Abstract
The metabolism of γ-tocotrienol (γ-TE) and γ-tocopherol (γ-T) was investigated in human A549 cells and in rats. Similar to γ-T, A549 cells metabolized γ-TE to sulfated 9′-, 11′-, and 13′-carboxychromanol and their unconjugated counterparts. After 72-h incubation with the cells, 90% of long-chain carboxychromanols in the culture media from γ-TE, but <45% from γ-T, were in the sulfated form. The formation of these metabolites was further investigated in rats gavaged by γ-TE at 10 or 50 mg/kg, γ-T at 10 mg/kg, or tocopherol-stripped corn oil in controls. Six hours after a single dosing, the supplemented rats had increased plasma concentrations of 13′-carboxychromanol and sulfated 9′-, 11′-, 13′-carboxychromanol, whereas none of these metabolites were detectable in the controls. Sulfated 11′-carboxychromanol was the most abundant long-chain metabolite in γ-TE–supplemented rats. Sulfatase/glucuronidase hydrolysis revealed for the first time that >88% 2-(β-carboxyethyl)-6-hydroxychroman (γ-CEHC), the terminal β-oxidation metabolite, was in the conjugated form in the plasma. In all groups, conjugated γ-CEHC accounted for >75% of total metabolites, whereas free CEHC was a minor metabolite. At 10 mg/kg, the plasma concentrations of total metabolites from γ-TE–supplemented rats were higher (P < 0.05) than those from γ-T–fed rats. These results demonstrate that in rats, conjugation such as sulfation occurs parallel to β-oxidation in the liver and is quantitatively important to vitamin E metabolism. Conjugated long-chain carboxychromanols may be novel excreted metabolites during supplementation. Our data also provide in vivo evidence that γ-TE is more extensively metabolized than γ-T.
Introduction
Vitamin E consists of 8 membrane antioxidants, i.e. α-, β-, γ-, or δ-tocopherol (α-, β-, γ-, or δ-T)4 and α-, β-, γ-, or δ-tocotrienol (α-, β-, γ-, or δ-TE). All vitamin E forms have a chromanol ring and a 13-carbon–long hydrophobic side chain. Compared with tocopherols, tocotrienols have an unsaturated side chain that contains 3 double bonds at the 3′, 7′, and 11′ position (1). Compared with α-T and γ-T, tocotrienols are poorly retained in tissues. Upon supplementation, only skin and adipose tissues have a detectable accumulation of tocotrienols, whereas tocopherols can be found in most tissues (1–7). These observations suggest that tocotrienols may be more extensively and quickly metabolized or excreted than tocopherols.
Tocopherols and tocotrienols are metabolized via oxidative degradation of the hydrophobic side chain without modification of the chromanol ring. The mechanism involves cytochrome P-450–catalyzed ω-hydroxylation and oxidation of the 13′-carbon to form 13′-carboxychromanol (13′-COOH or 13′), followed by a series of stepwise β-oxidations to cut off a 2- or 3-carbon moiety each cycle from the side chain (8–10). This mechanism was first proposed based on the discovery of the terminal urinary-excreted metabolite, 2-(β-carboxyethyl)-6-hydroxychroman (CEHC) (11–18), and was proven by the subsequent identification of a series of long-chain carboxychromanol intermediates in HepG2 cells incubated with vitamin E (8–10). Numerous studies have shown that CEHC and CEHC conjugates (sulfate or glucuronide) are excreted in the urine (11,15,16,19), but only a few reported intermediate metabolites in vivo, including 5′-carboxychromanol in the urine (20) and 5′-carboxychromanol, 13′-carboxychromanol, and 13′-hydroxychromanol in the liver (21–23).
We recently found that γ-T and δ-T are metabolized to novel metabolites, sulfated 9′, 11′, and 13′-carboxychromanol (9′S, 11′S, 13′S), in human lung epithelial A549 cells and in rats (21). This finding indicates that sulfation likely takes place parallel to β-oxidation during tocopherol metabolism. The present study aims to determine whether similar sulfated long-chain metabolites are generated from γ-TE and to compare the relative extent of metabolite formation between γ-TE and γ-T in A549 cells and in rats. In addition, we recently developed a sulfatase/glucuronidase hydrolysis procedure that allows complete deconjugation of conjugated CEHC in the plasma (H. Freiser and Q. Jiang, unpublished data). We used this procedure to quantify plasma-conjugated CEHC in the rat study.
Materials and Methods
Materials.
α-T (99%), γ-T (97–99%), and δ-T (97%) were purchased from Sigma. γ-CEHC (≥98%) was from Cayman Chemicals. γ-TE was a gift from Klaus Kramer at BASF. Tissue culture reagents were from Invitrogen. Helix promatia Type H-1 sufatase/glucuronidase (catalog no. S9626) (Sigma) used in this study is known to have both sulfatase (14.2 U/mg) and β-glucuronidase (>330 U/mg) activities. All other chemicals were purchased from Sigma.
