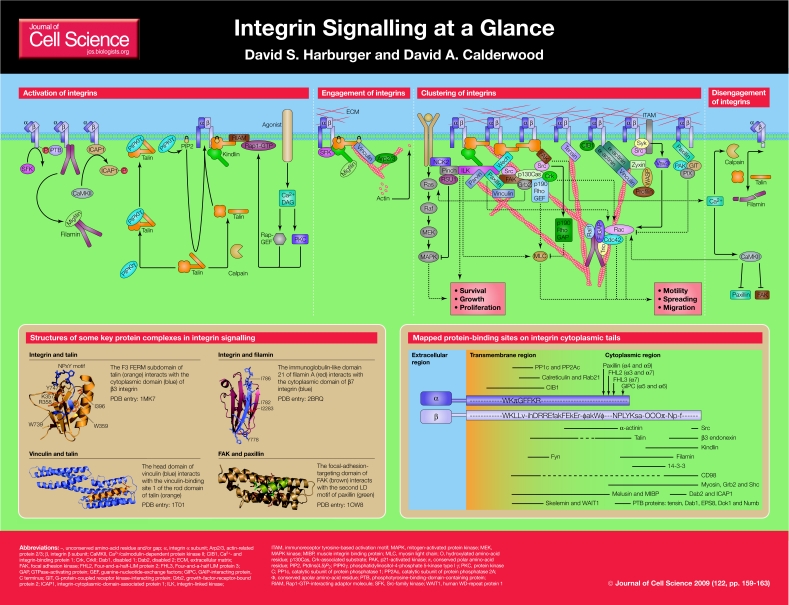
Integrins are a major family of cell-surface-adhesion receptors that are expressed in all metazoans. They are heterodimers of noncovalently associated α and β subunits, each of which is a single-pass type I transmembrane protein (Humphries et al., 2006; Hynes, 2002). The specific binding of the extracellular domains of integrins to extracellular-matrix (ECM) proteins or, in some cases, to counter-receptors on adjacent cells, supports cell adhesion and is crucial for embryonic development, tissue maintenance and repair, host defence and haemostasis. These processes rely on the linkage of integrins to the intracellular cytoskeleton through the generally short integrin cytoplasmic tails; such linkage permits the bi-directional transmission of force across the plasma membrane (Calderwood et al., 2000; Evans and Calderwood, 2007). In addition to their mechanical roles in anchorage, integrins transmit chemical signals into the cell (outside-in signalling), providing information on its location, local environment, adhesive state and surrounding matrix (Hynes, 2002; Miranti and Brugge, 2002). These signals determine cellular responses such as migration, survival, differentiation and motility, and provide a context for responding to other inputs, including those transmitted by growth-factor- or G-protein-coupled receptors. In addition to outside-in signalling, integrins can regulate their affinity for extracellular ligands. They do this by undergoing conformational changes in their extracellular domains that occur in response to signals that impinge upon the integrin cytoplasmic tails – a process that is termed inside-out signalling or activation (Calderwood, 2004).
Figure 1.
Outside-in and inside-out signalling require dynamic, and spatially and temporally regulated assembly and disassembly of multiprotein complexes that form around the cytoplasmic tails of integrins. Using the large literature on integrin signalling, Geiger and colleagues have recently described a network of 156 components (linked via 690 interactions) that make up the integrin `adhesome' (Zaidel-Bar et al., 2007). To summarise integrin signalling briefly, therefore, we must make generalisations and omit many reported interactions and pathways. Furthermore, the conservation of integrin β-tails means that their interactions can be generalised more readily than those of the more diverse α-tails, and this is likely to bias the view of integrin signalling that we present. We focus on integrin-proximal events because these allow us to illustrate signalling to and from integrins more easily. The specifics of extracellular ligand binding and the range of known integrin ligands are reviewed elsewhere (Humphries et al., 2006; Luo et al., 2007).
