Abstract
Studies in both healthy and diabetic subjects demonstrated that fructose produced a smaller postprandial rise in plasma glucose and serum insulin than other common carbohydrates. Substitution of dietary fructose for other carbohydrates produced a 13% reduction in mean plasma glucose in a study of type 1 and type 2 diabetic subjects. However, there is concern that fructose may aggravate lipemia. In 1 study, day-long plasma triglycerides in healthy men were 32% greater while they consumed a high-fructose diet than while they consumed a high-glucose diet. There is also concern that fructose may be a factor contributing to the growing worldwide prevalence of obesity. Fructose stimulates insulin secretion less than does glucose and glucose-containing carbohydrates. Because insulin increases leptin release, lower circulating insulin and leptin after fructose ingestion might inhibit appetite less than consumption of other carbohydrates and lead to increased energy intake. However, there is no convincing experimental evidence that dietary fructose actually does increase energy intake. There is also no evidence that fructose accelerates protein glycation. High fructose intake has been associated with increased risk of gout in men and increased risk of kidney stones. Dietary fructose appears to have adverse effects on postprandial serum triglycerides, so adding fructose in large amounts to the diet is undesirable. Glucose may be a suitable replacement sugar. The fructose that occurs naturally in fruits and vegetables provides only a modest amount of dietary fructose and should not be of concern.
Introduction
When ingested by humans, fructose is absorbed by an active transport system but at a slower rate than is glucose (1). Coingestion of glucose increases intestinal absorptive capacity for fructose. In the absence of glucose, human intestinal capacity to absorb fructose appears to be quite variable with some people unable to completely absorb 30- to 40-g quantities (1). Those individuals unable to completely absorb ingested fructose are at risk for diarrhea and other gastrointestinal side effects.
The first several steps in fructose and glucose metabolism differ significantly. Fructose stimulates only modest insulin secretion and does not require the presence of insulin to enter cells (2). Avidly taken up by hepatic cells, fructose is rapidly converted to fructose-1-phosphate and bypasses the early, rate-limiting steps of glucose metabolism. Fructose-1-phosphate is mainly converted to lactate, glucose, and glycogen (3). Gluconeogenesis from fructose is increased by starvation and poorly controlled diabetes. Fructose may also form acetyl-CoA, which is used in fatty acid synthesis. Enhanced activity of lipogenic enzymes with chronic fructose feeding may promote hepatic triglyceride production and output of VLDL particles. If energy intake is excessive, the potential for fructose to stimulate lipogenesis is presumably increased substantially.
Fructose is the sweetest-tasting naturally occurring carbohydrate. Advances in technology in the 1960s made possible the production of inexpensive high-fructose syrups from corn starch (4). The taste and sweetness of 55% high-fructose corn syrup are equivalent to those of sucrose. Because of sweetness and low cost, high-fructose syrups found commercial application. In the mid-1980s, 55% high-fructose syrup was adopted by the carbonated-beverage industry and became the predominant sweetener in soft drinks.
The United States has from the beginning been the world's largest producer of high-fructose corn syrups, but Japan, Canada, South Korea, China, Argentina, and other countries are also major producers (5). High-fructose syrups are widely used in soft drinks, fruit drinks, baked goods, jams, syrups, and candies. In 1977–1978, average fructose intake was estimated to be 37 g/d, accounting for ∼8% of total energy intake in the United States (6). In 1987–1988, fructose intake had increased to 39 g/d, accounting for ∼9% of energy intake (7), and in 1988–1994, it had further increased to 55 g/d, accounting for ∼10% of energy intake (8). Approximately one-third of fructose came from fruits, vegetables, and other natural sources, and two-thirds was added to beverages and foods in the diet. A similar trend toward substantial caloric sweetener and fructose consumption is occurring worldwide (9).
Metabolic effects of dietary fructose
There has long been interest in the metabolic effects of dietary fructose, particularly in people with metabolic syndrome and diabetes. Many studies attempting to describe these effects have been published. However, most of these studies have employed dietary instruction, sometimes with fructose supplementation, and have not established adequate control of nutrient intake. To define metabolic effects, investigators must provide foods to study participants and rigorously control the intake of all nutrients. Once metabolic effects have been defined, dietary instruction, menus, and supplements can be used to determine whether these effects can be translated into the population.
