Abstract
Soy isoflavones have functional similarity to human estrogens and may protect against breast cancer as a result of their antiestrogenic activity or increase risk as a result of their estrogen-like properties. We examined the relation between isoflavone supplementation and mammographic density, a strong marker for breast cancer risk, among postmenopausal women. The Osteoporosis Prevention Using Soy (OPUS) study, a multi-site, randomized, double-blinded, and placebo-controlled trial assigned 406 postmenopausal women to 80 or 120 mg/d of isoflavones each or a placebo for 2 y. Percent densities were assessed in digitized mammograms using a computer-assisted method. The mammogram reader did not know the treatment status and the time of mammograms. We applied mixed models to compare breast density by treatment while considering the repeated measures. The mammographic density analysis included 358 women, 88.2% of the OPUS participants; 303 had a complete set of 3 mammograms, 49 had 2, and 6 had only 1 mammogram. At baseline, the groups were similar in age, BMI, and percent density, but mean breast density differed by study site (P = 0.02). A model with all mammograms did not show a treatment effect on any mammographic measure, but the change over time was significant; breast density decreased by 1.6%/y across groups (P < 0.001). Stratification by age and BMI did not reveal any effects in subgroups. In this randomized 2-y trial, isoflavone supplements did not modify breast density in postmenopausal women. These findings offer reassurance that isoflavones do not act like hormone replacement medication on breast density.
Introduction
High mammographic densities confer a 4- to 6-fold risk to develop breast cancer (1). Factors that decrease breast density may, therefore, also decrease breast cancer risk. Breast density is strongly inversely associated with age and BMI despite the higher breast cancer risk associated with postmenopausal status and obesity (2). Due to the cumulative effect, a woman with high densities throughout life will have a higher breast cancer risk than a woman with low densities even after densities decrease with age (3). Several interventions have indicated that dietary and hormonal factors may modify breast density (4–6). As supported by epidemiological and experimental studies (7), soy isoflavones have been hypothesized to protect against breast cancer because of their antiestrogenic properties (8–10). The information on the relation between soy intake and breast density is limited and contradictory. A cross-sectional study in Hawaii showed a significant positive trend of percent densities by quartiles of soy intake (11). In contrast, a cross-sectional study among Singapore Chinese women observed an inverse relation between soy intake and breast density (12). Two interventions in premenopausal women, one with an isoflavone supplement and the other one with soy foods, did not observe a significant density change with treatment (13,14). As a result of the steep decline of endogenous estrogens, the interaction between isoflavones and estrogens may change after menopause and isoflavones may then have a different effect on breast density than during premenopausal years. Three intervention studies in postmenopausal women, 1 with red clover-derived isoflavones, 1 with black cohosh, and 1 with soy found no effects on breast density (15–17). The Osteoporosis Prevention Using Soy (OPUS) study offered the opportunity to examine the relation between different doses of an isoflavone supplement and mammographic density in a 2-y clinical trial designed to document the safety, efficacy, and optimal dosage of soy isoflavones to prevent bone loss after menopause.
Methods
Study population.
