Summary
Integrins are αβ heterodimeric adhesion receptors that relay signals bidirectionally across the plasma membrane between the extracellular matrix and cell-surface ligands, and cytoskeletal and signalling effectors. The physical and chemical signals that are controlled by integrins are essential for intercellular communication and underpin all aspects of metazoan existence. To mediate such diverse functions, integrins exhibit structural diversity, flexibility and dynamism. Conformational changes, as opposed to surface expression or clustering, are central to the regulation of receptor function. In recent years, there has been intense interest in determining the three-dimensional structure of integrins, and analysing the shape changes that underpin the interconversion between functional states. Considering the central importance of the integrin signalling nexus, it is perhaps no surprise that obtaining this information has been difficult, and the answers gained so far have been complicated. In this Commentary, we pose some of the key remaining questions that surround integrin structure-function relationships and review the evidence that supports the current models.
Keywords: Function, Integrins, Structure
Introduction
It is now almost 20 years since it was first demonstrated that the ligand-binding activity of integrins is regulated by conformational changes (Frelinger et al., 1990), and more than a decade since integrins were fully recognised as allosteric proteins (Mould, 1996). Integrins are known to adopt three major conformational states: `inactive' (low affinity), `primed' or `active' (high affinity), and ligand occupied. Major advances in our understanding of these states have taken place in the past decade, including the determination of the first crystal structures of integrin dimers (Xiong et al., 2001; Xiong et al., 2002; Xiao et al., 2004) (Fig. 1), but controversy remains. Specifically, there has been much debate concerning the precise nature of each conformational state and the key shape changes that distinguish their formation. In the `switchblade' model (Beglova et al., 2002; Takagi et al., 2002; Shimaoka et al., 2002; Nishida et al., 2006), the three states correspond to conformations that are bent, extended and extended with an open headpiece. Broadly speaking, this model is currently the most widely accepted paradigm, but there is also evidence that integrins can bind ligand in a bent (or partially unbent) conformation (Arnaout et al., 2007).
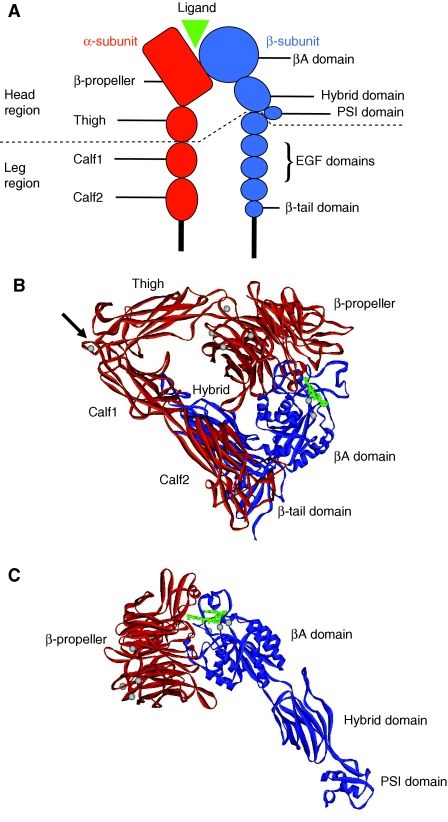
Fig. 1.
Integrin structure. (A) Schematic diagram of integrin structure. The overall structure is that of a head region [propeller and thigh domains of the α-subunit and the βA (also known as βI), hybrid and PSI domains of the β-subunit] supported on two legs that are made up of the calf1 and calf2 domains in the α-subunit and the EGF repeats and β-tail domain in the β-subunit. The binding of ligands takes place at an interface between the propeller domain and βA domain. (B) Ribbon diagram of the structure of the ectodomain of integrin αVβ3 in complex with the high-affinity ligand cyclic RGD peptide (Xiong et al., 2002). The α-subunit is shown in red, the β-subunit in blue; peptide is shown as a ball-and-stick model with atoms in green. Metal ions (silver spheres) occupy the base of the propeller and the top face of the βA domain. The protein is in a closed form, which is bent at the knees or `genu' (arrow). Some β-subunit domains are not visible in the structure. (C) Ribbon diagram of the structure of the head region of integrin αIIbβ3 in complex with the high-affinity ligand eptifibatide (Xiao et al., 2004). Colour coding is the same as in B. In this open structure the hybrid domain has swung outwards and the leg regions (not present) would be unbent so that the integrin is in an extended conformation, similar to that depicted in A.
