Abstract
Mutation of the gene drop-dead (drd) causes adult Drosophila to die within 2 weeks of eclosion and is associated with reduced rates of defecation and increased volumes of crop contents. In the current study, we demonstrate that flies carrying the strong allele drdlwf display a reduction in the transfer of ingested food from the crop to the midgut, as measured both as a change in the steady-state distribution of food within the gut and also in the rates of crop emptying and midgut filling following a single meal. Mutant flies have abnormal triglyceride (TG) and glycogen stores over the first four days post-eclosion, consistent with their inability to move food into the midgut for digestion and nutrient absorption. However, the lifespan of mutants was dependent upon food presence and quality, suggesting that at least some individual flies were able to digest some food. Finally, spontaneous motility of the crop was abnormal in drdlwf flies, with the crops of mutant flies contracting significantly more rapidly than those of heterozygous controls. We therefore hypothesize that mutation of drd causes a structural or regulatory defect that inhibits the entry of food into the midgut.
Keywords: Drosophila, crop, midgut, digestion, drop-dead
1. Introduction
The survival of any multicellular animal is dependent upon the proper functioning of the digestive system. In insects, the passage of food from the crop through the stomodaeal valve and into the midgut is an important regulatory step in controlling the movement of food through the gut (Gelperin, 1971; Stoffolano, Jr., 1995). Although some digestion can occur in the crop, nutrient absorption only takes place once the food reaches the midgut (Dimitriadis and Papamanoli, 1992; Maddrell and Gardiner, 1980; Treherne, 1957). Experiments in several insect species have shown that the rate of crop emptying is modulated by hemolymph sugar titers and osmolarity (Gelperin, 1966; Roces and Blatt, 1999; Treherne, 1957). Opening of the stomodaeal valve is controlled in part by neuronal signals (Davey and Treherne, 1963), which could potentially carry information about hemolymph composition. In addition, the spontaneous muscular contractions of the crop, which move ingested food down the crop duct and into the midgut, are sensitive to a variety of factors, including sugars, neuropeptides, and the volume of the crop itself (Duve et al., 2000; Predel et al., 2001; Richer et al., 2000; Thomson, 1975). Stretch receptors in the crop also provide inhibitory feedback to limit food intake once the crop becomes engorged (Bernays, 1985; Gelperin, 1967).
The control of adult feeding behavior and gut function in the fruit fly Drosophila melanogaster is relatively unstudied compared to larger insects. The Drosphila crop is innervated by neurons containing several peptides, some of which have been shown to alter the rate of spontaneous crop contractions (Duttlinger et al., 2002; Price et al., 2002). In a single study on the feeding behavior of adult Drosophila, it was reported that ad libitum fed flies never exhibited distended crops, suggesting that Drosophila eat small frequent meals when they have free access to food (Edgecomb et al., 1994). Maximally distended crops were only seen in flies that had been food-deprived for a significant period of time and then exposed to a high-sugar food to maximize the excitatory drive to feed.
We have previously reported that mutation of the Drosophila gene drd appears to cause a defect in the movement of food through the gut (Blumenthal, 2008). The drd gene encodes a large protein with multiple hydrophobic, presumably membrane-spanning, domains; the function of this protein and its pattern of expression are currently unknown. Flies carrying strong drd alleles appear normal, albeit with reduced body size, at eclosion, but then exhibit abnormal motor coordination and die within the first two weeks of adult life. Severe brain degeneration has been reported in mutant flies after the appearance of the motor defects (Benzer, 1971; Buchanan and Benzer, 1993). We have reported that, in addition to these phenotypes, drd mutant flies also show increased crop volumes and reduced rates of defecation; both of these phenotypes are evident during the first two days post-eclosion, before any of the flies have begun to die (Blumenthal, 2008). While mutation or misexpression of specific genes has been shown to affect feeding behavior in larval Drosophila (Lee and Park, 2004; Melcher and Pankratz, 2005; Wu et al., 2003; Wu et al., 2005), this was the first example of a mutation that altered food processing or digestive function in the adult.
The purpose of the current study is to examine in further detail the gut phenotype caused by mutation of drd. We wished to determine whether the blockage of food movement was at the level of crop emptying or elsewhere, whether crop motility was altered in mutant flies, and whether, as we had previously speculated, starvation as a result of the gut defect might be a contributing factor in the adult lethal phenotype.
