Abstract
Background: Higher intakes of fruit, vegetables, and dark fish may prevent sudden cardiac death and arrhythmias, but the exact mechanisms are not fully understood.
Objective: We examined whether high consumption of fruit, vegetables, and dark fish would be associated with beneficial changes in heart rate variability (HRV).
Design: HRV variables were measured among 586 older men with 928 total observations from November 2000 to June 2007 in the Normative Aging Study, a community-based longitudinal study of aging. Dietary intake was evaluated with a self-administered semiquantitative food-frequency questionnaire and categorized into quartiles.
Results: After controlling for potential confounders, intake of green leafy vegetables was positively associated with normalized high-frequency power and inversely associated with normalized low-frequency power (P for trend < 0.05). These significant associations were retained after further adjustment for healthy lifestyle factors, such as physical activity and use of multivitamins. No significant association was seen between HRV measures and intakes of other fruit and vegetables, vitamin C, carotenoids, tuna and dark-meat fish, or n–3 (omega-3) fatty acids. An effect modification of intake of noncitrus fruit by obesity and of total vegetables and cruciferous vegetables by cigarette smoking was seen, which warrants further investigation.
Conclusion: These findings suggest that higher intake of green leafy vegetables may reduce the risk of cardiovascular disease through favorable changes in cardiac autonomic function.
INTRODUCTION
Cardiovascular disease (CVD) is the first leading cause of death in developed countries (1, 2), and it is predicted that CVD will be the leading cause of death in developing countries as well (3). Improving diet, including higher consumption of fruit, vegetables, and oily fish, is an important strategy to reduce the risk of CVD (4). The benefits of high intake of fruit, vegetables, and fish are well known. Increased intake of fruit and vegetables may provide defense against oxidative stress. An overwhelming body of evidence has shown that higher intake of fruit and vegetables is associated with lower rates of all-cause, cancer, and CVD mortality (5–7). In addition, intake of dietary antioxidant nutrients, such as vitamin C and carotenoids that are found in vegetables and fruit, has been associated with decreased risk of CVD (8–10). Increased consumption of dark oily fish and n–3 (omega-3) polyunsaturated fatty acids [eicosapentaenoic acid (20:5n–3; EPA); and docosahexaenoic acid (22:6n–3; DHA] from dark fish appear to decrease the risk of sudden cardiac death and arrhythmia (11, 12). However, the underlying biological mechanisms by which high intakes of fruit, vegetables, and dark fish prevent CVD are not fully understood.
Heart rate variability (HRV) is a widely used noninvasive and quantitative marker of cardiac autonomic control. HRV is defined as beat-to-beat fluctuations in heart rate that are mainly determined by the activity of the cardiac sympathetic and parasympathetic nervous systems; it is analyzed in the time or frequency domains (13). Numerous randomized clinical trials have shown a beneficial effect of n–3 fatty acids on HRV in healthy men, the elderly, and in patients with ischemic heart disease (14–16). However, the doses of fish oil used in those studies (≥2 g/d) were much higher than amounts that average Americans consume (0.1–0.3 g/d). Therefore, the effect of dietary intake of dark fish and n–3 fatty acids on HRV is not clear. A recent population-based study found that dietary consumption of fish and n–3 fatty acids (0.3 g/d of n–3 for those who consumed 1–2 servings of fish/wk) was associated with HRV (17). Few studies have investigated associations between dietary antioxidants and HRV. Peeters et al (18) observed that supplementation with vitamin C stimulated the vagal activity of the autonomic nervous system in pigs. Because oxidative stress induced by environmental pollutants (eg, airborne particles) influences cardiac autonomic function (19), it is plausible to hypothesize that dietary intake of antioxidants may have beneficial effects on the autonomic nervous system. However, to our knowledge, no population study has been conducted to examine associations between the consumption of fruit, vegetables, or dietary antioxidant nutrients and HRV. The aim of the current study was to examine whether higher intake of fruit, vegetables, and dark fish was associated with beneficial changes in various HRV measures in a community-based, longitudinal study of an older population.
SUBJECTS AND METHODS
Study population
Study participants were from the Normative Aging Study (NAS), a longitudinal study of aging established by the Veterans Administration in 1963, when 2280 men from the greater Boston area, who were free of known chronic medical conditions, were enrolled (20). Participants have undergone detailed examinations every 3–5 y, including routine physical examinations, laboratory tests, collection of medical history information, and completion of questionnaires on smoking history, food intake, and other factors that may influence health. Between November 2000 and June 2007, HRV was measured for 683 active NAS subjects (1136 total observations). Of these, 189 observations were excluded because of problematic heart rate measurements (atrial fibrillation, atrial bigeminy and trigeminy, pacemakers, irregular rhythm, irregular sinus rhythm, frequent ventricular ectopic activity, ventricular bigeminy, multifocal atrial tachycardia, or measurement time < 3.5 min). We also excluded 15 observations without a complete food-frequency questionnaire (FFQ). An additional 4 observations with missing values for potential confounders were excluded. Hence, a total of 586 participants with 928 observations were available for the analyses. Among 306 subjects with ≥2 HRV measurements, the time interval between measurements was ≈3 y. All participants provided written informed consent. This study was reviewed and approved by the institutional review boards of all participating institutions.