Integrins are αβ heterodimers (Fig. 1), and their priming or activation from the inside out is thought to be initiated by separation of the two subunits at their cytoplasmic and transmembrane regions (Kim et al., 2003; Luo et al., 2004; Vinogradova et al., 2002), which then leads to unbending of the ligand-binding headpiece and conformational changes that increase ligand-binding affinity (Luo and Springer, 2006; Takagi and Springer, 2002). Inside-out integrin activation can be controlled by talin (Tadokoro et al., 2003), and crystal and nuclear magnetic resonance (NMR) structures of the complex comprising talin and the integrin β-subunit cytoplasmic domain have been solved (McCleverty et al., 2007; Wegener et al., 2007). There is also evidence that outside-in activation upon ligand binding stabilises an extended integrin conformer, which again leads to prolonged separation of the α- and β-subunit receptor legs, transmembrane regions and cytoplasmic domains (Luo and Springer, 2006).
Although the existence of conformational changes is incontrovertible, it is important to determine whether these changes are all-or-nothing responses or whether functional intermediate forms exist. Owing to a relative paucity of structural information, we do not have detailed insights into the structure-function relationship of different integrin dimers, and we do not know whether these vary between dimers. We also have limited information about the subcellular distribution of integrin conformations. Finally, because integrin activity and therefore function can be modulated in a variety of ways, including by ligand, divalent cations, reducing agents, monoclonal antibodies, the interaction of intracellular proteins (including talin), and engineered mutations, it is important to determine whether different agonists induce specific shape changes that result in different signalling responses. In this Commentary, we focus on the importance of transmembrane-leg separation and the bending and extension of integrins, pose some of the key remaining questions surrounding integrin structure-function relationships, and review the supporting evidence for the current models.
How do cytoplasmic factors control integrin conformation?
Binding of integrin β-subunit cytoplasmic domains to talin is a key convergence point for signals that regulate integrin activation (Tadokoro et al., 2003). It has been proposed that contact between the talin phosphotyrosine-binding (PTB) domain and the membrane-proximal region of the cytoplasmic domain is crucial for activation (Wegener et al., 2007). It has also been shown that Dok1 negatively regulates integrin activation (Oxley et al., 2008). The Dok1 PTB domain binds exclusively to the central NPLY motif of the β3 integrin cytoplasmic domain (Oxley et al., 2008), competes with talin for this site, but does not cause activation as it does not bind the membrane-proximal region. It has also been reported recently that kindlin-2 promotes integrin activation in a similar manner to that of talin (Shi et al., 2007b), and that kindlin-2 and talin share a common aspartic acid residue in the tyrosine-binding pocket. These reports imply that tyrosine phosphorylation may be a common mechanism for regulating inside-out signalling in integrins.
Most analyses of talin-integrin binding have used the isolated talin PTB domain, and it has been shown that the intact talin protein is a much less potent integrin activator. Recent structural studies, in combination with biochemical and mutational approaches, have provided an explanation for this discrepancy (Goksoy et al., 2008). The talin FERM domain, via its PTB domain, has been found to interact with the talin rod domain to restrain the molecule in an inactive conformation. The talin mutation M139A disrupts this PTB-rod interaction, but not the interaction of PTB with integrin, and therefore constitutively activates the platelet integrin αIIbβ3. Thus, in the closed state, the integrin membrane-proximal binding site of the talin head domain is masked by the rod domain. Following receipt of intracellular stimuli, talin undergoes a conformational change so that its PTB domain can then bind to the integrin membrane-proximal region. This, in turn, leads to separation of the integrin αβ cytoplasmic domain and inside-out activation. One possibility is that phosphatidylinositol (4,5)-bisphosphate (PIP2) binding serves as the trigger for the conformational change in talin. Using nuclear magnetic resonance (NMR), PIP2 has been shown to disrupt the inhibitory PTB-rod interaction (Goksoy et al., 2008). Taken together, these two studies (Goksoy et al., 2008; Oxley et al., 2008), along with other earlier structural studies (Garcia-Alvarez et al., 2003; Vinogradova et al., 2002; Wegener et al., 2007), have now provided the field with a clearer view of how conformational changes occur during talin-mediated integrin activation.
How are shape changes propagated across the plasma membrane?