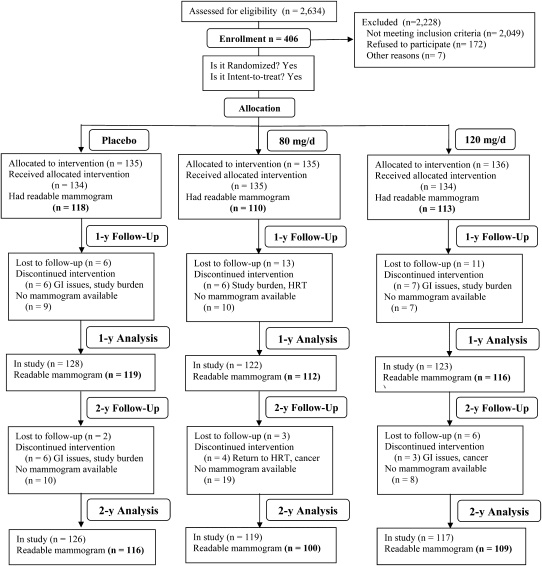
The OPUS study was a 2-y follow-up, multi-center, randomized, double-blind, placebo-controlled, and intent-to-treat clinical trial with bone density as the primary endpoint (Fig. 1). The study protocol was approved by the Institutional Review Boards of all participating institutions and all women signed informed consent forms. The details of the study design and recruitment will be described elsewhere (WW Wong, FM Steinberg, P Amato, MK Cramer, RD Lewis, MJ Murray, RL Young, unpublished data). In short, a group of 406 postmenopausal women in their early years of menopause were enrolled during 2003–4 at 3 collaborating sites. The USDA/Agricultural Research Services Children's Nutrition Research Center at Baylor College of Medicine in Houston, Texas was the coordination center. The University of Georgia at Athens, Georgia, in collaboration with the Athens Women's Clinic, was the second study site and the University of California at Davis in collaboration with the Kaiser Foundation Research Institute (Sacramento, CA) was the 3rd study site.
FIGURE 1 .
CONSORT Diagram for the OPUS trial.
To qualify for the study, women had to be postmenopausal (no menses for at least 12 mo and blood follicle stimulating hormone exceeding 30 IU/L) and between 40 and 60 y old. Women with allergic reactions to soy products and vegetarians were excluded. The latter may consume a large amount of soy in their habitual diets, which may affect the outcome variables. Soy food consumption of <1 serving/wk was considered acceptable. Participants were excluded for the following reasons, because these may affect bone loss or bone disease: smoking or having quit smoking <5 y prior to enrollment; highly physically active or completely sedentary; T-scores for lumbar spine bone mineral density outside the normal range; exercise or drug treatment for bone disease; BMI ≥30 kg/m2; and current use of bisphosphonates, calcitonin, fluoride, corticosteroids, Tamoxifen, Raloxifene, Farestron, Letrozole, Premarin, and any other hormone replacement therapy; current use of supplements, including black cohosh, blue cohosh, dong quai, Caltrate, 600+Soy, Estroven, ginseng, Healthy Women, Natural Estrogen, Opti-Soy, PhytoFem, Probalance, Promensil, Remifemin, Rimostil, or Trinovin; and medical illnesses, including osteoporosis, spine and/or hip fractures, cancer, as well as liver, kidney, gallbladder, and heart disease.
To account for potential differences and changes in isoflavone intakes across groups, each woman completed a FFQ before and after 12 and 24 mo of treatment. The questionnaire was developed and validated by the Fred Hutchinson Cancer Research Center Nutrition Assessment Shared Resource (Seattle, WA) (18). Dietary analyses were performed using a database comprised of manufacturer information and USDA and literature isoflavone values.
Intervention.
For each of the 3 study sites, the study participants were randomized within 9 time blocks of 15 so that 135 of the participants received 80 mg/d of isoflavone therapy, 134 received 120 mg/d of isoflavone therapy, and the remaining 135 received a placebo (Fig. 1). These isoflavone doses were equivalent to 300–500 g tofu or 500–1000 mL soy milk (19). In comparison, the typical isoflavone intake in Japan is estimated at 25–50 mg/d (20). The total duration of the study was 2 y for all groups. Soy-germ isoflavone tablets were used that contained 42% daidzin, 2% daidzein, 13% genistin, 1% genistein, 39% glycitin, and 3% glycitein by weight. Acatris Holding provided the soy-germ isoflavone materials and Pharma Consulting and Industries prepared the tablets. Compliance was assessed by pill counts as the participants returned to the study sites to pick up their next 6-mo supply of tablets and also confirmed by annual blood isoflavone measurements (WW Wong, FM Steinberg, P Amato, MK Cramer, RD Lewis, MJ Murray, RL Young, unpublished data). Compliance did not differ among the 3 study groups. To meet the adequate intake requirement of calcium recommended for women between 40 and 60 y of age, each study participant was supplemented with 1 g of calcium carbonate (Source Naturals). Also, all participants were asked to stop taking any vitamin or multivitamin supplementations during the study period and were provided a multivitamin supplement (Century Formula without iron from Swanson Health Products) with 10 μg cholecalciferol in a daily dose.
