Abstract
Nutritional rickets resulting from calcium insufficiency is common in Nigeria and high dietary phytate is thought to inhibit calcium and zinc absorption. We compared the effects of a high-phytate meal and enzymatic dephytinization on calcium and zinc absorption in Nigerian children with and without rickets. Nineteen children with rickets and 15 age-matched control children, aged 2–10 y, were given calcium (600 mg/d) and ergocalciferol (1250 μg/wk). After 6 wk, calcium and zinc absorption were measured in both groups with and without maize porridge using stable isotopes. One week later, absorption measurements were repeated to assess the effects of enzymatic dephytinization and fermentation of the maize porridge. The phytate concentration of maize porridge (3.87 ± 0.38 g/kg wet weight) was reduced by enzymatic dephytinization (2.83 ± 0.41 g/kg; P < 0.001) but not by fermentation (3.35 ± 0.27 g/kg; P = 0.08). Calcium and zinc absorption were unaffected by the presence of rickets or by fermentation of maize porridge. Calcium absorption was greater with a meal (61.3 ± 25.1%) than without (27.8 ± 14.6%; P < 0.001). Zinc absorption was lower with a meal (16.2 ± 8.0%) than without (63.4 ± 23.9%; P < 0.001). Enzymatic dephytinization increased relative zinc absorption from a meal by 101 ± 81% (P < 0.001) but did not affect calcium absorption. Rickets was not associated with impaired calcium or zinc absorption. Calcium absorption was enhanced by maize porridge, but zinc absorption was reduced. Enzymatic dephytinization increased zinc absorption. Multiple strategies may be required to optimize calcium and zinc absorption in deficient populations.
Introduction
Nutritional rickets is an increasingly recognized problem throughout the world (1) and there are concerns that dietary intakes of minerals may be insufficient to allow optimum skeletal development (2). In Nigeria, up to 9% of children may develop rickets despite prolonged periods of sun exposure and normal 25-hydroxyvitamin D levels (3). It has become increasingly clear that calcium deficiency is an important contributing factor in rickets in children (4–6) and calcium supplementation alone promotes healing of rickets (5). Nigerian children with rickets treated with supplemental calcium, with or without vitamin D, had significantly greater clinical improvement than children treated with vitamin D alone (7), confirming the importance of calcium deficiency as an etiological cause for rickets.
It is unclear why some Nigerian children develop rickets and others do not, because children with and without rickets have similar diets, largely comprised of grains and green leaves that contain inhibitors of mineral absorption (phytates, tannates, and oxalates). Dietary calcium intakes in Nigerian children are very low, averaging ∼200 mg/d (5). High dietary phytate has been postulated to contribute to the development of nutritional rickets (8,9). Maize has been demonstrated to lead to more rapid development of rickets in baboons, possibly due to inhibition of calcium absorption (10). We showed previously that fractional calcium absorption from a typical Nigerian meal containing a small (∼80 mg) calcium load is similarly high in rachitic and nonrachitic children (11).
Zinc is known to be important for bone maturation (12,13), particularly during the early stages of mineralization (14). The vitamin D receptor contains 2 zinc-finger domains (15) and zinc also increases the promoter response to 1,25-dihydroxyvitamin D in osteoblasts (16). Gelatinase B/metalloproteinase-9 is a zinc-dependent protease that is a key regulator of endochondral ossification (17). The grain-based, high-phytate diet of Nigerian children would be expected to limit the bioavailability of both zinc and calcium (2,18,19). It has been suggested that zinc deficiency may predispose children to calcium deficiency rickets by limiting bone mineralization in children with very low calcium intakes (20).
In the current study, we sought to examine 1) the effect of a typical Nigerian meal on the absorption of zinc and calcium 2), the effect of meal dephytinization on calcium and zinc absorption, and 3) whether the relationships between mineral absorption, meal consumption, and dephytinization were different in children with and without rickets. We hypothesized that a maize porridge meal would reduce calcium and zinc absorption, that calcium and zinc absorption would be significantly greater after dephytinization of maize porridge, and that mineral absorption would be similar in children with and without rickets. We examined 2 different methods of dephytinization, comparing a commercially available phytase with fermentation, a method traditionally used in some developing countries.
Methods
Recruitment of study participants.
Children age 2–10 y with rickets were recruited through posters and by word-of-mouth between February and September 2005 at the general outpatient department of the Jos University Teaching Hospital, Jos, Nigeria. The clinical diagnosis was made based on characteristic physical examination findings (including genu varum, genu valgum, and widened wrists) and confirmed by a radiological examination of the wrist and ankles. To be eligible for the study, participants required a radiological score >1.5 on a previously validated 10-point scoring scale for childhood rickets (21).
Age- and gender-matched controls were recruited wherever possible by the parent of each child with rickets, who were asked to recruit a neighbor or relative of the same gender and within 6 mo of age of the rachitic participant. If the parents of the rachitic child could not identify a control, we asked assistants to recruit healthy control children in the Jos community matched for age, gender, and similar socioeconomic status as the participant with rickets. We selected controls that were most closely matched with each child with rickets.
The ethical review committee of Jos University Teaching Hospital and the Institutional Review Board of Baylor College of Medicine and Affiliated Hospitals approved the protocol. The study protocol was explained to potential participants and their parents in Hausa or English (as they understood) and informed written consent was obtained in all cases. Participation in the study was voluntary and participants could withdraw from the study at any time. Parents whose children participated in the study were given a gift of toys and toiletries valued at $10.