Assessment of dietary intake
Since 1992, dietary intake was assessed with a self-administered semiquantitative FFQ adapted from the questionnaire used in the Nurses' Health Study. This scannable form, which requests participants to record the number of times they consume each of 126 food items per month, week, or day, was mailed to NAS participants before their examination visit and checked for completeness at the examination. Forms were processed through a nutrient database at the Channing Laboratory at Harvard University to obtain estimates of usual daily nutrient intake. On all dietary questionnaires, a commonly used unit or portion size for each food (eg, 1 tomato or 1/2 cup of strawberries) was specified, and the participant was asked how often on average during the past year he had consumed that amount of each food. Nine responses were possible, ranging from almost never or <1 time/mo to ≥6 times/d. For each participant, the average daily intake of each fruit and vegetable item was combined to compute total fruit and vegetable intake. Fruit questions included raisins or grapes, prunes, bananas, cantaloupe, avocado, fresh apples or pears, apple juice or cider, oranges, orange juice, grapefruit, grapefruit juice, other fruit juice, strawberries, blueberries, and peaches or apricots or plums. Individual vegetables included in the FFQ were tomatoes, tomato juice, tomato sauce, salsa, tofu or soybeans, string beans, broccoli, cabbage or cole slaw, cauliflower, brussels sprouts, carrots, beets, corn, peas or lima beans, mixed vegetables, beans or lentils, yellow squash, eggplant or zucchini, yams or sweet potatoes, spinach, kale or mustard greens, iceberg or head lettuce, romaine or leaf lettuce, celery, green peppers, and onions. We also created intake categories of composite fruit and vegetable groups (citrus fruit: oranges, orange juice, grapefruit, and grapefruit juice; noncitrus fruit: fresh apples or pears, apple juice or cider, avocado, bananas, blueberries, cantaloupe, peaches or apricots or plums, prunes, raisins or grapes; cruciferous vegetables: broccoli, cauliflower, Brussels sprouts, kale or mustard greens, and cabbage or cole slaw; green leafy vegetables: raw spinach, cooked spinach, kale or mustard greens, romaine or leaf lettuce). We did not include iceberg or head lettuce in the green leafy vegetables category because it does not have the nutrient content similar to other green leafy vegetables.
Fish questions included canned tuna, dark-meat fish (eg, mackerel, salmon, sardines, bluefish, and swordfish), and other fish (eg, cod, haddock, and halibut). We focused only on canned tuna and dark-meat fish, because these fish meals represent oily fish rich in n–3 fatty acids, whereas white fish, such as cod and haddock, is low in fat.
We also calculated daily intakes of dietary antioxidant nutrients (vitamin C and total carotenoids) and n–3 fatty acids from fish (EPA + DHA) by multiplying the frequency of consumption of each unit of food by the nutrient content of the specified portion. Vitamin C also included amounts from dietary supplements. Details on the reproducibility and validity of this FFQ for estimating daily nutrient intakes were published elsewhere (21, 22).
HRV measurement
HRV was measured between 0600 and 1300 (with most measurements occurring between 0700 and 1100) with the use of a 2-channel (5-lead) electrocardiogram monitor (Trillium 3000; Forest Medical, East Syracuse, NY). After a 5-min rest period, the participant's electrocardiogram was recorded (sampling rate: 256 Hz/channel) for ≈7 min with the subject seated. We used the best 4-consecutive-minute interval for the HRV calculations. The electrocardiogram digital recordings were processed, and heart rate and HRV measures were calculated with the use of personal computer-based software (Trillium 3000 PC Companion Software for MS Windows; Forest Medical), which conforms to established guidelines (13). We used only normal-to-normal (NN) beat intervals in the analysis, and the SD of NN (SDNN) intervals was calculated as a time-domain HRV measure. We also computed frequency-domain HRV measures with the use of a fast-Fourier transform: high-frequency (HF; 0.15–0.4 Hz) and low-frequency (LF; 0.04–0.15 Hz) powers and ratio of LF to HF (LF:HF). HF and LF were then included as normalized units (NHF and NLF), which reflect the relative value of each power component in proportion to the total power minus the very-low-frequency component. Because normalization may minimize the effect of LF and HF components on change in total power (13), we focused on NHF and NLF as frequency-domain HRV measures.
Statistical analysis
Because FFQs were collected between 1992 and 2004, multiple questionnaires were available for most subjects (median: 4). The correlations of food intakes during this period were relatively high (eg, Spearman's correlation coefficients ranged from 0.53 to 0.65 for total energy intake; 0.59–0.71 for total intake of fruit and vegetables; 0.51–0.65 for intake of tuna or dark fish). To best represent long-term dietary exposure and to reduce intraindividual variation, we used the cumulative average daily intakes of fruit, vegetables, or fish from all available questionnaires before the HRV measures (23). Each dietary variable was first adjusted for energy, using the residual method (24), and then categorized into quartiles with the lowest quartile as the reference group. SDNN and LF:HF were log10-transformed to improve normality and to stabilize the variance. We used hierarchical mixed-effects regression models to investigate the effect of each dietary variable on repeated measures of HRV. Age, body mass index (BMI; in kg/m2), fasting blood glucose, mean arterial pressure, smoking status (never, former, or current), alcohol consumption (≥2 drinks/d), and use of β-blockers, calcium channel blockers, or angiotensin-converting enzyme (ACE) inhibitors were included as fixed-effect covariates (model 1). Physical activity and vitamin supplement use were further adjusted to examine whether these healthy lifestyle factors confounded the associations (model 2). We also assessed whether resting heart rate might confound the associations. We considered an interaction between time elapsed from the first visit for HRV measurement and the dietary variable in the model to test whether the association between the individual nutrient variable and HRV changes over time, but this interaction was not included in the final model because it had little influence on the results. A random slope for the time elapsed from the first visit was initially considered to account for subject-specific variability of HRV over time, but a random intercept-only model was preferred, based on the likelihood ratio test comparing the 2 models. The hierarchical model we used is as follows:
where Yij is the logarithm of HRV in subject i at visit j, b0 is the overall intercept, and ui is a subject-specific intercept that reflects unexplained heterogeneity in subjects' overall HRV. For the diet model, b1 is the slope representing the overall effect of individual dietary intake. Antilogs of adjusted mean values of log-transformed HRV measures (SDNN and LF:HF) in each quartile of dietary intake were presented. Tests for trend were assessed by using ordinal terms for the quartiles of intake. We also conducted a sensitivity analysis that used only subjects with ≥2 HRV measures (n = 306).