2. Methods
2.1 Fly maintenance
Canton S and drdlwf/FM7a stocks were maintained on standard cornmeal-molasses-agar food at 24°C on a 12:12 light:dark cycle. For some survival experiments and for feeding with blue food, flies were placed on Instant Fly Food (Carolina Biological, Burlington, NC) prepared with 0.6 g food and 2 ml water or 0.5% acid blue 9 per vial. For starvation experiments, flies were placed in vials containing 1% Drosophila agar (Genesee Scientific, San Diego, CA).
2.2 Scoring of crops and midguts
Flies were dissected under PBS and scored for crop volume and the intensity of midgut color. Crops were given a score of 1–5 based on size as previously described (Edgecomb et al., 1994), with a score of 1 indicating a completely empty crop and 5 indicating a maximally distended crop. Midguts were given a score based on the level of blue dye ranging from 1 (colorless) to 5 (extremely dark blue).
2.3 Dye distribution experiments
In the first experiment, male flies were collected on the first or second day following eclosion (P1–P2) and placed on blue food for 22–24 hours. At the end of this time, flies were dissected and the crops and midguts scored for size and color. In the second experiment, male flies were collected on the day of eclosion and placed on agar for 48 hours. The flies were then placed on blue food for varying amounts of time before being dissected and scored.
2.4 Crop motility assays
Cold-anesthetized P1–2 drdlwf/drdlwf and drdlwf/FM7a females and P1 drdlwf and Canton S males were pinned in a dissecting dish under saline (5 mM HEPES, 128 mM NaCl, 36 mM sucrose, 4 mM MgCl2, 2 mM KCl, 1.8 mM CaCl2, pH 7.1) and the ventral cuticle was removed in order to view the crop. The contractions of a crop lobe were recorded from each fly for five 30 second intervals (with 30 second intervals between each recording period) and then the five counts were averaged as previously described (Duttlinger et al., 2002). For counting crop contractions in the intact fly, flies were fed for one day on blue food to allow for visualization of the crop. Flies were then pinned down under saline and contractions counted as above.
2.5 Survival assays
P0 male flies were collected and placed in vials containing regular food, instant food, or agar in groups of 6–27 flies. Surviving flies were counted daily.
2.6 Protein, TG, and glycogen assays
P0–P4 male flies were frozen and homogenized individually with a motor-driven pestle in 60μl of lysis buffer containing: 1% NP-40, 0.5% deoxycholate acid, 0.1% Triton-X 100, 100 mM NaCl, 0.1 mM CaCl2, and 2 mM MgCl2. The samples were incubated at 70°C for 5 minutes to inactivate lipases and then spun at 14,000 rpm for one minute. The supernatant of each sample was assayed for TG, glycogen, and protein as described below, and TG and glycogen levels were then normalized to protein concentration. All spectrophotometric assays were carried out with a Multiskan Ascent plate reader (ThermoFisher Scientific, Waltham, MA) and analyzed with Ascent v2.6 software (ThermoFisher). For each assay, homogenates were analyzed in triplicate and concentration standards in duplicate.
For the TG assay, standards of 0–0.1 μg/μl were prepared from a 2.5 mg/ml stock of Ultra Pure Glycerol (ICN Biomedicals, Inc.). 10 μl of standard or homogenate were mixed with 80 μl of Free Glycerol Reagent (Sigma-Aldrich, St. Louis, MO) on a 96-well plate, mixed at 960 rpm for 30 seconds, incubated for five minutes at 37°C, and the absorbances measured at 540 nm. 20μl of Triglyceride Reagent (Sigma-Aldrich) was then added to each well, and the samples were mixed at 960 rpm for 30 seconds, incubated for 15 minutes at 37°C, and measured at 540 nm. TG concentration was calculated from the difference in the two absorbances.
For the glycogen assay, standards of 0–0.3 μg/μl were prepared from a 0.3 mg/ml stock solution of glycogen (Sigma-Aldrich). 10μl of standard or 3-fold diluted homogenate were mixed with 10μl amyloglucosidase (0.8 mg/ml) (Sigma-Aldrich) and incubated overnight at room temperature. The following day 200 μl of Infinity Glucose Reagent (ThermoFisher) was added to each well. The plate was mixed at 960 rpm for 10 seconds, incubated for 10 minutes at 37°C and measured at 340 nm.
