Abstract
Background: The extent to which adipose tissue (AT) distribution is different between persons with type 2 diabetes (T2DM) and nondiabetic control subjects remains unclear.
Objective: The aim of this study was to establish whether total body adiposity and its distribution, quantified by using state-of-the-art whole-body magnetic resonance imaging, differs between these 2 groups.
Design: This cross-sectional evaluation included 93 participants (n = 56 women and 37 men) in the Look AHEAD (Action for HEAlth in Diabetes) Trial with T2DM who had a mean (±SD) age of 58.3 ± 6.6 y and body mass index (in kg/m2) of 31.6 ± 3.1 and 93 healthy non-T2DM control subjects (n = 64 women and 29 men) who had a mean (±SD) age of 60.6 ± 17.1 y and body mass index of 29.6 ± 3.0. All participants self-reported being of African American or white ancestry. Magnetic resonance imaging–derived in vivo measures of total-body AT (TAT) and its distribution, subcutaneous AT (SAT), visceral AT (VAT), and intermuscular AT (IMAT) were acquired. Linear regression models were developed for each AT compartment to adjust for important covariates of race, sex, age, height, and weight and to examine potential interactions of covariates.
Results: These models showed significantly less SAT (African American: −1.2 kg; white: −2.4 kg; both P = 0.001), including less femoral-gluteal SAT, more VAT (African American: 0.7 kg, P < 0.001; white: 1.8 kg, P = 0.007), and more IMAT (0.5 kg, P = 0.001) in the T2DM group.
Conclusion: We concluded that AT distribution is significantly altered in T2DM, ie, more VAT and IMAT—2 depots known to exacerbate insulin resistance—and less SAT in persons with T2DM than in healthy control subjects, a novel finding that we posit may compound the risk of insulin resistance.
INTRODUCTION
Most individuals with type 2 diabetes mellitus (T2DM) are overweight or obese. Obesity is a well-recognized risk factor for T2DM, especially if there is an upper-body-fat distribution pattern. Yet, apart from this general precept, there is no clearly delineated body habitus that is distinctive for individuals with T2DM, and it remains uncertain whether there are differences in body composition associated with this metabolic disease. Most, but not all studies indicate greater visceral adipose tissue (VAT) in T2DM, whether in adolescents, young to middle-aged adults, or the elderly (1). Most studies have found that VAT is strongly related to insulin resistance (IR) in T2DM, and that this relation also pertains to those without diabetes (2–4).
Some studies report increased fat content within liver and skeletal muscle in T2DM and perhaps it is these relatively small ectopic fat depots that are crucial to IR, rather than differences in adipose tissue distribution (5–7). Several recent studies have found that gluteal-femoral adiposity is less in persons with T2DM than in those without T2DM, despite a similar or overall increase in fat mass (8–12), and has led to the postulation that a relative decrement in a “metabolically-protective” depot, a role ascribed to gluteal-femoral adiposity, adds to the risk exerted by the accumulation of VAT. However, diminished gluteal-femoral adiposity has been shown by dual-energy X-ray absorptiometry (DXA) imaging—a modality that cannot discriminate between the layers or subcompartments of AT within regions (13). Lower-extremity AT consists of 2 compartments: subcutaneous AT (SAT), which account for the majority of AT, and AT interspersed within and surrounding skeletal muscle and yet located beneath a surrounding layer of muscle fascia. Of pertinence to IR, this subfascial AT of the lower extremity, commonly referred to as intermuscular AT (IMAT), is correlated with IR even though the large depot of lower-extremity SAT is not well correlated and has been observed to mitigate the risk of obesity-related IR (14). The method primarily used to assess IMAT is single cross-sectional computed tomography imaging (14). Although a more precise sense of the overall volume of IMAT in healthy persons has been documented (15), the amount in T2DM is unclear.
One general postulate is that the usual strong influences of sex on body composition are attenuated in association with T2DM. This concept is consistent with greater central adiposity in T2DM. Compared with men, women have more adiposity (16) and less muscle mass (17), adjusted for height and weight, and have proportionally less VAT (16). These differences are attenuated in postmenopausal women (18), and there are strong indications that this sex difference in VAT is less in T2DM (19). Moreover, women with T2DM may have proportionally less SAT than do nondiabetic women (20). Little is known about body composition in men with T2DM.
The goals of the current study were to use whole-body magnetic resonance imaging (MRI) (21) to obtain depot-specific quantitative determinations of total SAT, VAT, and IMAT in a cohort of overweight and obese adults with T2DM and compare these findings with those in nondiabetic adults. Empirically based linear regression models were formulated to account for the effects of age, race, sex, overall adiposity, height, and weight so that appropriate adjustments could be made before ascertaining potential differences in AT distribution between adults with and without T2DM. This is the first study to compare body composition by whole-body MRI between subjects with T2DM and matched control subjects. The findings confirm the presence of greater central fat deposits with less peripheral adiposity.