Enrollment visit.
A thorough history and physical examination was conducted in both rachitic and nonrachitic participants concentrating on those factors most related to vitamin D and calcium metabolism, including questions about birth weight, duration of breast-feeding, history of rickets in a first-degree relative, sunlight exposure as determined by the number of hours outside per day, and age at walking. Height and weight were measured and converted to Z-scores using Epi Info 3.3 (CDC). Dual energy X-ray absorptiometry was performed on the left radius and ulna by a single investigator (T.D.T.) with a Norland pDEXA (Model 476A110) portable bone densitometer. Measurement sites included the area of minimal bone density of the ultradistal radius and ulna (metaphysis) and the proximal one-third of the radius and ulna (diaphysis). The instrument was set at standard precision and calibrated daily.
Following enrollment, rachitic and control children were given chewable calcium carbonate to provide 600 mg/d elemental calcium (Goldline Laboratories). Calcium was given with meals in divided doses of 400 mg in the morning and 200 mg in the evening. Oral ergocalciferol (1250 μg/wk; Pliva) was given. Calcium and vitamin D were given for 6 wk prior to the first absorption study and during the week between the 2 absorption studies. Compliance was assessed by pill counts. Following the absorption studies, all participants with rickets were treated for a total of 6 mo with calcium supplementation. All but 2 of the participants completely healed their rickets after this duration of treatment.
First admission.
This admission included 2 stable isotope studies (4 h apart) to assess the effect of a typical Nigerian meal on calcium and zinc absorption. Following an overnight fast, participants were admitted and then voided to empty their bladders. Participants were given a typical Nigerian meal of 150 mL (233 g wet weight) of maize porridge and 50 mL of orange juice to which 120 mg calcium (as calcium glubionate), 5 mg zinc (as zinc acetate), 12 μg 46Ca (as calcium chloride), and 2 mg 67Zn (as zinc chloride) were added. The orange juice containing the isotopes was given after one-half of the porridge had been consumed and the cup was rinsed with an addition 20 mL of orange juice that the child drank. The remaining porridge was consumed after the orange juice. An i.v. butterfly needle was inserted to draw blood. After withdrawing blood, 0.8 mg 48Ca and 0.15 mg 70Zn were slowly infused, followed by flushing of the line with 5 mL of saline.
Approximately 4 h later, a second isotope study was carried out. Participants received the orange juice with the same amount of zinc and calcium added but with different zinc and calcium isotopes (1.6 mg of 42Ca and 2 mg of 68Zn). No meal was given with the orange juice and lunch was given 2 h later. Children were not given calcium or vitamin D supplements during the 24-h admission.
A complete urine collection was started immediately prior to the administration of the first doses of isotopes and continued until 24 h after the second oral isotopes were given. The children remained in the hospital until the urine collection was completed. After discharge, all participants collected spot urine samples 72 h after the first oral isotope for zinc absorption determination.
Second admission.
One week later, a second study was conducted to assess the effect of dephytinization on calcium and zinc bioavailability from a typical Nigerian meal. The protocol was similar to the first admission. Each child with rickets and their matched control were randomized by lottery to receive the same unfermented maize porridge as given during the first admission or to maize porridge that had been fermented by a traditional Nigerian method. The porridge was given in the same manner as the first admission (233 g of maize porridge and orange juice to which 120 mg calcium, 5 mg zinc, 12 μg 46Ca, and 2 mg 67Zn had been added). Four hours later they received an identical meal of maize porridge (fermented or unfermented) that had been enzymatically dephytinized (see below) with 120 mg calcium, 5 mg zinc, 1.6 mg of 42Ca, and 2 mg of 68Zn.
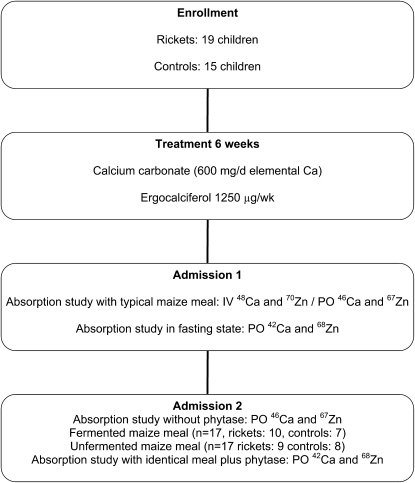
No i.v. isotopes were given during the second admission. As before, a complete 24-h urine collection was begun immediately before breakfast and a random urine sample collected ∼72 h after the first isotope was administered. The study design is depicted in Figure 1.
FIGURE 1 .
Flow chart of study.
Dietary methods.
Dietary data were obtained from all participants concerning the frequency and quantity of milk products consumed. Two 24-h dietary recalls were obtained to determine energy, phosphorus, and calcium intakes. Energy and mineral intakes were calculated using food composition tables for African foods (22–24).
Unfermented maize porridge was prepared by traditional methods using dry milled maize flour to which cold water was added (∼100 g flour/L water). Boiled water was added to the cold paste to make the porridge.