In this article, we provide an overview of integrin-based interaction networks and their role in signalling. For the sake of clarity, we depict signalling as an ordered series of events from integrin activation, to integrin engagement and initial signalling, to integrin clustering and focal adhesion assembly and, subsequently, to integrin inactivation. In most instances these steps are reversible and depend on the specific integrins that are involved, the nature, organisation and mechanical properties of the ECM, the cell type and its contractility, the presence of co-signalling receptors, and even the subcellular localisation of the integrin. These variables contribute both to the considerable diversity in integrin-based adhesions and to the flexibility in signalling that enables integrins to regulate a wide range of cellular processes.
Integrin activation
The regulation of the affinity of integrins for their extracellular ligands (integrin activation and inactivation) was first appreciated in blood cells, whose aggregation within the circulation or integrin-dependent adhesion to vessel walls must be strictly localised to appropriate sites (Miranti and Brugge, 2002). Platelet and leukocyte integrins remain the best-characterised systems for the study of integrin activation; however, integrin activation is a widespread phenomenon that is important in many cell types, in which it regulates matrix remodelling, angiogenesis, tissue form ation and cell migration (Calderwood, 2004).
The role of talin in integrin activation
Over the past 10 years, the binding of talin to the cytoplasmic tail of integrin-β subunits has been established to have a key role in integrin activation (Calderwood, 2004; Ginsberg et al., 2005; Tadokoro et al., 2003). Binding of the phosphotyrosine-binding (PTB)-domain-like subdomain of the protein 4.1, ezrin, radixin, moesin (FERM) domain of talin to the conserved WxxxNP(I/L)Y motif of the β-integrin tail permits additional weaker interactions between talin and the membrane-proximal region of the tail that trigger integrin activation, probably through the disruption of inhibitory interactions between α- and β-subunit cytoplasmic tails (Wegener et al., 2007). The central role of talin in integrin activation in vivo is supported by studies in transgenic mice that show the importance of talin-integrin interactions for platelet aggregation (Nieswandt et al., 2007; Petrich et al., 2007). Whether talin also activates invertebrate integrins is less clear – talin-integrin interactions are required for strengthening interactions between αPS2βPS integrin and the ECM in Drosophila embryos and integrin-activating mutations bypass the need for talin binding (Tanentzapf and Brown, 2006), but ligand-mimetic-antibody binding to a variety of cell lines suggests that talin is neither sufficient for αPS2βPS activation, nor necessary to maintain its basal activation state (Helsten et al., 2008). Talin also binds to actin and to numerous cytoskeletal and signalling proteins (Critchley and Gingras, 2008), thereby linking activated integrins directly to signalling and cytoskeletal systems. These findings have led to a search for the mechanisms that regulate talin-integrin interactions and so control activation.
Talin exists in an autoinhibited head-tail conformation that can be released by calpain-mediated proteolysis or by binding of phosphatidylinositol (4,5)-bisphosphate (PtdIns(4,5)P2) (Calderwood, 2004; Goksoy et al., 2008). Notably, even when in its autoinhibited conformation, talin binds to and activates one splice variant of the PtdIns(4,5)P2-producing enzyme phosphatidylinositol phosphate kinase type Iγ (PIPKIγ), which suggests that talin-PIPKIγ interactions initiate signalling that leads to enhanced PtdIns(4,5)P2 production, the release of talin autoinhibition, and binding and activation of integrins by talin (Goksoy et al., 2008). So far, the activation of the platelet integrin αIIbβ3 is the best-characterised pathway that leads to talin-mediated integrin activation. This pathway involves thrombin-receptor-triggered, protein kinase C (PKC)-dependent activation of the small GTPase Rap1. This results in formation of a complex that contains talin and the Rap1 effector Rap1-interacting molecule (RIAM) and that presumably activates talin and results in its targeting to integrins and integrin activation (Han et al., 2006; Watanabe et al., 2008).