Two recent papers that describe the three-dimensional structure of the integrin αIIb and β3-transmembrane segments have provided insights into how signals are transduced via the integrin transmembrane regions (Lau et al., 2008a; Lau et al., 2008b). These structures show that upon activation, the αβ extracellular domains connect to the transmembrane domains at different crossing angles. The structure of the αIIb transmembrane segment is characterised by a linear α-helix between I966 and K989, followed by a reversal of the backbone for residues F992-F993. This structure packs the two phenylalanine residues against the αIIb transmembrane helix and orientates the residues towards the lipid core of the membrane. Alanine substitution of either of these two residues leads to receptor activation (Hughes et al., 1996), implying that these residues are required for maintaining the inactive state. The authors conclude that the unusual G991-F993 motif of αIIb, which is almost completely conserved in integrin α-subunits, is a key element in the transduction of signals across the plasma membrane. Although the mechanism of inside-out signalling is now somewhat clearer, there are still a number of questions that remain to be answered concerning conformational states of the integrin extracellular domains.
Is bent integrin inactive?
In the crystal structure of integrin αVβ3, the bend in the integrin is at 135°, an obtuse angle (Fig. 1B). When αVβ3 and αIIbβ3 are engineered to be locked in this extreme conformation, they are unable to bind ligand, even though they are expressed on the cell surface (Takagi et al., 2002). In addition, negatively stained images of soluble αVβ3, αLβ2 and αXβ2 integrins show predominantly bent structures under conditions in which ligand binding is low, that is in which the C-termini are clasped, or when samples are prepared in Ca2+-containing buffer (Nishida et al., 2006; Takagi et al., 2002). However, electron microscopy (EM) images obtained under these conditions show that molecules exhibit varying degrees of bending, from 135° to almost right-angled forms. If this case were extrapolated to a cellular environment, the ligand-binding pocket might be positioned away from the cell membrane and available to bind ligand. This would produce a primed integrin that was not fully extended. Such a `breathing' movement has already been proposed as a mechanism for the conversion of bent to extended integrin (Beglova et al., 2002; Takagi et al., 2002), and would also allow stimulatory monoclonal antibodies to bind and exert their effect. In this context, modelling data combined with experimental hydrodynamic analyses of β3 integrins in solution (Rocco et al., 2008) suggest that a 20° head-tail unbending of αVβ3 integrin is necessary to achieve a good fit with the experimental transmission EM (TEM) and crystallographic data (Adair et al., 2005; Xiong et al., 2001).
There is also the question as to whether all integrin dimers are bent when inactive. Rotary shadowed images of integrin α5β1 with clasped C-termini reveal only extended conformers (Takagi et al., 2001) and, although the resolution of the images may not have been high enough to reveal bent conformers, this indicates that inactive integrin might not necessarily be bent. Furthermore, a cryo-EM reconstruction of unstimulated αIIbβ3 integrin indicated a partially extended, z-shaped conformation (Adair and Yeager, 2002) and, in the multi-resolution study mentioned above (Rocco et al., 2008), the same integrin was modelled to be fully extended at rest. Both structural and biochemical data indicate that inactive integrin adopts a more compact shape. However, the images of the very bent conformer were obtained with the extracellular domain in isolation, and the contribution of the transmembrane and cytoplasmic regions to the physiological conformation, as well as possible interaction of the integrin legs with other cell-surface receptors, remains poorly understood.
Is activated integrin extended?
Although current models of integrin function suggest that conversion to a high-affinity receptor requires extension, there is ample evidence to suggest that ligand-bound integrin can adopt a bent conformation. Crystallised αVβ3 integrin can bind a cyclic RGD-sequence-containing peptide while in the bent conformation (Xiong et al., 2002), and TEM images also show bent αVβ3 in complex with a fragment of fibronectin (Adair et al., 2005).
Further evidence has been gained from fluorescence resonance energy transfer (FRET) experiments using either integrin α4β1 (Chigaev et al., 2003; Chigaev et al., 2007) or αIIbβ3 (Coutinho et al., 2007). With α4β1, FRET between fluorescently tagged LDV-sequence-containing peptide, a ligand for α4β1, and labelled cell membrane was measured in the presence of various agonists. Mn2+ induces a 5 nm movement of the integrin away from the membrane and stimulation by chemokines produces a smaller 2.5 nm change. A fully extended integrin would require a 10 nm movement, therefore, the results not only provide evidence that an active or ligand-bound integrin does not need to straighten completely, but that different stimuli can induce apparently different conformations and that the degree of unbending of the receptor correlates with its activity. In addition, although the phorbol ester PMA was shown to activate α4β1, no accompanying extension of the receptor was detected (Chigaev et al., 2007). This finding might be explained by the effects of PMA on receptor clustering or trafficking.