In the preparation of fermented porridge, maize grain was soaked over 2 nights, rinsed, and then wet milled. The resulting slurry was wet sieved with muslin cloth to exclude the bran. The sediment was allowed to ferment for 24 h, after which excess water was drained. The sediment was then boiled with water to make the porridge.
Dephytinization and fermentation.
Dephytinization was carried out in 2 ways: enzymatically and by fermentation. We examined the effect of fermentation of the maize porridge on mineral bioavailability. The process of preparation of fermented porridge is detailed in the dietary methods section above. At the start of the study, participants were randomly assigned to receive fermented or unfermented porridge during the second admission.
During the second admission, each participant consumed an identical meal before and after enzymatic dephytinization. This was achieved by using a commercially available wheat phytase (Sigma-Aldrich P-1259). Prepared maize porridge was mixed with the enzyme in a ratio of 0.2 g phytase:120 g of porridge at 55°C for 15 min. Preparatory studies carried out in Houston using the same raw materials as used in the Nigerian studies had demonstrated that this would reduce phytate concentration in the maize porridge to undetectable levels.
Measurement of phytate concentration.
Samples of prepared porridge were collected and transported to Houston and phytate concentration was measured using a colorimetric method (25). Phytate was extracted from porridge samples with 2.4% HCl and the solution was eluted through AG1-X8 anion exchange resin (Bio-Rad Industries) to remove interfering compounds. The concentration of phytate was measured based on a color change of Wade reagent (0.03% FeCl 6H2O and 0.3% sulfosalicylic acid) (25,26). Phytate concentration was measured in 6 samples of the fermented and unfermented porridge before and after phytase treatment (n = 24).
Calcium and zinc absorption measurements.
Calcium and zinc isotope ratios were measured as previously described using thermal ionization magnetic sector MS following purification by oxalate precipitation and anion exchange chromatography, respectively (27). Fractional calcium and zinc absorption were calculated from the relative recovery of the oral and i.v. calcium isotope in the 24-h urine collection (for calcium) and the 72-h spot urine collection (for zinc) (28,29). No i.v. isotopes were administered during the second inpatient visit, so urinary fractional excretion of the i.v. calcium and zinc isotopes was assumed to be the same as at the first in-patient visit, because body pool masses would not be expected to change over a short period of time. We have found from previous studies (S. A. Abrams, unpublished data) that with comparable doses utilized in this study, there are no measurable residual concentrations of oral calcium and zinc isotopes 7 d after dosing. Thus, we considered it unnecessary to correct for residual isotope enrichment.
Biochemical methods.
Serum samples were obtained from children at their enrollment visit and 6 wk later during the first admission. All serum samples were stored at −70°C until they were transported on ice to the Children's Nutrition Research Center at the Texas Medical Center. Serum calcium, albumin, phosphorus, and alkaline phosphatase were analyzed using an ATAC 8000 chemistry analyzer (Vital Diagnostics) using standard procedures. Control samples were included for all measures and typical intra- and interassay CV for the analyses made were under 5%. Serum 25-hydroxyvitamin D was analyzed by radioimmunoassay (Diasorin). Serum intact parathyroid hormone (PTH) was analyzed using the Immulite 1000 (Siemens Healthcare Diagnostics Solutions); corresponding intra- and interassay CV for this method were 6.0 and 8.3%, respectively.
Statistical methods.
Statistical analyses were performed with Statview 5.01 for Macintosh (SAS Institute) and SPSS for windows version 13. Baseline calcium absorption (± SD) was expected to be 40 ± 10%, which is lower than we described in a previous study due to expected adaptation to the greater calcium intake (30). A sample size of 16 participants per group had a >90% power to detect a relative difference in calcium absorption of 30% (the smallest change we considered clinically significant) with 95% confidence.
The effect of fermentation and treatment with phytase on phytate concentration were assessed by 2-way ANOVA with phytate concentration as the dependent variable and fermentation and phytate treatment as independent variables. Unpaired t tests were used to determine differences in normally distributed continuous variables between children with and without rickets, whereas a chi-square test was used to assess differences in categorical variables. Differences in calcium and zinc absorption were assessed separately at each of the 2 in-patient visits by repeated-measures ANOVA with food matrix (meal vs. no meal for admission 1 and phytase vs. no phytase for admission 2) as the within-subject independent variable, and group (rachitic vs. nonrachitic) and type of porridge (fermented vs. unfermented) as the between-subject independent variables. Several urine samples in each batch were not included in the analysis due to contamination by stool or inability to measure isotopic enrichment. Differences were considered significant at P < 0.05. Data are given as mean ± SD, unless otherwise stated.
Results
Nineteen participants with rickets and 15 age- and sex-matched controls without rickets were recruited, of whom the majority (85%) were female. Symptoms of rickets were first noted when children were 24.9 ± 11.7 mo old, ∼18 mo before the age at presentation. The radiological score of children with rickets was 4.5 ± 2.4 (range 2–10). Compliance with vitamin D supplementation did not differ between children with and without rickets (Table 1), but compliance with calcium carbonate was greater in the rachitic children.
TABLE 1.