Studies in diabetic subjects done in the 1970s and 1980s demonstrated that fructose-containing test meals produced smaller postprandial increases in plasma glucose than test meals containing isocaloric amounts of sucrose, glucose, and starch (10–12). Jenkins et al. (13) greatly expanded our knowledge about the differences in response to dietary carbohydrates with the development of the glycemic index of foods. Glycemic index was defined as the increase in plasma glucose area from zero to 120 min after ingestion of 50 g of available carbohydrate from a test food compared with 50 g of carbohydrate from a reference food such as glucose. The glycemic indices of carbohydrate-containing foods vary substantially, with fructose having a particularly low glycemic index (Table 1).
TABLE 1.
Glycemic indices of selected carbohydrate-containing foods1
| Food | Glycemic Index | Food | Glycemic Index |
|---|---|---|---|
| Instant potato | 80 | Banana | 62 |
| White rice | 72 | Apple | 39 |
| White bread | 69 | Orange juice | 46 |
| Frozen peas | 51 | Glucose | 100 |
| Sweet corn | 59 | Sucrose | 59 |
| Carrots | 92 | Fructose | 20 |
| Lentils | 29 | Skim milk | 32 |
| Kidney beans | 29 | Ice cream | 36 |
Reference food was 50 g glucose. Reproduced from Jenkins et al. (13) with permission.
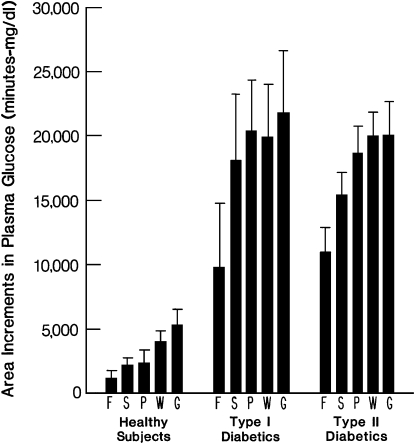
In an effort to further evaluate the potential for fructose to lower postprandial plasma glucose, we developed 5 test meals containing different carbohydrates and fed the meals to healthy and diabetic volunteers (14). The meals contained nearly identical amounts of carbohydrate, protein, and fat, but each had a different test carbohydrate, which accounted for 24–25% of total energy. The test carbohydrates were glucose, fructose, sucrose, potato starch, and wheat starch. Plasma glucose and serum insulin were measured before and at intervals for 240 min after the meals. In healthy volunteers, type 1 diabetic volunteers, and type 2 diabetic volunteers, the fructose meal produced the smallest postprandial increment in plasma glucose and the smallest increment in postprandial glucose area (Fig. 1). The fructose meals also produced the smallest increment in serum insulin in healthy and type 2 diabetic volunteers, but the differences among meals were not significant. Other investigators have clearly demonstrated that fructose test meals produced lesser insulin responses than sucrose or glucose test meals in both healthy and diabetic participants (15–17).
FIGURE 1 .
Area increments in plasma glucose (mean ± SEM) after test meals indicated as follows: F = fructose, S = sucrose, P = potato, W = wheat, and G = glucose; 10 healthy, 12 type 1 diabetic, and 10 type 2 diabetic subjects were studied. The area increment after F was significantly less than the area increments after W and G in healthy subjects and significantly less than after P, W, and G in type 2 diabetic subjects. Reproduced with permission from Bantle et al. (14).
