work indicated that vitamin E can reverse age-related defects in the redistribution of LAT to the immune synapse (29) and that the phosphorylation of LAT plays a critical role in its recruitment into signaling complexes (22). Therefore, we evaluated how aging and vitamin E influence the phosphorylation of LAT. Here, we confirm that the phosphorylation of LAT is significantly lower in CD4+ T cells from aged mice, even though the expression of LAT is unaltered with age (9). Using a phospho-LAT–specific antibody, we established that the age-associated defect in LAT phosphorylation extends to Tyrosine 191, which is required for T cell activation and is essential for the recruitment of the downstream adaptors Gads and SLP76 (14,34). In addition, the age-related decrease in LAT phosphorylation is observed in both naïve and memory cells, although the impact of aging on LAT phosphorylation is somewhat more pronounced in the naïve subset. In addition, we have identified a novel and beneficial function for vitamin E supplementation in aged immune cells: in vitro supplementation with vitamin E selectively reverses the age-associated defect in the phosphorylation of LAT on Tyrosine 191 in both naïve and memory CD4+ T cells. We hypothesize that the age-dependent defect in LAT phosphorylation causes the age-associated decline in effector function and immune synapse formation (28,29). By extension, the ability of vitamin E to reconstitute LAT phosphorylation in aged T cells may be directly related to the beneficial effects of vitamin E in vitro and in vivo indicators of the T cell function.
Because the total amount of LAT was unchanged with either age or vitamin E treatment, the changes in the overall phosphorylation of LAT must stem from reductions in the rate of LAT phosphorylation or increases in the rate of LAT dephosphorylation. Here, we were unable to detect age-related changes in the phosphorylation of Lck or Zap70, in accordance with previous reports (35,36). In addition, vitamin E selectively restored the phosphorylation of LAT in aged T cells without altering the phosphorylation of Lck or Zap70. Therefore, any changes in the rate of LAT phosphorylation are likely to stem from changes in the duration and thus the rate of encounter between LAT and its upstream kinases, rather than from changes in activities of these kinases.
Although the study of signaling in aged T cells has primarily focused on the activities of kinases, phosphatases profoundly influence the signals initiated by the TCR (37,38). Crucially, many TCR-initiated signaling cascades are antagonized by tyrosine phosphatases (39,40). In particular, SHP-1, a protein tyrosine phosphate with 2 SH2 domains, is a potent negative regulator of TCR-mediated signaling [reviewed in (41,42)]. Thus, the age- and vitamin E-induced changes in LAT phosphorylation could be due to changes in the expression, activation, or localization of SHP-1. Further studies are needed to explore the possible contribution of SHP-1 to the age- and vitamin E-induced changes in LAT phosphorylation.
Whether the age-related defect in LAT phosphorylation is caused by changes in the rates of phosphorylation or dephosphorylation, the ultimate cause of this defect remains unknown. One hypothesis is that aging alters the properties of substructures within the plasma membrane (e.g. lipid rafts), influencing the stability of LAT-containing signaling complexes and the exclusion of antagonistic phosphatases, such as SHP-1. This hypothesis is consistent with the observation that anergic T cells display a defect in the recruitment of LAT into lipid rafts. This defect is associated with impaired LAT phosphorylation and altered recruitment of LAT into the immune synapse (43). However, we confirmed that aging does not affect the association of LAT with the plasma membrane and Tamir et al. (9) showed that there is no age-related change in LAT association with lipid rafts. Therefore, aging is unlikely to grossly inhibit the recruitment of LAT into lipid rafts but instead may influence the biochemical properties of the lipid rafts themselves.
It is also interesting to note that vitamin E may regulate the properties of lipid rafts. Although vitamin E has been considered a regulator of phosphorylation [reviewed in (44)] and a regulator of gene expression [(45) and reviewed in (46)], vitamin E is also a modulator of membrane fluidity [reviewed in (47)]. In addition, vitamin E acts as a radical scavenger [reviewed in (48)] and may reverse specific age-dependent changes in the membrane redox environment (17,49,50). In this manner, vitamin E may influence the composition of lipid rafts by controlling the palmitoylation of critical raft components (51,52).
In future studies, we will explore how aging and vitamin E supplementation influence the phosphorylation of LAT by directly identifying the protein and lipid constituents of TCR- and LAT-containing signaling microclusters.
Supplementary Material
Acknowledgments
We are grateful for the assistance and helpful suggestions of Dr. K. Eric Paulson and Dr. Dayong Wu.
Supported by the USDA, Agriculture Research Service under contract number 58-1950-9-001, the National Institute on Aging grant R01-AG009140-10A1, Office of Dietary Supplement, and Unilever Health Institute fellowship.
Author disclosures: M. G. Marko, H-J. E. Pang, Z. Ren, A. Azzi, B. T. Huber, S. C. Bunnell, and S. N. Meydani, no conflicts of interest.