Mammographic density assessment.
The goal was to retrieve mammograms for all participants performed at baseline, after 1 y, and after 2 y. All films, including the 333 (33%) images obtained through digital mammography, were scanned at the Cancer Research Center of Hawaii using a Kodak LS85 Film Digitizer (absorbance range, 0.001–4.1; Eastman Kodak) with a pixel size of 260 μm. One of the authors (G. Maskarinec) quantified total breast area on the mammogram as well as the area of dense tissue within the breast using the Cumulus package version 3 (21). The single reader determined a threshold for the edges of the breast and the dense tissue in all mammograms from the 3 sites. Then the total number of pixels in the digitized image that constituted the total area and the dense area was calculated and the ratio between these 2 values, i.e. percent breast density, was computed.
Mammograms were assessed in 44 analytical batches that contained randomly selected participants from all 3 groups; all mammograms for the same woman were included in the same batch in random order. The reader did not know the study center, group allocation, or year of mammogram. A random sample of 239 craniocaudal images was read in duplicate to assess reliability. The correlation coefficients were 0.94 and 0.998 for the size of the dense and the total breast areas, respectively, resulting in a correlation of 0.95 for percent density. We computed means for the readings for the left and right view of the cranial caudal images.
Statistical analyses.
All analyses investigating the intervention effect followed the intent-to-treat principle, i.e. participants were analyzed as part of their assigned treatment group. We computed means ± SD by group at baseline and used ANOVA and chi-square tests to assess the differences for significance. A mixed general linear model using maximum likelihood estimation was applied to evaluate whether isoflavone supplementation modified mammographic density. The repeated measurements were modeled as random effects. The model included a fixed effect portion to test for a change in breast density over time and an interaction between group assignment and time. After the significant difference in breast density across study site was noted, we repeated the mixed models with study site and baseline density as covariates. We also assigned a variable to indicate the dosage level (0, 80, and 120) to test for a dose-response relation. Participants with missing mammograms were part of the overall analysis, but we repeated the models with the 303 women who had a complete set of 3 mammograms and again after exclusion of all 333 density readings from digital mammograms. To explore whether any subgroups responded differently to isoflavone exposure, we introduced an interaction term into the model and stratified by BMI (<25 and ≥25 kg/m2) and age group (<55 and ≥55 y). For all significance tests, an α of < 0.05 was considered significant. The statistical software package SAS 9.1 was used for all analyses.
Results
The mammographic density analysis included 358 women, 88% of the OPUS study participants, 303 of whom had a complete set of 3 mammograms, 49 had 2, and 6 had only 1 mammogram (Fig. 1). Of the 48 women without mammograms, 4 had films that could not be evaluated due to breast implants and the films could not be obtained from the clinics for the remaining women. The proportion of women without mammograms differed slightly by group: 9% for the placebo, 15% for the 80-mg/d group, and 12% for the 120-mg/d treatment arm (P = 0.05).
The original numbers by recruitment site were 102 at Baylor, 145 at UC Davis, and 111 at Georgia; the sites had the same proportion of women in the 3 treatment arms and did not differ significantly in age, BMI, and years since menopause. Mean percent density at baseline was lower at Baylor (26.6%) than at Davis and Georgia (32.2% and 33.5%; P = 0.02) due to the lower densities in the 333 digital (27%) compared with the 680 regular mammograms (31%; P = 0.0003) and the fact that 96% of mammograms at Baylor but only 3% at Davis and 17% at Georgia were digital (P < 0.0001). All mammograms were obtained with the same technique for 83% of women; for 95 participants, all mammograms were digital, whereas 201 women had only regular mammograms.
The mean age and BMI of all study participants were 54.8 ± 3.9 y and 25.1 ± 3.2 kg/m2, respectively. The date of the first mammogram preceded study entry by a mean of 23 ± 140 d, whereas the time between last mammogram and end of study was 73 ± 116 d. At baseline, characteristics predicting mammographic density, such as age, age at menopause, ethnicity, and BMI, did not differ among the 3 groups (Table 1). The majority of the 358 study participants were Caucasian. The 3 groups did not differ in any of the breast measures.