Incubation of A549 cells with vitamin E forms.
All cell culture studies were conducted as previously described (21). Briefly, the human alveolar epithelial A549 cells (ATCC) were maintained in RPMI-1640 with 10% fetal bovine serum. γ-T, γ-TE, and δ-T were dissolved in dimethyl sulfoxide and then diluted in fatty acid-free bovine serum albumin (10 g/L) prior to the addition to culture media. A549 cells were incubated with vitamin E forms or dimethyl sulfoxide (0.05% in controls) in the presence of 1% fetal bovine serum for 24–72 h. Media were collected, frozen immediately, and stored at −20°C until use.
Metabolite extraction from cell culture media and enzymatic hydrolysis.
The metabolites in culture media were extracted and analyzed by HPLC as previously described (21). Briefly, 400 μL of media was mixed with ascorbic acid (8 μL, 60 mmol/L), ethanol (10 μL), and hexane (500 μL). After a brief centrifugation (11,200 × g; 5 min), the aqueous phase was acidified to pH 3–4 using 14 μL of acetic acid and extracted twice with 1 mL of ethyl acetate. The dried ethyl acetate layer was reconstituted in methanol:water (7:3) and injected onto the HPLC column. In the study with sulfatase/glucuronidase hydrolysis, the extracted metabolites were reconstituted in 0.1 mol/L sodium acetate (pH 5) and were hydrolyzed by the Type H-1 sulfatase/glucuronidase at 0.2 mg/sample at 37°C for 90 min. The hydrolyzed samples were extracted twice with ethyl acetate after acidification and analyzed using HPLC.
Metabolite extraction from the plasma.
One hundred microliters of plasma was mixed with 140 μL of methanol and kept on ice for 5 min, which was then added with 8 μL ascorbic acid (60 mmol/L) and 200 μL PBS. The mixture was acidified to pH 3–4 with 20 μL acetic acid and extracted twice with 1 mL of ethyl acetate. The dried ethyl acetate layer was reconstituted in 100 μL of methanol:water (7:3) and injected onto the HPLC column. This extraction procedure yielded a recovery of 90 and 83% spiked γ-CEHC and 11′S in the plasma, respectively.
Enzyme digestion of conjugated CEHC and sulfated long-chain carboxychromanols in the plasma.
One hundred microliters of plasma was mixed with ascorbic acid (8 μL, 60 mmol/L), 2 mL methanol, 100 μL of water, and 5 mL of hexane. After a brief centrifugation (2000 × g; 10 min), 1.8 mL methanol layer was collected and dried under N2 stream. The residues were reconstituted in 100 μL water and 125 μL enzyme solution (pH 5), which was prepared using sodium acetate (9.45 g) and acetic acid (1.725 mL) in 500 mL water. Samples were incubated with the sulfatase/glucuronidase (1.25 mg/sample) at 37°C for 18–24 h for the analysis of conjugated CEHC or for 4 h for the partial deconjugation of 9′S and 11′S (the result of Fig. 3B). After the enzymatic hydrolysis, samples were acidified to pH 3–4 by 15 μL of acetic acid, subsequently extracted twice with ethyl acetate, and analyzed using HPLC. During the overnight enzymatic hydrolysis, γ-CEHC was stable with a recovery yield of 81% (H. Freiser and Q. Jiang, unpublished data).