Inhibitors of integrin activation
Several proteins can inhibit integrin activation by competing with talin for binding to the β-integrin tail. Structural analyses have revealed an overlap between talin- and filamin-binding sites on β-integrin tails, and competition for β-tail binding can regulate integrin activation (Garcia-Alvarez et al., 2003; Kiema et al., 2006). Integrins also bind to many PTB-domain-containing proteins (Calderwood et al., 2003) – including Dok1 and integrin-cytoplasmic-domain-associated protein 1 (ICAP1) – and these can compete with talin for binding to integrin and so can impair activation (Millon-Fremillon et al., 2008; Wegener et al., 2007). Threonine phosphorylation of the β-integrin tail (β7 residues 783, 784 or 785 or β2 residue 758), possibly mediated by PKC, inhibits filamin binding without altering talin binding, whereas Src-mediated tyrosine phosphorylation of the conserved integrin NP(I/L)Y motif, inhibits talin binding but enhances the binding of other PTB-domain-containing proteins (Kiema et al., 2006; Oxley et al., 2008; Takala et al., 2008); both of these examples indicate that competition can be modulated by integrin phosphorylation. With the exception of ICAP1, which reduces integrin affinity by antagonising the effects of talin and, consequently, slows down focal-adhesion assembly and modulates matrix surface-density sensing (Millon-Fremillon et al., 2008), the in vivo significance of competition with talin remains unclear. It is not known whether inhibitors bind constitutively to inactive integrins, or whether their major role is to inactivate integrins and thereby promote the turnover of adhesions and the termination of signalling. In addition, competitor proteins often have positive signalling or adaptor roles downstream of integrins, which complicates loss-of-function experiments.
Beyond talin – other activators of integrins
Despite the evidence that talin is required for integrin activation, several observations have indicated that other activating factors might cooperate with talin. These include the observation of differential sensitivity among integrins to activation by talin, the involvement of additional talin domains in integrin activation and sub-maximal integrin activation by talin (Bouaouina et al., 2008; Ma et al., 2008). In recent major advances, the first set of these factors – the proteins of the kindlin family – have been identified (Ma et al., 2008; Montanez et al., 2008; Moser et al., 2008). The PTB-like subdomain within the kindlin FERM domain is similar to that of talin (Kloeker et al., 2003) but binds to the second NPxY motif in β-integrin tails, whereas talin binds to the first motif. Inhibition of kindlin binding inhibits integrin activation, whereas co-expression of kindlin and talin activates integrins. Whether this observation applies to all integrins remains to be determined, as do its molecular basis and regulation. Nonetheless, inside-out integrin signalling appears to be a complex process that involves more interactions than those between talin and integrin.
Integrin engagement and signalling
Following the interaction of activating proteins with the β-integrin tail, conformational changes are propagated across the membrane to the extracellular domains of integrins, increasing their affinity for ligands. The exact nature of these conformational changes remains controversial; it is clear, however, that the packing of the α- and β-transmembrane domains changes (the domains separate, rotate or change their relative position within the membrane), and this is followed by alteration in the ligand-binding site in the integrin ectodomain (Ginsberg et al., 2005; Luo et al., 2007; Wegener et al., 2007). The binding of individual integrins, or small clusters of integrins, to ligand forms an initial talin-mediated connection between the cytoskeleton and the ECM; forces that are transmitted through these nascent adhesions contribute to the reinforcement of the ECM-cytoskeleton link and to the recruitment of additional cytoskeletal and signalling proteins (Giannone and Sheetz, 2006; Ginsberg et al., 2005).
As adhesions mature, multiprotein complexes assemble at the cytoplasmic face of clustered, ligand-bound integrins. These complexes are responsible for connecting integrins to the actin cytoskeleton and transmitting signals into the cell. Many elements of the outside-in integrin signalling network have now been identified, but there is considerable variability in the molecular make-up of integrin-containing adhesions, and how the dynamics of their assembly and turnover are determined remains poorly understood. Indeed, it is still unclear how the clustering of integrins, and the binding of ECM proteins, triggers signalling (Ginsberg et al., 2005). In this article, we highlight several key nodes in the network; we refer readers to Zaidel-Bar et al. (Zaidel-Bar et al., 2007) and Liu et al. (Liu et al., 2000) for more information on the many interactions that occur.