SUBJECTS AND METHODS
Type 2 diabetes
Participants enrolled in the Look AHEAD (Action for HEAlth in Diabetes) Trial at the New York and Pittsburgh sites were invited to enroll in this ancillary study after randomization and before initiation of any intervention. Recruitment began in January 2002. This clinical trial of weight loss for the prevention of cardiovascular disease in T2DM [men and women aged 45–74 y with T2DM with a body mass index (BMI; in kg/m2) of ≥25] was described previously (22) and only preintervention or baseline data were used in this analysis
Non–type 2 diabetic control subjects
The subjects were a convenience sample, recruited through advertisements in the New York City local newspapers and flyers posted in the community, who had participated in clinical trials involving healthy participants. This group was compiled from the investigators' (DG and JA) archived databases, and the inclusion criteria were race (white or African American), age ≥18 y, and BMI ≥25 and <38. Each subject underwent a medical examination and provided blood samples after fasting overnight for standard hematology and blood chemistry testing. Subjects with diabetes mellitus, with malignant or catabolic conditions, who had undergone joint replacement, or were currently taking estrogen replacements or medications that could potentially influence body composition were excluded from the study.
Inclusion was restricted to African Americans and whites for both the T2DM and control groups because archived data included these 2 race groups only. Persons who were claustrophobic and who did not fit within the field-of-view for MRI (BMI > 38) were excluded. All studies were approved by the Institutional Review Board of St Luke's–Roosevelt Hospital or the University of Pittsburgh (where appropriate), and all subjects gave written consent to participate.
Body-composition measures
Body weight was measured to the nearest 0.1 kg (Weight Tronix, New York, NY; and Scale-Tronix, Wheaton, IL) and height to the nearest 0.5 cm with a stadiometer (Holtain, Crosswell, United Kingdom).
MRI
Total AT (TAT)—including total SAT, VAT, and IMAT—were measured by using whole-body multislice MRI as previously described (21, 23). Subjects at both sites (New York and Pittsburgh) were placed on a 1.5-T scanner platform (6× Horizon; General Electric, Milwaukee, WI) with their arms extended above their heads. The protocol involved the acquisition of ≈40 axial images, 10 mm in thickness, and at 40-mm intervals across the whole body. SliceOmatic 4.2 image analysis software (Tomovision, Montreal, CA) was used to analyze images on a PC workstation (Gateway, Madison, WI). Estimates of MRI volume were converted to mass by using the assumed density of 0.92 kg/L for AT (24). All T2DM scans were read by the same technician in the Image Analysis Laboratory of the New York Obesity Research Center. The archived scans for the control group had been read by 3 different technicians. The technical errors for 3 repeated readings of the same scan by the same observer for SAT, VAT, and IMAT volumes in our laboratory were 0.96%, 1.97%, and 0.65%, respectively. The intraclass correlation coefficients among the 3 analysts (who each read the same scans twice, separated by a 3-mo interval) for SAT, VAT, and IMAT in our laboratory were 0.99, 0.95, and 0.97, respectively (25).
Total-body SAT was subdivided by a single observer (J-EY) into subcompartments as previously described (26): 1) lower leg AT from the toes to the superior margin of the patella, 2) upper leg AT as femoral-gluteal AT from the superior margin of the patella to the level of the greater trochanter, 3) trunk AT from the level of the greater trochanter to the level of shoulder, and 4) arm AT from the level of the shoulder to the finger tips.
Statistical analysis
General linear models were used to determine whether there were statistically significant differences in TAT and the distribution of its components between the T2DM and control subjects after adjustment for significant covariates, including race, sex, age, height, weight (or TAT), and interactions, as well as quadratic terms for nonlinear components of age, height, and weight (or TAT). Models were developed by using a backward elimination stepwise procedure. Residuals were checked for equal variance and normality. When violations of assumptions for linear models were encountered, the model and the significance and magnitude of the terms were confirmed by 1) excluding a small number of cases that were responsible for violations and rerunning the model, 2) conducting a robust regression, which uses an iteratively reweighted least-squares algorithm to discount the influence of outlying cases (SAS PROC ROBUSTREG; 27).
Descriptive subject data are expressed as means ± SDs. Data were analyzed by using SAS version 9.1 (Statistical Analysis Systems, Cary, NC). Statistical significance was set at P < 0.05, 2-tailed.
RESULTS
Demographic and body-composition characteristics
Subjects with a BMI between 25 and ≤38 were retained in the analysis (n = 186), except as indicated. Demographic characteristics of the subjects and unadjusted mean values for AT compartments, by sex, for the T2DM and control groups are shown in Table 1.
TABLE 1.