Effects in the diabetic diet
Because fructose has an agreeable taste similar to that of sucrose, and because fructose produces a smaller postprandial rise in plasma glucose than other common carbohydrates, fructose seemed to be an excellent candidate for a sweetening agent in the diabetic diet. To test this possibility, we studied 12 type 1 and 12 type 2 diabetic participants who were fed 3 isocaloric diets for 8 d, each using a randomized, crossover design (18). The 3 diets provided, respectively, 21% of energy as fructose, 23% of energy as sucrose, and almost all carbohydrate energy as starch with <5% of energy derived from fructose and sucrose. All meals were prepared in a metabolic kitchen and provided to participants. The fructose diet resulted in significantly lower 1- and 2-h postprandial plasma glucose levels, overall mean plasma glucose, and urinary glucose excretion than did the starch diet. The reductions in mean plasma glucose with the fructose diet were 24% in type 1 diabetic participants and 7% in type 2 diabetic participants. Of note, the fructose diet increased postprandial lactate. There were no differences between the sucrose and starch diets in any of the measures of glycemic control in either group.
It next seemed important to extend the period of dietary intervention with fructose to see if beneficial effects on glycemia persisted and to look for potential adverse effects. Accordingly, we compared isocaloric high-fructose (20% of energy derived from fructose) and high-starch diets (<3% of energy derived from fructose) in 6 type 1 and 12 type 2 diabetic participants using a crossover design (19). Both study diets were composed of common foods. All meals were prepared in a metabolic kitchen and provided to participants for 28 d. The diets were well received by all participants. Mean plasma glucose, urine glucose, and serum glycosylated albumin were all lower during the fructose diet than during the starch diet. On d 28 of the fructose diet, mean plasma glucose was 13% lower than on d 28 of the starch diet. However, of concern, fasting serum LDL-cholesterol on d 28 of the fructose diet was 11% higher than the corresponding value for LDL-cholesterol on d 28 of the starch diet.
In an outpatient study, Osei et al. (20) compared dietary instruction in weight maintenance diets with and without fructose supplementation in 2 groups of type 2 diabetic participants. After 12 wk, fasting serum glucose and glycosylated hemoglobin were both lower in the group that received fructose supplementation. There were no differences between the groups in fasting serum cholesterol or triglycerides. In 2 other outpatient studies with diet instruction and fructose supplementation in type 2 diabetic participants, fructose did not have any effect on fasting plasma glucose or fasting serum lipids (21,22). However, in all 3 of these studies, postprandial plasma glucose and serum triglycerides were not measured. More importantly, in all 3 studies, participants were given diet instruction and fructose supplements, so nutrient intake was not adequately controlled.
Effects on plasma lipids
The potential for dietary fructose to raise serum LDL-cholesterol in diabetic participants created concern about the potential effects of fructose in the general population because, in the United States and many other countries, fructose is an important source of dietary energy (9). Several studies did not find adverse effects of dietary fructose on serum lipids in healthy participants (23–25). However, these studies either compared fructose to sucrose or were outpatient studies that did not provide meals to participants. Because sucrose is composed of 50% fructose, it is not an optimal reference. Moreover, rigorous control of nutrient intake requires the provision of meals. Thus, these studies were probably not reliable for assessing the effects of dietary fructose on serum lipids.
Two studies that compared a high-fructose diet to a diet nearly devoid of fructose and established rigorous control of nutrient intake by providing all food to participants both reported adverse effects of fructose on serum lipids (26,27). Hallfrish et al. (26) reported that 7.5% and 15% fructose diets consumed for 5 wk both increased fasting plasma LDL cholesterol in healthy and hyperinsulinemic men and increased fasting plasma triglycerides in hyperinsulinemic men. Reiser et al. (27) found that a 20% fructose diet consumed for 5 wk increased fasting plasma LDL-cholesterol in healthy men and fasting plasma triglycerides in both healthy and hyperinsulinemic men. These 2 well-done studies both suggested that dietary fructose does adversely affect serum lipids, at least in men. Women were not included in either study.