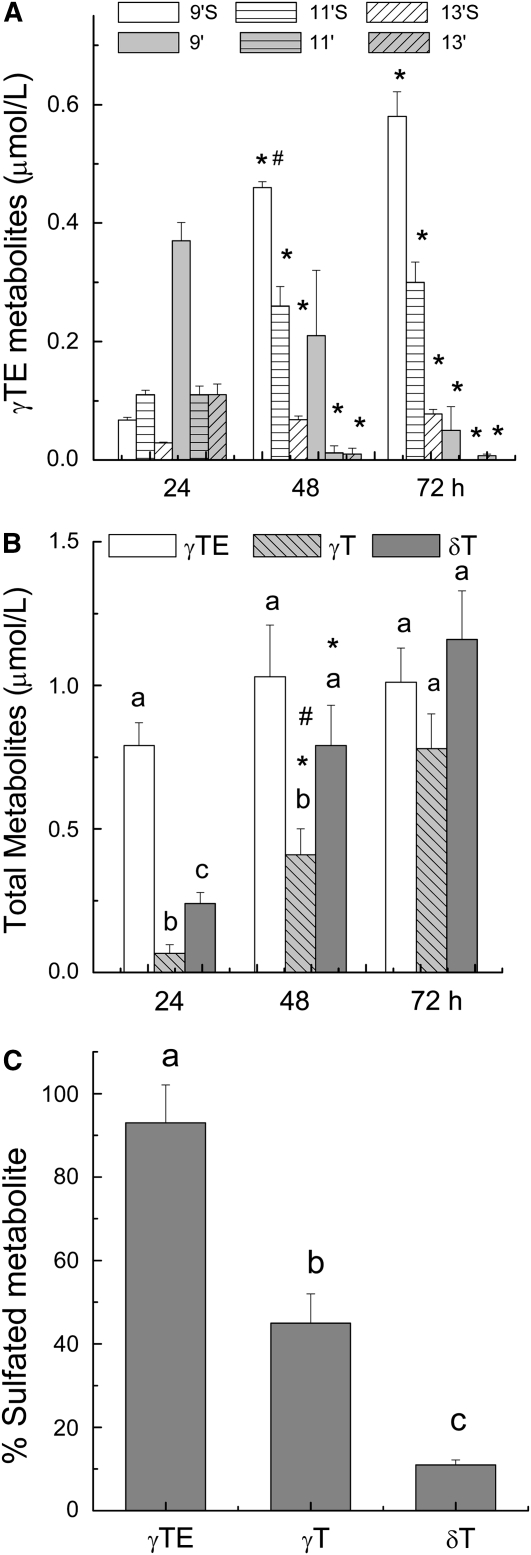
FIGURE 3 .
Time-dependent changes of individual metabolites in culture media in which 10 μmol/L of γ-TE was incubated with A549 cells for 24, 48, and 72 h (A). Time-dependent changes of total metabolites (the sum of sulfated and unconjugated metabolites) in culture media in which 10 μmol/L of γ-TE, γ-T, or δ-T was incubated with A549 cells for 24, 48, and 72 h (B). Percentage of the sulfated metabolites in media in which 10 μmol/L of γ-TE, γ-T, or δ-T was incubated with A549 cells for 72 h (C). Values are means ± SEM, n = 3–4. *Different from 24 h; #different from 72 h, P < 0.05 (A,B). Means at a time without a common letter differ, P < 0.05 (B,C).
Analysis of vitamin E metabolites by HPLC.
γ-CEHC and all the long-chain metabolites were quantified using a sensitive HPLC assay with fluorescent detection at the excitation and emission of 292 and 327 nm, respectively (21). The metabolites were separated on a 5μ Supelcosil LC-18-DB column at a flow rate of 1.0 mL/min with the following gradient: 100% A (35% acetonitrile, 65% 10 mmol/L ammonium acetate at pH 4.3) for 8 min and then linearly increasing from 8 to 30 min to 100% B (96% acetonitrile, 4% 10 mmol/L ammonium acetate at pH 4.3). γ-CEHC was quantified using the authentic standard as the external standard. Long-chain metabolites were quantified using γ-T or γ-TE as the external standard, which have similar fluorescent intensity to γ-CEHC (our unpublished data). The signal of the standard under a specific mobile-phase composition (% B) was calculated by a linear relationship between fluorescent intensity and solvent content, i.e. y = 0.187 x – 6.34 (R2 = 0.995) [x = % B (70–100%) and y = peak area (x 10−6) of 1 μmol/L of the standard] (21). Sulfated long-chain carboxychromanols were calculated from the concentrations of long-chain carboxychromanols multiplied by 1.4 (21), a suppression factor that is the same for all the long-chain metabolites from γ-T and γ-TE (our unpublished data).
To study their susceptibility to oxidation, metabolites were analyzed by HPLC with electrochemical detection at 450 mV using a Model of 5011 analytical cell (ESA).
Electrospray ionization MS.
The liquid chromatography/MS experiments were conducted using negative ion electrospray ionization (ESI) on an LCQ Classic (ThermoFinnigan) mass spectrometer system as previously described (21).
Quantitation of tocopherols and tocotrienols.
α-T, γ-T, and γ-TE were measured by an HPLC assay with electrochemical detection (24).
Animal studies.
All the animal studies were approved by the Purdue Animal Care and Use Committee. Male Wistar rats (230–260 g) (Charles River) were caged singly and routinely consumed ad libitum a 2018 Teklad Global 18% Protein Rodent Maintenance diet (Harlan Teklad) and tap water. Rats were randomly grouped according to body weight. Rats were gavaged with γ-TE at 10 and 50 mg/kg, or γ-T at 10 mg/kg, body weight using tocopherol-stripped corn oil (0.5 mL) as the vehicle (n = 3–4 in each group). Control groups received 0.5 mL of tocopherol-stripped corn oil. Six hours later, rats were killed, plasma was collected, and the metabolites were extracted and analyzed by HPLC.
Statistical analysis.