Subject characteristics and adipose tissue distribution1
| T2DM group |
Control group |
|||
| Women | Men | Women | Men | |
| (n = 56) | (n = 37) | (n = 64) | (n = 29) | |
| African Americans (n) | 27 | 4 | 37 | 11 |
| Whites (n) | 29 | 33 | 27 | 18 |
| Age (y) | 58.2 ± 6.0 | 58.4 ± 7.6 | 62.8 ± 15.7 | 55.8 ± 19.2 |
| (45–75)2 | (45–75) | (25–88) | (19–83) | |
| Body weight (kg) | 83.7 ± 10.3 | 97.4 ± 10.3 | 77.9 ± 9.3 | 85.6 ± 11.3 |
| (63–106) | (76–118) | (57–97) | (64–109) | |
| Height (m) | 1.62 ± 0.07 | 1.76 ± 0.07 | 1.60 ± 0.06 | 1.75 ± 0.09 |
| (1.48–1.80) | (1.62–1.90) | (1.42–1.74) | (1.55–1.93) | |
| BMI (kg/m2) | 31.8 ± 3.3 | 31.4 ± 2.6 | 30.4 ± 3.0 | 27.8 ± 2.3 |
| (25.7–38.0) | (26.9–36.2) | (25.2–37.0) | (25.0–33.5) | |
| TAT (kg) | 37.8 ± 7.3 | 34.2 ± 7.2 | 35.5 ± 7.3 | 23.0 ± 7.2 |
| (24.7–54.0) | (19.5–51.1) | (22.1–49.5) | (11.8–38.0) | |
| SAT (kg) | 31.5 ± 6.8 | 25.7 ± 6.2 | 31.5 ± 6.6 | 18.8 ± 5.6 |
| (16.5–48.0) | (13.6–44.5) | (19.1–44.3) | (10.0–30.2) | |
| VAT (kg) | 4.1 ± 1.7 | 6.1 ± 1.7 | 2.6 ± 1.3 | 2.9 ± 1.8 |
| (1.1–8.1) | (2.2–9.7) | (0.6–6.0) | (0.6–6.2) | |
| IMAT (kg) | 2.2 ± 0.9 | 2.4 ± 0.8 | 1.5 ± 0.5 | 1.3 ± 0.8 |
| (0.8–4.9) | (1.1–4.5) | (0.7–3.4) | (0.4–4.1) | |
T2DM, type 2 diabetes mellitus; TAT, total adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; IMAT, intramuscular adipose tissue. The fitted model showed significant contributions of race (African Americans compared with whites; P = 0.027), sex (male compared with female; P < 0.001), group (T2DM compared with control; P < 0.001), weight (P < 0.001), and height (P < 0.001) and 2-factor interactions of group with weight (P = 0.001), age (P = 0.020), and sex (P < 0.001) and accounted for 78% of the variance in TAT. Because of the significant interactions, main effects for variables were not interpreted when there were also interactions with those variables.
Unadjusted mean ± SD; range in parentheses (all such values).
Adipose tissue mass
The fitted model showed significant contributions of race (African American compared with white; P = 0.027), sex (male compared with female; P < 0.001), group (T2DM compared with control group; P < 0.001), weight (P < 0.001), and height (P < 0.001) and 2-factor interactions of group with weight (P = 0.001), age (P = 0.020), and sex (P < 0.001) accounted for 78% of the variance in total AT mass (TAT). Because of the significant interactions, main effects for variables were not interpreted when there were also interactions with those variables.
The means for the sex-by-group interaction are shown in Table 2. At the mean value of covariates in the sample (mean ± SD: 84.7 ± 12.2 kg, 1.66 ± 0.1 m, 59.4 ± 13.0 y) the simple-effects analysis indicated that there were reliable differences in the amount of TAT between the T2DM and control groups for both men and women. The difference in women was relatively small (1.6 ± 0.8 kg; P = 0.05), and the T2DM group had less TAT than did the control group, whereas the difference in men was larger: the T2DM group had 3.4 ± 1.2 kg (P = 0.005) more TAT than did the control group (P for interaction = 0.001). In the T2DM group, the between sex difference in TAT was 7.8 ± 1.2 kg (P < 0.001), which was significantly less than the difference of 12.8 ± 1.2 kg (P < 0.001) in the control group.
TABLE 2.
Group interactions of race and sex with diabetes status by adipose tissue compartments, adjusted for weight, height, and age1
| Group-by-sex interaction |
Group-by-race interaction |
|||
| Men | Women | African Americans | Whites | |
| kg | kg | |||
| T2DM group | ||||
| TAT | 29.2 ± 0.92 [35] | 37.0 ± 0.63 [54] | — | — |
| SAT | — | — | 27.6 ± 0.32 [31] | 26.0 ± 0.24 [62] |
| VAT | — | — | 3.7 ± 0.25 [31] | 5.5 ± 0.24 [60] |
| Control group | ||||
| TAT | 25.8 ± 0.9 [29] | 38.6 ± 0.6 [64] | — | — |
| SAT | — | — | 28.7 ± 0.2 [48] | 28.4 ± 0.2 [45] |
| VAT | — | — | 3.0 ± 0.2 [48] | 3.7 ± 0.2 [45] |
All values are means ± SEs; no. of subjects in brackets. T2DM, type 2 diabetes mellitus; TAT, total adipose tissue; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
General linear models were used to test differences between groups for adipose tissue compartments within race or sex: 2 P < 0.01, 3 P < 0.05, 4 P < 0.001, 5 P = 0.012.