Focal adhesion kinase
One of the first integrin signalling molecules to be identified was focal adhesion kinase (FAK), which acts as a phosphorylation-regulated signalling scaffold and is important for adhesion turnover, Rho-family GTPase activation, cell migration and cross-talk between growth-factor signalling and integrins (Mitra et al., 2005). This ubiquitously expressed, essential protein contains an N-terminal FERM domain, a central kinase domain, proline-rich regions and a C-terminal focal-adhesion-targeting (FAT) domain that interacts with paxillin (see below) and talin. The FAK homologue Pyk2 shares many of these features, but Pyk2 and FAK have unique activities and are only partially redundant. In response to integrin clustering, the autophosphorylation of FAK generates docking sites for SH2-domain-containing proteins; these include Src kinases, which in turn become activated and phosphorylate FAK, promoting its kinase activity and its interaction with other proteins (see below). Structural analyses have revealed the mechanism of interaction between FAK and paxillin, and how FAK is inhibited by interactions between its FERM and kinase domains; they have also elucidated a role for PtdIns(4,5)P2 in FAK activation (Hayashi et al., 2002; Lietha et al., 2007; Mitra et al., 2005). Continuing studies seek to integrate this information into a comprehensive picture and to understand how FAK interactions are remodelled during adhesion turnover.
Src-family kinases
Src-family protein tyrosine kinases (SFKs) are rapidly activated following integrin-ligand interactions and SFK activity contributes to reinforcement of initial integrin-mediated adhesions by activating downstream kinases and adaptors (Giannone and Sheetz, 2006; Ginsberg et al., 2005). SFKs can bind directly to β-integrin tails in a tail- and SFK-specific manner, and this interaction contributes to activation of kinase activity and controls cell spreading (Arias-Salgado et al., 2003; Reddy et al., 2008). SFKs also bind to and phosphorylate FAK and FAK-binding proteins.
Integrin-linked kinase
The integrin-linked kinase (ILK) is another key node in integrin signalling (Legate et al., 2006). Similar to FAK, ILK is an essential protein that has a major role as a signalling scaffold at integrin adhesions. ILK forms a heterotrimeric complex with the LIM-domain protein PINCH and the actin- and paxillin-binding protein parvin. This complex serves as a hub in integrin signaling networks and, in mammals, formation of the complex precedes, and is required for, correct targeting of its components to integrin-mediated adhesions; complex formation also protects its components from proteasomal degradation (Legate et al., 2006). ILK contains an N-terminal ankyrin-repeat domain that mediates protein interactions with PINCH1 or PINCH2, and a C-terminal kinase domain that supports interactions with α-, β- or γ-parvin, paxillin (see below) and, possibly, β-integrin tails (Hannigan et al., 2005; Legate et al., 2006). The kinase domain lacks catalytic residues that are normally conserved among protein kinases, and whether ILK has kinase activity remains highly controversial (Hannigan et al., 2005; Legate et al., 2006). Nonetheless, ILK clearly has a central role in integrin signalling and cytoskeletal connections, and this role is conserved from invertebrates to mammals. In contrast to FAK, there are only few structural data available for ILK and this remains an impediment to a detailed understanding of its regulation and activity. ILK also interacts with kindlin proteins (Mackinnon et al., 2002; Montanez et al., 2008), and this might account for observations that implicate ILK in integrin activation (Tucker et al., 2008).
Paxillin
Paxillin, a FAK- and ILK-binding protein, is another essential signalling scaffold that is recruited early to integrin adhesions (Deakin and Turner, 2008). Paxillin contains several protein-protein interaction modules (leucine-rich repeats, a proline-rich region and LIM domains) and its numerous phosphorylation sites provide additional regulated sites of protein-protein interaction. Together, they mediate the binding of kinases (e.g. FAK, Src and ILK), phosphatases (e.g. PTP-PEST), actin-binding proteins (e.g. vinculin and the parvins) and regulators and effectors of the Rho family of small GTPases (e.g. the CrkII-DOCK180-ELMO complex and PIX). Paxillin also binds directly to the α4-integrin cytoplasmic tail, and this accounts for the ability of α4 integrin to promote cell migration and the recruitment of leukocytes to inflammatory sites (Kummer and Ginsberg, 2006). Some interactions of paxillin are understood at the structural level [e.g. see the X-ray crystal structure of a FAK-paxillin complex (Hoellerer et al., 2003) on the accompanying poster], and competition between potential binding partners, regulation by conformational changes and signal-dependent phosphorylation probably explain the ability of paxillin to coordinate multiple interactions and to regulate dynamic processes such as adhesion turnover and migration.