We assessed effect modification by preexisting conditions (obesity, diabetes, and hypertension), use of antihypertensive medications (β-blockers, calcium channel blockers, ACE inhibitors), and smoking status (never compared with ever smokers). Diabetes was defined as fasting blood glucose of ≥126 mg/dL, a physician's diagnosis of type 2 diabetes, or use of a diabetes medication. For obesity, BMI of 30 was used as the cutoff. Hypertension was defined as reported use of hypertension medication, systolic blood pressure of ≥140 mm Hg, or diastolic blood pressure of ≥90 mm Hg. To test for multiplicative interaction, the main effect terms for these dichotomous dietary variables and the modifying factor, along with the cross-product term, were included in the model. All statistical analyses were performed with the use of SAS software (version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
The distribution of the characteristics of the participants at their first visit for the HRV measurement by quartile of total fruit and vegetable intake (n = 586) is shown in Table 1. Persons who consumed fruit and vegetables more frequently were older and were less likely to drink alcohol (P for trend < 0.05). The proportion of current smokers gradually decreased with consumption of fruit and vegetables (P < 0.01). Statistically significant increasing and decreasing trends were observed in NHF and NLF, respectively, across quartiles of total fruit and vegetable intake. All dietary intake variables adjusted for total energy, including fruit, vegetables, dietary antioxidant nutrients, tuna or dark fish, and n–3 fatty acids, were highly associated with total fruit and vegetable intake. When stratified by quartile of consumption of tuna or dark fish, a significant decreasing trend was observed in heart rate with increasing intake (data not shown). The other trends were similar to those stratified by quartile of total fruit and vegetable intake.
TABLE 1.
Characteristics of study participants at their first visits for heart rate variability measurement (n = 586) by quartile (Q) of fruit and vegetable intake in the Normative Aging Study1
| Total fruit and vegetable intake |
||||
| Q1 (n = 146) | Q2 (n = 147) | Q3 (n = 147) | Q4 (n = 146) | |
| Age (y) | 71.3 ± 6.22 | 73.4 ± 6.8 | 72.8 ± 6.7 | 74.4 ± 6.83 |
| BMI (kg/m2) | 27.9 ± 3.8 | 28.4 ± 3.7 | 28.2 ± 4.5 | 27.9 ± 4.5 |
| SBP (mm Hg) | 128.5 ± 15.7 | 130.1 ± 16.1 | 129.2 ± 16.8 | 130.6 ± 16.4 |
| DBP (mm Hg) | 74.2 ± 9.5 | 74.7 ± 9.7 | 74.2 ± 10.9 | 74.2 ± 9.6 |
| MAP (mm Hg) | 92.3 ± 10.2 | 93.2 ± 10.5 | 92.5 ± 11.8 | 93.0 ± 10.2 |
| Heart rate (beats/min) | 70.8 ± 7.0 | 71.8 ± 7.1 | 71.0 ± 6.6 | 70.2 ± 7.3 |
| Fasting glucose (mg/dL) | 108.7 ± 23.2 | 107.3 ± 24.0 | 106.5 ± 30.0 | 108.7 ± 31.6 |
| Total cholesterol (mg/dL) | 193.3 ± 38.3 | 197.5 ± 35.2 | 189.0 ± 36.9 | 195.3 ± 40.8 |
| HDL (mg/dL) | 48.4 ± 13.1 | 51.2 ± 15.2 | 48.9 ± 12.5 | 49.8 ± 12.3 |
| Triglyceride (mg/dL) | 134.3 ± 63.4 | 132.6 ± 77.1 | 123.2 ± 62.8 | 130.7 ± 76.2 |
| Physical activity (kcal/wk)4 | 2116 ± 2045 | 2062 ± 1610 | 2508 ± 1971 | 2891 ± 2120 |
| Use of multivitamin (%)5 | 45.5 | 55.3 | 51.2 | 57.4 |
| Smoking status (%) | ||||
| Never smoker | 24.0 | 26.5 | 36.1 | 30.1 |
| Former smoker | 66.4 | 66.7 | 61.2 | 69.2 |
| Current smoker | 9.6 | 6.8 | 2.7 | 0.7 |
| Alcohol intake ≥ 2 drinks/d (%) | 23.3 | 21.8 | 16.3 | 15.16 |
| Diabetes mellitus (%) | 21.9 | 21.8 | 15.7 | 21.9 |
| Obesity (%) | 21.9 | 28.6 | 27.9 | 25.3 |
| Hypertension (%) | 69.9 | 67.4 | 69.4 | 73.3 |
| Use of β-blocker (%) | 37.7 | 24.5 | 36.1 | 37.7 |
| Use of calcium channel blocker (%) | 13.7 | 16.3 | 8.8 | 15.8 |
| Use of ACE inhibitor (%) | 23.3 | 21.1 | 18.4 | 26.7 |
| Heart rate variability | ||||
| SDNN (ms) | 42.0 (36.0)7 | 37.4 (31.0) | 36.6 (32.0) | 38.5 (34.0) |
| HF (ms2) | 316 (75) | 245 (62) | 234 (71) | 317 (72.5) |
| LF (ms2) | 252 (108) | 171 (88) | 171 (88) | 191 (73) |
| NHF (%) | 43.3 (41.2) | 43.3 (38.2) | 46.3 (44.7) | 49.9 (44.3)6 |
| NLF (%) | 56.8 (58.8) | 56.7 (62.0) | 53.6 (55.3) | 49.9 (54.9)6 |
| LF:HF | 2.17 (1.43) | 2.16 (1.63) | 2.26 (1.24) | 1.63 (1.24) |
| Dietary intake8 | ||||
| Total fruit and vegetables (servings/d) | 3.7 (1.2) | 5.0 (0.58) | 6.5 (0.90) | 8.7 (2.1)9 |
| Total fruit (servings/d) | 1.4 (0.75) | 2.3 (0.93) | 2.8 (1.0) | 3.7 (1.5)9 |
| Total vegetables (servings/d) | 2.0 (0.95) | 2.8 (0.80) | 3.6 (1.2) | 4.9 (1.8)9 |
| Total noncitrus fruit (servings/d) | 0.61 (0.62) | 1.2 (0.70) | 1.5 (0.77) | 2.2 (0.94)9 |
| Total citrus fruit (servings/d) | 0.62 (0.72) | 0.91 (0.66) | 1.1 (0.72) | 1.2 (0.72)9 |
| Cruciferous vegetables (servings/d) | 0.19 (0.23) | 0.29 (0.20) | 0.33 (0.23) | 0.50 (0.45)9 |
| Green leafy vegetables (servings/d) | 0.12 (0.15) | 0.22 (0.18) | 0.26 (0.26) | 0.46 (0.41)9 |
| Vitamin C (mg/d) | 156 (227) | 218 (266) | 242 (288) | 297 (441)9 |
| Carotenoids (IU/d) | 4993 (3508) | 7003 (3114) | 8970 (4401) | 13475 (8227)9 |
| Tuna or dark fish (servings/d) | 0.14 (0.12) | 0.16 (0.13) | 0.19 (0.14) | 0.24 (0.21)9 |
| n–3 Fatty acids (g/d) | 0.23 (0.16) | 0.27 (0.17) | 0.30 (0.18) | 0.38 (0.33)9 |
| Total energy (kcal/d) | 1937 (915) | 1732 (856) | 1900 (816) | 1944 (981) |
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; ACE, angiotensin-converting enzyme; SDNN, SD of normal-to-normal intervals; HF, high frequency; LF, low frequency; NHF, normalized high-frequency power; NLF, normalized low-frequency power; LF:HF, ratio of low to high frequency.