The interaction of group by age indicated that TAT was larger with increasing age (0.13 ± 0.03 kg/y; P < 0.001) in the control group but not in the T2DM group (−0.04 ± 0.07 kg/y). The interaction of group by weight indicated that, in the range of weights in this sample, each additional 1 kg of weight was associated with an additional 0.8 ± 0.05 kg of TAT in the control group compared with 0.6 ± 0.06 kg of TAT in the T2DM group (P for interaction = 0.001). A main effect for height indicates that, adjusted for other variables in the model, taller subjects had less TAT (−0.35 ± 0.05 kg/cm; P < 0.001).
Adipose tissue distribution
Further analyses were performed to assess whether group differences existed in AT distribution for an amount of TAT, where TAT represents the sum of total-body SAT, VAT, and IMAT.
Subcutaneous adipose tissue
With whole-body SAT as the dependent variable, there were main effects of race, sex, group, TAT, TAT squared, age, and interactions of group by race and sex by race. The model accounted for 97% of the variance in SAT. Main effects for variables were not interpreted when interactions with those variables were present.
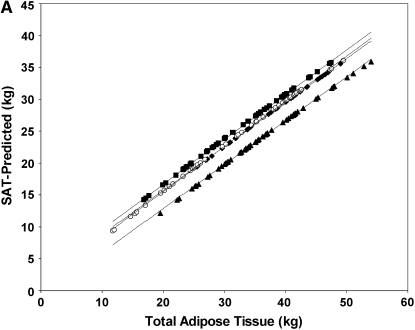
The group-by-race interaction is shown in Table 2. At the mean value of covariates in the sample (34 ± 8.7 kg TAT; age 59 y) the simple-effects analysis showed that there was less SAT in the T2DM group than in the control group in both African American (P < 0.001) and white (P < 0.001) subjects; however, the difference between the T2DM and control groups was significantly larger in the white subjects (1.1 ± 0.3 compared with 2.4 ± 0.3 kg; P = 0.003; Figure 1A).
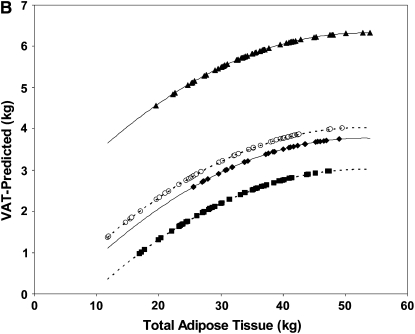
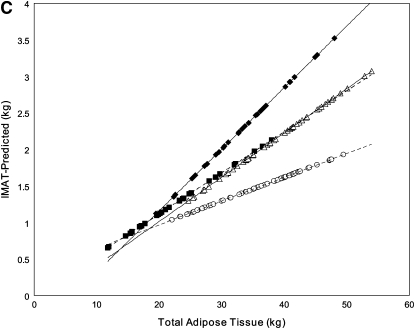
FIGURE 1.
Linear regression model–derived curves for sex-, age-, and height-adjusted subcutaneous adipose tissue (SAT; A), visceral adipose tissue (VAT; B), and intermuscular adipose tissue (IMAT; C) over a range of total adipose tissue for subjects with type 2 diabetes mellitus (T2DM) and control subjects. A and B: T2DM (solid lines: ♦, African Americans; ▴, whites) and control (dashed lines: ▪, African Americans; ○, whites) subjects. C: T2DM (solid lines: ▵, women; ♦, men) and control (dashed lines: ○, women; ▪, men) subjects. A: The SEM of the adjusted mean value was 0.3 kg for African Americans with T2DM (n = 31), 0.2 kg for whites with T2DM (n = 63), 0.2 kg for African American control subjects (n = 48), and 0.2 kg for white control subjects (n = 45). SAT was lower in the T2DM group than in the control group, for both African Americans (P < 0.001) and whites (P < 0.001); however, the difference between the T2DM and control groups was significantly larger in the white subjects (P = 0.003). B: The SEM of the adjusted mean value was 0.2 kg for African Americans with T2DM (n = 31), 0.2 kg for whites with T2DM (n = 60), 0.2 kg for African American control subjects (n = 48), and 0.2 kg for white control subjects (n = 45). VAT was greater in the T2DM group than in the control group for both African Americans (P = 0.011) and whites (P < 0.001), but the difference was greater in whites than in African Americans (P for interaction < 0.001). C: The SEM of the adjusted mean value was 0.09 kg for women with T2DM (n = 54), 0.12 kg for men with T2DM (n = 35), 0.08 kg for control women (n = 64), and 0.13 kg for control men (n = 29). Both men and women had more IMAT with greater amounts of TAT, and the amount increased in men more rapidly than in women (β = 0.059 ± 0.011 and β = 0.027 ± 0.009, respectively; both P < 0.001; P for interaction with TAT = 0.004). At the mean value of covariates in the sample, men had more IMAT than did women (P < 0.001).
Although not of direct interest to the question of fat distribution in T2DM, we also noted a sex-by-race interaction (P = 0.047) in SAT distribution such that, whereas African Americans tended to have more SAT overall than did whites, the difference was larger and was statistically significant for the women (29.6 ± 0.2 compared with 28.1 ± 0.2 kg; P < 0.001) but not for the men (26.7 ± 0.4 compared with 26.2 ± 0.2 kg; P = 0.23).
