Abstract
Phytochemicals may reduce chronic inflammation and cancer risk in part by modulating T-cell nuclear factor-κB (NF-κB) activation. Therefore, we examined the effects of curcumin (Cur) and limonin (Lim) feeding on NF-κB–dependent CD4+ T-cell proliferation. DO11.10 transgenic mice (n = 5–7) were fed diets containing 1% Cur or 0.02% Lim combined with either (n-6) PUFA [5% corn oil (CO)] or (n-3) PUFA [4% fish oil+1% corn oil (FO)] for 2 wk, followed by splenic CD4+ T-cell isolation and stimulation with ovalbumin peptide 323–339 (OVA) and antigen-presenting cells from mice fed a conventional nonpurified rodent diet. Both Cur and Lim diets suppressed (P < 0.05) NF-κB p65 nuclear translocation in activated CD4+ T-cells. In contrast, activator protein-1 (c-Jun) and nuclear factor of activated T-cells c1 were not affected compared with the CO control diet (no Cur or Lim). CD4+ T-cell proliferation in response to either mitogenic anti-CD3/28 monoclonal antibodies (mAb) or antigenic stimulation by OVA was also suppressed (P < 0.05) by Cur as assessed by carboxyfluorescein succinimidyl ester staining. In contrast, interleukin-2 production was not directly associated with NF-κB status. Interestingly, dietary combination with FO enhanced the suppressive effects (P < 0.05) of Cur or Lim with respect to CD4+ T-cell proliferation in response to anti-CD3/28 mAb. These results suggest that combination chemotherapy (FO+Cur or Lim) may favorably modulate CD4+ T-cell–mediated inflammation.
Introduction
Inflammation is an imperative host defense response involving both innate and adaptive immune systems. However, chronic inflammation is a disease state that is associated with a higher risk of cancer development (1–4). With respect to the adaptive immune response, CD4+ T-cells regulate inflammatory responses in part by clonal expansion into effector T helper cell subsets with distinct cytokine profiles. The function of CD4+ T-cells is affected by a variety of factors such as genetic background, antigen (Ag)10 peptide, adjuvant, Ag-presenting cell (APC) subset, as well as nutrients (5–9).
With respect to the heterogeneous plasma membrane phospholipid bilayer, cholesterol/sphingomyelin-rich microdomains, i.e. lipid rafts (10–12), are thought to form platforms for receptor residence and signaling molecule migration (13). In addition, signaling molecules are recruited to the immunological synapse where T-cells and APC make physical contact after T-cell receptor (TCR)/CD3 ligation to the major histocompatibility complex/Ag complex. Following the induction of signaling cascades proximal to TCR ligation, nuclear factors translocate from the cytosol into the nucleus to elicit the expression of an array of cytokines. It is now appreciated that nuclear factor-κB (NF-κB) delivers a “life signal” by stimulating proinflammatory cytokines such as interleukin-2 (IL-2) and interferon-γ in T-cells (14–16). IL-2 gene transcription, a hallmark feature of T-cell activation, is also regulated by activator protein-1 (AP-1) and nuclear factor of activated T-cell (NFAT) (17). Interestingly, dietary fish oil (FO), which is enriched in antiinflammatory (n-3) PUFA such as eicosapentaenoic acid [20:5(n-3)] and docosahexaenoic acid [DHA; 22:6(n-3)], suppressed the recruitment of signaling molecules into lipid rafts, nuclear factor-κB (NF-κB)/AP-1 activation, and IL-2 production in mice, linking alteration in lipid raft composition to reduced IL-2 production (18). Kim et al. (19) reported that (n-3) PUFA enhanced the formation of lipid rafts at the immunological synapse, which was associated with suppressed signaling protein translocation and proliferation in CD4+ T-cells from fat-1 transgenic mice. In contrast, dietary lipids rich in (n-6) PUFA, e.g. linoleic acid [18:2(n-6)] and arachidonic acid [20:4(n-6)], found in vegetable oils and animal fats can be deleterious with respect to some inflammatory diseases [reviewed in (20)].
Phytochemicals have been investigated for decades as chemotherapeutic agents for cancer and chronic inflammatory diseases. Curcumin (Cur) (diferuloylmethane), a major active component of turmeric (Curcuma longa Linn), exhibited antiinflammatory effects by suppression of the NF-κB signaling pathway in T-cells (21) as well as ovarian and pancreatic cancer cell-line models (22–24). In addition, our laboratory has shown that Lim (7,16-dioxo-7,16-dideoxylimondiol), a compound extracted from citrus fruit, downregulates inducible nitric oxide synthase and cyclooxygenase-2, which are regulated in part by NF-κB, in a rat mucosal cancer model (25). These data suggest that limonin (Lim) may also be a putative NF-κB inhibitor and therefore capable of modulating CD4+ T-cell function. However, the function of dietary Cur and Lim with regard to CD4+ T-cell activation has not been examined.