TABLE 1.
| Treatment
|
|||
|---|---|---|---|
| Placebo | 80 mg/d | 120 mg/d | |
| n | 123 | 115 | 120 |
| Study center, % | |||
| Baylor College | 271 | 30 | 28 |
| UC Davis | 41 | 40 | 41 |
| University of Georgia | 32 | 30 | 31 |
| Age, y | 54.8 ± 3.6 | 55.2 ± 4.0 | 54.7 ± 3.8 |
| Ethnicity, % | |||
| Asian | 3 | 5 | 11 |
| Black | 4 | 4 | 2 |
| Caucasian | 78 | 84 | 79 |
| Hispanic | 6 | 4 | 3 |
| Other/unknown | 9 | 3 | 5 |
| BMI, kg/m2 | 25.0 ± 2.7 | 25.5 ± 3.8 | 24.7 ± 3.1 |
| Age at menopause, y | 48.3 ± 5.2 | 48.5 ± 5.7 | 47.9 ± 6.2 |
| Time since menopause, y | 6.5 ± 5.2 | 6.7 ± 5.4 | 6.9 ± 6.7 |
| Breast measures at baseline | |||
| Breast area, cm2 | 124.3 ± 50.4 | 120.6 ± 56.4 | 123.1 ± 44.8 |
| Dense area, cm2 | 33.8 ± 24.4 | 35.6 ± 24.1 | 36.2 ± 20.8 |
| Nondense area, cm2 | 90.6 ± 46.6 | 85.0 ± 54.5 | 86.9 ± 45.2 |
| Percent density, % | 28.9 ± 17.4 | 32.3 ± 19.0 | 32.0 ± 17.5 |
Values are means ± SD or percent.
None of the ANOVA or chi-square tests had a P < 0.10.
Group assignment and the interaction of group with time were not significant for any of the 4 breast measures (Table 2). Although percent density was somewhat lower in the 80-mg/d supplement group than in the other 2 groups, the interaction term indicated that the intervention did not affect the 3 treatment groups differently (P = 0.85). On the other hand, both measures of mammographic density, i.e. the dense area and percent density, decreased significantly over time, whereas the size of the total breast area and the nondense area increased significantly. Based on the parameter estimates in the mixed models, the respective annual decreases in dense area and percent density were 2.0 ± 0.6 cm2 and 1.6 ± 0.3%, resulting in ∼3% lower percent density after 2 y of study. The annual decrease in densities was similar for the placebo, the 80-mg/d, and the 120-mg/d arm: 1.4 ± 0.3, 1.6 ± 0.3, and 1.3 ± 0.3%. Breast area and nondense area increased by 1.4 ± 1.8 and 3.4 ± 1.5 cm2/y, respectively. To test for a dose-response relation, we repeated the percent density model with the dose of isoflavones (0, 80, and 120 mg/d) as independent variable, but there was no effect. Site (P = 0.04) and baseline percent density (P < 0.001) were both significant when added to the model.
TABLE 2.