Mean ± SD (all such values).
P for trend < 0.001.
Reduced numbers of subjects with physical activity because of missing information, from the left column to the right, were 113, 120, 125, and 118.
Reduced numbers of subjects with multivitamin intake because of missing information, from the left column to the right, were 110, 123, 123, and 115.
P for trend < 0.05.
Mean; median in parentheses (all such values).
Median (adjusted for energy except for total energy); interquartile range in parentheses (all such values).
P for trend < 0.0001.
The associations of intakes of fruit, vegetables, composite fruit and vegetable groups (noncitrus fruit, citrus fruit, cruciferous vegetables, and green leafy vegetables), and dietary antioxidant nutrients (vitamin C and total carotenoids) with NHF and NLF are shown in Table 2. See Table S1 under “Supplemental Data” in the online issue for the results of SDNN and LF:HF. After adjustment for potential confounders, including physical activity and vitamin supplements, green leafy vegetable intake was positively associated with NHF (P for trend: 0.04) and inversely associated with NLF (P = 0.03). Noncitrus fruit intake and total fruit and vegetable intake were also borderline significantly associated with increasing trends in NHF and decreasing trends in NLF (model 1), but those associations were no longer observed after controlling for physical activity and vitamin supplement use (model 2). No associations were found between HRV and intakes of citrus fruit, cruciferous vegetables, or dietary vitamin C and carotenoid.
TABLE 2.
Adjusted heart rate variability according to intakes of fruit and vegetables by quartile (Q)1
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Fruit and vegetables | |||||
| Median (servings/d) | 3.67 | 5.05 | 6.50 | 8.72 | |
| Model 1 [n (total obs)]2 | 146 (218) | 147 (249) | 147 (232) | 146 (229) | |
| NHF (%)3 | 49.1 (1.83) | 47.2 (1.80) | 49.9 (1.89) | 52.1 (1.94) | 0.08 |
| NLF (%)3 | 51.0 (1.83) | 52.8 (1.80) | 49.8 (1.89) | 47.7 (1.94) | 0.06 |
| Model 2 [n (total obs)]4 | 110 (172) | 119 (210) | 122 (200) | 115 (185) | |
| NHF (%)3 | 48.9 (2.08) | 46.6 (1.96) | 49.4 (2.03) | 50.1 (2.14) | 0.38 |
| NLF (%)3 | 51.1 (2.08) | 53.3 (1.96) | 50.5 (2.03) | 49.6 (2.14) | 0.33 |
| Fruit | |||||
| Median (servings/d) | 1.33 | 2.11 | 2.85 | 3.98 | |
| Model 1 [n (total obs)]2 | 146 (235) | 147 (229) | 147 (232) | 146 (232) | |
| NHF (%)3 | 47.8 (1.81) | 49.8 (1.85) | 50.6 (1.89) | 49.5 (1.95) | 0.38 |
| NLF (%)3 | 52.2 (1.81) | 50.2 (1.85) | 49.4 (1.89) | 50.2 (1.95) | 0.36 |
| Model 2 [n (total obs)]4 | 114 (194) | 115 (182) | 121 (201) | 116 (190) | |
| NHF (%)3 | 47.2 (2.03) | 48.8 (2.02) | 49.6 (2.05) | 49.2 (2.13) | 0.39 |
| NLF (%)3 | 52.7 (2.02) | 51.2 (2.02) | 50.4 (2.04) | 50.5 (2.13) | 0.34 |
| Vegetables | |||||
| Median (servings/d) | 1.89 | 2.75 | 3.65 | 5.20 | |
| Model 1 [n (total obs)]2 | 146 (229) | 147 (232) | 147 (236) | 146 (231) | |
| NHF (%)3 | 47.9 (1.82) | 48.6 (1.83) | 50.9 (1.88) | 50.5 (1.91) | 0.13 |
| NLF (%)3 | 52.2 (1.82) | 51.3 (1.83) | 49.0 (1.88) | 49.2 (1.91) | 0.10 |
| Model 2 [n (total obs)]4 | 117 (192) | 117 (194) | 112 (186) | 120 (195) | |
| NHF (%)3 | 47.1 (2.00) | 48.5 (2.02) | 50.7 (2.06) | 48.3 (2.07) | 0.44 |
| NLF (%)3 | 52.9 (2.00) | 51.4 (2.02) | 49.1 (2.05) | 51.5 (2.07) | 0.38 |
| Noncitrus fruit | |||||
| Median (servings/d) | 0.50 | 1.08 | 1.62 | 2.39 | |
| Model 1 [n (total obs)]2 | 146 (225) | 147 (234) | 147 (246) | 146 (223) | |
| NHF (%)3 | 48.3 (1.78) | 46.9 (1.86) | 51.6 (1.86) | 51.2 (1.96) | 0.05 |
| NLF (%)3 | 51.6 (1.78) | 53.0 (1.86) | 48.5 (1.87) | 48.6 (1.96) | 0.05 |
| Model 2 [n (total obs)]4 | 113 (183) | 117 (190) | 118 (205) | 118 (189) | |
| NHF (%)3 | 47.9 (1.99) | 46.1 (2.03) | 51.2 (2.03) | 49.8 (2.13) | 0.17 |
| NLF (%)3 | 52.0 (1.99) | 53.8 (2.03) | 48.9 (2.03) | 50.0 (2.12) | 0.17 |