In this study, using Ag-specific or mitogenic stimulation of CD4+ T-cells from DO11.10 Rag2−/− TCR transgenic mice, which respond specifically to ovalbumin peptide 323–339 (OVA) to mimic physiological Ag stimulation (26), we hypothesized that dietary Cur and Lim would suppress CD4+ T-cell function by modulating NF-κB activity and that dietary “combination chemotherapy” using (n-3) PUFA plus either Cur or Lim would enhance the suppression of IL-2 secretion and cell proliferation in mice.
Materials and Methods
Animals, diets, and cell purification.
All procedures followed guidelines approved by the Public Health Service and the Institutional Animal Care and Use Committee at Texas A&M University. TCR transgenic DO11.10 Rag2−/− mice were purchased from Taconic Farms and bred and maintained at Texas A&M University. Lim was purified by the Vegetable and Fruit Improvement Center at Texas A&M University from crude grapefruit extract as previously described (27). The composition of experimental diets is shown (Table 1) and met the NRC nutrition requirements (28,29). Briefly, diets differed in fat source and the addition of Cur or Lim. The FO diet contained 4% fish oil + 1% corn oil (CO) to meet the essential fatty acids requirement, whereas the 5% CO diet served as a control. 1% Cur or 0.02% Lim was added to both CO and FO diets (CO+Cur; CO+Lim; FO+Cur; FO+Lim) at the expense of cellulose. Mice were fed a wash-out CO diet for 1 wk followed by a 2-wk experimental diet feeding. CD4+ T-cells from mice were isolated from spleens by a magnetic microbead positive selection method (Miltenyi Biotec) according to the manufacturer's protocol. For antigenic stimulation, splenic monocytes from standard mouse diet (Teklad 9F Sterilizable Rodent diet)-fed BALB/c mice were used as APC. Briefly, lymphocytes were isolated by density gradient centrifugation using Lympholyte-M (Cedarlane Labs) and incubated in the presence of 25 mg/L Mitomycin C (Sigma) for 20 min followed by washing to remove excess Mitomycin C.
TABLE 1.
Experimental diet composition
| Ingredients | CO | CO+1% Cur | CO+0.02% Lim | FO | FO+1% Cur | FO+0.02% Lim |
|---|---|---|---|---|---|---|
| g/100 g | ||||||
| Casein1 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Sucrose | 42.0 | 42.0 | 42.0 | 42.0 | 42.0 | 42.0 |
| Corn starch | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 | 22.0 |
| Cellulose | 6.0 | 5.0 | 5.98 | 6.0 | 5.0 | 5.98 |
| AIN-76 mineral mix2 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| AIN-76 vitamin mix | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| dl-Methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| CO3 | 5.0 | 5.0 | 5.0 | 1.0 | 1.0 | 1.0 |
| FO4 | — | — | — | 4.0 | 4.0 | 4.0 |
| Cur5 | — | 1.0 | — | — | 1.0 | — |
| Lim6 | — | — | 0.02 | — | — | 0.02 |
Ingredients were purchased from Bio-serv unless otherwise indicated.
AIN-76 mineral mix and vitamin mix formula were previously reported (29).
CO was supplied by Dyets, Inc.
Fish oil was supplied by Omega Protein, Inc.
Cur was supplied by Sabinsa, Co and Dr. Bharat B. Aggarwal at University of Texas M.D. Anderson Cancer Center.
Lim was purified by the Vegetable and Fruit Improvement Center, Texas A&M University.
T-cell activation using Ag or mitogen.
To test either cell proliferation or IL-2 production and nuclear factor activation, 1 × 109 DO11.10 CD4+ T-cells/L were cultured in 200 μL medium in an U-bottomed 96-well culture plate or in 2 mL medium in a flat-bottom 24-well culture plate. Cells were cultured in 5% CO2 at 37°C for 72 h. For Ag-specific stimulation, CD4+ T-cells were cocultured with 2.5 × 109 APC/L in the presence of 0.1 μmol/L OVA (Biosource) in complete medium [RPMI 1640 medium with 25 mmol/L HEPES (Irvine Scientific) supplemented with 5% heat-inactivated fetal bovine serum (Invitrogen), 100 kU/L penicillin, 100 mg/L streptomycin (Gibco), 2 mmol/L l-glutamine (Glutamax, Gibco), and 10 μmol/L 2-mercaptoethanol (Sigma)]. Plate-bound CD3-specific monoclonal antibody (mAb; 1 mg/L) and soluble CD28-specific mAb (5 mg/L, BD Pharmingen) (anti-CD3/28 mAb) were used for mitogenic stimulation using our previously published protocol (18).
Nuclear extraction and quantification of NF-κB, NFAT, and AP-1 activation.