Data were analyzed by 1-way ANOVA and post hoc Tukey's multiple comparisons when overall group effects were significant and log transformation was performed to normalize unequal variances between groups. For metabolites accumulated in the culture media, time-dependent changes were compared (Fig. 3A,B). Total metabolites from each vitamin E form were also compared at each time point (Fig. 3B). Differences of P < 0.05 were considered significant.
Results
Identification of the metabolites generated from incubation of γ-TE with A549 cells.
In the culture media obtained after γ-TE was incubated with A549 cells for 24–48 h, compared with solvent controls, new peaks appeared with retention times at 17.8, 19.8, 22.7, 27.3, 29.4, and 33.1 min (Fig. 1A), each of which was identified by ESI-MS. For example, the metabolites eluted at 22.7 and 33.1 min showed [M-H]− at 521.27 and 441.27 (Supplemental Fig. 1), corresponding to 2,6,10-trimethyl-13-(2,7,8-trimethyl-6-sulfooxy-chroman-2-yl)-tridecanoic acid (13′S) and 13-(6-hydroxy-2,7,8-trimethyl-chroman-2-yl)-2,6,10-trimethyl-tridecanoic acid (13′-COOH) (Fig. 2), respectively. Like tocopherols (21), the molecular mass of sulfated metabolites is 80 mass units (SO3) more than their unconjugated counterparts, e.g. mass:charge ratio 521.27 (13′S) → mass:charge ratio 441.27 (13′-COOH).
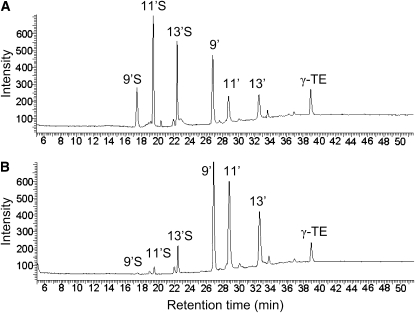
FIGURE 1 .
A HPLC-fluorescent chromatogram of metabolites detected in the culture medium from A549 cells incubated with 20 μmol/L of γ-TE for 48 h (A) and that of the same medium after being treated with sulfatase/glucuronidase (B).
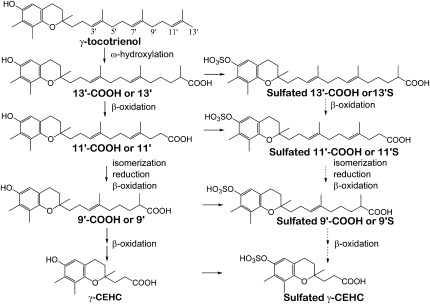
FIGURE 2 .
Proposed metabolism of γ-TE based on the identified metabolites. Based on the previous studies, γ-TE is metabolized via ω-hydroxylation and β-oxidation (8–10). Our previous study (21) and the current study suggest that sulfation may occur parallel to β-oxidation in the liver. Dash-line arrows indicate hypothesized β-oxidation of sulfated intermediate metabolites, which needs to be further validated. 9′-, 11′-, and 13′-COOH from γ-TE are (E)-9-(6-hydroxy-2,7,8-trimethylchroman-2-yl)-2,6-dimethylnon-6-enoic acid, (4E,8E)-11-(6-hydroxy-2,7,8-trimethylchroman-2-yl)-4,8-dimethylundeca-4,8-dienoic acid, and (6E,10E)-13-(6-hydroxy-2,7,8-trimethylchroman-2-yl)-2,6,10-trimethyltrideca-6,10-dienoic acid, respectively.
Similar to the previous studies by Birringer et al. (8) and You et al. (25), compared with tocopherols, the mass of 9′-COOH and 13′-COOH and their sulfated counterparts from γ-TE were 2 mass units greater than the predicted products generated from direct β-oxidation. These data suggest that 9′-COOH or 9′S, and 13′-COOH or 13′S, do not have α, β-unsaturated double bonds that are conjugated with the carboxylic acid group but are instead enoic acids and dienoic acids, respectively (Fig. 2). These metabolites suggest that auxiliary enzymes, including reductases and isomerases, are likely to be involved in acting on preexisting double bonds for tocotrienols during β-oxidation (8,26,27).
Sulfation of long-chain carboxychromanols was further confirmed by sulfatase/glucuronidase digestion experiments. Enzymatic hydrolysis of metabolite-containing media resulted in a marked decrease of 9′S, 11′S, and 13′S, and the corresponding increase in 9′-, 11′-, and 13′-COOH, respectively (Fig. 1B). The fact that the sulfate group is removed by sulfatase suggests that sulfation is likely to occur at the hydroxyl group on the chromanol ring. Consistently, the sulfated carboxychromanols were resistant to electrochemical oxidation, whereas unconjugated carboxychromanols were readily oxidized (Supplemental Fig. 2), indicating that sulfated metabolites lack antioxidant activities.