Protein concentrations were determined using the BCA™ Protein Assay Kit (ThermoFisher). Standards ranging in concentration from 0 μg/ml to 400 μg/ml were prepared from a 2.0 mg/ml stock of Alubumin Standard. 25 μl standard or 9-fold diluted homogenate were mixed with 200 μl Working Reagent, mixed at 960 rpm for 30 seconds, incubated for 30 minutes at 37°C, cooled to room temperature, and measured at 570 nm.
2.7 Statistics and data analysis
Data were graphed and analyzed using either Origin 7.5 for Windows (OriginLab, Northampton, MA) or GraphPad Prism 4.03 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com) software. All statistical analyses and calculations of median survival were performed with GraphPad Prism 4.03 software. Survival curves were compared in a pair-wise fashion using a Mantel-Haenszel logrank test.
3. Results
3.1 Distribution and movement of food within the gut
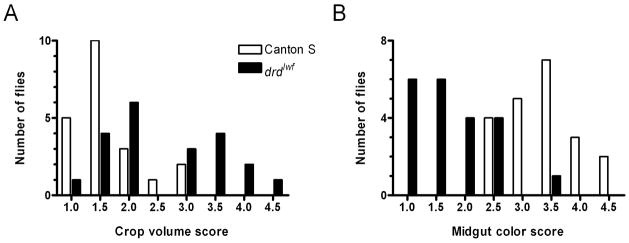
We studied the distribution of food within the digestive tracts of Canton S (wild-type) flies and flies carrying the strong drd allele drdlwf, in which a nonsense mutation in codon 181 (out of 827) results in a severely truncated protein. After 22–24 hours on blue food, there were significant differences between the genotypes in both the size of the crop (fig. 1a) and the intensity of blue color in the midgut (fig. 1b) (Mann-Whitney test, p=0.0013 for crop scores and p<0.0001 for midgut scores) with mutant flies having larger crops and less dye in their midguts. It should be noted that while there was a clear separation in the midgut scores between the two genotypes (20/21 mutant flies had scores of ≤2.5 while 21/21 wild-type flies had scores of ≥2.5), the distribution of crop sizes in the mutant flies was biphasic, with 10/21 flies having scores of ≥3 and 11/21 having scores of ≤2 that overlapped with the wild-type distribution (see discussion).
Figure 1.
Distribution of crop volume scores (A) and midgut color scores (B) for flies maintained on blue food for 22–24 hours. See text for descriptions of the scoring systems.
Figure 2 shows the localization of a single meal in flies that had been starved for 48 hours on agar after eclosion. In wild-type flies (fig. 2a), crops became engorged within three minutes of the flies being placed on food, as has previously been reported (Edgecomb et al., 1994). No dye was seen in the midgut until 15 minutes after the beginning of feeding, but after this delay we observed a steady increase in the intensity of staining in the midgut for the remainder of the experiment (the slope of a midgut score vs. time linear regression was non-zero, F-test, p<0.0001). At the same time, crop volume scores steadily decreased (F-test, p=0.0006). The data from the mutant flies are strikingly different from the wild-type data. First, 6 of the 26 flies, distributed throughout the time course of the experiment, had empty crops, something never seen in the wild-type flies. Interestingly, another 6 flies had full crops but no blue dye, suggesting that they had become engorged on water from the agar during the starvation period and did not eat following the return to food. Thus nearly half of the mutant flies (12/26) did not eat after 48 hours of starvation. For the flies that did eat, as evidenced by blue dye in their crops, there was no significant transfer of food to the midgut over the 90-minute duration of the experiment (F-test, p=0.56). This subset of mutant flies did show a weak but significant change in crop score over time (F-test, p=0.048), but unlike in wild-type flies, it was a gradual increase in crop volume. Thus, while the 24-hour data demonstrate that mutant flies are able to move at least some food into the midgut, that movement is severely delayed or restricted, even under conditions of extreme starvation.
Figure 2.