Following a 72-h stimulation period, CD4+ T-cell nuclei were pelleted and extracted using a Nuclear Extraction kit (Active Motif). The levels of activated NF-κB, NFAT, and AP-1 were measured using ELISA-based Trans AM NF-κB p65, NFATc1, and AP-1 c-Jun Transcription Factor Assay kits (Active Motif), respectively, as previously described (18). Briefly, induction of NF-κB, NFAT, or AP-1 in the nuclear extracts was quantified by nucleotide (5′-GGGACTTTCC-3′ for NF-κB, 5′-T/AGGAAA-3′ for NFAT, or 5′-TGA(C/G)TCA-3′ for AP-1) binding using specific antibodies to NF-κBp65, NFATc1, or c-Jun followed by secondary horseradish peroxidase-conjugated antibodies and chromogenic substrate. A sensitive colorimetric readout was quantified by either chemiluminescence (NF-κB) or spectrophotometry at 450 nm with a reference wavelength of 655 nm (NFAT and AP-1). Competition experiments were performed by incubating selected extracts with labeled probe in the presence of excess (20 pmol/L) unlabeled wild-type NF-κB, NFAT, or AP-1 oligonucleotide. Phorbol-12-myristate-13-acetate and calcium ionophore-stimulated Jurkat T-cell extracts were used as a positive control, resulting in an 18-fold activation of NF-κB compared with the negative control (Jurkat T-cell extract plus NF-κB wild-type oligonucleotide competitor). Similarly, 4-fold (NFAT) and 9-fold (AP-1) levels of activation were observed in the positive controls (phytohemagglutinin-stimulated Jurkat T-cell nuclear extract and phorbol-12-myristate-13-acetate–stimulated K-562 nuclear extract, respectively) relative to the negative controls (Jurkat nuclear extract plus NFAT wild-type oligonucleotide competitor and K-562 nuclear extract plus AP-1 wild-type oligonucleotide competitor). Serial dilution of control nuclear extract revealed a dose-dependent sensitivity of the assays (Supplemental Fig. 1). The induction of nuclear factors was expressed as fold of unstimulated control, which was derived by (OD of stimulated cells ÷ OD of unstimulated cells).
IL-2 quantification.
Following a 72-h cell stimulation period, culture media was harvested and stored at −80°C until analysis by Quantikine mouse IL-2 ELISA kit (R&D Systems) as previously described (18). The amount of IL-2 in the media was calculated using linear regression of serially diluted standards provided in the kit.
Cell proliferation assay.
For carboxyfluorescein succinimidyl ester (CFSE) profile analysis, CD4+ T-cells were pretreated with 5 μmol/L CFSE (Molecular Probes) in PBS supplemented with 5% fetal bovine serum for 10 min (30). After 72 h of stimulation with Ag or mitogen, CFSE was analyzed by flow cytometry (FACSCalibur, BD) as we have previously reported (31). Briefly, cells were harvested by centrifugation (200 × g; 10 min at room temperature) and resuspended in PBS. To determine viability, propidium iodide (PI) (1 mg/L; Sigma) was added to each sample immediately prior to analysis. CFSE fluorescence was detected using a 530/30 band pass filter and PI fluorescence through a 650LP filter. CFSE fluorescence was sufficiently intense to be detected through a 650LP filter. Viable cells were determined using a plot of CFSE compared with PI fluorescence based on the fluorescence patterns of a sample with CFSE but no PI. This gate also excluded most of the APC because of their higher level of autofluorescence (data not shown). An additional lymphocyte gate was set based on forward and side light-scattering properties. CFSE profiles were analyzed by FlowJo (Tree Star). Data were expressed as difference of percentage compared with CO control (Δ percentage) at each daughter cell generation. For [3H]-thymidine incorporation, 148 kBq [3H]-thymidine/well (New England Nuclear) was added to the cultures for the final 6 h. Cells were harvested using a 96-well cell harvester (Packard Instrument) and thymidine uptake was measured using liquid scintillation counting (Beckman Coulter).
Statistics.
The Proc GLM procedure (SAS 9.2 for Windows) was used to determine the effect of dietary fat, phytochemical, and the interaction on nuclear transcription factor activation, IL-2 production, and thymidine uptake. These data are reported as least squares means ± SEM with differences detected using Fisher's least significant difference. For full model analysis, where interactions were examined, P < 0.1 was considered significant. In the case of an interaction, differences among individual values were tested using P < 0.1. In the absence of an interaction, differences among main effects were considered different at P < 0.05. To compare daughter cell percentage from CFSE staining experiments at each generation to CO control, we used a Student's t test (SPSS 15.0 for Windows).
Results
Dietary Cur and Lim suppress NF-κB activation.
With respect to the physiological relevance of the diets used in this study, FO (4%), Lim (0.02%), and Cur (1.0%) diets are within the estimated range consumed by humans (31–34). Body weight gains of mice were not affected by the experimental diets following a 2-wk feeding period (data not shown).