Quantification of the metabolites from vitamin E forms in cell culture media.
We characterized and compared the metabolite generation from γ-TE, γ-T, and δ-T in A549 cells. Like γ-T and δ-T (21), incubation of γ-TE with A549 cells released long-chain carboxychromanols and the sulfated counterparts to culture media in a time-dependent manner (Fig. 3A). During the initial 24-h incubation, 9′-, 11′-, and 13′-COOH and their sulfated counterparts were generated. When the incubation was prolonged to 48 or 72 h, sulfated carboxychromanols increased with a parallel decrease in free carboxychromanols (Fig. 3A), whereas the total metabolites slightly increased (Fig. 3B). Compared with γ-T or δ-T, γ-TE was more rapidly metabolized, as indicated by quicker accumulation of total metabolites at 24 h from γ-TE than that from tocopherols (Fig. 3B). In addition, consistent with our recent study (28), the metabolites from γ-TE were much more extensively sulfated; therefore, after 72 h of incubation, >90% metabolites from γ-TE, but only 45% from γ-T and 10% from δ-T, were sulfated (21) (Fig. 3C).
γ-TE and γ-T were primarily metabolized to γ-CEHC conjugates and sulfated long-chain carboxychromanols in rats.
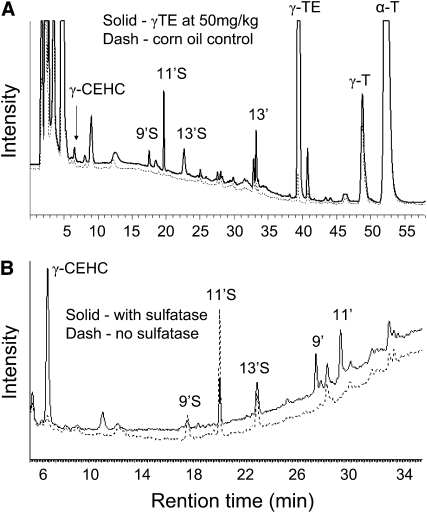
Using the HPLC-fluorescent assay, we found that like γ-T (21), the plasma of γ-TE–supplemented rats had several new peaks, which showed the same retention times as those of 9′S, 11′S, 13′S, and 13′-COOH (Fig. 4A). To further confirm the identity of these metabolites, plasma samples were treated with the sulfatase/glucuronidase. The enzymatic digestion resulted in a decrease of 9′S and 11′S and a corresponding increase of 9′-COOH and 11′-COOH that were not present in the original sample (Fig. 4B). Sulfatase/glucuronidase digestion also led to a substantial increase of γ-CEHC (Fig. 4B), indicating the existence of relatively high levels of conjugated CEHC in the plasma, although the exact nature of the conjugation, e.g. sulfation vs. glucuronidation, was not revealed due to the dual activities (sulfatase and glucuronidase) of the enzyme.
FIGURE 4 .
HPLC-fluorescent chromatograms of the metabolites in the plasma of rats that were gavaged with a single dose of γ-TE at 50 mg/kg or tocopherol-stripped corn oil alone (A) and those in the plasma of rats supplemented with γ-TE at 10 mg/kg treated with or without the sulfatase/glucuronidase (B).
In both control and supplemented rats, conjugated γ-CEHC was quantitatively the most abundant among all the metabolites (Table 1). Except for γ-CEHC and conjugated CEHC, no detectable amounts of any other metabolites were found in the controls. In γ-TE–supplemented rats, 11′S was significantly higher than any other long-chain metabolite in the plasma. When γ-TE was increased from 10 to 50 mg/kg, the concentrations of most metabolites increased, whereas noticeably, there was no significant increase of γ-CEHC (Table 1). γ-TE and γ-T supplementation led to a significant increase in the corresponding vitamin E form in the plasma but did not affect α-T (Table 1). At 10 mg/kg, plasma concentrations of all the metabolites in γ-TE–supplemented rats were significantly higher than those from γ-T–fed rats, with the exception of γ-CEHC and 13′S.
TABLE 1.