Vinculin
Vinculin does not directly bind to β-integrin tails, but interacts with many other focal-adhesion proteins including F-actin, talin, α-actinin, paxillin, VASP and Arp2/3. Vinculin is important for integrin-mediated cell adhesion and vinculin-null cells exhibit reduced spreading, enhanced focal-adhesion turnover and increased random migration (Ziegler et al., 2006). The Arp2/3-vinculin interaction might account for the role of vinculin in cell spreading. The crystal structure of vinculin has been solved and reveals an autoinhibited conformation; some controversy remains, however, as to the exact mechanism for release of autoinhibition (Ziegler et al., 2006).
Other integrin-signalling proteins
Many other important integrin-signalling proteins have been, and continue to be, identified. Numerous knockouts have been generated and analysed, and the structural basis of more interactions is being elucidated. These should yield insights into the regulatory steps in integrin signalling, the roles of competition for binding, as well as the hierarchy of dependency of interprotein interactions. In addition, integrins make many important direct or indirect interactions with other transmembrane signalling proteins, including those from the growth-factor receptor, syndecan and tetraspanin families. We refer readers to several reviews (Alam et al., 2007; Morgan et al., 2007; Berditchevski, 2001) for a detailed discussion of these interactions.
Integrin disengagement
Integrins must disengage from their ligands and disassemble intracellular multiprotein complexes to terminate adhesion signalling or facilitate cell migration. Depending on the conditions, adhesions can entirely disassemble, remodel or slide. This is achieved by altering the extent of association of proteins with the adhesion complex through competition, phosphorylation and proteolysis, and might be regulated by applied forces. As discussed above, integrin phosphorylation and competitor binding might displace talin from β-integrin tails, favouring inactive conformations of the integrin and altering adhesion formation or turnover (Millon-Fremillon et al., 2008). Proteolysis of talin by the intracellular protease calpain also enhances adhesion turnover (Franco et al., 2004) and calpain proteolysis of β-integrin tails modulates integrin signalling, favouring retraction rather than cell spreading (Flevaris et al., 2007). As mentioned above, FAK and paxillin signalling also have roles in adhesion turnover (Deakin and Turner, 2008; Mitra et al., 2005).
Conclusions and perspectives
A complete understanding of the complex and dynamic process of integrin activation is some way off, although significant progress has been made. Additional structural studies are likely to resolve remaining questions about the nature of the conformational changes in integrin ectodomains upon activation, and recent advances in characterising the membrane-spanning regions of integrins (Lau et al., 2008a; Lau et al., 2008b) will be key to building a complete picture of how information is passed across the membrane. Further characterisation of the pathways that regulate integrin-talin interactions, and of the structural basis for this regulation, is needed. The structural basis of integrin-kindlin interactions, and perhaps the interactions of integrins with other yet-to-be-identified co-activators, must also be addressed.
Many of the players in outside-in signalling have been identified, but an improved understanding of the specificity and dynamics of interactions is needed. The continuing structural analysis of crucial multiprotein complexes, as well as recent technical advances in imaging the localisation and relative motion of proteins during adhesion formation and turnover (Brown et al., 2006; Hu et al., 2007), are indicators of progress in these areas. The effect of matrix organisation on signalling is now being investigated, and it is thought that three-dimensional matrices elicit different, and often more physiologically relevant, signalling activities in many cell types (Green and Yamada, 2007). In addition, the impact of matrix stiffness and mechanical or shear forces on integrin signalling, and the molecular basis for these effects will continue to be important areas for research (Evans and Calderwood, 2007; Giannone and Sheetz, 2006). Finally, the importance of integrin trafficking in regulating cell adhesion, migration and signalling is now being appreciated (Caswell and Norman, 2008) and must be integrated into our picture of integrin signalling. In summary, inside-out and outside-in signalling through integrins involves complex, dynamic, regulated interactions that link intracellular and extracellular protein networks and serve to tune cellular responses to extracellular cues. Our understanding of the molecular basis of these processes continues to advance, bringing with it the potential for therapies that modulate integrin signalling for the treatment of cancer, and cardiovascular and inflammatory diseases.