To investigate the effect of Cur and Lim on nuclear transcription factor activation, nuclei were extracted from CD4+ T-cells following a 72-h stimulation period with OVA+APC or mAb. Both Cur and Lim suppressed NF-κB activation in both Ag- and mitogen-stimulated cells (P < 0.001) but did not affect the dietary oil or the interaction of the phytochemical and the oil (Table 2). These data indicate that FO did not influence NF-κB activation, as opposed to Cur and Lim, which suppressed activation (Table 2). Because the promoter region of the gene encoding a key T-cell growth-promoting cytokine, IL-2, also contains NFAT and AP-1 response elements (17), we measured the effect of Cur and Lim on NFAT and AP-1 activation. The results demonstrate that NFAT activation was affected by the interaction of fat and phytochemicals (P = 0.072, anti-CD3/28; P = 0.082, OVA+APC), although individual values did not differ compared with the CO control diet (Table 2). AP-1 activation was not affected by dietary fat, phytochemicals, or their interaction (Table 2).
TABLE 2.
Activation of nuclear factors in CD4+ T-cells from mice fed FO or CO diets with or without phytochemicals for 2 wk1
| Nuclear factor
|
CO
|
FO
|
Main effects (P-values)
|
Interaction of variables (P-values)
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation | Control | Cur | Lim | Control | Cur | Lim | Fat | Phyto chemical | ||
| Fold of unstimulated control | ||||||||||
| NF-κB2 | Anti-CD3/28 | 146.19 ± 22.19a | 9.43 ± 22.19b | 17.56 ± 23.97b | 111.23 ± 23.97a | 17.78 ± 22.19b | 17.29 ± 23.97b | 0.638 | <0.001 | 0.610 |
| OVA+APC | 183.58 ± 35.63a | 12.63 ± 35.63b | 19.11 ± 35.63b | 138.85 ± 35.63a | 23.82 ± 35.63b | 18.70 ± 35.63b | 0.700 | <0.001 | 0.712 | |
| NFAT3 | Anti-CD3/28 | 45.28 ± 9.27cd | 25.17 ± 8.46d | 33.27 ± 9.23cd | 30.49 ± 10.36d | 54.07 ± 8.46c | 37.49 ± 8.46cd | 0.417 | 0.882 | 0.072 |
| OVA+APC | 34.83 ± 8.95cd | 14.92 ± 8.17cd | 22.46 ± 8.17d | 24.00 ± 8.95cd | 40.18 ± 7.56c | 18.71 ± 8.17d | 0.605 | 0.545 | 0.082 | |
| AP-1 | Anti-CD3/28 | 100.02 ± 47.23 | 101.09 ± 47.23 | 131.34 ± 47.23 | 238.57 ± 55.93 | 57.58 ± 47.23 | 140.00 ± 47.23 | 0.337 | 0.203 | 0.196 |
| OVA+APC | 21.12 ± 19.48 | 23.60 ± 19.48 | 39.70 ± 19.48 | 94.09 ± 19.48 | 61.50 ± 18.23 | 31.51 ± 19.48 | 0.064 | 0.444 | 0.126 | |
Values are means ± SEM, n = 5–7.
For a significant main effect, labeled means in a row with a,b superscripts without a common letter differ, P < 0.05.
For a significant interaction, labeled means in a row with c,d superscripts without a common letter differ, P < 0.1.
Dietary Cur and Lim reduce IL-2 production.
To link suppression of NF-κB to T-cell function, we measured IL-2 secretion into the culture medium. IL-2 production was not altered by the experimental diets in mitogen-stimulated cultures (Table 3). In contrast, in response to antigenic OVA+APC stimulation, IL-2 production was suppressed (P < 0.05) by Cur (29.4% of control) and Lim (31.7%) supplementation only in the presence of CO, whereas there was an interaction between fat source and phytochemical. However, Cur and Lim supplementation with FO did not further suppress IL-2 secretion (Table 3).
TABLE 3.
IL-2 production by CD4+ T-cells from mice fed FO or CO diets with or without phytochemicals for 2 wk 1
| CO
|
FO
|
Main effects (P-values)
|
Interaction of variables (P-values)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulation | Control | Cur | Lim | Control | Cur | Lim | Fat | Phyto chemical | |
| mg/L | |||||||||
| Anti-CD3/28 | 66.93 ± 7.69 | 70.77 ± 7.69 | 67.03 ± 7.02 | 80.83 ± 8.59 | 59.09 ± 7.69 | 58.04 ± 7.02 | 0.720 | 0.324 | 0.227 |
| μg/L | |||||||||
| OVA+APC2 | 845.60 ± 148.81a | 248.73 ± 148.81b | 268.24 ± 135.84b | 250.45 ± 135.84b | 362.38 ± 148.81b | 239.26 ± 166.37b | 0.179 | 0.127 | 0.056 |
Values are means ± SEM, n = 6–7.
For a significant interaction, labeled means in a row with a,b superscripts without a common letter differ, P < 0.1.
Dietary Cur and Lim suppress CD4+ cell proliferation.