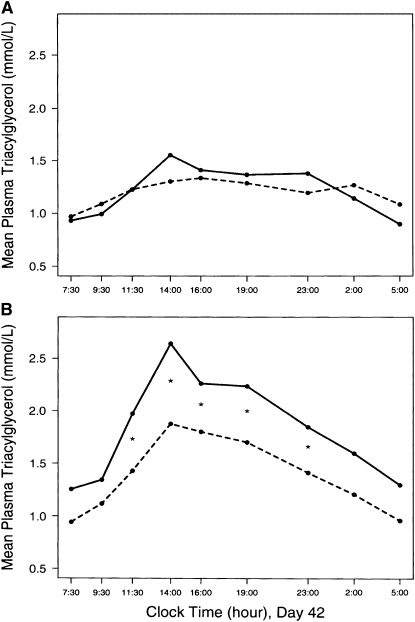
In an effort to gain additional insight into the effects of fructose on plasma lipids, we compared high- and low-fructose diets in 24 healthy volunteers (12 men and 12 women; 6 of each gender aged <40 y and 6 of each gender aged >40 y) (28). All participants consumed 2 isocaloric diets for 6 wk. One diet provided 17% of energy as fructose. The other diet was sweetened with glucose and was nearly devoid of fructose. Diet order was assigned randomly using a balanced crossover design. Both diets were composed of common foods and contained nearly identical amounts of carbohydrate, protein, fat, fiber, cholesterol, and saturated, monounsaturated, and polyunsaturated fatty acids. All meals were prepared in the metabolic kitchen of the University of Minnesota General Clinical Research Center. The fructose diet resulted in higher fasting total and LDL plasma cholesterol at d 28, but this effect did not persist at d 42 (Table 2). The plasma triglyceride responses to the diets differed by gender. The fructose diet had no significant effect on fasting or postprandial plasma triglycerides in women (Table 2, Fig. 2). However, in men, the fructose diet produced significantly higher fasting and postprandial plasma triglycerides. This effect persisted through d 42. On d 42 of the fructose diet, daylong plasma triglycerides (estimated by determining the area under the response curves) in men were 32% greater than during the glucose diet. A more recent study by Teff et al. (16) demonstrated an acute elevation in plasma triglycerides in women fed high-fructose test meals. Thus, the effect of dietary fructose to raise plasma triglycerides appears to occur in both men and women.
TABLE 2.
Effects of the 2 study diets on mean fasting plasma lipids1
| Day
|
|||
|---|---|---|---|
| 14 | 28 | 42 | |
| Plasma cholesterol | |||
| Fructose diet | 4.53 | 4.61 | 4.30 |
| Glucose diet | 4.43 | 4.30 | 4.22 |
| P2 | 0.154 | <0.001 | 0.169 |
| Plasma LDL-cholesterol | |||
| Fructose diet | 2.67 | 2.69 | 2.49 |
| Glucose diet | 2.59 | 2.49 | 2.49 |
| P2 | 0.256 | <0.001 | 0.756 |
| Plasma HDL-cholesterol | |||
| Fructose diet | 1.35 | 1.37 | 1.30 |
| Glucose diet | 1.40 | 1.37 | 1.30 |
| P2 | 0.077 | 0.897 | 0.965 |
| Plasma triglycerides | |||
| Women | |||
| Fructose diet | 0.97 | 1.02 | 0.93 |
| Glucose diet | 0.88 | 0.99 | 0.97 |
| P2 | 0.298 | 0.810 | 0.631 |
| Men | |||
| Fructose diet | 1.32 | 1.30 | 1.25 |
| Glucose diet | 1.12 | 1.03 | 0.95 |
| P2 | 0.018 | 0.001 | <0.001 |
The means for each endpoint have a common SE based on the appropriate repeated-measures ANOVA error term; to convert cholesterol to mg/dL, multiply by 38.6; to convert triglycerides to mg/dL, multiply by 88.5. Reproduced from Bantle et al. (28) with permission.
Because 6 paired comparisons of this endpoint were made (not all data shown), only P < 0.008 (0.05/6) should be considered significant at the 0.05 level.
FIGURE 2 .
Mean plasma triacylglycerol (triglyceride) concentrations in 12 healthy women (A) and 12 healthy men (B) during the 24-h metabolic profiles on d 42 of the fructose (solid line) and glucose (dashed line) diets from 07:30 to 14:00; *significant difference between the 2 points, P < 0.006 (0.05/9, Bonferroni adjustment for multiple comparisons). To convert triglycerides to mg/dL, multiply by 88.5. Reproduced from Bantle et al. (28) with permission.