Acknowledgments
The work of D.S.H. and D.A.C. on integrin signalling is supported by grants from the National Institutes of Health (RO1 GM068600; R21 HL089433), the US-Israel Binational Science Foundation and a Swebelius Cancer Research Award from the Yale Cancer Center. D.S.H. is supported by a National Science Foundation Graduate Research Fellowship. We thank Tony Koleske, Yatish Lad and members of D.A.C.'s laboratory for helpful discussions and comments on the manuscript. Deposited in PMC for release in 12 months.
References
- Alam, N., Goel, H. L., Zarif, M. J., Butterfield, J. E., Perkins, H. M., Sansoucy, B. G., Sawyer, T. K. and Languino, L. R. (2007). The integrin-growth factor receptor duet. J. Cell Physiol. 213, 649-653. [DOI] [PubMed] [Google Scholar]
- Arias-Salgado, E. G., Lizano, S., Sarkar, S., Brugge, J. S., Ginsberg, M. H. and Shattil, S. J. (2003). Src kinase activation by direct interaction with the integrin β cytoplasmic domain. Proc. Natl. Acad. Sci. USA 100, 13298-13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski, F. (2001). Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 114, 4143-4151. [DOI] [PubMed] [Google Scholar]
- Bouaouina, M., Lad, Y. and Calderwood, D. A. (2008). The N-terminal domains of talin cooperate with the phosphotyrosine binding-like domain to activate β1 and β3 integrins. J. Biol. Chem. 283, 6118-6125. [DOI] [PubMed] [Google Scholar]
- Brown, C. M., Hebert, B., Kolin, D. L., Zareno, J., Whitmore, L., Horwitz, A. R. and Wiseman, P. W. (2006). Probing the integrin-actin linkage using high-resolution protein velocity mapping. J. Cell Sci. 119, 5204-5214. [DOI] [PubMed] [Google Scholar]
- Calderwood, D. A. (2004). Integrin activation. J. Cell Sci. 117, 657-666. [DOI] [PubMed] [Google Scholar]
- Calderwood, D. A., Shattil, S. J. and Ginsberg, M. H. (2000). Integrins and actin filaments: reciprocal regulation of cell adhesion and signaling. J. Biol. Chem. 275, 22607-22610. [DOI] [PubMed] [Google Scholar]
- Calderwood, D. A., Fujioka, Y., de Pereda, J. M., Garcia-Alvarez, B., Nakamoto, T., Margolis, B., McGlade, C. J., Liddington, R. C. and Ginsberg, M. H. (2003). Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. USA 100, 2272-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell, P. and Norman, J. (2008). Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 18, 257-263. [DOI] [PubMed] [Google Scholar]
- Critchley, D. R. and Gingras, A. R. (2008). Talin at a glance. J. Cell Sci. 121, 1345-1347. [DOI] [PubMed] [Google Scholar]
- Deakin, N. O. and Turner, C. E. (2008). Paxillin comes of age. J. Cell Sci. 121, 2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E. A. and Calderwood, D. A. (2007). Forces and bond dynamics in cell adhesion. Science 316, 1148-1153. [DOI] [PubMed] [Google Scholar]
- Flevaris, P., Stojanovic, A., Gong, H., Chishti, A., Welch, E. and Du, X. (2007). A molecular switch that controls cell spreading and retraction. J. Cell Biol. 179, 553-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, S. J., Rodgers, M. A., Perrin, B. J., Han, J., Bennin, D. A., Critchley, D. R. and Huttenlocher, A. (2004). Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6, 977-983. [DOI] [PubMed] [Google Scholar]
- Garcia-Alvarez, B., de Pereda, J. M., Calderwood, D. A., Ulmer, T. S., Critchley, D. R., Campbell, I. D., Ginsberg, M. H. and Liddington, R. C. (2003). Structural determinants of integrin recognition by talin. Mol. Cell 11, 49-58. [DOI] [PubMed] [Google Scholar]
- Giannone, G. and Sheetz, M. P. (2006). Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16, 213-223. [DOI] [PubMed] [Google Scholar]
- Ginsberg, M. H., Partridge, A. and Shattil, S. J. (2005). Integrin regulation. Curr. Opin. Cell Biol. 17, 509-516. [DOI] [PubMed] [Google Scholar]