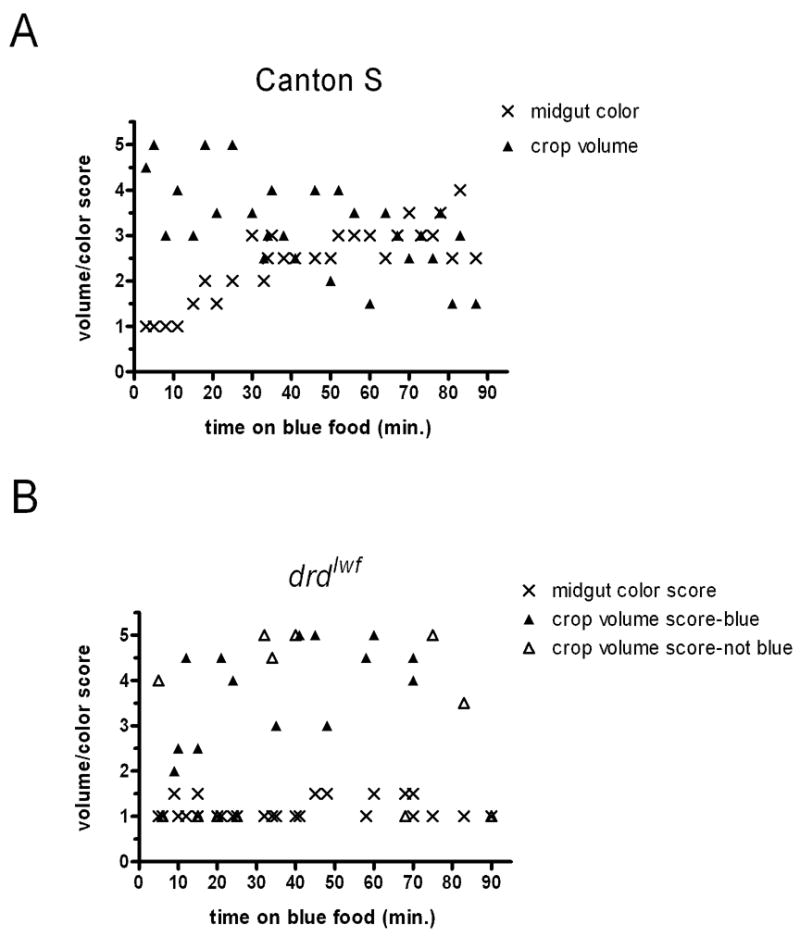
Crop volume and midgut color scores of wild-type (A) and drdlwf mutant (B) flies that were starved for 48 hours and then placed on blue food for various times. Crop scores of mutant flies with no blue dye in their crops are indicated by open triangles (this was never observed in wild-type flies).
3.2 Measurement of energy stores
Glycogen and TG levels were measured in wild-type and mutant males during the first five days of adult life. Results of the TG assays are shown in figure 3a. In wild-type flies, TG stores remain constant over the five days of the assay period (1-way ANOVA, p=0.10). Mutant TG stores are identical to wild-type on the day of eclosion and the next day, but they are significantly lower than wild-type on day 2 and the difference is more pronounced on days 3 and 4 (see figure legend for statistics). Glycogen stores are also abnormal in the mutants (fig. 3b). In wild-type flies, glycogen levels are low on the day of eclosion but increase the following day and remain constant thereafter (p<0.01 day 0 vs. all other days, p<0.05 for all other pairwise comparisons, 1-way ANOVA/Tukey’s post-hoc test). Mutants have identical glycogen stores to wild-type at eclosion, but are significantly lower than wild-type for the next four days (see figure legend for statistics). An analysis of the mutant data alone shows that glycogen stores remain at a constant low level for days 0–3 but then rise significantly on day 4 (p<0.05 day 4 vs. all other days, p>0.05 for all other pairwise comparisons, 1-way ANOVA/Tukey’s post-hoc test). Our interpretation of this rise will be discussed below.
Figure 3.