Supplemental Figure 1 is available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AEBSF, 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride; FBS, fetal bovine serum; IL, interleukin; LAT, Linker for Activation of T cells; TCR, T cell receptor.
References
- 1.Patel HR, Miller RA. Age-associated changes in mitogen-induced protein phosphorylation in murine T lymphocytes. Eur J Immunol. 1992;22:253–60. [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Miller RA. Tyrosine-specific protein phosphorylation in response to anti-CD3 antibody is diminished in old mice. J Gerontol. 1992;47:B147–53. [DOI] [PubMed] [Google Scholar]
- 3.Grossmann A, Rabinovitch PS, Kavanagh TJ, Jinneman JC, Gilliland LK, Ledbetter JA, Kanner SB. Activation of murine T-cells via phospholipase-C gamma 1-associated protein tyrosine phosphorylation is reduced with aging. J Gerontol A Biol Sci Med Sci. 1995;50:B205–12. [DOI] [PubMed] [Google Scholar]
- 4.Garcia GG, Miller RA. Differential tyrosine phosphorylation of zeta chain dimers in mouse CD4 T lymphocytes: effect of age. Cell Immunol. 1997;175:51–7. [DOI] [PubMed] [Google Scholar]
- 5.Miller RA, Jacobson B, Weil G, Simons ER. Diminished calcium influx in lectin-stimulated T cells from old mice. J Cell Physiol. 1987;132:337–42. [DOI] [PubMed] [Google Scholar]
- 6.Grossmann A, Maggio-Price L, Jinneman JC, Rabinovitch PS. Influence of aging on intracellular free calcium and proliferation of mouse T-cell subsets from various lymphoid organs. Cell Immunol. 1991;135:118–31. [DOI] [PubMed] [Google Scholar]
- 7.Gorgas G, Butch ER, Guan KL, Miller RA. Diminished activation of the MAP kinase pathway in CD3-stimulated T lymphocytes from old mice. Mech Ageing Dev. 1997;94:71–83. [DOI] [PubMed] [Google Scholar]
- 8.Kirk CJ, Miller RA. Analysis of Raf-1 activation in response to TCR activation and costimulation in murine T-lymphocytes: effect of age. Cell Immunol. 1998;190:33–42. [DOI] [PubMed] [Google Scholar]
- 9.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–51. [DOI] [PubMed] [Google Scholar]
- 10.Garcia GG, Miller RA. Single-cell analyses reveal two defects in peptide-specific activation of naive T cells from aged mice. J Immunol. 2001;166:3151–7. [DOI] [PubMed] [Google Scholar]
- 11.Chan AC, Desai DM, Weiss A. The role of protein tyrosine kinases and protein tyrosine phosphatases in T cell antigen receptor signal transduction. Annu Rev Immunol. 1994;12:555–92. [DOI] [PubMed] [Google Scholar]
- 12.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–61. [DOI] [PubMed] [Google Scholar]
- 15.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem J. 2001;356:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seminario MC, Bunnell SC. Signal initiation in T-cell receptor microclusters. Immunol Rev. 2008;221:90–106. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–46. [DOI] [PubMed] [Google Scholar]
- 18.Bonello G, Blanchard N, Montoya MC, Aguado E, Langlet C, He HT, Nunez-Cruz S, Malissen M, Sanchez-Madrid F, et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J Cell Sci. 2004;117:1009–16. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. Int Immunol. 1999;11:943–50. [DOI] [PubMed] [Google Scholar]
- 20.Hartgroves LC, Lin J, Langen H, Zech T, Weiss A, Harder T. Synergistic assembly of linker for activation of T cells signaling protein complexes in T cell plasma membrane domains. J Biol Chem. 2003;278:20389–94. [DOI] [PubMed] [Google Scholar]
- 21.Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, Samelson LE. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 2006;13:798–805. [DOI] [PubMed] [Google Scholar]
- 22.Bunnell SC, Singer AL, Hong DI, Jacque BH, Jordan MS, Seminario MC, Barr VA, Koretzky GA, Samelson LE. Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol. 2006;26:7155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meydani SN, Meydani M, Verdon CP, Shapiro AA, Blumberg JB, Hayes KC. Vitamin E supplementation suppresses prostaglandin E1(2) synthesis and enhances the immune response of aged mice. Mech Ageing Dev. 1986;34:191–201. [DOI] [PubMed] [Google Scholar]
- 24.Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990;52:557–63. [DOI] [PubMed] [Google Scholar]
- 25.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–6. [DOI] [PubMed] [Google Scholar]
- 26.Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93:59–77. [DOI] [PubMed] [Google Scholar]
- 27.Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol. 1998;275:C661–8. [DOI] [PubMed] [Google Scholar]
- 28.Adolfsson O, Huber BT, Meydani SN. Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2-producing capacity. J Immunol. 2001;167:3809–17. [DOI] [PubMed] [Google Scholar]