Recently, Chong et al. (17) described what is probably the mechanism whereby dietary fructose raises plasma triglycerides. These investigators fed healthy volunteers high-fructose and high-glucose test meals that had added to them stable isotopes of fructose, glucose, and palmitate. As expected, fructose test meals produced smaller excursions in plasma glucose and plasma insulin and a greater increase in plasma triglycerides than glucose test meals. Fructose also produced a greater rise in VLDL triglycerides and a greater rise and delayed peak in chylomicron triglycerides. Only ∼0.1% of fructose was converted to fatty acids at 240 min. Taken together, these results suggest that the lower insulin excursion after fructose resulted in less activation of adipose tissue lipoprotein lipase and impaired triglyceride clearance. Because type 1 diabetic participants are unable and type 2 diabetic participants are poorly able to augment insulin secretion after meals, this would explain why differences between dietary fructose- and glucose-induced increases in triglycerides have not been demonstrated in diabetic participants.
Other metabolic effects of dietary fructose
Other concerns about dietary fructose include the possibilities that fructose accelerates protein glycation, increases uric acid production, and increases energy intake leading to weight gain. Glucose nonenzymatically attaches to proteins and other macromolecules and forms advanced glycosylation end products, which may be important in the pathogenesis of diabetic complications (29). Other monosaccharides are more reactive with proteins than glucose, perhaps explaining why glucose is the universal metabolic fuel (30). Fructose is approximately a 7 times more rapid glycating agent in vitro than is glucose (30), raising concern that replacing dietary glucose with fructose might lead to increased protein glycation. However, the rise in plasma fructose after a high-fructose test meal peaks at 0.3–0.6 mmol/L (5–10 mg/dL) and returns to near 0 in 4 h (17,18). In contrast, plasma glucose circulates at a concentration of ∼5.0 mmol/L and may rise to 8.0 mmol/L after a meal. Thus, rapid clearance of plasma fructose after dietary fructose ingestion makes it unlikely that fructose would have time to affect protein glycation significantly. Consistent with this are the reductions with fructose feeding of glycosylated albumin in 1 of our studies (19) and glycosylated hemoglobin in a study by Osei et al. (20).
Concern has also been expressed about the potential for dietary fructose to raise serum uric acid levels and cause gout. Fructose infusion results in rapid phosphorylation of fructose in the liver, depletion of ATP and increased uric acid production (31). Dietary fructose may increase serum uric acid in children with hereditary fructose intolerance and hyperinsulinemic men (2). Intake of large amounts of oral fructose increased serum uric acid in healthy volunteers (32), and chronic fructose consumption has been associated with increased risk of gout in men (33). However, a cause-and-effect relation between fructose consumption and gout has not been established. Similarly, an association has been demonstrated between high fructose intake and kidney stones (34), but a cause-and-effect relation has not been established.
There is also concern that fructose may be associated with increased energy intake and obesity. Worldwide trends in per capita consumption of caloric sweeteners (of which high-fructose syrups are a major component) demonstrated an increase from 970 kJ/d (232 kcal/d) in the year 1962 to 1280 kJ/d (306 kcal/d) in the year 2000 (9). In the United States, caloric sweeteners accounted for 16% of energy intake in the year 1996 (9). About 43% of the caloric sweeteners came from soft drinks and fruit drinks.
Several authors have suggested that dietary fructose may play a role in the worldwide increase in obesity prevalence (35,36). Their reasons for implicating fructose are principally 2. The first is the association, mentioned above, between increasing consumption of fructose and increasing obesity. The second is the theoretical possibility that dietary fructose increases energy intake. Clearly dietary fructose stimulates insulin secretion less than glucose and glucose-containing carbohydrates. Insulin stimulates leptin release from adipocytes (37), and circulating insulin and leptin concentrations were thus lower after ingestion of fructose-containing meals than after ingestion of glucose-containing meals in healthy women (16). However, energy intake by the women was not greater during the fructose-containing meals. Nevertheless, lower circulating insulin and leptin after fructose consumption might inhibit appetite less than consumption of other carbohydrates and lead to an increase in food intake.