Baseline characteristics of children with and without rickets1
| Characteristic | Rickets, n = 19 | Control, n = 15 | P-value |
|---|---|---|---|
| Age, mo | 52 ± 24 | 57 ± 24 | 0.59 |
| Males/females, n/n | 3/16 | 2/13 | 0.84 |
| Religion (Muslim/Christian) | 4/15 | 1/14 | 0.35 |
| Duration of breast-feeding, mo | 18 ± 7 | 17 ± 6 | 0.87 |
| Age started walking, mo | 16 ± 6 | 14 ± 4 | 0.28 |
| Rickets in first-degree relative, n (%) | 6 (32) | 0 | 0.017 |
| Sunlight exposure, h/d | 2.5 ± 1.9 | 2.3 ± 1.0 | 0.66 |
| Father's education, y | 8.1 ± 4.4 | 8.7 ± 4.5 | 0.68 |
| Mother's education, y | 6.2 ± 3.8 | 6.5 ± 3.7 | 0.78 |
| Anthropometric characteristics | |||
| Weight, kg | 12.7 ± 3.2 | 16.2 ± 5.0 | 0.019 |
| Weight-for-age Z-score | −2.1 ± 1.1 | −0.7 ± 0.9 | <0.001 |
| Height, cm | 88 ± 11 | 102 ± 16 | 0.005 |
| Height-for-age Z-score | −3.4 ± 1.6 | −0.6 ± 1.1 | <0.001 |
| Weight-for-height Z-score | −0.2 ± 0.8 | −0.2 ± 0.6 | 0.96 |
| Dietary characteristics | |||
| Calcium intake, mg/d | 183 ± 63 | 256 ± 110 | 0.037 |
| Phosphorus intake, mg/d | 650 ± 200 | 693 ± 239 | 0.59 |
| Energy intake, kJ/d | 4458 ± 1160 | 4726 ± 1026 | 0.40 |
| Milk calcium intake,2mg/d | 8 (0–100) | 29 (7–72) | 0.38 |
| Milk intake < once/wk, % | 8 (42) | 3 (20) | 0.17 |
| Supplementation compliance, % | |||
| Compliance with calcium | 92 ± 15 | 86 ± 20 | 0.06 |
| Compliance with vitamin D | 100.0 | 98 ± 10 | 0.13 |
| Serum biochemistry | |||
| Calcium, mmol/L | 2.3 ± 0.2 | 2.6 ± 0.2 | <0.001 |
| Albumin, g/L | 4.8 ± 0.4 | 5.0 ± 0.6 | 0.33 |
| Phosphorus, mmol/L | 1.2 ± 0.3 | 2.0 ± 0.3 | <0.001 |
| Alkaline phosphatase, U/L | 778 ± 371 | 250 ± 51 | <0.001 |
| 25-hydroxyvitamin D, nmol/L | 27.8 ± 11.3 | 55.8 ± 8.3 | <0.001 |
| Parathyroid hormone, ng/L | 342 ± 157 | 71 ± 33 | <0.001 |
| Forearm bone densitometry2 | |||
| Ultradistal bone mass, g | 0.256 (0.213–0.300) | 0.304 (0.245–0.449) | 0.05 |
| Ultradistal bone area, cm2 | 2.046 (1.826–2.374) | 2.079 (1.898–2.649) | 0.88 |
| Distal 1/3 bone mass, g | 0.350 (0.290–0.429) | 0.432 (0.362–0.624) | 0.03 |
| Distal 1/3 bone area, cm2 | 1.878 (1.767–1.990) | 1.755 (1.680–2.024) | 0.32 |
| Forearm bone densitometry change3 | |||
| Ultradistal bone mass, g/6 wk | +0.029 ± 0.096 | +0.003 ± 0.065 | 0.39 |
| Ultradistal bone area, cm2/6 wk | +0.039 ± 0.360 | −0.066 ± 0.259 | 0.35 |
| Distal 1/3 bone mass, g/6 wk | +0.043 ± 0.056 | +0.004 ± 0.040 | 0.03 |
| Distal 1/3 bone area, cm2/6 wk | +0.107 ± 0.377 | −0.028 ± 0.110 | 0.33 |
Values are means ± SD unless otherwise noted.
Values are medians (interquartile ranges).
Change after 6 wk of calcium and vitamin D supplementation.
The 2 groups were generally well matched except that the rachitic children more frequently had a history of rickets in a first-degree family member. Furthermore, the rachitic children were significantly shorter and lighter, although this may be partly explained by the bony abnormalities in rickets. Dietary calcium intake was very low in both groups and was lower in rachitic children (183 ± 63 mg) than in controls (256 ± 110 mg/d; P = 0.037).
Phytate concentrations.
The phytate concentration of the maize porridge, 3.87 ± 0.38 g/kg wet weight, was reduced by treatment with phytase (2.83 ± 0.41 g/kg; P < 0.001) but not by fermentation (3.35 ± 0.27 g/kg; P = 0.08). There was no interaction between phytase and fermentation (P = 0.21), indicating that enzymatic dephytinization had a similar effect on phytate concentration irrespective of porridge fermentation.
Calcium absorption.
Calcium absorption did not differ significantly between children with and without rickets for any permutation of meal, fermentation, or phytase effects. For example, calcium absorption with a meal in children with rickets was 64 ± 23% compared with 59 ± 28% in children without rickets (P = 0.65). Assuming a calcium intake for the day of study of 200 mg from the diet and 240 mg of calcium given with the isotopes, we calculated the overall calcium absorption by multiplying the mean of fractional absorption with and without a meal by the total calcium intake of 440 mg. Overall calcium absorption was estimated to be 199 ± 61 mg in children with rickets compared with 190 ± 65 mg in children without rickets during the first study (P = 0.73). Therefore, data from children with and without rickets were combined for examination of meal, fermentation, and phytase effects on calcium absorption (Table 2).