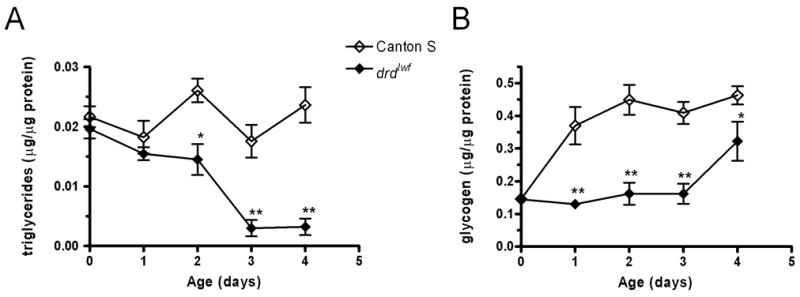
Changes in TG (A) and glycogen (B) levels in Canton S and drdlwf males during the first four days of adult life. Error bars represent SEM. n=10–12 flies/timepoint. *: significant difference between genotypes, p<0.05, **: p<0.001, 2-way ANOVA/Bonferroni post-hoc test.
3.3 Survival of flies on different foods
To determine whether drdlwf mutant flies derive any nutritional support from their food, we generated survival curves from populations of mutant and wild-type males maintained from the day of eclosion on agar, instant fly food, or standard cornmeal-molasses food (figure 4). As is clear from the figure, the presence and quality of the food affects the survival of some, but not all, of the mutant flies. When starved on agar, both mutant and wild-type flies had a median survival of 3 days, with no difference between the curves of the two genotypes (see figure legend for statistics). Mutants kept on instant food also had a median survival time of 3 days; however, there was a clear tail on the survival curve with 40% of the flies surviving for an additional 1–3 days such that the survival curves of the starved and instant food populations were significantly different. This tail was even more pronounced when mutant flies were maintained on standard food. Over 60% of the flies died within 4 days, but the survivors then died more gradually over the ensuing week, and the survival curve for the flies kept on standard food differed from those of the other two populations. Wild-type flies did not display any significant mortality on either instant or standard food during the time course of the experiment.
Figure 4.
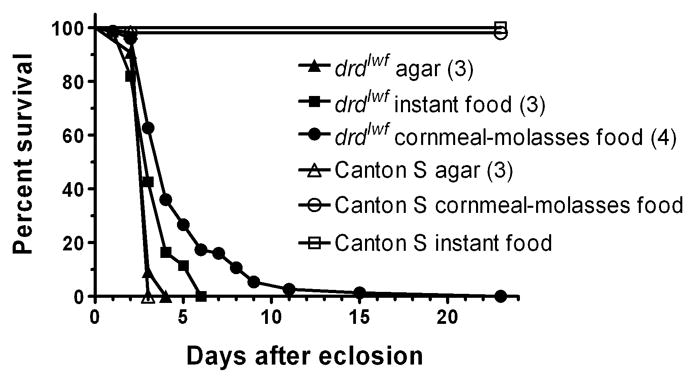
Survival curves of Canton S and drdlwf males collected on the day of eclosion and placed on agar (n=55 wild-type and 65 mutant flies), instant food (n=52 wild-type and 61 mutant), or cornmeal-molasses food (n=51 wild-type and 75 mutant). Median survival for each condition is shown in parentheses. Pairwise comparisons by the Mantel-Haenszel logrank test showed that drdlwf survival curves under the three conditions were different from each other: p=0.0014 (agar vs instant), p<0.0001 (agar vs cornmeal), p=0.0002 (instant vs cornmeal). Survival curves of drdlwf and Canton S males on agar did not differ (p=0.71).
3.4 Crop motility
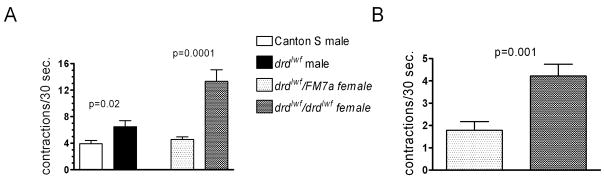
The rate of spontaneous crop contractions was measured to determine whether a defect in crop motility might explain the failure of mutant flies to move food into their midguts. In semi-intact preparations, with the cuticle opened to expose the crop, we observed significantly higher rates of contractions in drdlwf males compared to Canton S and in homozygous drdlwf females compared to heterozygous sibling controls (fig. 5a). Because opening the cuticle will alter the environment of the crop and potentially dilute circulating factors that could affect crop motility, we also measured contractions in intact flies which had been fed blue food to enhance the visibility of the crop through the cuticle. As shown in figure 5b, crop motility in homozygous females remained significantly higher than in heterozygotes even in these intact preparations. All of the contraction rates observed in our study are slower than the 47 contractions/minute previously reported (Kaminski et al., 2002). This difference could result from the use of older flies (P2–4) from another strain (Oregon R) in the previous study.