- Goksoy, E., Ma, Y. Q., Wang, X., Kong, X., Perera, D., Plow, E. F. and Qin, J. (2008). Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. A. and Yamada, K. M. (2007). Three-dimensional microenvironments modulate fibroblast signaling responses. Adv. Drug Deliv. Rev. 59, 1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., Lim, C. J., Watanabe, N., Soriani, A., Ratnikov, B., Calderwood, D. A., Puzon-McLaughlin, W., Lafuente, E. M., Boussiotis, V. A., Shattil, S. J. et al. (2006). Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 16, 1796-1806. [DOI] [PubMed] [Google Scholar]
- Hannigan, G., Troussard, A. A. and Dedhar, S. (2005). Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat. Rev. Cancer 5, 51-63. [DOI] [PubMed] [Google Scholar]
- Hayashi, I., Vuori, K. and Liddington, R. C. (2002). The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat. Struct. Biol. 9, 101-106. [DOI] [PubMed] [Google Scholar]
- Helsten, T. L., Bunch, T. A., Kato, H., Yamanouchi, J., Choi, S. H., Jannuzi, A. L., Feral, C. C., Ginsberg, M. H., Brower, D. L. and Shattil, S. J. (2008). Differences in regulation of Drosophila and vertebrate integrin affinity by talin. Mol. Biol. Cell 19, 3589-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoellerer, M. K., Noble, M. E., Labesse, G., Campbell, I. D., Werner, J. M. and Arold, S. T. (2003). Molecular recognition of paxillin LD motifs by the focal adhesion targeting domain. Structure 11, 1207-1217. [DOI] [PubMed] [Google Scholar]
- Hu, K., Ji, L., Applegate, K. T., Danuser, G. and Waterman-Storer, C. M. (2007). Differential transmission of actin motion within focal adhesions. Science 315, 111-115. [DOI] [PubMed] [Google Scholar]
- Humphries, J. D., Byron, A. and Humphries, M. J. (2006). Integrin ligands at a glance. J. Cell Sci. 119, 3901-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. [DOI] [PubMed] [Google Scholar]
- Kiema, T., Lad, Y., Jiang, P., Oxley, C., Baldassarre, M., Wegener, K. L., Campbell, I. D., Ylanne, J. and Calderwood, D. A. (2006). The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 21, 337-347. [DOI] [PubMed] [Google Scholar]
- Kloeker, S., Major, M. B., Calderwood, D. A., Ginsberg, M. H., Jones, D. A. and Beckerle, M. C. (2003). The Kindler syndrome protein is regulated by TGFβ and involved in integrin-mediated adhesion. J. Biol. Chem. 279, 6824-6833. [DOI] [PubMed] [Google Scholar]
- Kummer, C. and Ginsberg, M. H. (2006). New approaches to blockade of alpha4-integrins, proven therapeutic targets in chronic inflammation. Biochem. Pharmacol. 72, 1460-1468. [DOI] [PubMed] [Google Scholar]
- Lau, T. L., Dua, V. and Ulmer, T. S. (2008a). Structure of the integrin αIIb transmembrane segment. J. Biol. Chem. 283, 16162-16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, T. L., Partridge, A. W., Ginsberg, M. H. and Ulmer, T. S. (2008b). Structure of the integrin β3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry 47, 4008-4016. [DOI] [PubMed] [Google Scholar]
- Legate, K. R., Montanez, E., Kudlacek, O. and Fassler, R. (2006). ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell. Biol. 7, 20-31. [DOI] [PubMed] [Google Scholar]
- Lietha, D., Cai, X., Ceccarelli, D. F., Li, Y., Schaller, M. D. and Eck, M. J. (2007). Structural basis for the autoinhibition of focal adhesion kinase. Cell 129, 1177-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Calderwood, D. A. and Ginsberg, M. H. (2000). Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113, 3563-3571. [DOI] [PubMed] [Google Scholar]
- Luo, B. H., Carman, C. V. and Springer, T. A. (2007). Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y. Q., Qin, J., Wu, C. and Plow, E. F. (2008). Kindlin-2 (Mig-2): a co-activator of β3 integrins. J. Cell Biol. 181, 439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackinnon, A. C., Qadota, H., Norman, K. R., Moerman, D. G. and Williams, B. D. (2002). C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr. Biol. 12, 787-797. [DOI] [PubMed] [Google Scholar]