TABLE 2.
Effects of meals, enzymatic dephytinization, and fermentation on fractional calcium and zinc absorption in 34 Nigerian children1
| n | % | |
|---|---|---|
| Fractional calcium absorption | ||
| With meal | 25 | 61.3 ± 25.1 |
| Without meal | 29 | 27.8 ± 14.6* |
| Maize porridge | ||
| Untreated | 21 | 50.4 ± 17.8 |
| Phytase-treated | 25 | 44.5 ± 17.3 |
| Fermented | 11 | 50.7 ± 19.1 |
| Nonfermented | 10 | 50.1 ± 17.3 |
| Fractional zinc absorption | ||
| With meal | 31 | 16.2 ± 8.0 |
| Without meal | 30 | 61.7 ± 22.5* |
| Maize porridge | ||
| Untreated | 31 | 32.2 ± 14.8 |
| Phytase-treated | 30 | 55.5 ± 18.0* |
| Fermented | 15 | 30.8 ± 12.0 |
| Nonfermented | 16 | 33.5 ± 17.4 |
Values are means ± SD. *Different from corresponding mean, P < 0.001.
Calcium absorption was greater from a traditional Nigerian maize meal (61.3 ± 25.1%) than that measured in fasting participants (27.8 ± 14.6%; P < 0.001). There was no interaction between the effect of the meal and the presence of rickets (P = 0.40) or fermentation of the porridge (P = 0.45). Calcium absorption with fermented porridge (50.7 ± 19.1%) did not differ from unfermented porridge (50.1 ± 17.3%; P = 0.94). Calcium absorption was paradoxically lower from the phytase-treated porridge (44.5 ± 17.3%) than without phytase (50.4 ± 17.8%; P = 0.05).
Fractional calcium absorption with a meal was unrelated to age (P = 0.97), gender (P = 0.06), sun exposure (0.97), weight (P = 0.55), height (P = 0.62), usual dietary calcium intake (P = 0.99), radiographic severity of rickets (P = 0.90), serum calcium (P = 0.48), parathyroid hormone (P = 0.92), 25-hydroxyvitamin D (P = 0.63), or ultradistal (P = 0.36) or distal 1/3 (P = 0.27) bone mineral content of the radius and ulna.
Zinc absorption.
In the first study, zinc absorption was significantly lower with a meal (16.2 ± 8.0%) than without a meal (63.4 ± 23.9%; P < 0.001). Zinc absorption was similar in children with (40.5 ± 29.6%) and without (38.7 ± 30.2%) rickets (P = 0.65) and rickets did not interact with the effect of the meal on zinc absorption (P = 0.83). Fermentation of the porridge did not affect zinc absorption (37.3 ± 28.3% vs. 41.9 ± 31.0%; P = 0.52) and did not modify the effect of the meal on zinc absorption (P = 0.83 and P = 0.88, respectively).
Enzymatic dephytinization increased zinc absorption during the second absorption study (55.5 ± 18.0% vs. 32.2 ± 14.8%; P < 0.001). Dephytinization resulted in a mean relative increase in zinc absorption of 101 ± 88%. The presence or absence of rickets (46.2 ± 21.8% vs. 40.0 ± 17.2%; P = 0.25) and fermentation of the porridge (45.8 ± 20.3% vs. 46.2 ± 20.3%; P = 0.47) did not affect zinc absorption, nor did these factors modify the effect of phytase on zinc absorption (P = 0.94 and P = 0.11 for rickets and fermentation, respectively).
Zinc absorption was not significantly related to calcium absorption in any of the dietary comparisons. Fractional zinc absorption without a meal was unrelated to age (P = 0.38), gender (P = 0.18), sun exposure (0.80), weight (P = 0.44), height (P = 0.69), usual dietary calcium intake (P = 0.59), radiographic severity of rickets (P = 0.39), serum calcium (P = 0.10), parathyroid hormone (P = 0.72), 25-hydroxyvitamin D (P = 0.09), or ultradistal (P = 0.80) or distal 1/3 (P = 0.88) bone mineral content of the radius and ulna.
Discussion
Calcium and zinc absorption did not differ between children with rickets and healthy control children. Calcium intake was very low in both groups but was significantly lower in children with rickets. A phytate-rich maize meal augmented calcium absorption but reduced zinc absorption. Enzymatic dephytinization of the maize meal increased zinc absorption, but this effect was not achieved with maize fermentation.
Nutritional rickets has been associated with increased intake of dietary fiber, which is rich in phytate, presumably because phytate binds with calcium in the gut, inhibiting its absorption (9,31). However, we did not observe a significant effect of phytate in maize porridge on calcium absorption. This is likely due to the limited effect of phytate on calcium absorption, although we cannot exclude the possibility that further dephytinization would have increased calcium absorption. However, further dephytinization would be impractical in the home setting and thus was not a goal of our study. The improved absorption of calcium with a meal more than compensated for any inhibitory effect of phytate. Taking calcium with a meal has been previously demonstrated to increase its absorption by 10–30% in studies in adults (32).