Figure 5.
Crop contractions in Canton S and drdlwf males and drdlwf heterozygous and homozygous females. A. Contractions were measured in a lobe of the crop in dissected flies. n=16–46 flies/genotype. B. Contractions measured in intact females. n=15–16 flies/genotype. Error bars represent SEM. p-values are from unpaired t-tests (with Welch’s correction for unequal variances where necessary) comparing wild-type and mutant males or heterozygous and homozygous females.
4. Discussion
We have shown that disruption of the drd gene results in a severe defect in the movement of food through the Drosophila gut. During the ninety minutes following a meal, the amount of food transferred into the midguts of mutant flies is undetectable (fig. 2). Over the longer term however, the majority of mutant flies are able to move enough food into their midguts for blue dye to be visible (fig. 1b). This result is consistent with our previous finding that defecation rates in mutant flies are severely reduced but not eliminated (Blumenthal, 2008).
As predicted, the reduction in the amount of food being digested in the midgut is correlated with a drop in stored nutrients in the mutants. On the day of eclosion, both TG and glycogen stores in the mutant flies are equal to those of wild-type flies. By day 3, when the flies begin to die, TG stores have been essentially exhausted. Glycogen levels in the mutants remain at the same low level through the first three days post-eclosion. In contrast, glycogen levels in wild-type flies rise significantly on the day after eclosion and remain constant thereafter. This post-eclosion increase in glycogen stores is likely the result of feeding, as has been shown in the mosquito, Aedes taeniorhynchius (Nayar and Sauerman, Jr., 1974); thus the failure of mutant flies to increase their glycogen stores is further evidence that the normal movement of food is disrupted.
Although all drdlwf mutant flies have a short lifespan and reduced amounts of dye in their midguts, other parameters such as age at death and crop volume at steady state and after starvation and refeeding show marked variability. In previous work (Blumenthal, 2008), we observed variability in the crop volumes of the mutants and had speculated that those flies with small crops might be the most severely affected and be too weak to feed. Our current data are consistent with this interpretation. In particular, 11 out of 21 of the flies shown in figure 1 have both low crop scores and low midgut scores, suggesting that these flies are not ingesting significant amounts of food; the same fraction of flies (40–60%) kept on either instant or regular food die on day 3, the same day that almost all of the starved flies die. Thus, approximately half of the mutants phenotypically resemble starved flies. At the other extreme, a significant fraction of the mutant flies survive well past day 3 when maintained on cornmeal-molasses food (figure 4). It is likely that this phenotypic variability explains the rise in mean glycogen stores seen on day 4 in figure 3b. By this day, the most severely affected mutant flies have died, so while earlier data points were derived from a random selection of mutant flies, the measurement on day 4 is only derived from the longer-lived individuals. The rise in glycogen stores in this subpopulation suggests that these flies are able to digest enough food to maintain a positive energy balance, at least temporarily. Why this energy would be stored only as glycogen and not also as TG is uncertain, although the storage and utilization of glycogen and TG in the adult fly are known to be genetically separable processes (Gronke et al., 2007). It remains to be determined what physiological variables underlie the spectrum of phenotypic severity in the mutant flies and what specific components of the cornmeal-molasses food support extended survival compared with instant food.