- 29.Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-associated decline in effective immune synapse formation of CD4(+) T cells is reversed by vitamin E supplementation. J Immunol. 2007;178:1443–9. [DOI] [PubMed] [Google Scholar]
- 30.Ren Z, Guo Z, Meydani SN, Wu D. White button mushroom enhances maturation of bone marrow-derived dendritic cells and their antigen presenting function in mice. J Nutr. 2008;138:544–50. [DOI] [PubMed] [Google Scholar]
- 31.Meydani SN, Meydani M, Blumberg JB, Leka LS, Pedrosa M, Diamond R, Schaefer EJ. Assessment of the safety of supplementation with different amounts of vitamin E in healthy older adults. Am J Clin Nutr. 1998;68:311–8. [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Marko M, Claycombe K, Paulson KE, Meydani SN. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J Biol Chem. 2003;278:10983–92. [DOI] [PubMed] [Google Scholar]
- 33.Gringhuis SI, Papendrecht-van der Voort EA, Leow A, Nivine Levarht EW, Breedveld FC, Verweij CL. Effect of redox balance alterations on cellular localization of LAT and downstream T-cell receptor signaling pathways. Mol Cell Biol. 2002;22:400–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goda S, Quale AC, Woods ML, Felthauser A, Shimizu Y. Control of TCR-mediated activation of beta 1 integrins by the ZAP-70 tyrosine kinase interdomain B region and the linker for activation of T cells adapter protein. J Immunol. 2004;172:5379–87. [DOI] [PubMed] [Google Scholar]
- 35.Garcia GG, Miller RA. Increased Zap-70 association with CD3zeta in CD4 T cells from old mice. Cell Immunol. 1998;190:91–100. [DOI] [PubMed] [Google Scholar]
- 36.Larbi A, Douziech N, Dupuis G, Khalil A, Pelletier H, Guerard KP, Fulop T Jr. Age-associated alterations in the recruitment of signal-transduction proteins to lipid rafts in human T lymphocytes. J Leukoc Biol. 2004;75:373–81. [DOI] [PubMed] [Google Scholar]
- 37.Desai DM, Sap J, Silvennoinen O, Schlessinger J, Weiss A. The catalytic activity of the CD45 membrane-proximal phosphatase domain is required for TCR signaling and regulation. EMBO J. 1994;13:4002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 1998;273:13367–70. [DOI] [PubMed] [Google Scholar]
- 39.Schade AE, Levine AD. Signal transduction through the T cell receptor is dynamically regulated by balancing kinase and phosphatase activities. Biochem Biophys Res Commun. 2002;296:637–43. [DOI] [PubMed] [Google Scholar]
- 40.Schade AE, Levine AD. Phosphatases in concert with kinases set the gain for signal transduction through the T cell receptor. Mol Immunol. 2003;40:531–7. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol. 2000;12:361–78. [DOI] [PubMed] [Google Scholar]
- 42.Neel BG. Role of phosphatases in lymphocyte activation. Curr Opin Immunol. 1997;9:405–20. [DOI] [PubMed] [Google Scholar]
- 43.Hundt M, Tabata H, Jeon MS, Hayashi K, Tanaka Y, Krishna R, De Giorgio L, Liu YC, Fukata M, et al. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–22. [DOI] [PubMed] [Google Scholar]
- 44.Azzi A. Molecular mechanism of alpha-tocopherol action. Free Radic Biol Med. 2007;43:16–21. [DOI] [PubMed] [Google Scholar]
- 45.Barella L, Muller PY, Schlachter M, Hunziker W, Stocklin E, Spitzer V, Meier N, de Pascual-Teresa S, Minihane AM, et al. Identification of hepatic molecular mechanisms of action of alpha-tocopherol using global gene expression profile analysis in rats. Biochimica et biophysica acta. 2004;1689:66–74. [DOI] [PubMed] [Google Scholar]
- 46.Claycombe KJ, Meydani SN. Vitamin E and genome stability. Mutat Res. 2001;475:37–44. [DOI] [PubMed] [Google Scholar]
- 47.Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44:739–64. [DOI] [PubMed] [Google Scholar]
- 48.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu M, Shen S, Liu Y, Granillo O, Zhang W. Cutting edge: localization of linker for activation of T cells to lipid rafts is not essential in T cell activation and development. J Immunol. 2005;174:31–5. [DOI] [PubMed] [Google Scholar]
- 50.Shogomori H, Hammond AT, Ostermeyer-Fay AG, Barr DJ, Feigenson GW, London E, Brown DA. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–42. [DOI] [PubMed] [Google Scholar]
- 51.Parat MO, Stachowicz RZ, Fox PL. Oxidative stress inhibits caveolin-1 palmitoylation and trafficking in endothelial cells. Biochem J. 2002;361:681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adam L, Bouvier M, Jones TL. Nitric oxide modulates beta(2)-adrenergic receptor palmitoylation and signaling. J Biol Chem. 1999;274:26337–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.