High dietary phytate intakes have frequently been cited as a potential contributor to nutritional rickets (8,9,33). This study is the first to evaluate the role of phytate interference with calcium absorption in children with nutritional rickets. Phytate inhibition of calcium absorption is unlikely to play an important role in the pathogenesis of calcium deficiency rickets. Calcium absorption was similar in the rachitic and nonrachitic children but was significantly greater when consumed with a meal. This agrees with evidence in the literature that light meals can increase calcium absorption from various sources (9,32). The meals used in our study contained large amounts of phytate that we hypothesized would reduce calcium absorption (34). However, the beneficial “meal effect” on calcium absorption seems to have outweighed any possible adverse effect of phytate on calcium absorption, so that calcium absorption was higher with a meal than without it. This confirms our previous finding of high calcium absorption in Nigerian children with and without rickets from a typical Nigerian meal (11). We did not explore the mechanism of the meal effect on calcium absorption, but we speculate that glucose from the digestion of carbohydrate enhanced calcium absorption (35). The effect of a meal to increase gastric acid secretion and to slow gastric emptying could also increase intestinal calcium absorption (32).
Neither method of dephytinization (enzymatic or fermentation) significantly affected calcium absorption. The relatively modest reduction in phytate content achieved by enzymatic dephytinization was less than expected, based on preliminary studies in Houston, in which the phytate content was reduced to almost zero with an identical quantity of phytase. This difference may have arisen from a partial loss of phytase activity during transport and storage or from the relative resistance to phytate degradation of Nigerian maize compared with U.S. maize. In our study, enzymatic dephytinization reduced the phytate concentration of porridge from 3.87 to 2.83 g/kg. This contrasts with the results of enzymatic dephytinization of porridge in Malawi, in which a reduction of phytate content from 1.21 to 0.25 g/kg was achieved (36). It is possible that with a greater reduction in phytate concentration, we might have observed greater improvement in calcium absorption. However, it is notable that even with the relatively high phytate concentration of maize porridge used in our study, fractional calcium absorption was high. Our results are consistent with data that near-complete dephytinization of soybean protein with a low native phytate concentration of 0.3 g/kg did not significantly change calcium absorption but increased zinc absorption (37).
In contrast to the effects on calcium absorption, a typical Nigerian meal significantly reduced zinc absorption in rachitic and nonrachitic children. It is likely that this effect was the result of phytate, which is known to reduce zinc absorption (34,38–40). Enzymatic dephytinization led to increased zinc absorption from the meal, despite the relatively modest reduction in phytate concentration. Our findings contrast with a study in Malawi, where dietary phytate reduction improved zinc absorption in children recovering from tuberculosis (41 vs. 24%) but not in healthy children (36). The effect of phytate to reduce zinc absorption but not calcium in our study is consistent with the relatively minor effect of phytate on absorption of calcium compared with zinc (41), probably reflecting a more avid binding activity of phytate with zinc than with calcium to produce insoluble complexes unavailable for absorption.
We compared enzymatic dephytinization with a nonenzymatic method using natural lactic acid fermentation. Several nonenzymatic methods of dephytinization have been described (42), including hydrothermal treatment (43), fermentation with Aspergillus usamii (44), soaking grain in water (45), leavening of breads (46), and malting (47). We found that fermentation resulted in a nonsignificant reduction (P = 0.08) in the phytate concentration, which was inferior to enzymatic dephytinization and did not affect calcium or zinc absorption. It is important to note that the group sample sizes used to evaluate the effect of fermentation were one-half those used to evaluate meal and phytase effects, thereby reducing the power to detect an effect of fermentation. However, the effect of fermentation was likely negligible given that it did not significantly reduce the phytate concentration.
We noted that approximately one-third of the children with rickets had first-degree relatives with a history consistent with rickets. An increased risk of rickets in siblings of affected children suggests a possible impact of genotype on the ability to adequately mineralize bone in the face of similar dietary restrictions. However, our study design did not permit us to differentiate unmeasured shared environmental effects within a family from those caused by genetic effects.
Another limitation of our study deserves mention. For logistic reasons, we did not randomize the order of testing the effects of meals or dephytinization. This could potentially give biased results if calcium absorption measured with the breakfast meal differed from that measured with a meal later in the day. However, we are not aware of any evidence that this was the case, unless the calcium content varied widely between meals. Children in this study did not consume Western diets with widely varying calcium content at each meal. Because each meal had a similar calcium content, we think that measured calcium absorption was unlikely to be significantly altered by test order.
Calcium and zinc absorption were similar in children with and without rickets and rickets did not modify the effect of meal consumption or dephytinization on calcium or zinc absorption. Impaired calcium or zinc absorption, or an exaggerated effect of phytate, do not appear to explain the development of rickets in Nigerian children. In the treatment of rickets with calcium, supplemental calcium should be consumed with a meal. Multiple strategies may be required to optimize absorption of calcium and zinc, as well as other nutrients, in deficient populations.
This study was funded by NIH Fogarty grant R03 TW006428.
Author disclosures: T. D. Thacher, O. Aliu, I. J. Griffin, S. D. Pam, K. O. O'Brien, G. E. Imade, and S. A. Abrams, no conflicts of interest.