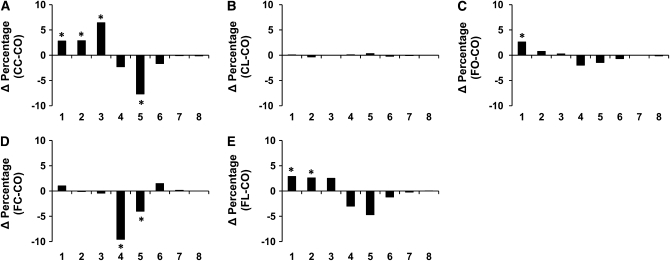
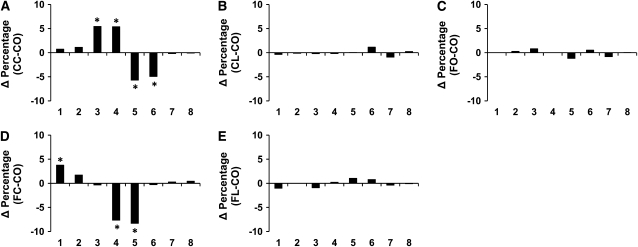
To investigate the effect of suppressed NF-κB activation and IL-2 on CD4+ T-cell function, cell proliferation was measured using 2 independent methods. Analysis of CFSE profiles (Supplemental Fig. 2) indicated that CD4+ T-cell division was suppressed (P < 0.05) by dietary Cur and Lim addition to CO. Specifically, compared with the CO control diet group, Cur-fed animals had more cells following anti-CD3/28 mAb stimulation at generations 1, 2, and 3 (Δ percentage = 2.83, 2.89, and 6.45, respectively; P < 0.05) and fewer cells at generation 5 (Δ percentage = −7.69; P < 0.05; Fig. 1A). Similarly, Cur supplementation with FO (Fig. 1D) reduced the percentage of cells at generations 4 and 5 (Δ percentage = −9.58 and −4.02, respectively; P < 0.05). In contrast, Lim supplementation did not alter the proliferation of CD4+ T-cells (Fig. 1B) and the FO diet had little effect, except in generation 1 (Δ percentage = 2.65; P < 0.05; Fig. 1C). Of interest, the Lim and FO combination (Fig. 1E) exhibited a synergistic suppressive effect on CD4+ T-cell proliferation with more cells in generations 1 and 2 (Δ percentage = 2.88 and 2.61, respectively; P < 0.05). Similarly, following stimulation with OVA+APC, Cur supplementation to the CO diet (Fig. 2A) resulted in a greater number of cells at generations 3 and 4 (Δ percentage = 5.46 and 5.40, respectively; P < 0.05), whereas fewer cells were observed at generations 5 and 6 (Δ percentage = −5.71 and −4.94, respectively; P < 0.05). Cur+FO treatment increased the percentage of cells at generation 1 (Δ percentage = 3.79; P = 0.059), with fewer cells at generations 4 and 5 (Δ percentage = −7.67 and −8.31, respectively; P < 0.05, Fig. 2D). However, neither Lim nor FO supplementation altered Ag-specific CD4+ T-cell proliferation (Fig. 2B,C,E).
FIGURE 1 .
Suppression of CD4+ T-cell division in mitogen-stimulated cultures by feeding mice with FO diets with or without phytochemicals as assessed by CFSE analysis. Data are presented as the difference of percentage (Δ percentage, y-axis) of cells in each daughter generation (x-axis) compared with the CO control diet group, *P < 0.05. Values are means, n = 10–12 (duplicate cultures per mouse).
FIGURE 2 .
Suppression of CD4+ T-cell division in Ag-stimulated cultures by feeding mice with FO diets with or without phytochemicals as assessed by CFSE analysis. Data are presented as the difference of percentage (Δ percentage, y-axis) of cells in each daughter generation (x-axis) compared with the CO control diet group, *P < 0.05. Values are means, n = 10–12 (duplicate cultures per mouse).
In parallel experiments, cell proliferation was quantified by uptake of [3H]-thymidine (Table 4). In contrast to CFSE analyses, thymidine uptake in response to mitogenic anti-CD3/28 mAb was not affected by dietary fat, phytochemicals, or their interaction. However, Ag-specific OVA+APC-induced [3H]-thymidine uptake was suppressed (P < 0.001) by Cur supplementation.
TABLE 4.
Proliferation of CD4+ T-cells from mice fed CO or FO diets with or without phytochemicals as assessed by thymidine incorporation1
| CO
|
FO
|
Main effects (P-values)
|
Interaction of variables (P-values)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Stimulation | Control | Cur | Lim | Control | Cur | Lim | Fat | Phyto chemical | |
| kBq | |||||||||
| Anti-CD3/28 | 1.53 ± 0.21 | 1.27 ± 0.21 | 1.83 ± 0.21 | 1.89 ± 0.21 | 1.47 ± 0.19 | 1.42 ± 0.19 | 0.758 | 0.219 | 0.149 |
| OVA+APC | 1.70 ± 0.15a | 1.17 ± 0.15b | 1.49 ± 0.15ab | 1.69 ± 0.15a | 0.94 ± 0.13b | 1.56 ± 0.13a | 0.633 | <0.001 | 0.595 |
Values are means ± SEM, n = 10–12 (duplicate cultures per mouse). Labeled means in a row with a, b superscripts without a common letter differ, P < 0.05.