The concentrations of the major metabolites detected in the plasma of rats that were gavaged with a single dose of γ-TE at 10 or 50 mg/kg or γ-T at 10 mg/kg body weight, or tocopherol-stripped corn oil in controls1
| Control | γ-TE (10 mg/kg) | γ-TE (50 mg/kg) | γ-T (10 mg/kg) | |
|---|---|---|---|---|
| μmol/L | ||||
| Conjugated γ-CEHC | 0.48 ± 0.11a** | 3.5 ± 1.7b** | 9.7 ± 1.7c** | 1.4 ± 0.66b** |
| γ-CEHC | 0.065 ± 0.028a | 0.17 ± 0.10a | 0.20 ± 0.051a | 0.083 ± 0.066a |
| 9′S | nd | 0.23 ± 0.043a | 0.36 ± 0.19a | 0.058 ± 0.021b |
| 11′S | nd | 0.61 ± 0.19a* | 1.2 ± 0.42a* | 0.048 ± 0.013b |
| 13′S | nd | 0.021 ± 0.014a | 0.12 ± 0.014b | 0.030 ± 0.012a |
| 13′ | nd | 0.052 ± 0.013a | 0.14 ± 0.012b | 0.017 ± 0.0043c |
| Total metabolites | 0.55 ± 0.11a | 4.6 ± 2.0b | 12 ± 1.7c | 1.7 ± 0.75d |
| γ-TE | 0.21 ± 0.11a | 2.6 ± 0.42b | 8.2 ± 1.3c | 0.11 ± 0.071a |
| γ-T | 1.0 ± 0.31a | 1.2 ± 0.22a | 0.72 ± 0.22a | 5.1 ± 1.2b |
| α-T | 31 ± 7.0a | 31 ± 1.7a | 24 ± 6.7a | 24 ± 3.3a |
Values are means ± SD, n = 3–4. Means in a row without a common letter differ, P < 0.05. **Different from all other individual metabolites in a column, P < 0.05. *Different from 9′S, 13′S, and 13′ in a column, P < 0.05. nd, Nondetectable.
Discussion
One novel finding of the current study is that in both control and supplemented rats, conjugated γ-CEHC was by far the most abundant among all the vitamin E metabolites in the plasma, whereas γ-CEHC appeared to be a minor metabolite. Chiku et al. (11) previously reported that 90% δ-CEHC was excreted as CEHC sulfate in rat urine. Most subsequent studies on conjugated CEHC have also focused on the urinary excretion (13,15,16,19). Leonard et al. (22) reported that 30–43% CEHC is in the conjugated form in rat liver. We recently found that direct sulfatase/glucuronidase digestion of the plasma homogenate only converted 30–40% CEHC conjugates to CEHC and therefore underestimated the amount of conjugated CEHC (H. Freiser and Q. Jiang, unpublished data). Here, we employed a newly developed protocol, including methyl/hexane extraction and overnight enzyme hydrolysis of plasma samples, to ensure complete deconjugation (H. Freiser and Q. Jiang, unpublished data). Using this method, we showed that 88–98% plasma γ-CEHC was in the conjugated form. The presence of high levels of conjugated CEHC in the plasma indicates that a high degree of conjugation reactions take place in the liver to conjugate the CEHC immediately upon its generation. The fact that γ-CEHC is a minor metabolite explains the observation that its plasma concentrations were not responsive to the increased dose of γ-TE from 10 to 50 mg/kg (Table 1). The similar lack of response of γ-CEHC to an enhanced supplementation of γ-T was previously reported by Leonard et al. (22) and us (21).
Similar to our previous findings with tocopherols (21), γ-TE was metabolized to sulfated 9′-, 11′-, and 13′-carboxychromanols and the unconjugated counterparts in human A549 cells (Fig. 2). Like γ-T (21) in supplemented but not control rats, 9′S and 11′S but not free 9′-COOH or 11′-COOH from γ-TE are detectable in the plasma, whereas both 13′-COOH and 13′S are found in the plasma. The lack of 9′-COOH and 11′-COOH in vivo is not due to poor detection of the assay (21). These data along with previous studies (8–10) suggest that when vitamin E intake is relatively low, tocopherols and tocotrienols are mainly metabolized by β-oxidation to CEHC, most of which is immediately conjugated in the liver. Supplementation of γ-T or γ-TE likely leads to increased formation of the intermediate metabolites including 9′, 11′, and 13′-COOH, some of which may be “scavenged” by sulfotransferases in the liver (29). The lack of detectable 9′- and 11′-COOH underscores high efficiency of the sulfation reactions in rats. Whether β-oxidation of sulfated intermediates may also occur (Fig. 2, right) remains to be determined. Regardless, the current study also suggests that under supplementation conditions, sulfated long-chain carboxychromanols and 13′-COOH may be novel excreted metabolites. Consistent with this, 13′-COOH was found in rat feces in response to γ-T supplementation (28). Further investigation is needed to detect excretion of conjugated long-chain metabolites in the urine or feces.