Mammographic measures during a 2-y soy isoflavone intervention among 358 postmenopausal women1
| Time
|
P-value2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Treatment | n | Baseline | y 1 | y 2 | Group | Time | Interaction |
| Breast area, cm2 | Placebo | 123 | 123.1 ± 44.81 | 124.5 ± 48.3 | 127.7 ± 47.7 | |||
| 80 mg/d | 115 | 124.3 ± 50.4 | 126.0 ± 47.0 | 127.0 ± 48.3 | ||||
| 120 mg/d | 120 | 120.6 ± 56.4 | 131.5 ± 88.8 | 123.7 ± 56.7 | 0.91 | 0.006 | 0.59 | |
| Dense area, cm2 | Placebo | 123 | 36.2 ± 20.8 | 33.8 ± 20.7 | 34.3 ± 23.0 | |||
| 80 mg/d | 115 | 33.8 ± 24.4 | 31.9 ± 22.5 | 29.9 ± 21.7 | ||||
| 120 mg/d | 120 | 35.6 ± 24.1 | 37.2 ± 27.4 | 33.9 ± 23.6 | 0.62 | <0.001 | 0.26 | |
| Nondense area, cm2 | Placebo | 123 | 86.9 ± 45.1 | 90.6 ± 48.7 | 93.4 ± 48.6 | |||
| 80 mg/d | 115 | 90.6 ± 46.6 | 94.1 ± 47.0 | 97.1 ± 49.4 | ||||
| 120 mg/d | 120 | 85.0 ± 54.5 | 94.4 ± 77.7 | 89.8 ± 57.5 | 0.69 | <0.001 | 0.79 | |
| Percent density, % | Placebo | 123 | 32.0 ± 17.5 | 30.0 ± 17.2 | 29.3 ± 18.0 | |||
| 80 mg/d | 115 | 28.9 ± 17.4 | 27.2 ± 17.6 | 25.8 ± 17.4 | ||||
| 120 mg/d | 120 | 32.3 ± 19.0 | 31.4 ± 18.4 | 30.6 ± 19.2 | 0.17 | <0.001 | 0.85 | |
Values are means ± SD or percent.
Based on mixed models showing P-values for treatment group, effect of time, and interaction between treatment and time.
Restricting the analysis to the 303 women with a complete set of 3 mammograms or excluding the 333 films obtained through digital mammography did not indicate a treatment effect on percent density (Table 3). Stratification by age group indicated a nonsignificant smaller effect of time on percent density among younger women (P = 0.65); the respective parameter estimates were 1.2 ± 0.7% annually for women <55 y and 1.7 ± 0.4% annually for those ≥55 y. However, the treatment had no effect in either age group. Although normal weight women had ∼3% higher percent densities than overweight participants (P < 0.001), neither group experienced a treatment effect and the interaction term between treatment and weight status was not significant. The difference by treatment group was significant among overweight women (P = 0.02), because the overall densities were lower for the 120-mg/d group (21.4%) than for the placebo (28.7%) and the 80-mg/d group (24.8%), but the interaction between treatment and time was not significant.
TABLE 3.
Percent breast density during a 2-y soy isoflavone intervention among subgroups of postmenopausal women1
| Time
|
P-value2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | Treatment | n | Baseline | y 1 | y 2 | Group | Time | Interaction |
| 3 Mammograms | Placebo | 107 | 32.0 ± 17.91 | 29.9 ± 17.5 | 29.1 ± 18.5 | |||
| 80 mg/d | 97 | 29.3 ± 17.5 | 27.4 ± 18.1 | 26.2 ± 17.5 | ||||
| 120 mg/d | 99 | 32.4 ± 19.2 | 31.7 ± 18.7 | 29.9 ± 19.0 | 0.40 | <0.001 | 0.79 | |
| No digital films | Placebo | 90 | 35.3 ± 17.3 | 32.3 ± 18.0 | 30.7 ± 18.8 | |||
| 80 mg/d | 86 | 30.4 ± 16.3 | 28.8 ± 18.1 | 26.8 ± 16.9 | ||||
| 120 mg/d | 87 | 35.1 ± 19.4 | 31.7 ± 18.8 | 30.1 ± 19.0 | 0.34 | <0.001 | 0.79 | |
| Age <55 y | Placebo | 62 | 34.2 ± 16.8 | 33.6 ± 18.4 | 34.8 ± 17.5 | |||
| 80 mg/d | 42 | 31.0 ± 18.7 | 27.1 ± 18.3 | 28.1 ± 19.5 | ||||
| 120 mg/d | 56 | 33.0 ± 19.7 | 34.3 ± 18.9 | 33.0 ± 18.8 | 0.65 | 0.001 | 0.91 | |
| Age ≥55 y | Placebo | 61 | 29.5 ± 18.2 | 27.8 ± 16.1 | 26.9 ± 17.8 | |||
| 80 mg/d | 73 | 27.7 ± 16.5 | 27.3 ± 17.5 | 25.2 ± 16.8 | ||||