Consistent with the idea that dietary fructose might increase energy intake are data from Ludwig et al. (38) that demonstrated an association between consumption of sugar-sweetened drinks and obesity in children. Further evidence is provided by Raben et al. (39), who fed overweight participants supplements of either sucrose or artificial sweeteners for 10 wk. The participants who consumed sucrose demonstrated increases in energy intake, body weight, fat mass, and blood pressure. However, it is important to point out that participants in the sucrose group were “instructed” to consume 2 g sucrose/kg body weight daily (∼23% of energy intake) and were provided with the necessary sucrose-sweetened beverages and foods to do so. Thus, the increased sucrose intake was not spontaneous. These 2 studies were the main evidence cited by the World Health Organization in implicating sugars as a cause of obesity and used to justify their recommendation that free sugar consumption be <10% of total daily energy intake (40).
Although increasing fructose consumption is temporally associated with the increasing worldwide prevalence of obesity, there is little or no evidence proving cause and effect. In the United States, increasing energy intake was associated with increased restaurant and fast-food meals and increased consumption of salty snacks and pizza as well as soft drinks (41). Decreased physical activity is also almost certainly a factor in the increasing prevalence of obesity. To prove that dietary fructose is important in causing obesity, it would be necessary to conduct a clinical trial demonstrating that fructose caused a spontaneous increase in energy intake. Given fructose's availability, low cost, and pleasant taste, such a clinical trial might provide important information.
Summary
Fructose is a naturally occurring sugar with a pleasant taste. Fructose produces a smaller postprandial rise in plasma glucose than other common carbohydrates and thus might be a useful sweetening agent in the diabetic diet. However, dietary fructose appears to have adverse effects on plasma lipids in both diabetic and healthy populations. There is also concern that dietary fructose may stimulate energy intake and promote weight gain and obesity. However, there is no compelling evidence that this is true. Nevertheless, adding large amounts of fructose to the diet may be undesirable because of adverse effects on lipemia. Glucose may be a suitable replacement sugar. Concern about fructose should not extend to the naturally occurring fructose in fruits and vegetables. These are healthy foods that provide only a modest amount of fructose in most people's diets.
Other articles in this supplement include references (42–51).
Published in a supplement to The Journal of Nutrition. Presented at the conference “The State-of-the-Science on Dietary Sweeteners Containing Fructose,” held in Beltsville, MD, March 18–19 2008. The conference was cosponsored by the Technical Committee on Carbohydrates of the International Life Sciences Institute North America (ILSI North America) and the USDA, Agricultural Research Service. The views expressed in these papers are not necessarily those of the USDA, the Agricultural Research Service, or the Supplement Coordinators. Supplement Coordinators for this supplement were David M. Klurfeld, USDA, Agricultural Research Service, and Molly Kretsch, USDA, Agricultural Research Service. Supplement Coordinator Disclosure: D. Klurfeld and M. Kretsch: no relationships to disclose.
Supported by grant M01RR00400 from the Division of Research Resources, NIH; Archer-Daniels-Midland Foundation; American Diabetes Association; and the International Fructose Foundation.
Author disclosure: J. P. Bantle, funding to support travel to the 18–19 March 2008 workshop “The State of the Science on Dietary Sweeteners Containing Fructose” and the writing of this resulting manuscript was provided by the International Life Sciences Institute, North American Branch.