References
- 1.Thacher TD, Fischer PR, Strand MA, Pettifor JM. Nutritional rickets around the world: causes and future directions. Ann Trop Paediatr. 2006;26:1–16. [DOI] [PubMed] [Google Scholar]
- 2.Prentice A, Bates CJ. Adequacy of dietary mineral supply for human bone growth and mineralisation. Eur J Clin Nutr. 1994;48 Suppl 1:S161–76. [PubMed] [Google Scholar]
- 3.Pfitzner MA, Thacher TD, Pettifor JM, Zoakah AI, Lawson JO, Isichei CO, Fischer PR. Absence of vitamin D deficiency in young Nigerian children. J Pediatr. 1998;133:740–4. [DOI] [PubMed] [Google Scholar]
- 4.Oginni LM, Worsfold M, Oyelami OA, Sharp CA, Powell DE, Davie MW. Etiology of rickets in Nigerian children. J Pediatr. 1996;128:692–4. [DOI] [PubMed] [Google Scholar]
- 5.Thacher T, Glew RH, Isichei C, Lawson JO, Scariano JK, Hollis BW, VanderJagt DJ. Rickets in Nigerian children: response to calcium supplementation. J Trop Pediatr. 1999;45:202–7. [DOI] [PubMed] [Google Scholar]
- 6.DeLucia MC, Mitnick ME, Carpenter TO. Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for reexamining the role of dietary calcium intake in North American infants. J Clin Endocrinol Metab. 2003;88:3539–45. [DOI] [PubMed] [Google Scholar]
- 7.Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, Chan GM. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N Engl J Med. 1999;341:563–8. [DOI] [PubMed] [Google Scholar]
- 8.Sanders TA, Reddy S. Vegetarian diets and children. Am J Clin Nutr. 1994;59:S1176–81. [DOI] [PubMed] [Google Scholar]
- 9.Wills MR, Phillips JB, Day RC, Bateman EC. Phytic acid and nutritional rickets in immigrants. Lancet. 1972;1:771–3. [DOI] [PubMed] [Google Scholar]
- 10.Sly MR, van der Walt WH, du Bruyn DB, Pettifor JM, Marie PJ. Exacerbation of rickets and osteomalacia by maize: a study of bone histomorphometry and composition in young baboons. Calcif Tissue Int. 1984;36:370–9. [DOI] [PubMed] [Google Scholar]
- 11.Graff M, Thacher TD, Fischer PR, Stadler D, Pam SD, Pettifor JM, Isichei CO, Abrams SA. Calcium absorption in Nigerian children with rickets. Am J Clin Nutr. 2004;80:1415–21. [DOI] [PubMed] [Google Scholar]
- 12.Lowe NM, Lowe NM, Fraser WD, Jackson MJ. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc Nutr Soc 2002;61:181–5. [DOI] [PubMed] [Google Scholar]
- 13.Leek JC, Keen CL, Vogler JB, et al. Long-term marginal zinc deprivation in rhesus monkeys. IV. Effects on skeletal growth and mineralization. Am J Clin Nutr 1988;47:889–95. [DOI] [PubMed] [Google Scholar]
- 14.Vukicevic S, Bagi C, Vujicic G, Krempien B, Stavljenic A, Herak M. The influence of 1 alpha,25- and 24(R),25-dihydroxyvitamin D3 on bone constituents during early mineralization in the rat. Bone Miner. 1986;1:383–96. [PubMed] [Google Scholar]
- 15.Berg JM. Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules. J Biol Chem. 1990;265:6513–6. [PubMed] [Google Scholar]
- 16.Lutz W, Burritt MF, Nixon DE, Kao PC, Kumar R. Zinc increases the activity of vitamin D-dependent promoters in osteoblasts. Biochem Biophys Res Commun 2000;271:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Tong A, Reich A, Genin O, Pines M, Monsonego-Ornan E. Expression of chicken 75-kDa gelatinase B-like enzyme in perivascular chondrocytes suggests its role in vascularization of the growth plate. J Bone Miner Res. 2003;18:1443–52. [DOI] [PubMed] [Google Scholar]
- 18.Fredlund K, Isaksson M, Rossander-Hulthen L, Almgren A, Sandberg AS. Absorption of zinc and retention of calcium: Dose-dependent inhibition by phytate. J Trace Elem Med Biol 2006;20:49–57. [DOI] [PubMed] [Google Scholar]
- 19.Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr 2000;130:1374S–7S. [DOI] [PubMed] [Google Scholar]
- 20.Combs GF, Hassan N. The Chakaria food system study: household-level, case-control study to identify risk factor for rickets in Bangladesh. Eur J Clin Nutr. 2005;59:1291–301. [DOI] [PubMed] [Google Scholar]
- 21.Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Manaster BJ, Reading JC. Radiographic scoring method for the assessment of the severity of nutritional rickets. J Trop Pediatr. 2000;46:132–9. [DOI] [PubMed] [Google Scholar]
- 22.Latham MC. Human nutrition in tropical Africa. Rome: FAO of the United Nations; 1979. p. 264–76.
- 23.Food composition table for use in Africa. Agricultural Extension and Research Liaison Services. Soyabeans in the Nigerian diet. Extension bulletin no 21. Home economics series no 1. Zaria (Nigeria): Ahmadu Bello University; 1985. p. 62–71.
- 24.Gouws E, Langenhoven ML, editors. NRIND food composition tables. 2nd ed. Parow (South Africa): South African Medical Research Council; 1986.