In all subjects, SAT mass was larger with greater amounts of TAT (β = 0.66 ± 0.08, P < 0.001), but the proportion was nonlinear (TAT squared: β = 0.003 ± 0.001, P < 0.02). The amount of SAT was smaller with greater age (β = −0.033 ± 0.008, P < 0.001).
Subcutaneous adipose tissue distribution
Because of the finding of less SAT in the T2DM group, an analysis was performed in which SAT was partitioned into trunk, arm, upper leg (as femoral-gluteal), and lower leg regions as described previously (27) for the purpose of investigating where the SAT deficit was located. The dependent variable in the regression model (SAT) was successively replaced with each of the constituents of SAT, ie, arm, trunk, upper leg, and lower leg SAT. There were no significant differences in arm or trunk SAT by diabetes status. There was significantly less upper leg SAT in the T2DM group than in the control group (8.1 ± 0.2 compared with 9.3 ± 0.2 kg; P < 0.001), in men than in women (8.0 ± 0.3 compared with 9.4 ± 0.2 kg; P < 0.001), and in whites than in African Americans (8.4 ± 0.2 compared with 9.0 ± 0.3 kg; P = 0.042). There were no significant interactions between these variables. For lower leg SAT, there was an interaction. In African Americans, those with T2DM had more lower leg SAT than did the control group (2.6 ± 0.1 compared with 2.5 ± 0.1 kg; P = 0.5). In whites, the T2DM group had significantly less lower leg SAT than did the control group (2.3 ± 0.1 compared with 2.7 ± 0.1 kg; P = 0.008).
Visceral adipose tissue
With total VAT as the dependent variable, there were main effects for race, sex, group, TAT, TAT squared, and age and a race-by-group interaction. The equation accounted for 70% of the variance in VAT. Two cases with large residuals were excluded from this analysis. At the mean value of TAT and age in the sample, VAT was larger in men than in women by 2.0 kg (4.9 ± 0.02 compared with 2.9 ± 0.01 kg; P < 0.001). VAT was greater in older subjects (β = 0.02 ± 0.007, P < 0.001) and in subjects with more TAT (TAT: β = 0.31 ± 0.07; TAT squared: β = −0.003 ± 0.001; both P < 0.001). The nonlinear relation of VAT with TAT was such that, for subjects with a 10-kg difference in TAT at mean TAT values of 20–30 kg, the difference in VAT was 1.6 kg; for subjects with mean TAT values of 40–50 kg, a similar 10-kg difference in TAT contributed a 0.5-kg difference in VAT.
The race-by-group interaction effect shown in Table 2 indicates that there was more VAT in the T2DM group than in the control group for both the African Americans (P = 0.011) and whites (P < 0.001), but the difference was larger in the white group than in the African American group (P for interaction < 0.001; Figure 1B).
Intermuscular adipose tissue
Four cases were excluded from this analysis to bring the distribution of residuals to normality. With whole-body IMAT as the dependent variable, there were main effects of race, TAT, and age and interactions of race by height, sex by TAT, and group by TAT. The model accounted for 56% of the variance in IMAT.
The main effect of age indicated larger amounts of IMAT in older subjects (β = 0.010 ± 0.003, P = 0.002). The group-by-TAT interaction was such that, whereas there was more IMAT when there was more TAT in both the T2DM and control groups (β = 0.054 ± 0.010 and β = 0.027 ± 0.009, respectively; both P < 0.001), the amount increased more rapidly in the T2DM group (interaction P = 0.006; Figure 1C). At the mean value of covariates in the sample, the T2DM group had 0.5 kg more IMAT than did the control group (2.2 ± 0.1 compared with 1.7 ± 0.1 kg; P < 0.001). The interaction of sex-by-TAT indicated that, whereas both men and women had more IMAT with greater amounts of TAT, the amount increased in men more rapidly than in women (β = 0.059 ± 0.011 and β = 0.027 ± 0.009, respectively; both P < 0.001; P for interaction = 0.004). At the mean value of covariates in the sample, men had more IMAT than did women (2.2 ± 0.1 compared with 1.7 ± 0.1 kg; P < 0.001). The race-by-height interaction indicated that IMAT was unrelated to or tended to decrease with height in whites (β = −1.23 ± 0.69 m, P = 0.08), but increased with height in African Americans (β = 2.20 ± 0.89 m, P < 0.001).
DISCUSSION
The purpose of this study was to use whole-body MRI to determine whether differences exist in total adiposity and AT distribution between persons with and without T2DM. Women with T2DM had less TAT, whereas men with T2DM had more TAT; the between-sex differences in TAT were attenuated in T2DM. VAT was greater in the T2DM group than in the control group for both the African American and white subjects, but the difference between groups was larger for whites. At low levels of adiposity, the T2DM and control groups had similar amounts of IMAT, but the rate of increase in IMAT with greater TAT was more rapid in the T2DM group; at high levels of adiposity, the T2DM group had more IMAT than did the control group. Interestingly, there was less SAT in the T2DM group than in the control group, and the difference between the T2DM and control groups was significantly greater in the white subjects. The group differences in SAT were found to be primarily in the femoral-gluteal region. These findings indicated significant differences in body composition between the T2DM and control groups in a direction consistent with previous findings of an AT distribution favoring IR (greater VAT and IMAT and less femoral-gluteal SAT). The finding of less SAT in the T2DM group is novel and the overall findings highlight the importance of using appropriate methods to measure phenotypes (ie, whole-body MRI) when investigating body composition at the level of specific tissues and depots.