| Citrus fruit | |||||
| Median (servings/d) | 0.35 | 0.79 | 1.13 | 1.60 | |
| Model 1 [n (total obs)]2 | 146 (231) | 147 (233) | 147 (230) | 146 (234) | |
| NHF (%)3 | 48.5 (1.86) | 49.8 (1.83) | 49.6 (1.86) | 49.2 (1.93) | 0.81 |
| NLF (%)3 | 51.4 (1.86) | 50.0 (1.83) | 50.5 (1.86) | 50.4 (1.93) | 0.71 |
| Model 2 [n (total obs)]4 | 109 (185) | 119 (197) | 119 (188) | 119 (197) | |
| NHF (%)3 | 46.8 (2.08) | 49.0 (1.98) | 50.2 (2.04) | 48.2 (2.10) | 0.48 |
| NLF (%)3 | 53.3 (2.07) | 50.8 (1.97) | 49.8 (2.03) | 51.5 (2.09) | 0.42 |
| Cruciferous vegetables | |||||
| Median (servings/d) | 0.11 | 0.25 | 0.37 | 0.62 | |
| Model 1 [n (total obs)]2 | 146 (236) | 147 (242) | 147 (222) | 146 (228) | |
| NHF (%)3 | 48.8 (1.87) | 48.0 (1.81) | 51.5 (1.83) | 48.8 (1.92) | 0.61 |
| NLF (%)3 | 51.2 (1.87) | 52.0 (1.81) | 48.4 (1.83) | 51.0 (1.92) | 0.55 |
| Model 2 [n (total obs)]4 | 123 (207) | 114 (194) | 113 (179) | 116 (187) | |
| NHF (%)3 | 48.9 (1.99) | 47.1 (1.99) | 51.6 (2.06) | 47.0 (2.09) | 0.86 |
| NLF (%)3 | 51.1 (1.99) | 52.8 (1.99) | 48.3 (2.05) | 52.9 (2.08) | 0.91 |
| Green leafy vegetables | |||||
| Median (servings/d) | 0.07 | 0.18 | 0.31 | 0.56 | |
| Model 1 [n (total obs)]2 | 146 (232) | 147 (225) | 147 (233) | 146 (238) | |
| NHF (%)3 | 46.5 (1.86) | 48.0 (1.82) | 51.7 (1.84)5 | 51.2 (1.88)5 | 0.008 |
| NLF (%)5 | 53.6 (1.86) | 51.8 (1.82) | 48.1 (1.84)5 | 48.7 (1.88)5 | 0.007 |
| Model 2 [n (total obs)]4 | 110 (188) | 114 (179) | 123 (201) | 119 (199) | |
| NHF (%)3 | 45.9 (2.04) | 47.7 (2.04) | 50.7 (1.99)5 | 50.1 (2.05)6 | 0.04 |
| NLF (%)3 | 54.1 (2.03) | 52.1 (2.04) | 49.2 (1.99)5 | 49.7 (2.05)6 | 0.03 |
| Vitamin C | |||||
| Median (mg/d) | 112 | 189 | 302 | 713 | |
| Model 1 [n (total obs)]2 | 141 (222) | 141 (228) | 142 (222) | 141 (230) | |
| NHF (%)3 | 47.0 (1.90) | 49.5 (1.86) | 50.8 (1.91)6 | 48.5 (1.87) | 0.42 |
| NLF (%)3 | 52.9 (1.90) | 50.5 (1.86) | 49.1 (1.91)6 | 51.4 (1.87) | 0.42 |
| Model 2 [n (total obs)]4 | 113 (188) | 119 (197) | 118 (189) | 116 (193) | |
| NHF (%)3 | 46.6 (2.23) | 48.8 (1.99) | 50.6 (2.14) | 48.5 (2.03) | 0.48 |
| NLF (%)3 | 53.3 (2.22) | 51.1 (1.98) | 49.3 (2.13) | 51.5 (2.03) | 0.49 |
| Carotenoids | |||||
| Median (IU/d) | 3845 | 6815 | 9703 | 14277 | |
| Model 1 [n (total obs)]2 | 141 (225) | 141 (217) | 142 (242) | 141 (218) | |
| NHF (%)3 | 47.9 (1.82) | 48.7 (1.90) | 50.8 (1.90) | 48.7 (1.94) | 0.50 |
| NLF (%)3 | 52.1 (1.82) | 51.3 (1.90) | 49.1 (1.90) | 51.1 (1.94) | 0.44 |
| Model 2 [n (total obs)]4 | 116 (193) | 110 (175) | 117 (203) | 123 (196) | |
| NHF (%)3 | 47.9 (2.02) | 47.6 (2.08) | 50.7 (2.05) | 48.5 (2.06) | 0.53 |
| NLF (%)3 | 52.1 (2.02) | 52.3 (2.07) | 49.2 (2.04) | 51.4 (2.05) | 0.49 |
Each dietary intake was adjusted first for energy and then was categorized into quartiles (residual method). NHF, normalized high-frequency power; NLF, normalized low-frequency power; obs, observations.
Adjusted for age, BMI, fasting blood glucose, mean arterial pressure, cigarette smoking, alcohol consumption, use of β-blockers, use of calcium channel blockers, and use of angiotensin-converting enzyme inhibitors.
Values are adjusted means; SEs in parentheses.
As in model 1 with additional adjustment for use of multivitamins and physical activity.
Significantly different from subjects in quartile 1: 5P < 0.05, 6P < 0.1.
To examine whether the significant associations between green leafy vegetable intake and HRV were mediated by dietary folate and α-linolenic acid (18:3n–3), we included these variables in final models. These did not change the results (data not shown).
Adjusted associations between NHF and NLF and intakes of tuna or dark fish and n–3 fatty acids are shown in Table 3. See Table S2 under “Supplemental Data” in the online issue for the results of SDNN and LF:HF. Intakes of dark fish and n–3 fatty acids were not statistically associated with HRV.
TABLE 3.