There are three potential explanations for the midgut-filling defect in drd mutant flies. First, there could be some blockage in the foregut or cardia which physically prevents ingested food from entering the midgut. Second, the regulation of the stomodaeal valve in the cardia could be abnormal, such that the valve remains closed and does not allow the passage of food. Finally, the mutation could affect the contractions of the crop musculature, preventing the normal propulsion of food up the crop duct and into the midgut. While our data do not differentiate between the first two mechanisms, they are not consistent with the third. Our direct measurements of crop motility show that mutant flies actually have a higher rate of spontaneous contractions then do wild-type flies, making drd the first Drosophila gene known to alter crop motility, directly or indirectly, when mutated. It has previously been reported that crop motility is enhanced by engorgement of the crop in the blowfly, Phormia regina (Thomson, 1975) and by a reduction in hemolymph carbohydrate levels in the honeybee, Apis mellifera (Roces and Blatt, 1999). As we have shown that both crop engorgement and reduced carbohydrate stores are caused by mutation of drd, the observed enhancement in crop motility could be a consequence rather than a cause of the defective food movement. Further evidence for a defect in the cardia and not the crop comes from our observation that a significant number (6/26) of mutant flies had engorged crops after being starved for 48 hours on agar. In Phormia, it has been shown that ingested water moves directly into the midgut, while only food with high caloric content is stored in the crop (Stoffolano, Jr., 1995). This regulation of food destination is thought to prevent the fly from expending energy carrying large amounts of non-nutritive material. If such a regulatory mechanism is also operational in other dipteran species, including Drosophila, then the engorgement of mutant flies’ crops with water could not be explained by a defect in crop motility, as the water normally would never have entered the crop. Rather, such engorgement is more consistent with a physical or regulatory defect in the cardia that inhibits the passage of food or water into the midgut.
In an earlier study of flies that were mosaic for an allele of drd, Hotta and Benzer determined that the survival phenotype of individual mosaic flies was most closely linked to the genotype of the head, which the authors interpreted as indicating a role for drd within the brain (Hotta and Benzer, 1972). This result is consistent with one interpretation of our current data, that drd mutants exhibit misregulation of food transit through the cardia. While there are no data currently available about the control of the stomodaeal valve by the brain in Drosophila, the cardia is known to be innervated by the subesophageal ganglion via the stomodaeal nerve (Miller, 1950). Thus, it is plausible that death or dysfunction of neurons within the brain could be responsible for the gut phenotypes described in the current study. Future resolution of this issue should be possible using tissue-specific rescue of drd expression. We believe that drd mutant flies represent an excellent model for advancing our understanding of the regulation of insect feeding behavior and digestive function.
Acknowledgments
We wish to thank Dr. Deborah Hoshizaki for TG, protein, and glycogen assay protocols. Research was supported by NIH 1R15 GM080682 to E.M. Blumenthal. E.M. Bacon was a student in the Marquette Dept. of Biological Sciences Undergraduate Summer Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benzer S. From the gene to behavior. Journal of the American Medical Association. 1971;218:1015–1022. [PubMed] [Google Scholar]
- 2.Bernays EA. Regulation of feeding behavior. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry, and pharmacology. Regulation: digestion, nutrition, excretion. Vol. 4. Pergamon Press; Oxford: 1985. pp. 1–32. [Google Scholar]
- 3.Blumenthal EM. Cloning of the neurodegeneration gene drop-dead and characterization of additional phenotypes of its mutation. Fly. 2008;2:180–188. doi: 10.4161/fly.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- 5.Davey KG, Treherne JE. Studies on crop function in the cockroach (Periplaneta americana L.). II. The nervous control of crop-emptying. Journal of Experimental Biology. 1963;40:775–780. [Google Scholar]
- 6.Dimitriadis VK, Papamanoli E. Functional morphology of the crop of Drosophila auraria. Cytobios. 1992;69:143–152. [PubMed] [Google Scholar]