Discussion
To investigate the antiinflammatory properties of dietary Cur and Lim, we assessed NF-κB activity in mitogen (anti-CD3/28 mAb) or Ag (OVA+APC) stimulated murine CD4+ T-cells. NF-κB p65 nuclear translocation was suppressed by both dietary Cur and Lim. Because NFAT and AP-1 nuclear factors regulate not only IL-2 gene expression but also T-cell proliferation/function (17,35–37), NFATc1 and AP-1 c-Jun nuclear activation were measured. Of interest, dietary combination of either Cur and Lim with CO or FO diets did not suppress NFATc1 and c-Jun activation in response to either mitogen or Ag. The selective suppression of NF-κB by dietary Cur and Lim can be explained in part by the mechanism of protein activation. In a resting state, NF-κB family proteins, i.e. c-Rel, p50 (NF-κB1), p52 (NF-κB2), p65 (RelA), and RelB, form homo- or heterodimers that are bound to the inhibitor of NF-κB (IκB). Once T-cells are activated, IκB is phosphorylated by IκB kinases (IKK) and detaches from the NF-κB dimer. Phospho-IκB is degraded by the proteasomal pathway in an ubiquitin-dependent manner (38). The NF-κB dimer migrates into the nucleus to bind to the response element of target genes including IL-2. In contrast, NFAT activation requires direct dephosphorylation of the nuclear factor by calcineurin (17) and AP-1 c-Jun is upregulated by phosphorylation and formation of a heterodimer with newly synthesized c-Fos. This is noteworthy, because Milacic et al. (39) recently reported that Cur directly binds to and inhibits proteasomes, resulting in suppressed dissociation of IκB from NF-κB and therefore NF-κB suppression in a human colon cancer cell line. In addition, Aggarwal et al. (34) demonstrated that Cur also inhibits IKK activity. With respect to dietary FO, our laboratory previously reported that DHA, a major active fatty acid in FO, suppresses PKCθ, which also has IKK activity. Hence, we hypothesized that FO, Cur, and Lim affect different molecular targets; FO alters proximal signaling, whereas the phytochemicals antagonize NF-κB. To test this hypothesis, FO was combined with either Cur or Lim.
We further examined the consequence of the suppression of NF-κB p65 by Cur and Lim with respect to T-cell function. IL-2 production was suppressed in Ag-stimulated cultures, whereas mitogen-stimulated cells produced the same amounts of IL-2 across the dietary treatments. The function of NF-κB subunits in T-cell activation has been well studied (35). Liou et al. (40) reported that c-Rel is required for lymphocyte proliferation. Rao et al. (41) observed that IL-2 gene transcription in CD4+ T-cells is dependent on c-Rel, but not p65. In contrast, Lederer et al. reported that the p65-p50 heterodimer accounts for the NF-κB-mediated IL-2 upregulation (42). IL-2 secretion data from this study suggest that NF-κB p65 does not directly regulate IL-2 production in CD4+ T-cells. In addition to transcriptional regulation of IL-2, mRNA degradation in activated T-cells was also reported (43). These previous findings suggest further studies are required to elucidate the mechanism by which diet influences IL-2 production and NF-κB inhibition.
The functional result of dietary modulation was assessed by examining CD4+ T-cell proliferation using 2 different methods. Purified CD4+ T-cells were labeled by CFSE and stimulated for 72 h. Cells were analyzed by either flow cytometry or [3H]-thymidine incorporation. CFSE analysis has been shown to be more sensitive than the [3H]-thymidine assay to quantify cell division (44–46). Thymidine incorporation analysis demonstrated that T-cell proliferation was significantly suppressed by Cur supplementation when T-cells were stimulated by OVA+APC. Interestingly, T-cell proliferation induced by mitogenic stimulation was not affected by dietary intervention. In contrast, CFSE data revealed that dietary Cur supplementation to CO and FO diets suppressed T-cell proliferation following both mitogenic and antigenic stimulation compared with the CO control diet. To our knowledge, this is the first study to investigate the antiinflammatory effects of FO supplementation with either Cur or Lim on CD4+ T-cells.
We next tested the hypothesis that dietary “combination therapy” with Cur and Lim supplementation to FO enhanced the suppression of CD4+ T-cell function. Interestingly, the FO diet alone (4% FO+1% CO) did not suppress NF-κB activation. In contrast, our laboratory previously demonstrated that dietary FO, as well as purified DHA, suppressed NF-κB nuclear translocation in part by affecting fatty acid composition of the plasma membrane including lipid rafts (18). These disparate outcomes may be explained by the different mouse strain and stimuli used in the 2 studies. In addition, the use of homologous mouse serum during the long-term, 72-h culture in the previous study may have augmented the lipid effect by maintaining an (n-3) PUFA-enriched membrane microenvironment (18).