One study measured changes in FRET between antibodies directed against the head-piece and leg regions of αIIbβ3 on platelets (Coutinho et al., 2007). On resting platelets, a separation of 7.0-7.5 nm between the headpiece and membrane was measured, which only slightly increased upon activation with ADP or thrombin receptor-activating peptide (TRAP), again suggesting that activated integrin can still be bent. Cryoelectron tomographic studies also indicate that αIIbβ3 remains the same height in reconstituted membranes after activation by Mn2+ (Ye et al., 2008).
Experiments investigating competition between a panel of antibodies directed against the calf2 domain and the βA domain (Fig. 1) of the same integrin also suggest that different agonists induce specific conformations (Calzada et al., 2002). On resting platelets, antibodies that bind to the βA domain cross-compete with those that recognise the calf2 domain of the α-subunit, suggesting a bent conformation. Surprisingly, activation using ADP or TRAP induces a conformational change that was interpreted as a closing of the bent conformation, in agreement with the FRET data above. Conversely, stimulation with arachidonic acid induces a different conformational change involving an opening of the αβ interface, which is consistent with extension of the integrin.
It is well known that different integrins within the same family differ in their response to agonists. Integrin αXβ2 is harder to activate than other β2 integrins (Lu et al., 2001), αIIbβ3 is more resistant to the effects of Mn2+ than αVβ3 (Kamata et al., 2005), and there is a differential response of β1 integrins to both Mn2+ and ligand as measured by the exposure of epitopes on the β-subunit (Bazzoni et al., 1998). How these differences relate to structure has not been resolved, but it is clear that the same agonist can have diverse influences on integrin function. It is also important to point out that the energy barrier between different conformational states is likely to vary for different integrins – for example, it has been shown that interactions between leg domains are stronger for αIIbβ3 than for αVβ3, which may help to explain why the default state of αIIbβ3 integrin on platelets is completely inactive, whereas αVβ3 integrin is constitutively active on many cell types (Kamata et al., 2005).
Recent structural data using two constructs of the integrin β2 subunit have revealed a possible level of complexity to the straightening of the receptor. A construct comprising the plexin, semaphorin and integrin (PSI) domain, the hybrid domain and epidermal growth factor (EGF)-like repeats 1 and 2 crystallised in a bent conformation; however, following the addition of EGF-3, they adopt an extended form. The differences in the two structures showed that rather than a simple unbending in one plane, the transition involves a twisting of EGF-2 relative to EGF-1, a change of angle of a disulphide bond, and disordering of an α-helix (Shi et al., 2007a). As most EM images of integrins show very poor resolution of the lower β-subunit leg, it seems likely that this region is extremely flexible and can adopt a variety of positions relative to the α-subunit, which remains fixed and stable. Electron tomographic analysis of αIIbβ3 integrin also shows the β3 lower leg in many different conformations (Iwasaki et al., 2005), and this raises the possibility that a twisting of the β-subunit around the stable α-subunit could yield intermediate conformers that are agonist-specific. Indeed, such a twisting could reveal activating epitopes in the β-subunit leg without the receptor necessarily fully extending.
In summary, upon activation, integrins certainly undergo a conformational change that involves unbending of the receptor. However, evidence is beginning to emerge that the degree of extension is both agonist- and integrin-specific. In addition, active integrin might not be necessarily fully extended. This would also suggest that a rapid conversion from the bent to extended conformation does not occur and that intermediate forms are functionally relevant. In addition, most of the current structural data are restricted to β2 and β3 integrins, the activation of which, by necessity, must be precisely controlled, and it remains to be seen whether this information can be extrapolated to β1 integrins on adherent cells.
How far does the hybrid domain move?
Although opinion on receptor extension is still varied, there is general agreement that ligand binding is accompanied by an outwards movement of the β-hybrid domain, that this represents the key conformational change in the switch from inactive to active integrin, and that this is a clear indicator of receptor affinity (Rocco et al., 2008; Takagi et al., 2003; Xiao et al., 2004). However, the extent of this swing-out is unclear, with reports varying from 10° to 80° (Adair and Yeager, 2002; Mould et al., 2003b; Xiao et al., 2004). This variation could be interpreted as evidence for intermediate conformations, particularly as the α7 helix in both the αA and βA domains has been shown to have a degree of elasticity (Jin et al., 2004; Shimaoka et al., 2003; Yang et al., 2004). However, it might also relate to the nature of the material used, because the smaller the integrin fragment – and therefore the lower the constraint imposed upon the hybrid domain – the larger the reported movement.