| 120 mg/d | 64 | 31.7 ± 18.5 | 29.7 ± 19.4 | 29.5 ± 19.4 | 0.35 | <0.001 | 0.99 | |
| BMI <25 kg/m2 | Placebo | 67 | 35.2 ± 18.0 | 32.4 ± 15.6 | 32.6 ± 18.3 | |||
| 80 mg/d | 51 | 36.8 ± 17.9 | 36.0 ± 18.9 | 35.4 ± 17.7 | ||||
| 120 mg/d | 70 | 38.7 ± 20.2 | 36.4 ± 18.2 | 35.9 ± 20.0 | 0.75 | <0.001 | 0.91 | |
| BMI ≥25 kg/m2 | Placebo | 56 | 28.3 ± 16.4 | 28.1 ± 18.3 | 26.6 ± 17.5 | |||
| 80 mg/d | 64 | 22.4 ± 14.0 | 20.2 ± 12.9 | 17.4 ± 11.9 | ||||
| 120 mg/d | 50 | 24.0 ± 13.5 | 24.4 ± 16.6 | 23.3 ± 15.4 | 0.02 | <0.001 | 0.95 | |
Values are means ± SD or percent.
Based on mixed models showing P-values for treatment group, effect of time, and interaction between treatment and time.
Discussion
In a 2-y randomized trial among postmenopausal women, 2 doses of an isoflavone supplement did not influence mammographic density. Stratification by age and BMI also did not indicate an effect of the treatment for any subgroup of women. However, percent density decreased by ∼3% over the study period, which reflects the normal decline of fibroglandular tissue in the aging breast. The findings contradict our hypothesis that isoflavone treatment would decrease mammographic density, but they are in agreement with 5 previous intervention studies that observed no treatment-related changes in mammographic densities (13–17). Three of those studies were conducted in postmenopausal women. Atkinson et al. (15) administered red clover-derived isoflavones to 205 participants 49–65 y old for 12 mo and saw no change. A smaller study (n = 74) with an isoflavone dosage of 40 mg from black cohosh detected no significant difference in mammographic patterns after 6 mo (16). A recent Dutch study also used a computer-assisted density assessment method and found no differences in change of mammographic density over 1 y (17), although the treatment with 99 mg of isoflavones achieved an isoflavone intake similar to the current study and above the habitual isoflavone intake in Asian countries (20). Two trials among premenopausal women, 1 with an isoflavone supplement and 1 with soy foods for 2 y, reported no difference in breast density between intervention and control groups (13,14). The contradicting evidence from the 2 cross-sectional reports may be chance findings (11,12).
The present study was well designed to examine possible effects of isoflavones and overcame some of the limitations of the previous trials. The study had a 2-y duration, the sample size was larger than in previous trials (n = 358), loss to follow-up was low (10%), and 2 doses of isoflavones (80 and 120 mg/d) allowed us to study a possible dose response. The age range of the participants was optimal to study effects on breast density, because, just like for bone density, the change is greatest around menopause and becomes more modest after age 60 y (3,22). Unfortunately, 12% of the randomized OPUS participants had to be excluded from the analysis due to missing data on mammographic density. Nevertheless, the drop-out rate in combination with missing mammograms was very reasonable and probably did not bias the findings. Inclusion of 333 digital mammograms with different technical characteristics than regular films (23) was not a major problem, because density changes were assessed in mammograms obtained with the same technique for 83% of the participants. Also, the exclusion of digital films did not change the results of the intervention analysis.