Total-body adipose tissue
Compared with the control group, women with T2DM had less TAT, whereas men with T2DM had more TAT. Thus, the usual strong between-sex differences in TAT in persons without diabetes is attenuated in T2DM. Previous studies, most of which used DXA to measure body composition, have reported greater fat mass in women with T2DM (28), greater percentage body fat in women and men (29), or similar percentages of body fat in women with T2DM and in normoglycemic control subjects (30, 31). Differences in the sex-specific relevance of measures of body fat distribution in predicting the risk of T2DM have been reported, ie, BMI, waist circumference, and waist-hip ratio had similar predictive powers in men, whereas WC was more strongly predictive than BMI in women, which suggests that abdominal fat is more important than total fatness in women (32). Because TAT is the sum of different AT depots, the implications of between-group differences in TAT must be considered in light of the differences observed in subdepots, as discussed later.
Subcutaneous adipose tissue
The finding of less total-body SAT in the subjects with T2DM than in the control subjects, for both African Americans and whites, is a novel finding where the difference between the T2DM and control groups was significantly larger for the white subjects. We partitioned SAT into subdepots (arms, trunk, upper legs, and lower legs), and the results confirm the expected pattern of deficits in femoral-gluteal SAT depots in T2DM and in men compared with women. Between-race differences in upper leg SAT were found; whites had less femoral-gluteal SAT than did African Americans. Among whites, the implications of the finding of less lower leg SAT in the T2DM group than in the control group are unclear.
Several recent studies have found that femoral-gluteal adiposity measured by DXA or computed tomography is reduced in T2DM, despite similar or an overall greater fat mass (8, 10–12, 32), and greater thigh SAT in obese adults was shown to be associated with a lower prevalence of the metabolic syndrome (33). In the third National Health and Nutrition Examination Survey population that underwent a fasting oral-glucose-tolerance test, the relation between BMI, thigh skinfold thickness, and IR was associated with a greater degree of IR in the obese, who had relatively small lower body fat depots, whereas those with large accumulations of lower body subcutaneous fat were less likely to have IR or T2DM (34). A smaller hip circumference is associated with increased metabolic risk and mortality (9). The inference is that hip circumference reflects femoral-gluteal AT (which is predominantly SAT), with greater femoral-gluteal AT being protective in relation to IR and dyslipidemia (10).
We acknowledge that the potential role of abdominal SAT in IR is unclear. Large adipocytes within abdominal SAT are a risk factor for T2DM, even after the adjustment for overall and central adiposity (35). Abdominal SAT has been found to correlate as strongly, or more strongly, than VAT with IR (1, 36). Because the relation between abdominal SAT and IR was not investigated with control for VAT, it is therefore unknown whether a remaining association for SAT existed after adjustment for VAT. It is postulated that a paucity of small adipocytes and a predominance of large ones in SAT limits the capacity for efficient energy storage and disposes to VAT and fat accumulation within liver and muscle (37). Intriguingly, treatment with peroxisome proliferator–activated receptor-γ agonists induces a modest redistribution of adiposity away from VAT toward SAT (38), which is associated with improvement in IR, including within SAT itself (39, 40). Thus, a relative deficiency of SAT and altered composition of SAT may combine with a relative predominance of VAT as a body composition alteration in T2DM, but this remains uncertain.
Visceral adipose tissue
VAT was greater in the T2DM group than in the control group for both the African American and white subjects. Growing evidence suggests that VAT accumulation may play an important role in the etiology of T2DM. Most previous studies have used surrogate measures of abdominal adiposity (waist circumference and/or waist-hip ratio), which have proven to be stronger predictors of disease development than BMI (a surrogate for total fatness) (41). Moreover, a central (VAT) deposition of fat is more strongly linked to IR and T2DM than is a peripheral (gluteal and subcutaneous) deposition of fat (42).
At the cellular level, attempts have been made to compare omental and subcutaneous adipocyte development (43) to ascertain whether regional differences in the capacity for differentiation, proliferation, and cellular growth might contribute to patterns of AT distribution in obesity. The findings suggest that regional differences in AT may exist with regard to proliferation capacity between subcutaneous and omental preadipocytes, whereas differences were not observed in differentiation capacity. Cell proliferation was found to decrease with aging in subcutaneous but not in omental depots, which suggests that the latter could contribute to the preferential expansion of the omental adipose depot with age. In the current study, we observed that aging was associated with less SAT and more VAT in both the T2DM and control groups.
The difference in VAT between the T2DM and control groups was greater in whites than in African Americans. This suggests that T2DM is associated with a greater central deposition of AT in whites, although the reasons for this race difference are unknown.