References
- 1.Riby JE, Fujisawa T, Kretchmer N. Fructose absorption. Am J Clin Nutr. 1993;58:748S–53S. [DOI] [PubMed] [Google Scholar]
- 2.Henry RR, Crapo PA. Current issues in fructose metabolism. Annu Rev Nutr. 1991;11:21–39. [DOI] [PubMed] [Google Scholar]
- 3.Mayes PA. Intermediary metabolism of fructose. Am J Clin Nutr. 1993;58:754S–65S. [DOI] [PubMed] [Google Scholar]
- 4.Hanover LM, White JS. Manufacturing, composition and applications of fructose. Am J Clin Nutr. 1993;58:724S–32S. [DOI] [PubMed] [Google Scholar]
- 5.Vuilleumier S. Worldwide production of high-fructose syrup and crystalline fructose. Am J Clin Nutr. 1993;58:733S–6S. [DOI] [PubMed] [Google Scholar]
- 6.Park YK, Yetley EA. Intake and food sources of fructose in the United States. Am J Clin Nutr. 1993;58:737S–47S. [DOI] [PubMed] [Google Scholar]
- 7.Sigman-Grant M, Keast DR. Addendum to Am J Clin Nutr 1995;62 (Suppl):178S–194S. Am J Clin Nutr. 1997;65:1572–4. [DOI] [PubMed] [Google Scholar]
- 8.Bialostosky K, Wright JD, Kennedy-Stephenson J, McDowell M, Johnson CL. Dietary intake of macronutrients, micronutrients and other dietary constituents: United States 1988–94. National Center for Health Statistics. Vital Health Stat 11. 2002;245:1–58. [PubMed] [Google Scholar]
- 9.Popkin BM, Nielsen SJ. The sweetening of the world's diet. Obes Res. 2003;11:1325–32. [DOI] [PubMed] [Google Scholar]
- 10.Akerblom HK, Siltanen I, Kallio AK. Does dietary fructose affect the control of diabetes in children? Acta Med Scand Suppl. 1972;542:195–202. [DOI] [PubMed] [Google Scholar]
- 11.Crapo PA, Kolterman OG, Olefsky JM. Effects of oral fructose in normal, diabetic, and impaired glucose tolerance subjects. Diabetes Care. 1980;3:575–81. [DOI] [PubMed] [Google Scholar]
- 12.Akgun S, Ertel NH. A comparison of carbohydrate metabolism after sucrose, sorbital, and fructose meals in normal and diabetic subjects. Diabetes Care. 1980;3:582–5. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DJA, Wolever TMS, Tayler RH, Barker H, Fielden H, Baldwin JM, Bowling AC, Newman HC, Jenkins AS, Goff DV. Glycemic index of foods: a physiologic basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–6. [DOI] [PubMed] [Google Scholar]
- 14.Bantle JP, Laine DC, Castle GW, Thomas W, Hoogwerf BJ, Goetz FC. Postprandial glucose and insulin responses to meals containing different carbohydrates in normal and diabetic subjects. N Engl J Med. 1983;309:7–12. [DOI] [PubMed] [Google Scholar]
- 15.Crapo PA, Scarlett JA, Kolterman OG. Comparison of the metabolic responses to fructose and sucrose sweetened foods. Am J Clin Nutr. 1982;36:256–61. [DOI] [PubMed] [Google Scholar]
- 16.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D'Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–72. [DOI] [PubMed] [Google Scholar]
- 17.Chong MFF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–20. [DOI] [PubMed] [Google Scholar]
- 18.Bantle JP, Laine DC, Thomas W. Metabolic effects of dietary fructose and sucrose in types 1 and 2 diabetic subjects. JAMA. 1986;256:3241–6. [PubMed] [Google Scholar]
- 19.Bantle JP, Swanson JE, Thomas W, Laine DC. Metabolic effects of dietary fructose in diabetic subjects. Diabetes Care. 1992;15:1468–76. [DOI] [PubMed] [Google Scholar]
- 20.Osei K, Falko J, Bossetti BM, Holland GC. Metabolic effects of fructose as a natural sweetener in the physiologic meals of ambulatory obese patients with type 2 diabetes. Am J Med. 1987;83:249–55. [DOI] [PubMed] [Google Scholar]
- 21.McAteer EJ, O'Reilly G, Hadden DR. The effects of one month high fructose intake on plasma glucose and lipid levels in non-insulin-dependent diabetes. Diabet Med. 1987;4:62–4. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JW, Story LJ, Zettwoch NC, Gustafson NJ, Jefferson BS. Metabolic effects of fructose supplementation in diabetic individuals. Diabetes Care. 1989;12:337–44. [DOI] [PubMed] [Google Scholar]
- 23.Crapo PA, Kolterman OG. The metabolic effects of 2-wk fructose feeding in normal subjects. Am J Clin Nutr. 1984;39:525–34. [DOI] [PubMed] [Google Scholar]