The null results of this clinical trial may be due to several reasons. The duration, the sample size, or the dose may have been insufficient to affect breast density. The latter would be unlikely, because the dose of isoflavones was considerably higher than among Asian populations (20). Given the negative findings in 5 previous studies (13–17), it is likely that isoflavones do not modify breast density in adult women. However, this would not rule out a protective effect of isoflavones against breast cancer supported by the large difference in breast cancer incidence between Asian and Western countries (24) and by evidence from case-control studies (25). One possibility is that the exposure has to occur earlier in life, as shown by 2 case-control studies that detected a stronger beneficial effect of soy consumption during adolescence than adulthood (26,27). The other explanation could be that soy isoflavones affect breast cancer through a different pathway than mammographic densities, similar to the effect of endogenous steroid hormones (28,29) and postmenopausal obesity (30) on breast cancer risk, which does not appear to be mediated by breast density.
The fact that hormone replacement therapy interventions, primarily those with progestins, and not those with estrogens alone, modify breast density (6,31) while soy isoflavones do not, offers some reassurance to those who have been concerned about adverse effects of soy supplementation on breast cell proliferation (32–35). Furthermore, when adult soy exposure was analyzed in relation to breast density, women reporting regular soy intake had a faster decline in mammographic densities than those who did not consume soy foods (3). A Dutch report found that a faster decline in densities was protective against breast cancer (36).
In conclusion, although we did not observe a beneficial effect of soy-derived isoflavones on mammographic densities during a 2-y randomized trial with >300 women, there was also no sign of any adverse effects. These findings do not exclude the possibility that breast cancer risk may be reduced as a result of isoflavone exposure earlier in life or through alternate mechanisms of action than through mammographic densities.
Supported by the Initiative for Future Agriculture and Food Systems grant no. 2001-52102-11255 from the USDA Cooperative State Research, Education, and Extension Service and R03 CA121879 from the National Cancer Institute.
Author disclosures: G. Maskarinec, M. Verheus, F. M. Steinberg, P. Amato, M. K. Cramer, R. D. Lewis, M. J. Murray, R. L. Young, and W. W. Wong, no conflicts of interest.
References
- 1.Boyd NF, Lockwood GA, Martin LJ, Knight JA, Byng JW, Yaffe MJ, Tritchler DL. Mammographic densities and breast cancer risk. Breast Dis. 1998;10:113–26. [DOI] [PubMed] [Google Scholar]
- 2.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. [DOI] [PubMed] [Google Scholar]
- 3.Maskarinec G, Pagano I, Lurie G, Kolonel LN. A longitudinal investigation of mammographic density: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:732–9. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Greenberg C, Lockwood G, Little L, Martin L, Byng J, Yaffe M, Tritchler D. Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. J Natl Cancer Inst. 1997;89:488–96. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Martin LJ, Stone J, Greenberg C, Minkin S, Yaffe MJ. Mammographic densities as a marker of human breast cancer risk and their use in chemoprevention. Curr Oncol Rep. 2001;3:314–21. [DOI] [PubMed] [Google Scholar]
- 6.Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density. J Natl Cancer Inst. 2003;95:30–7. [DOI] [PubMed] [Google Scholar]
- 7.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adlercreutz H, Goldin BR, Gorbach SL, Höckerstedt KAV, Watanabe S, Hämäläinen E, Markkanen MH, Mäkelä TK, Wähäläm KT, et al. Soybean phytoestrogen intake and cancer risk. J Nutr. 1995;125:S757–70. [DOI] [PubMed] [Google Scholar]
- 9.Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. 1991;83:541–6. [DOI] [PubMed] [Google Scholar]
- 10.Setchell KDR. Naturally occurring non-steroidal estrogens of dietary origin. McLachlan JA, editor. Estrogens in the environment. New York: Elsevier Science Publishing Co., Inc.; 1985. p. 69–83.