The presence of less VAT and more SAT in African Americans would be expected to predispose to less risk of IR, yet African Americans are more insulin resistant. This paradox suggests that race differences in IR are not only related to SAT and VAT. Albu et al (44) have shown that race differences in the acute insulin response to glucose was independent of AT depot (VAT, SAT, or IMAT) and was related to greater skeletal muscle volume in healthy nondiabetic premenopausal African Americans. The hypothesis is that some characteristic of MRI-measured skeletal muscle is associated with greater IR in African American women, such as higher amounts of muscle lipid, both in nonvisible adipocytes (extramyocellular lipid) and inside the myocytes (intramyocellular lipid).
Intermuscular adipose tissue
At low levels of adiposity, the T2DM and control groups had similar amounts of IMAT; at high levels of adiposity, the T2DM group had a greater amount of IMAT than did the controls. The increase in IMAT with greater TAT was more rapid in the T2DM group, which suggests that more obese persons with T2DM have more AT interspersed in their muscle groups. Thus, similar to VAT, IMAT in T2DM increases proportionately more with increasing adiposity, whereas increases in SAT are attenuated compared with controls. It is possible that more IMAT and VAT contribute to greater amounts of ectopic fat in muscle and liver in T2DM (5, 14).
Study strengths and limitations
No previous study has measured whole-body AT distribution in vivo and differentiated between its subdepots in T2DM using imaging techniques (21, 45). The protocol described herein was not exhaustive in its choice of AT depots, for example, liver fat (5) was not quantified, which is prevalent in obese T2DM. Increases in liver fat (nonalcoholic steatohepatitis; 46) are associated with decreases in IR and increases in hepatic glucose output (47).
An assumed tissue AT density (24) was used, and it is unclear whether this value can be accurately applied across the adult age span and race-ethnicity groups. The T2DM and control samples were not matched on many relevant demographic and body-composition characteristics and, hence, comparisons were made on values adjusted by regression models. It is possible that the models did not adequately adjust for these differences.
Conclusions
These findings expand our knowledge of body composition in a cohort with T2DM. Adjusted for differences in covariates between samples, VAT and IMAT were confirmed to be greater in T2DM, and, for the first time, SAT was found to be lower in T2DM.
Acknowledgments
We thank Else Ruts, Jowanda Green, Carol Kelley, and Qing He (project coordinators); Isaiah Janumala (MRI analyst); Elli Ioannidou (DXA analyst); and Mark Punyanitya (MRI analysis quality control).
The authors' responsibilities were as follows— DG: study design, data collection for T2DM in New York and archived data from DK 42618-Project 4, data analysis, writing of manuscript, administrative support, and supervision; DEK: principal investigator of the Pittsburgh Look AHEAD site, coinvestigator and responsible for T2DM data collection in Pittsburgh, and writing of manuscript; SH: data analysis and writing of manuscript; JA: data collection for archived studies (RR-00645 and DK-40414); FXP-S: principal investigator of the New York Look AHEAD site; LB: MRI quality control for T2DM data collected at both sites; NS: participated in data collection and data analyses for T2DM in New York during her minority fellowship period; and J-EY: MRI-derived SAT distribution analysis. Each author declared that she or he had no conflict of financial or personal interests in any company or organization sponsoring this study.
REFERENCES
- 1.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes 1996;45:1684–93 [DOI] [PubMed] [Google Scholar]
- 2.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 1997;46:456–62 [DOI] [PubMed] [Google Scholar]
- 3.Banerji MA, Chaiken RL, Gordon D, Kral JG, Lebovitz HE. Does intra-abdominal adipose tissue in black men determine whether NIDDM is insulin-resistant or insulin-sensitive?. Diabetes 1995;44:141–6 [DOI] [PubMed] [Google Scholar]
- 4.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care 2001;24:933–41 [DOI] [PubMed] [Google Scholar]
- 5.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab 2003;285:E906–16 [DOI] [PubMed] [Google Scholar]
- 6.Perseghin G, Scifo P, De Cobelli F, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999;48:1600–6 [DOI] [PubMed] [Google Scholar]
- 7.Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 2000;49:749–58 [DOI] [PubMed] [Google Scholar]
- 8.Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE. and the Look AHEAD Adipose Research Group Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2007;293:E435–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seidell JC, Han TS, Feskens EJ, Lean ME. Narrow hips and broad waist circumferences independently contribute to increased risk of non-insulin-dependent diabetes mellitus. J Intern Med 1997;242:401–6 [DOI] [PubMed] [Google Scholar]
- 10.Snijder MB, Dekker JM, Visser M, et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res 2003;11:104–11 [DOI] [PubMed] [Google Scholar]
- 11.Snijder MB, Dekker JM, Visser M, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 2004;27:372–7 [DOI] [PubMed] [Google Scholar]