- 7.Duttlinger A, Berry K, Nichols R. The different effects of three Drosophila melanogaster dFMRFamide-containing peptides on crop contractions suggest these structurally related peptides do not play redundant functions in gut. Peptides. 2002;23:1953–1957. doi: 10.1016/s0196-9781(02)00179-1. [DOI] [PubMed] [Google Scholar]
- 8.Duve H, Audsley N, Weaver RJ, Thorpe A. Triple co-localisation of two types of allatostatin and an allatotropin in the frontal ganglion of the lepidopteran Lacanobia oleracea (Noctuidae): innervation and action on the foregut. Cell and Tissue Research. 2000;300:153–163. doi: 10.1007/s004410000192. [DOI] [PubMed] [Google Scholar]
- 9.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of feeding behavior in adult Drosophila melanogaster varies with feeding regime and nutritional state. Journal of Experimental Biology. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 10.Gelperin A. Control of crop emptying in the blowfly. Journal of Insect Physiology. 1966;12:331–345. doi: 10.1016/0022-1910(66)90034-5. [DOI] [PubMed] [Google Scholar]
- 11.Gelperin A. Stretch receptors in the foregut of the blowfly. Science. 1967;157:208–210. doi: 10.1126/science.157.3785.208. [DOI] [PubMed] [Google Scholar]
- 12.Gelperin A. Regulation of feeding. Annual Review of Entomology. 1971;16:365–378. [Google Scholar]
- 13.Gronke S, Muller G, Hirsch J, Fellert S, Andreou A, Haase T, Jackle H, Kuhnlein RP. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007;5:e137. doi: 10.1371/journal.pbio.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hotta Y, Benzer S. Mapping of behaviour in Drosophila mosaics. Nature. 1972;240:527–535. doi: 10.1038/240527a0. [DOI] [PubMed] [Google Scholar]
- 15.Kaminski S, Orlowski E, Berry K, Nichols R. The effects of three Drosophila melanogaster myotropins on the frequency of foregut contractions differ. Journal of Neurogenetics. 2002;16:125–134. doi: 10.1080/01677060213156. [DOI] [PubMed] [Google Scholar]
- 16.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddrell SHP, Gardiner BOC. The permeability of the cuticular linnig of the insect alimentary canal. Journal of Experimental Biology. 1980;85:227–237. [Google Scholar]
- 18.Melcher C, Pankratz MJ. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biology. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller A. The internal anatomy and histology of the imago of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. Cold Spring Harbor Laboratory Press; Plainview, NY: 1950. pp. 420–534. [Google Scholar]
- 20.Nayar JK, Sauerman DM., Jr Long-term regulation of sucrose intake by the female mosquito, Aedes taeniorhynchius. Journal of Insect Physiology. 1974;20:1203–1208. doi: 10.1016/0022-1910(74)90226-1. [DOI] [PubMed] [Google Scholar]
- 21.Predel R, Rapus J, Eckert M. Myoinhibitory neuropeptides in the American cockroach. Peptides. 2001;22:199–208. doi: 10.1016/s0196-9781(00)00383-1. [DOI] [PubMed] [Google Scholar]
- 22.Price MD, Merte J, Nichols R, Koladich PM, Tobe SS, Bendena WG. Drosophila melanogaster flatline encodes a myotropin orthologue to Manduca sexta allatostatin. Peptides. 2002;23:787–794. doi: 10.1016/s0196-9781(01)00649-0. [DOI] [PubMed] [Google Scholar]
- 23.Richer S, Stoffolano JG, Jr, Yin CM, Nichols R. Innervation of dromyosuppressin (DMS) immunoreactive processes and effect of DMS and benzethonium chloride on the Phormia regina (Meigen) crop. Journal of Comparative Neurology. 2000;421:136–142. [PubMed] [Google Scholar]
- 24.Roces F, Blatt J. Haemolymph sugars and the control of the proventriculus in the honey bee Apis mellifera. Journal of Insect Physiology. 1999;45:221–229. doi: 10.1016/s0022-1910(98)00116-4. [DOI] [PubMed] [Google Scholar]
- 25.Stoffolano JG., Jr . Regulation of a carbohydrate meal in the adult Diptera, Lepidoptera, and Hymenoptera. In: Chapman RF, de Boer G, editors. Regulatory mechanisms in insect feeding. Chapman & Hall; New York: 1995. pp. 210–247. [Google Scholar]
- 26.Thomson AJ. Regulation of crop contraction in the blowfly Phormia regina Meigen. Canadian Journal of Zoology. 1975;53:451–455. doi: 10.1139/z75-058. [DOI] [PubMed] [Google Scholar]
- 27.Treherne JE. Glucose absorption in the cockroach. Journal of Experimental Biology. 1957;34:478–485. [Google Scholar]
- 28.Wu Q, Wen T, Lee G, Park JH, Cai HN, Shen P. Developmental control of foraging and social behavior by the Drosophila neuropeptide Y-like system. Neuron. 2003;39:147–161. doi: 10.1016/s0896-6273(03)00396-9. [DOI] [PubMed] [Google Scholar]