- Millon-Fremillon, A., Bouvard, D., Grichine, A., Manet-Dupe, S., Block, M. R. and Albiges-Rizo, C. (2008). Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent β1-integrin affinity. J. Cell Biol. 180, 427-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranti, C. K. and Brugge, J. S. (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83-E90. [DOI] [PubMed] [Google Scholar]
- Mitra, S. K., Hanson, D. A. and Schlaepfer, D. D. (2005). Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell. Biol. 6, 56-68. [DOI] [PubMed] [Google Scholar]
- Montanez, E., Ussar, S., Schifferer, M., Bosl, M., Zent, R., Moser, M. and Fassler, R. (2008). Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 22, 1325-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, M. R., Humphries, M. J. and Bass, M. D. (2007). Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell. Biol. 8, 957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, M., Nieswandt, B., Ussar, S., Pozgajova, M. and Fassler, R. (2008). Kindlin-3 is essential for integrin activation and platelet aggregation. Nat. Med. 14, 325-330. [DOI] [PubMed] [Google Scholar]
- Nieswandt, B., Moser, M., Pleines, I., Varga-Szabo, D., Monkley, S., Critchley, D. and Fassler, R. (2007). Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J. Exp. Med. 204, 3113-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxley, C. L., Anthis, N. J., Lowe, E. D., Vakonakis, I., Campbell, I. D. and Wegener, K. L. (2008). An integrin phosphorylation switch: the effect of β3 integrin tail phosphorylation on Dok1 and talin binding. J. Biol. Chem. 283, 5420-5426. [DOI] [PubMed] [Google Scholar]
- Petrich, B. G., Fogelstrand, P., Partridge, A. W., Yousefi, N., Ablooglu, A. J., Shattil, S. J. and Ginsberg, M. H. (2007). The antithrombotic potential of selective blockade of talin-dependent integrin αIIbβ3 (platelet GPIIb-IIIa) activation. J. Clin. Invest. 117, 2250-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, K. B., Smith, D. M. and Plow, E. F. (2008). Analysis of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling. J. Cell Sci. 121, 1641-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., de Pereda, J. M., Ginsberg, M. H. and Calderwood, D. A. (2003). Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103-106. [DOI] [PubMed] [Google Scholar]
- Takala, H., Nurminen, E., Nurmi, S. M., Aatonen, M., Strandin, T., Takatalo, M., Kiema, T., Gahmberg, C. G., Ylanne, J. and Fagerholm, S. C. (2008). Integrin β2 phosphorylation on THR758 acts as a molecular switch to regulate 14-3-3 and filamin binding. Blood 112, 1853-1856. [DOI] [PubMed] [Google Scholar]
- Tanentzapf, G. and Brown, N. H. (2006). An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat. Cell Biol. 8, 601-606. [DOI] [PubMed] [Google Scholar]
- Tucker, K. L., Sage, T., Stevens, J. M., Jordan, P. A., Jones, S., Barrett, N. E., St Arnaud, R., Frampton, J., Dedhar, S. and Gibbins, J. M. (2008). A dual role for integrin linked kinase in platelets: regulating integrin function and α-granule secretion. Blood 112, 4523-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., Bodin, L., Pandey, M., Krause, M., Coughlin, S., Boussiotis, V. A., Ginsberg, M. H. and Shattil, S. J. (2008). Mechanisms and consequences of agonist-induced talin recruitment to platelet integrin αIIbβ3. J. Cell Biol. 181, 1211-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener, K. L., Partridge, A. W., Han, J., Pickford, A. R., Liddington, R. C., Ginsberg, M. H. and Campbell, I. D. (2007). Structural basis of integrin activation by talin. Cell 128, 171-182. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar, R., Itzkovitz, S., Ma'ayan, A., Iyengar, R. and Geiger, B. (2007). Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, W. H., Liddington, R. C. and Critchley, D. R. (2006). The structure and regulation of vinculin. Trends Cell Biol. 16, 453-460. [DOI] [PubMed] [Google Scholar]