- 12.Snijder MB, Visser M, Dekker JM. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005;48:301–8 [DOI] [PubMed] [Google Scholar]
- 13.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr 1990;51:1106–12 [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–92 [DOI] [PubMed] [Google Scholar]
- 15.Gallagher D, Kuznia P, Heshka S, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 2005;81:903–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res 2005;13:66–74 [DOI] [PubMed] [Google Scholar]
- 17.He Q, Heo M, Heshka S, et al. Total body potassium differs by sex and race across the adult age span. Am J Clin Nutr 2003;78:72–7 [DOI] [PubMed] [Google Scholar]
- 18.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 1992;55:950–4 [DOI] [PubMed] [Google Scholar]
- 19.Sosenko JM, Kato M, Soto R, Goldberg RB. A comparison of adiposity measures for screening non-insulin dependent diabetes mellitus. Int J Obes Relat Metab Disord 1993;17:441–4 [PubMed] [Google Scholar]
- 20.Stoney RM, Walker KZ, Best JD, Ireland PD, Giles GG, O'Dea K. Do postmenopausal women with NIDDM have a reduced capacity to deposit and conserve lower-body fat?. Diabetes Care 1998;21:828–30 [DOI] [PubMed] [Google Scholar]
- 21.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 2004;79:874–80 [DOI] [PubMed] [Google Scholar]
- 22.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–28 [DOI] [PubMed] [Google Scholar]
- 23.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol 1996;74:778–85 [PubMed] [Google Scholar]
- 24.Snyder WS, Cook MJ, Nasset ES, Karhaussen LR, Howells GP, Tipton IH. Report of the task group on reference men. International Commission on Radiological Protection No. 23. Oxford, United Kingdom: Pergamon, 1975 [Google Scholar]
- 25.Heshka S, Punyanitya M, Shen W, et al. Inter-reader reliability in reconstructing tissue volumes from magnetic resonance images. FASEB J 2004;18:139 [Google Scholar]
- 26.Yim JE, Heshka S, Albu JB, Heymsfield SB, Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol (Epub ahead of print December 13 2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huber PJ. Robust estimation of a location parameter. Ann Math Statist 1964;35:73–101 [Google Scholar]
- 28.Svendsen OL, Hassager C. Body composition and fat distribution measured by dual-energy X-ray absorptiometry in premenopausal and postmenopausal insulin-dependent and non-insulin-dependent diabetes mellitus patients. Metabolism 1998;47:212–6 [DOI] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–9 [DOI] [PubMed] [Google Scholar]
- 30.Maiolo C, Mohamed EI, Di DN, Pepe M, Perriello G, De LA. Body composition and pulmonary function in obese type 2 diabetic women. Diabetes Nutr Metab 2002;15:20–5 [PubMed] [Google Scholar]
- 31.Poynten AM, Markovic TP, Maclean EL, et al. Fat oxidation, body composition and insulin sensitivity in diabetic and normoglycaemic obese adults 5 years after weight loss. Int J Obes Relat Metab Disord 2003;27:1212–8 [DOI] [PubMed] [Google Scholar]
- 32.Meisinger C, Doring A, Thorand B, Heier M, Lowel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr 2006;84:483–9 [DOI] [PubMed] [Google Scholar]
- 33.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–83 [DOI] [PubMed] [Google Scholar]
- 34.Livingston EH. Lower body subcutaneous fat accumulation and diabetes mellitus risk. Surg Obes Relat Dis 2006;2:362–8 [DOI] [PubMed] [Google Scholar]
- 35.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000;43:1498–506 [DOI] [PubMed] [Google Scholar]
- 36.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest 1995;96:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Danforth E., Jr Failure of adipocyte differentiation causes type II diabetes mellitus?. Nat Genet 2000;26:13. [DOI] [PubMed] [Google Scholar]
- 38.Ciaraldi TP, Kong AP, Chu NV, et al. Regulation of glucose transport and insulin signaling by troglitazone or metformin in adipose tissue of type 2 diabetic subjects. Diabetes 2002;51:30–6 [DOI] [PubMed] [Google Scholar]
- 39.Virtanen KA, Lonnroth P, Parkkola R, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 2002;87:3902–10 [DOI] [PubMed] [Google Scholar]
- 40.Virtanen KA, Hallsten K, Parkkola R, et al. Differential effects of rosiglitazone and metformin on adipose tissue distribution and glucose uptake in type 2 diabetic subjects. Diabetes 2003;52:283–90 [DOI] [PubMed] [Google Scholar]
- 41.Wei M, Gaskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio and other anthropometric measurements in Mexican Americans—a 7 year prospective study. Obes Res 1997;5:16–23 [DOI] [PubMed] [Google Scholar]
- 42.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994;74:761–811 [DOI] [PubMed] [Google Scholar]
- 43.Van Harmelen V, Rohrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism 2004;53:632–7 [DOI] [PubMed] [Google Scholar]
- 44.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 2005;82:1210–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallagher D, Kovera AJ, Clay-Williams G, et al. Weight loss in postmenopausal obesity: no adverse alterations in body composition and protein metabolism. Am J Physiol Endocrinol Metab 2000;279:E124–31 [DOI] [PubMed] [Google Scholar]
- 46.Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diabetes Rep 2002;2:210–5 [DOI] [PubMed] [Google Scholar]
- 47.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002;87:3023–8 [DOI] [PubMed] [Google Scholar]