Adjusted heart rate variability according to intakes of tuna or dark fish and n–3 fatty acids by quartile (Q)1
| Q1 | Q2 | Q3 | Q4 | P for trend | |
| Tuna or dark fish | |||||
| Median (servings/d) | 0.06 | 0.14 | 0.21 | 0.35 | |
| Model 1 [n (total obs)]2 | 146 (227) | 147 (235) | 147 (236) | 146 (230) | |
| NHF (%)3 | 49.8 (1.87) | 48.5 (1.80) | 47.9 (1.87) | 51.2 (1.88) | 0.57 |
| NLF (%)3 | 50.2 (1.87) | 51.4 (1.80) | 52.1 (1.87) | 48.6 (1.88) | 0.54 |
| Model 2 [n (total obs)]4 | 113 (188) | 117 (190) | 117 (194) | 119 (195) | |
| NHF (%)3 | 48.6 (2.09) | 48.5 (1.95) | 46.4 (2.05) | 50.9 (2.05) | 0.51 |
| NLF (%)3 | 51.4 (2.09) | 51.4 (1.95) | 53.6 (2.04) | 48.9 (2.04) | 0.47 |
| n–3 Fatty acids | |||||
| Median (g/d) | 0.14 | 0.25 | 0.33 | 0.57 | |
| Model 1 [n (total obs)]2 | 146 (228) | 147 (232) | 147 (237) | 146 (231) | |
| NHF (%)3 | 48.9 (1.84) | 49.8 (1.87) | 47.2 (1.87) | 51.2 (1.84) | 0.53 |
| NLF (%)3 | 51.1 (1.84) | 50.1 (1.87) | 52.8 (1.87) | 48.6 (1.84) | 0.49 |
| Model 2 [n (total obs)]4 | 117 (193) | 117 (189) | 116 (193) | 116 (192) | |
| NHF (%)3 | 48.7 (2.02) | 48.7 (2.08) | 45.9 (2.05) | 50.7 (2.01) | 0.66 |
| NLF (%)3 | 51.4 (2.01) | 51.0 (2.08) | 54.1 (2.05) | 49.1 (2.00) | 0.61 |
Each dietary intake was adjusted first for energy and then categorized into quartiles (residual method). NHF, normalized high-frequency power; NLF, normalized low-frequency power; obs, observations.
Adjusted for age, BMI, fasting blood glucose, mean arterial pressure, cigarette smoking, alcohol consumption, use of β-blockers, use of calcium channel blockers, and use of angiotensin-converting enzyme inhibitors.
Values are adjusted means; SEs in parentheses.
As in model 1 with additional adjustment for use of multivitamins and physical activity.
Further adjustment for resting heart rate in the models of all dietary variables did not change the results. In a sensitivity analysis using subjects with ≥2 HRV measurements, the overall trends were similar but less significant. No statistically significant associations were found even in green leafy vegetables (see Table S3 under “Supplemental Data” in the online issue).
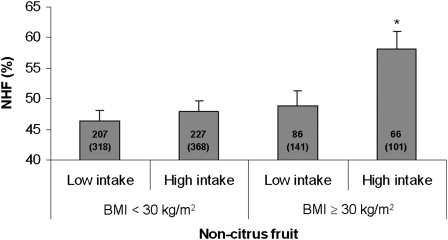
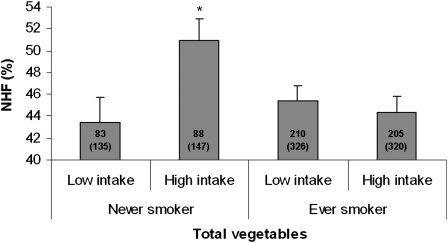
We assessed whether the associations of fruit, vegetable, and dark fish intakes with HRV measures in model 2 were modified by preexisting conditions, use of antihypertensive medication, or smoking status. To increase the number of subjects in each category, we combined 2 lower quartiles and 2 upper quartiles and compared low with high intake of dietary variables. We found that the high-intake group of noncitrus fruit had a significantly higher NHF than did the low-intake group only among obese persons; no difference was observed between the low- and high-intake groups among nonobese subjects (P for interaction: 0.04; Figure 1). Similar effect modification by obesity was observed in association with NLF (data not shown). We also found statistically significant interactions (P = 0.02–0.04) between smoking status and intakes of total vegetables and cruciferous vegetables for NHF and NLF. For brevity, we present the associations between NHF and total vegetable intakes by smoking status in Figure 2. In this case, a beneficial association with total vegetables intake was observed only among never smokers. No apparent modification was observed of dietary associations by diabetes, hypertension, or use of β-blockers, calcium channel blockers, or ACE inhibitors or in relation to intakes of other fruit and vegetables, tuna or dark fish, and n–3 fatty acids (data not shown).
FIGURE 1.
Adjusted mean (±SE) of the normalized high frequency (NHF) of heart rate variability by consumption of noncitrus fruit and obesity [BMI (in kg/m2) ≥ 30 or <30]. The median value for calorie-adjusted noncitrus fruit consumption (1.34 servings/d) was used to divide participants into low- and high-intake groups. The numbers inside the bars indicate the number of subjects (total observations) in each category. The model was adjusted for age, fasting blood glucose, mean arterial pressure, cigarette smoking, alcohol consumption, use of β-blockers, use of calcium channel blockers, use of angiotensin-converting enzyme inhibitors, use of multivitamins, and physical activity. *Significantly different from the low-intake group, P < 0.005.
FIGURE 2.
Adjusted mean (±SE) of normalized high-frequency (NHF) of heart rate variability by consumption of total vegetables and smoking status. The median value for calorie-adjusted total vegetable consumption (3.08 servings/d) was used to divide participants into low- and high-intake groups. The numbers inside the bars indicate the number of subjects (total observations) in each category. The model was adjusted for age, BMI, fasting blood glucose, mean arterial pressure, alcohol consumption, use of β-blockers, use of calcium channel blockers, use of angiotensin-converting enzyme inhibitors, use of multivitamins, and physical activity. *Significantly different from the low-intake group, P < 0.02.