- 24.Bossetti BM, Kocher LM, Moranz JF, Falko JM. The effects of physiologic amounts of simple sugars on lipoprotein, glucose and insulin levels in normal subjects. Diabetes Care. 1984;7:309–12. [DOI] [PubMed] [Google Scholar]
- 25.Koh ET, Ard NF, Mendoza F. Effects of fructose feeding on blood parameters and blood pressure in impaired glucose-tolerant subjects. J Am Diet Assoc. 1988;88:932–8. [PubMed] [Google Scholar]
- 26.Hallfrisch J, Reiser S, Prather ES. Blood lipid distribution of hyperinsulinemic men consuming three levels of fructose. Am J Clin Nutr. 1983;37:740–8. [DOI] [PubMed] [Google Scholar]
- 27.Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylase cornstarch. Am J Clin Nutr. 1989;49:832–9. [DOI] [PubMed] [Google Scholar]
- 28.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72:1128–34. [DOI] [PubMed] [Google Scholar]
- 29.Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835–43. [DOI] [PubMed] [Google Scholar]
- 30.Bunn HF, Higgins PJ. Reaction of monosaccharides with proteins: possible evolutionary significance. Science. 1981;213:222–4. [DOI] [PubMed] [Google Scholar]
- 31.Narins RG, Weisberg JS, Myers AR. Effects of carbohydrates on uric acid metabolism. Metabolism. 1974;23:455–65. [DOI] [PubMed] [Google Scholar]
- 32.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974;33:276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor EN, Curhan GC. Fructose consumption and the risk of kidney stones. Kidney Int. 2008;73:207–12. [DOI] [PubMed] [Google Scholar]
- 35.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain and the insulin resistance syndromne. Am J Clin Nutr. 2002;76:911–22. [DOI] [PubMed] [Google Scholar]
- 36.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 37.Saad MF, Khan A, Sharma A, Michael R, Riad-Gabriel MG, Boyadjian R, Jinagouda SD, Steil GM, Kamdar V. Physiological insulinemia acutely modulates plasma leptin. Diabetes. 1998;47:544–9. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–8. [DOI] [PubMed] [Google Scholar]
- 39.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 40.Waxman A. The WHO global strategy on diet, physical activity and health: The controversy on sugar. Development. 2004;47:75–82. [Google Scholar]
- 41.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10:370–8. [DOI] [PubMed] [Google Scholar]
- 42.Jones JM. Dietary sweeteners containing fructose: overview of a workshop on the state of the science. J Nutr. 2009;139:1210S–3S. [DOI] [PubMed] [Google Scholar]
- 43.Borra ST, Bouchoux A. Effects of science and the media on consumer perceptions about dietary sugars. J Nutr. 2009;139:1214S–8S. [DOI] [PubMed] [Google Scholar]
- 44.White JS. Misconceptions about high-fructose corn syrup: is it uniquely responsible for obesity, reactive dicarbonyl compounds, and advanced glycation endproducts? J Nutr. 2009;139:1219S–27S. [DOI] [PubMed] [Google Scholar]
- 45.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr. 2009;139:1228S–35S. [DOI] [PubMed] [Google Scholar]
- 46.Stanhope KL, Havel PJ. Fructose consumption: considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J Nutr. 2009;139:1236S–41S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angelopoulos TJ, Lowndes J, Zukley L, Melanson KJ, Nguyen V, Huffman A, Rippe JM. The effect of high-fructose corn syrup consumption on triglycerides and uric acid. J Nutr. 2009;139:1242S–5S. [DOI] [PubMed] [Google Scholar]
- 48.Livesey G. Fructose ingestion: dose-dependent responses in health research. J Nutr. 2009;139:1246S–52S. [DOI] [PubMed] [Google Scholar]
- 49.Moran TH. Fructose and satiety. J Nutr. 2009;139:1253S–6S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer EJ, Gleason JA, Dansinger ML. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J Nutr. 2009;139:1257S–62S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy SP. The state of the science on dietary sweeteners containing fructose: summary and issues to be resolved. J Nutr. 2009;139:1269S–70S. [DOI] [PubMed] [Google Scholar]