- 11.Maskarinec G, Meng L. An investigation of soy intake and mammographic characteristics in Hawaii. Breast Cancer Res. 2001;3:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakes RW, Duffy SW, Ng FC, Gao F, Ng EH, Seow A, Lee HP, Yu MC. Mammographic parenchymal patterns and self-reported soy intake in Singapore Chinese women. Cancer Epidemiol Biomarkers Prev. 2002;11:608–13. [PubMed] [Google Scholar]
- 13.Maskarinec G, Williams AE, Carlin L. Mammographic densities in a one-year isoflavone intervention. Eur J Cancer Prev. 2003;12:165–9. [DOI] [PubMed] [Google Scholar]
- 14.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–94. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, Healey CS, Runswick S, Day NE, Bingham SA. Red clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial. Breast Cancer Res. 2004;6:R170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschberg AL, Edlund M, Svane G, Azavedo E, Skoog L, von Schoultz B. An isopropanolic extract of black cohosh does not increase mammographic breast density or breast cell proliferation in postmenopausal women. Menopause. 2007;14:89–96. [DOI] [PubMed] [Google Scholar]
- 17.Verheus M, van Gils CH, Kreijkamp-Kaspers S, Kok L, Peeters PH, Grobbee DE, van der Schouw YT. Soy protein containing isoflavones and mammographic density in a randomized controlled trial in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:2632–8. [DOI] [PubMed] [Google Scholar]
- 18.Frankenfeld CL, Patterson RE, Horner NK, Neuhouser ML, Skor HE, Kalhorn TF, Howald WN, Lampe JW. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. Am J Clin Nutr. 2003;77:674–80. [DOI] [PubMed] [Google Scholar]
- 19.USDA. USDA-Iowa State University database on the isoflavone content of foods [cited 2005. Jun 23]. Available from: http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html.
- 20.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. [DOI] [PubMed] [Google Scholar]
- 21.Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ. The quantitative analysis of mammographic densities. Phys Med Biol. 1994;39:1629–38. [DOI] [PubMed] [Google Scholar]
- 22.Kelemen LE, Pankratz VS, Sellers TA, Brandt KR, Wang A, Janney C, Fredericksen ZS, Cerhan JR, Vachon CM. Age-specific trends in mammographic density: the Minnesota Breast Cancer Family Study. Am J Epidemiol. 2008;167:1027–36. [DOI] [PubMed] [Google Scholar]
- 23.Yaffe MJ. Measurement of mammographic density. Breast Cancer Res. 2008;10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: cancer incidence, mortality and prevalence worldwide. Lyon: IARC Press; 2001.
- 25.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–71. [DOI] [PubMed] [Google Scholar]
- 26.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23:1491–6. [DOI] [PubMed] [Google Scholar]
- 27.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–8. [PubMed] [Google Scholar]
- 28.Tamimi RM, Hankinson SE, Colditz GA, Byrne C. Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2005;14:2641–7. [DOI] [PubMed] [Google Scholar]
- 29.Boyd NF, Martin LJ, Li Q, Sun L, Chiarelli AM, Hislop G, Yaffe MJ, Minkin S. Mammographic density as a surrogate marker for the effects of hormone therapy on risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:961–6. [DOI] [PubMed] [Google Scholar]
- 30.Boyd NF, Martin LJ, Sun L, Guo H, Chiarelli A, Hislop G, Yaffe M, Minkin S. Body size, mammographic density, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2086–92. [DOI] [PubMed] [Google Scholar]
- 31.McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, Wang CY, Brunner RL, Johnson KC, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: women's health initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–76. [DOI] [PubMed] [Google Scholar]
- 32.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, Lee MM, Miike R, Kirk M, Coward L. Stimulatory influence of soy protein isolate on breast secretion in pre-and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–94. [PubMed] [Google Scholar]
- 33.Hargreaves DF, Potten CS, Harding C, Shaw LE, Morton MS, Roberts SA, Howell A, Bundred NJ. Two-week dietary soy supplementation has an estrogenic effect on normal premenopausal breast. J Clin Endocrinol Metab. 1999;84:4017–24. [DOI] [PubMed] [Google Scholar]
- 34.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:S3095–108. [DOI] [PubMed] [Google Scholar]
- 35.Messina M, Caskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–84. [DOI] [PubMed] [Google Scholar]
- 36.van Gils CH, Hendriks JH, Holland R, Karssemeijer N, Otten JD, Straatman H, Verbeek AL. Changes in mammographic breast density and concomitant changes in breast cancer risk. Eur J Cancer Prev. 1999;8:509–15. [DOI] [PubMed] [Google Scholar]