DISCUSSION
In this community-based, longitudinal study of older men, we found that high intakes of green leafy vegetables may reduce the risk of CVD through favorable changes in cardiac autonomic function. Positive associations with NHF and inverse associations with NLF and LF:HF suggest that high consumption of fruit and vegetables may enhance the parasympathetic (vagal) response and may reduce the sympathetic response, which prevents future sudden cardiac death and arrhythmia (25, 26). We believe that this is the first study to evaluate the association between the intakes of fruit, vegetables, and dietary antioxidant nutrients and HRV.
The strongest associations we observed were between green leafy vegetables and HRV. Green leafy vegetables such as spinach and collards are widely considered healthful foods abundant in vitamin C, carotenoids, folate, and α-linolenic acid (27). Few studies have reported the association between green leafy vegetable consumption and cardiovascular incidence or mortality, but those that did have consistently shown strong associations. The Nurses' Health Study, which followed >80,000 women for 14 y, and the Health Professionals' Follow-Up Study, which included >40,000 men followed for 8 y, showed that a 1-serving/d increment in green leafy vegetable consumption showed a relative risk of 0.77 (95% CI: 0.64, 0.93) for coronary heart disease (28). Hung et al (29) found a relative risk of 0.89 (95% CI: 0.83, 0.96) for CVD in relation to a 1-serving/d increase in green leafy vegetables in the same cohort. A case-control study conducted in India observed a significant dose-dependent inverse association; subjects who consumed >3 servings/wk of green leafy vegetables had a 66% lower risk of ischemic heart disease than did subjects who consumed <1 serving/wk (30).
Our findings suggest that these cardioprotective effects of green leafy vegetable intake may be acting through a mechanistic pathway mediated by increased vagal and decreased sympathetic activities. It is not clear what specific substances in green leafy vegetables have beneficial effects. In our study, subjects in the third quartile of vitamin C intake had borderline significantly higher parasympathetic HRV measures and lower sympathetic HRV measures than did subjects in the lowest quartile, but those beneficial effects were reduced in the highest quartile. Because that specific vitamin did not exhibit the same pattern as observed for our intake variables, several components of green leafy vegetables may act to reduce the risk of cardiac sudden death, and recommending more frequent intake of these foods is appropriate.
We found no association of HRV with intake of dark oily fish or n–3 fatty acids. This finding is inconsistent with previous evidence that high frequency of dark oily fish or n–3 fatty acid intake is associated with favorable changes in HRV (14–17). Potential mechanisms by which dark oily fish intake may increase HRV and thus reduce the risk of CVD include antiarrhythmic, hypotriglyceridemic and antithrombogenic effects, inhibition of atherosclerotic plaque growth, and promotion of nitric oxide–induced endothelial relaxation (11, 31). A recent population-based cohort of older US adults showed that consumption of tuna or other broiled or baked fish was associated with increased NHF and reduced NLF, LF:HF ratio, as well as measures of erratic heart rate patterns [detrended fluctuation analysis for short-term R-R intervals (DFA1) and Poincaré ratio] (17). They also found similar associations with dietary n–3 fatty acids. It is not clear why we did not observe these associations in the NAS cohort. Misclassification of exposure, because of limitations in the questions about type of fish, along with small sample size, may have limited our ability to detect associations. However, this FFQ has been used to assess n–3 fatty acids successfully in other studies (32). Another possibility is that the consumption of n–3 fatty acids from dark oily fish in this population might be too low to allow us to detect a significant association. In fact, no differences in consumption of n–3 fatty acids and dark oily fish on HRV were seen through the third quartile (median: 0.33 g/d and 0.21 serving/d, respectively), but a tendency toward beneficial measures appeared in the highest quartile (median: 0.57 g/d and 0.35 serving/d, respectively). We could not evaluate how higher intakes of n–3 fatty acids, as used in several randomized controlled trials (≥2 g/d of fish oil, with 1.5 g of EPA + DHA), associate with HRV (14–16), because of the small number of subjects in this range (n = 7). However, the aforementioned US population–based study found significant associations in a similar range of the intake of n–3 fatty acids in NAS (17).
Obesity modified the association between noncitrus fruit and NHF and NLF (significant only in those with BMI ≥ 30). This may be because obese subjects were more likely to have low NHF and high NLF and therefore had greater ranges in these variables. Therefore, the antioxidant (or other) protective properties of noncitrus fruit are more easily detected in this higher risk group. However, we found that beneficial effects of intake of total vegetables or cruciferous vegetables appeared only among never smokers, but not among ever smokers. This might be because autonomic imbalance because of tobacco smoke is too high to be modified by dietary intake of vegetables. However, because of multiple comparisons, our results should be viewed with caution until replicated in future studies.
Important strengths of this study include the community-based longitudinal design with a large number of subjects, providing sufficient power to detect small changes in HRV measures. Because this is an older population and some participants died before the second visit, not all subjects had repeated measures of HRV. However, more than one-half of participants had repeated measures that allowed us to adjust for subject-specific variations in HRV with the use of longitudinal analyses. We treated age, BMI, fasting blood glucose, blood pressure, smoking status, alcohol consumption, and use of medications as time-dependent confounding factors, affording better control for them relative to cross-sectional studies. In addition, repeated measures of the FFQ allowed us to calculate cumulative average intakes. Use of the cumulative averages is more likely to reduce measurement error because of intraindividual variations over time and is more relevant causatively than is using either baseline only or most recent diet (23).
This study has several limitations. We cannot exclude the possibility that the observed associations are attributable to other factors that correlate with fruit and vegetable intakes. Persons who eat more fruit and vegetables are less likely to be overweight or obese or to be cigarette smokers (33, 34). However, regression analyses with and without BMI or cigarette smoking or both did not provide different results (data not shown). Further, all analyses were adjusted for total energy intake. In addition, because HRV is a sensitive marker, it can be influenced by environmental factors, such as air pollution. Our group has reported a significant association between ambient particle exposure and HRV (35). Additional adjustment for ambient particle exposure did not change the results. We cannot rule out uncontrolled confounding by other nonmeasured variables, and some of our findings may have occurred by chance. However, with few exceptions, the pattern of results presented here are consistent with our hypotheses. Finally, our findings may not be generalizable to women. This study cohort consists of all men, and sex may be an important effect modifier in association of intake of fruit, vegetables, and fish with CVD (36).