- 25.Latta M, Eskin M. A simple and rapid colorimetric method for phytate determination. J Agric Food Chem. 1980;28:1313–15. [Google Scholar]
- 26.Wade HE, Morgan DM. Fractionation of phosphates by paper ionophoresis and chromatography. Biochem J. 1955;60:264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrams SA. Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr. 1999;70:955–64. [DOI] [PubMed] [Google Scholar]
- 28.Abrams SA, Griffin IJ, Hicks PD, Gunn SK. Pubertal girls only partially adapt to low dietary calcium intakes. J Bone Miner Res. 2004;19:759–63. [DOI] [PubMed] [Google Scholar]
- 29.Herman S, Griffin IJ, Suwarti S, Ernawati F, Permaesih D, Pambudi D, Abrams SA. Cofortification of iron-fortified flour with zinc sulfate, but not zinc oxide, decreases iron absorption in Indonesian children. Am J Clin Nutr. 2002;76:813–7. [DOI] [PubMed] [Google Scholar]
- 30.Oramasionwu GE, Thacher TD, Pam SD, Pettifor JM, Abrams SA. Adaptation of calcium absorption during treatment of nutritional rickets in Nigerian children. Br J Nutr. 2008;100:387–92. [DOI] [PubMed] [Google Scholar]
- 31.Dunnigan MG, Henderson JB, Hole DJ, Barbara Mawer E, Berry JL. Meat consumption reduces the risk of nutritional rickets and osteomalacia. Br J Nutr 2005;94:983–91. [DOI] [PubMed] [Google Scholar]
- 32.Heaney RP, Smith KT, Recker RR, Hinders SM. Meal effects on calcium absorption. Am J Clin Nutr. 1989;49:372–6. [DOI] [PubMed] [Google Scholar]
- 33.Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Chan GM. Case-control study of factors associated with nutritional rickets in Nigerian children. J Pediatr. 2000;137:367–73. [DOI] [PubMed] [Google Scholar]
- 34.Fredlund K, Isaksson M, Rossander-Hulthen L, Almgren A, Sandberg AS. Absorption of zinc and retention of calcium: dose-dependent inhibition by phytate. J Trace Elem Med Biol. 2006;20:49–57. [DOI] [PubMed] [Google Scholar]
- 35.Morgan EL, Mace OJ, Affleck J, Kellett GL. Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol. 2007;580:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Arnold T, Broadhead RL, Hambidge KM. Dietary phytate reduction improves zinc absorption in Malawian children recovering from tuberculosis but not in well children. J Nutr. 2000;130:2959–64. [DOI] [PubMed] [Google Scholar]
- 37.Davidsson L, Ziegler EE, Kastenmayer P, van Dael P, Barclay D. Dephytinisation of soyabean protein isolate with low native phytic acid content has limited impact on mineral and trace element absorption in healthy infants. Br J Nutr. 2004;91:287–94. [DOI] [PubMed] [Google Scholar]
- 38.Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:S1378–83. [DOI] [PubMed] [Google Scholar]
- 39.Turnlund JR, King JC, Keyes WR, Gong B, Michel MC. A stable isotope study of zinc absorption in young men: effects of phytate and alpha-cellulose. Am J Clin Nutr. 1984;40:1071–7. [DOI] [PubMed] [Google Scholar]
- 40.Sandstrom B, Sandberg AS. Inhibitory effects of isolated inositol phosphates on zinc absorption in humans. J Trace Elem Electrolytes Health Dis. 1992;6:99–103. [PubMed] [Google Scholar]
- 41.Hurrell RF. Influence of vegetable protein sources on trace element and mineral bioavailability. J Nutr. 2003;133:S2973–7. [DOI] [PubMed] [Google Scholar]
- 42.Egli I, Davidsson L, Zeder C, Walczyk T, Hurrell R. Dephytinization of a complementary food based on wheat and soy increases zinc, but not copper, apparent absorption in adults. J Nutr. 2004;134:1077–80. [DOI] [PubMed] [Google Scholar]
- 43.Fredlund K, Bergman EL, Rossander-Hulthen L, Isaksson M, Almgren A, Sandberg AS. Hydrothermal treatment and malting of barley improved zinc absorption but not calcium absorption in humans. Eur J Clin Nutr. 2003;57:1507–13. [DOI] [PubMed] [Google Scholar]
- 44.Hirabayashi M, Matsui T, Yano H. Fermentation of soybean flour with Aspergillus usamii improves availabilities of zinc and iron in rats. J Nutr Sci Vitaminol (Tokyo). 1998;44:877–86. [DOI] [PubMed] [Google Scholar]
- 45.Hotz C, Gibson RS, Temple L. A home-based method to reduce phytate content and increase zinc bioavailability in maize-based complementary diets. Int J Food Sci Nutr. 2001;52:133–42. [PubMed] [Google Scholar]
- 46.Navert B, Sandstrom B, Cederblad A. Reduction of the phytate content of bran by leavening in bread and its effect on zinc absorption in man. Br J Nutr. 1985;53:47–53. [DOI] [PubMed] [Google Scholar]
- 47.Larsson M, Rossander-Hulthen L, Sandstrom B, Sandberg AS. Improved zinc and iron absorption from breakfast meals containing malted oats with reduced phytate content. Br J Nutr. 1996;76:677–88. [DOI] [PubMed] [Google Scholar]