The FO diet suppressed IL-2 secretion, which was not further reduced by Cur or Lim supplementation. We further investigated the combination effect on T-cell proliferation. In accordance with the NF-κB data, CD4+ T-cell proliferation was modestly affected in the FO control diet. This is likely explained in part by the loss of (n-3) PUFA from the plasma membrane in a long-term culture (47,48). Lim supplementation to the CO control diet did not suppress T-cell proliferation either. Of interest, FO+Lim supplementation significantly suppressed T-cell proliferation in anti-CD3/28–stimulated cultures. Taken together, these data suggest that (n-3) PUFA indirectly affect NF-κB activation by altering membrane composition, but Cur and Lim directly suppress NF-κB translocation. The combination diets were shown to augment the antiinflammatory effects.
In summary, the antiinflammatory effects of dietary Cur and Lim on CD4+ T-cell proliferation are attributed in part to the suppression of NF-κB. Interestingly, there was no obvious association between NF-κB status and IL-2 secretion. We further show that the combination of FO and Cur or Lim elicits a maximal suppressive effect with respect to CD4+ T-cell activation. Further studies are required to elucidate the relationship of dietary dose of active components with respect to mechanism of action.
Supplementary Material
Acknowledgments
We thank Dr. Bharat B. Aggarwal for providing Cur.
Supported by NIH grants DK 071707 and P30ES09106 and USDA Cooperative State Research, Education, and Extension Service Special Grant “Designing Foods for Health”, 2006-34402-17121.
Author disclosures: W. Kim, Y. Fan, R. Smith, B. Patil, G. Jayaprakasha, D. N. McMurray, and R. S. Chapkin, no conflicts of interest.
Supplemental Figures 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: Ag, antigen; AP-1, activator protein-1; APC, antigen-presenting cell; CFSE, carboxyfluorescein succinimidyl ester; CO, 5% corn oil; Cur, curcumin; DHA, docosahexaenoic acid; FO, 4% fish oil+1% CO; IκB, inhibitor of NF-κB; IKK, inhibitor of NF-κB kinase; IL-2, interleukin-2; Lim, limonin; mAb, monoclonal antibody; NFAT, nuclear factor of activated T-cell; NF-κB, nuclear factor-κB; OVA, ovalbumin peptide 323-339; PI, propidium iodide; TCR, T-cell receptor; TPA, phorbol-12-myristate-13-acetate.
References
- 1.Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J Nutr. 2007;137:S200–4. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Dubois RN. Pro-inflammatory prostaglandins and progression of colorectal cancer. Cancer Lett. 2008;267:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels EA. Inflammation in the development of lung cancer: epidemiological evidence. Expert Rev Anticancer Ther. 2008;8:605–15. [DOI] [PubMed] [Google Scholar]
- 4.Maeda S, Omata M. Inflammation and cancer: role of nuclear factor-κB activation. Cancer Sci. 2008;99:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J Exp Med. 1995;181:713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. [DOI] [PubMed] [Google Scholar]
- 7.Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, Trezza RP, Heinzel FP, Forsthuber T, et al. Adjuvant-guided type-1 and type-2 immunity: Infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–9. [PubMed] [Google Scholar]
- 8.Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via Retinoid X Receptor pathway. J Immunol. 2002;168:4495–503. [DOI] [PubMed] [Google Scholar]
- 9.Calder PC. The relationship between the fatty acid composition of immune cells and their function. Prostaglandins Leukot Essent Fatty Acids. 2008;79:101–8. [DOI] [PubMed] [Google Scholar]
- 10.Pike LJ. Rafts defined: a report on the keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–8. [DOI] [PubMed] [Google Scholar]
- 11.Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protoc. 2007;2:2159–65. [DOI] [PubMed] [Google Scholar]
- 13.Jury EC, Flores-Borja F, Kabouridis PS. Lipid rafts in T cell signalling and disease. Semin Cell Dev Biol. 2007;18:608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park KR, Lee JH, Choi C, Liu KH, Seog DH, Kim YH, Kim DE, Yun CH, Yea SS. Suppression of interleukin-2 gene expression by isoeugenol is mediated through down-regulation of NF-AT and NF-κB. Int Immunopharmacol. 2007;7:1251–8. [DOI] [PubMed] [Google Scholar]
- 15.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375–80. [DOI] [PubMed] [Google Scholar]
- 16.Pahl HL. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–66. [DOI] [PubMed] [Google Scholar]
- 17.Katsiari CG, Tsokos GC. Transcriptional repression of interleukin-2 in human systemic lupus erythematosus. Autoimmun Rev. 2006;5:118–21. [DOI] [PubMed] [Google Scholar]
- 18.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–60. [DOI] [PubMed] [Google Scholar]