Our results suggest that consumption of fruit and vegetables, in particular green leafy vegetables, may have beneficial effects on cardiac autonomic dysfunction. It is well known that a healthy diet is an important way to prevent CVD. Although more research is needed, our data suggest that diets high in fruit and vegetables may help to prevent sudden cardiac death and arrhythmia through favorable changes in HRV.
Supplementary Material
Acknowledgments
We thank Elaine R Dibbs and Jordan D Awerbach for their invaluable assistance in conducting the HRV measurements and other contributions to the VA Normative Aging Study.
The authors' responsibilities were as follows—SKP and JS: conceived and designed the research; DS and PSV: acquired the data; SKP, KLT, MSO, HH, and JS: analyzed and interpreted the data; SKP: performed statistical analysis; HH and JS: handled funding and supervision; SKP: drafted the manuscript; and KLT, MSO, DS, HH, and JS: provided critical revisions of the manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Allender S, Scarborough P, Peto V, et al. European cardiovascular disease statistics. Brussels, Belgium: European Heart Network, 2008 [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–146 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Cardiovascular disease: prevention and control. 2008. Available from: http://www.who.int/dietphysicalactivity/publications/facts/cvd/en/ (cited 24 June 2008)
- 4.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 5.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 2004;160:1223–33 [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Branch LG, Lipnick RJ, et al. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am J Clin Nutr 1985;41:32–6 [DOI] [PubMed] [Google Scholar]
- 7.Bazzano LA, He J, Ogden LG, et al. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am J Clin Nutr 2002;76:93–9 [DOI] [PubMed] [Google Scholar]
- 8.Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr 2004;80:1508–20 [DOI] [PubMed] [Google Scholar]
- 9.Nam CM, Oh KW, Lee KH, et al. Vitamin C intake and risk of ischemic heart disease in a population with a high prevalence of smoking. J Am Coll Nutr 2003;22:372–8 [DOI] [PubMed] [Google Scholar]
- 10.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Carotenoids and cardiovascular health. Am J Clin Nutr 2006;83:1265–71 [DOI] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 12.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n–3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n–3 fish oils. Circulation 2003;107:2646–52 [DOI] [PubMed] [Google Scholar]
- 13.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–65 [PubMed] [Google Scholar]
- 14.Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content of blood cell membranes: a dose-response study with n–3 fatty acids. Am J Clin Nutr 1999;70:331–7 [DOI] [PubMed] [Google Scholar]
- 15.Holguin F, Tellez-Rojo MM, Lazo M, et al. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest 2005;127:1102–7 [DOI] [PubMed] [Google Scholar]
- 16.Villa B, Calabresi L, Chiesa G, Rise P, Galli C, Sirtori CR. Omega-3 fatty acid ethyl esters increase heart rate variability in patients with coronary disease. Pharmacol Res 2002;45:475–8 [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish and omega-3 fatty acid consumption and heart rate variability in US adults. Circulation 2008;117:1130–7 [DOI] [PubMed] [Google Scholar]
- 18.Peeters E, Neyt A, Beckers F, De Smet S, Aubert AE, Geers R. Influence of supplemental magnesium, tryptophan, vitamin C, and vitamin E on stress responses of pigs to vibration. J Anim Sci 2005;83:1568–80 [DOI] [PubMed] [Google Scholar]
- 19.Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, Gonzalez-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim Biophys Acta 2005;1725:305–13 [DOI] [PubMed] [Google Scholar]
- 20.Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist 1966;6:179–84 [DOI] [PubMed] [Google Scholar]
- 21.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 22.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26(discussion 1127–36) [DOI] [PubMed] [Google Scholar]
- 23.Willett WC. Issues in analysis and presentation of dietary data. Willett WC. Nutritional epidemiology. 2nd ed New York, NY: Oxford University Press, 1998:321–46 [Google Scholar]
- 24.Willett WC, Stampfer MJ. Implications of total energy intake for epidemiologic analyses. Willett WC. Nutritional epidemiology. 2nd ed New York, NY: Oxford University Press, 1998:273–301 [Google Scholar]
- 25.Tsuji H, Larson MG, Venditti FJ, et al. Impact of reduced heart rate variability on risk for cardiac events: The Framingham Heart Study. Circulation 1996;94:2850–5 [DOI] [PubMed] [Google Scholar]
- 26.Tapanainen JM, Thomsen PE, Kober L, et al. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol 2002;90:347–52 [DOI] [PubMed] [Google Scholar]
- 27.Van Duyn MA, Pivonka E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. J Am Diet Assoc 2000;100:1511–21 [DOI] [PubMed] [Google Scholar]
- 28.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 2001;134:1106–14 [DOI] [PubMed] [Google Scholar]
- 29.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst 2004;96:1577–84 [DOI] [PubMed] [Google Scholar]
- 30.Rastogi T, Reddy KS, Vaz M, et al. Diet and risk of ischemic heart disease in India. Am J Clin Nutr 2004;79:582–92 [DOI] [PubMed] [Google Scholar]
- 31.Connor WE. Importance of n–3 fatty acids in health and disease. Am J Clin Nutr 2000;71(suppl):171S–5S [DOI] [PubMed] [Google Scholar]
- 32.Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol 2006;63:1545–50 [DOI] [PubMed] [Google Scholar]
- 33.Galan P, Viteri FE, Bertrais S, et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr 2005;59:1181–90 [DOI] [PubMed] [Google Scholar]
- 34.Wallstrom P, Wirfalt E, Lahmann PH, Gullberg B, Janzon L, Berglund G. Serum concentrations of beta-carotene and α-tocopherol are associated with diet, smoking, and general and central adiposity. Am J Clin Nutr 2001;73:777–85 [DOI] [PubMed] [Google Scholar]
- 35.Park SK, O'Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect 2005;113:304–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Nagata C, Oba S, Takatsuka N, Shimizu H. Fruit and vegetable intake and mortality from cardiovascular disease are inversely associated in Japanese women but not in men. J Nutr 2008;138:1129–34 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.