Previous studies have shown that tocotrienols such as γ-TE are not retained as well as γ-T in most tissues even when the intake is high (3,4,6). Sontag and Parker (30) recently demonstrated that γ-TE is more rapidly metabolized by tocopherol-ω-hydroxylase than γ-T and more metabolites were accumulated from tocotrienols than tocopherols in HepG2 cells. The present study provides direct evidence that in rats, γ-TE is metabolized much more rapidly and extensively than γ-T. In the plasma, the concentrations of most metabolites in γ-TE–supplemented rats were higher than those from γ-T–fed rats, whereas plasma γ-TE was lower than γ-T. Consistent with the higher rate of metabolism, the ratio of total metabolites:γ-TE (1.77) was much higher than that to γ-T (0.33). In addition, it is interesting to note that the amounts of sulfated carboxychromanols formed varied markedly among vitamin E forms. Compared with tocopherols, metabolites from γ-TE were more extensively sulfated in A549 cells. These results suggest a possible distinct substrate specificity of sulfotransferase(s) (31). Further investigation is necessary to elucidate the role of sulfation in the metabolism of different forms of vitamin E by using sulfation inhibitors or modulation of sulfotransferase/sulfatase by genetic means.
It remains to be determined whether conjugated CEHC is the predominant metabolite in human plasma and whether long-chain metabolites exist in human tissues. Previous work on conjugated CEHC in human urine reported both sulfation and glucuronidation (15,16,19). Future studies are required to evaluate the plasma levels of conjugated CEHC and investigate potential formation of unconjugated and conjugated (both glucuronide and sulfate) long-chain carboxychromanols following vitamin E supplementation in humans. In addition, we have recently shown that 13′-COOH is a novel and competitive inhibitor of cyclooxygenases and inhibits these enzymes much more potently than vitamin E or shorter-chain carboxychromanols (28). Emerging evidence shows that sulfated and glucuronidated metabolites could play a role in regulation of specific biological activities (32–34). Considering micromolar concentrations of conjugated CEHC and 11′S are found in the plasma, it may be worth investigating any potentially interesting bioactivities of these compounds.
Supplementary Material
Acknowledgments
We thank Karl V. Wood at the Purdue University Campus-Wide Mass Spectrometry Center for the help in ESI-MS analyses. We also thank Xinmin Yin for the help with cell-culture and rat studies.
Supported by NIH grant R01AT001821 and R21CA133651.
Author disclosures: H. Freiser and Q. Jiang, no conflicts of interest.
Supplemental Figures 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: CEHC, 2-(β-carboxyethyl)-6-hydroxychroman; 9′-, 11′- and 13′-COOH or 9′, 11′ and 13′, 9′-, 11′- and 13′-carboxychromanol; ESI, electrospray ionization; 9′S, 11′S, and 13′S, sulfated 9′-, 11′- and 13′-carboxychromanol; α-T, β-T, γ-T, or δ-T, α, β, γ, or δ-tocopherol; α-TE, β-TE, γ-TE, or δ-TE, α, β, γ, or δ-tocotrienol.
References
- 1.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–55. [PubMed] [Google Scholar]
- 2.Hayes KC, Pronczuk A, Liang JS. Differences in the plasma transport and tissue concentrations of tocopherols and tocotrienols: observations in humans and hamsters. ProcSoc Exp Biol Med. 1993;202:353–9. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda S, Niwa T, Yamashita K. Selective uptake of dietary tocotrienols into rat skin. J Nutr Sci Vitaminol (Tokyo). 2000;46:141–3. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda S, Toyoshima K, Yamashita K. Dietary sesame seeds elevate alpha- and gamma-tocotrienol concentrations in skin and adipose tissue of rats fed the tocotrienol-rich fraction extracted from palm oil. J Nutr. 2001;131:2892–7. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-Tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–22. [DOI] [PubMed] [Google Scholar]
- 6.Okabe M, Oji M, Ikeda I, Tachibana H, Yamada K. Tocotrienol levels in various tissues of Sprague-Dawley rats after intragastric administration of tocotrienols. Biosci Biotechnol Biochem. 2002;66:1768–71. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita K, Ikeda S, Iizuka Y, Ikeda I. Effect of sesaminol on plasma and tissue alpha-tocopherol and alpha-tocotrienol concentrations in rats fed a vitamin E concentrate rich in tocotrienols. Lipids. 2002;37:351–8. [DOI] [PubMed] [Google Scholar]
- 8.Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J Nutr. 2002;132:3113–8. [DOI] [PubMed] [Google Scholar]