- 19.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremer JM. n-3 Fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:S349–51. [DOI] [PubMed] [Google Scholar]
- 21.Cipriani B, Borsellino G, Knowles H, Tramonti D, Cavaliere F, Bernardi G, Battistini L, Brosnan CF. Curcumin inhibits activation of Vγ9Vδ2 T cells by phosphoantigens and induces apoptosis involving apoptosis-inducing factor and large scale DNA fragmentation. J Immunol. 2001;167:3454–62. [DOI] [PubMed] [Google Scholar]
- 22.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM, et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-κB pathway. Clin Cancer Res. 2007;13:3423–30. [DOI] [PubMed] [Google Scholar]
- 23.Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-κB-regulated gene products. Cancer Res. 2007;67:3853–61. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Aggarwal BB, Shishodia S, Abbruzzese J, Kurzrock R. Nuclear factor-κB and IκB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer. 2004;101:2351–62. [DOI] [PubMed] [Google Scholar]
- 25.Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis. 2006;27:1257–65. [DOI] [PubMed] [Google Scholar]
- 26.Mittler JN, Lee WT. Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment. I. The primary T cell response is unaffected. Mech Ageing Dev. 2004;125:47–57. [DOI] [PubMed] [Google Scholar]
- 27.Mandadi K, Ramirez M, Jayaprakasha GK, Faraji B, Lihono M, Deyhim F, Patil BS. Citrus bioactive compounds improve bone quality and plasma antioxidant activity in orchidectomized rats. Phytomedicine. Epub 2008. Oct 18. [DOI] [PubMed]
- 28.NRC. Nutrient requirements of laboratory animals. Washington, DC: Academy of Sciences; 1995. p. 80–102.
- 29.Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–8. [DOI] [PubMed] [Google Scholar]
- 30.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–56. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Kim W, Zhou L, Wang N, Ly LH, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–8. [DOI] [PubMed] [Google Scholar]
- 32.Manners GD, Jacob RA, Breksa AP III, Schoch TK, Hasegawa S. Bioavailability of citrus limonoids in humans. J Agric Food Chem. 2003;51:4156–61. [DOI] [PubMed] [Google Scholar]
- 33.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, Marczylo TH, Morgan B, Hemingway D, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. [DOI] [PubMed] [Google Scholar]
- 35.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr Opin Immunol. 1995;7:333–42. [DOI] [PubMed] [Google Scholar]
- 36.Calame K. Activation-dependent induction of blimp-1. Curr Opin Immunol. 2008;20:259–64. [DOI] [PubMed] [Google Scholar]
- 37.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–89. [DOI] [PubMed] [Google Scholar]
- 38.Magnani M, Crinelli R, Bianchi M, Antonelli A. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-κB (NF-κB). Curr Drug Targets. 2000;1:387–99. [DOI] [PubMed] [Google Scholar]
- 39.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, Dou QP. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liou HC, Jin Z, Tumang J, Andjelic S, Smith KA, Liou ML. c-Rel is crucial for lymphocyte proliferation but dispensable for T cell effector function. Int Immunol. 1999;11:361–71. [DOI] [PubMed] [Google Scholar]
- 41.Rao S, Gerondakis S, Woltring D, Shannon MF. c-Rel is required for chromatin remodeling across the IL-2 gene promoter. J Immunol. 2003;170:3724–31. [DOI] [PubMed] [Google Scholar]
- 42.Lederer JA, Liou JS, Todd MD, Glimcher LH, Lichtman AH. Regulation of cytokine gene expression in T helper cell subsets. J Immunol. 1994;152:77–86. [PubMed] [Google Scholar]
- 43.Umlauf SW, Beverly B, Lantz O, Schwartz RH. Regulation of interleukin 2 gene expression by CD28 costimulation in mouse T-cell clones: Both nuclear and cytoplasmic RNAs are regulated with complex kinetics. Mol Cell Biol. 1995;15:3197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulcher D, Wong S. Carboxyfluorescein succinimidyl ester-based proliferative assays for assessment of T cell function in the diagnostic laboratory. Immunol Cell Biol. 1999;77:559–64. [DOI] [PubMed] [Google Scholar]
- 45.Hilchey SP, Bernstein SH. Use of CFSE to monitor ex vivo regulatory T-cell suppression of CD4+ and CD8+ T-cell proliferation within unseparated mononuclear cells from malignant and non-malignant human lymph node biopsies. Immunol Invest. 2007;36:629–48. [DOI] [PubMed] [Google Scholar]
- 46.Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, Stinissen P. A cfse based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods. 2007;322:1–11. [DOI] [PubMed] [Google Scholar]
- 47.Switzer KC, Fan YY, Wang N, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids promote activation-induced cell death in Th1-polarized murine CD4+ T-cells. J Lipid Res. 2004;45:1482–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.