- 9.Parker RS, Sontag TJ, Swanson JE, McCormick CC. Discovery, characterization, and significance of the cytochrome P450 omega-hydroxylase pathway of vitamin E catabolism. Ann N Y Acad Sci. 2004;1031:13–21. [DOI] [PubMed] [Google Scholar]
- 10.Sontag TJ, Parker RS. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J Biol Chem. 2002;277:25290–6. [DOI] [PubMed] [Google Scholar]
- 11.Chiku S, Hamamura K, Nakamura T. Novel urinary metabolite of d-delta-tocopherol in rats. J Lipid Res. 1984;25:40–8. [PubMed] [Google Scholar]
- 12.Hattori A, Fukushima T, Yoshimura H, Abe K, Imai K. Production of LLU-alpha following an oral administration of gamma-tocotrienol or gamma-tocopherol to rats. Biol Pharm Bull. 2000;23:1395–7. [DOI] [PubMed] [Google Scholar]
- 13.Lodge JK, Ridlington J, Leonard S, Vaule H, Traber MG. Alpha- and gamma-tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine. Lipids. 2001;36:43–8. [DOI] [PubMed] [Google Scholar]
- 14.Saito H, Kiyose C, Yoshimura H, Ueda T, Kondo K, Igarashi O. Gamma-tocotrienol, a vitamin E homolog, is a natriuretic hormone precursor. J Lipid Res. 2003;44:1530–5. [DOI] [PubMed] [Google Scholar]
- 15.Schultz M, Leist M, Petrzika M, Gassmann B, Brigelius-Flohe R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am J Clin Nutr. 1995;62:S1527–34. [DOI] [PubMed] [Google Scholar]
- 16.Swanson JE, Ben RN, Burton GW, Parker RS. Urinary excretion of 2,7, 8-trimethyl-2-(beta-carboxyethyl)-6-hydroxychroman is a major route of elimination of gamma-tocopherol in humans. J Lipid Res. 1999;40:665–71. [PubMed] [Google Scholar]
- 17.Traber MG, Elsner A, Brigelius-Flohe R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998;437:145–8. [DOI] [PubMed] [Google Scholar]
- 18.Wechter WJ, Kantoci D, Murray ED Jr, D'Amico DC, Jung ME, Wang WH. A new endogenous natriuretic factor: LLU-α. Proc Natl Acad Sci USA. 1996;93:6002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pope SA, Burtin GE, Clayton PT, Madge DJ, Muller DP. Synthesis and analysis of conjugates of the major vitamin E metabolite, alpha-CEHC. Free Radic Biol Med. 2002;33:807–17. [DOI] [PubMed] [Google Scholar]
- 20.Parker RS, Swanson JE. A novel 5′-carboxychroman metabolite of gamma-tocopherol secreted by HepG2 cells and excreted in human urine. Biochem Biophys Res Commun. 2000;269:580–3. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J Lipid Res. 2007;48:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard SW, Gumpricht E, Devereaux MW, Sokol RJ, Traber MG. Quantitation of rat liver vitamin E metabolites by LC-MS during high-dose vitamin E administration. J Lipid Res. 2005;46:1068–75. [DOI] [PubMed] [Google Scholar]
- 23.Mustacich DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG. Alpha-tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Radic Biol Med. 2006;41:1069–78. [DOI] [PubMed] [Google Scholar]
- 24.Christen S, Jiang Q, Shigenaga MK, Ames BN. Analysis of plasma tocopherols alpha, gamma, and 5-nitro-gamma in rats with inflammation by HPLC coulometric detection. J Lipid Res. 2002;43:1978–85. [DOI] [PubMed] [Google Scholar]
- 25.You CS, Sontag TJ, Swanson JE, Parker RS. Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J Nutr. 2005;135:227–32. [DOI] [PubMed] [Google Scholar]
- 26.Kunau WH, Dommes V, Schulz H. beta-Oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog Lipid Res. 1995;34:267–342. [DOI] [PubMed] [Google Scholar]
- 27.Mukherji M, Schofield CJ, Wierzbicki AS, Jansen GA, Wanders RJ, Lloyd MD. The chemical biology of branched-chain lipid metabolism. Prog Lipid Res. 2003;42:359–76. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci USA. 2008;105:20464–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LQ, James MO. Inhibition of sulfotransferases by xenobiotics. Curr Drug Metab. 2006;7:83–104. [DOI] [PubMed] [Google Scholar]
- 30.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their {omega}-oxidation by tocopherol-{omega}-hydroxylase. J Lipid Res. 2007;48:1090–8. [DOI] [PubMed] [Google Scholar]
- 31.Chapman E, Best MD, Hanson SR, Wong CH. Sulfotransferases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew Chem Int Ed Engl. 2004;43:3526–48. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RG, Barua AB, Olson JA, Moon RC. Effects of retinoid glucuronides on mammary gland development in organ culture. Oncology. 1991;48:505–9. [DOI] [PubMed] [Google Scholar]
- 33.Strott CA, Higashi Y. Cholesterol sulfate in human physiology: what's it all about? J Lipid Res. 2003;44:1268–78. [DOI] [PubMed] [Google Scholar]
- 34.Totta P, Acconcia F, Virgili F, Cassidy A, Weinberg PD, Rimbach G, Marino M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J Nutr. 2005;135:2687–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.