Molecular dynamics modelling has predicted that the hybrid domain of integrin αVβ3 swings open about 20° when the leg-region constraints are lifted (Puklin-Faucher et al., 2006), and that this also involves an inward movement of the α1 helix as previously shown (Mould et al., 2003a). These changes might be enough to induce alterations in receptor affinity, and were suggested to represent outside-in integrin priming (Puklin-Faucher et al., 2006). Further molecular dynamics analysis revealed that conversion to the state in which the hybrid domain was fully extended (an 80° movement) required the application of force to overcome the energy barrier needed for this change to occur. Thus, there is evidence to suggest that different positions of the hybrid domain relative to the βA domain are physiologically relevant. These findings also raise the question of whether full extension of an integrin with a concomitant full outward movement of the hybrid domain is only achieved once the cell is under tension, and thus represents a post-ligand-binding event. In addition, this Commentary highlights that we might have to be more precise in what we term `active' integrin, because it seems likely that different agonists have wide-ranging effects that are integrin-specific and what `activates' one receptor may not apply to all receptors.
How are conformational changes coupled?
The pathway of conformational change from the interior of the cell to the ligand-binding site of the integrin (a long-range distance of more than 20 nm) is a poorly researched topic, but it probably underpins cellular control of integrin activity. A major restraint that holds integrins in an inactive state appears to be the interaction between α- and β-cytoplasmic domains. As mentioned above, disruption of these interactions through the binding of proteins such as talin is proposed to be the first step in the pathway (Han et al., 2006). Consistent with this proposal, mutations that disrupt the interactions between the cytoplasmic domains lead to constitutively active integrins, whereas locking the legs of the integrin together prevents activation. A weakening of the interactions between the leg regions of the α- and β-subunits allows unbending of the receptor.
In the bent state, interactions between the hybrid domain and the leg domains prevent the outward swing of the hybrid domain, but this constraint is removed in the extended state and the hybrid domain is free to move. It should be noted that extension does not by itself cause increased affinity, although extension of the integrin on the cell surface is likely to result in increased capture efficiency of ligands (Chigaev et al., 2007). Instead, conformational changes in the head are the key determinant of ligand-binding activity, specifically the conformation of the βA domain, which, in turn, is determined by the position of the hybrid domain. Thus, a swing-out of the hybrid domain away from the α-subunit pulls downwards on the α7 helix of the βA domain and favours the upward movement of the α1 helix (Xiao et al., 2004). The motion of these two helices shifts the βA domain from a low-affinity into a high-affinity conformation (Mould et al., 2003a). Mutations that favour a downward shift of the α7 helix (Cheng et al., 2007; Hato et al., 2006; Mould et al., 2003a) also result in a high-affinity state.
How does force affect integrin activation?
Normally, receptor-ligand bonds are weakened by applied force because the receptor and ligand are pulled apart (these types of bonds are known as slip bonds). By contrast, catch bonds are interactions that are strengthened by tensile force. The nature of these interactions can be explained by allostery: force promotes the formation of a higher-affinity conformation (Thomas et al., 2008). Recently, the adhesion molecule P-selectin has been proposed to form catch bonds with its ligand sialyl-Lewis-X because force promotes an unbending of the molecule that leads to enhanced carbohydrate recognition (Phan et al., 2006; Thomas, 2006). Is there evidence that integrin-ligand interactions are enhanced by force? First, it has been shown that that moderate shear forces can activate leukocyte integrins (Astrof et al., 2006). Second, molecular dynamics predicts that applying tensile force to the integrin-ligand interface pulls on the α1 helix and leads to an opening of the hybrid-domain hinge – this opening would reinforce the bond by stabilising the active conformation of the βA domain (Puklin-Faucher et al., 2006).
Until recently, integrin catch bonds have not been observed directly, however, our atomic force spectroscopy experiments have shown that the lifetimes of α5β1-integrin-fibronectin interactions are increased by forces in the range of 20-40 pN (F. Kong, A. J. Garcia, A.P.M., M.J.H. and C. Zhu, unpublished data). This ability of integrin-ligand bonds to strengthen with force might be of importance, not only for leukocyte trafficking, but also for the migration of many cell types.
Conclusion
We previously proposed that multiple intermediate conformations of integrins exist, based on flexible joints and hinges in the receptor, particularly at the knees and the interface of the hybrid and βA domains (Mould and Humphries, 2004). Experimental evidence is now emerging suggesting that a spectrum of conformations is possible, with variations in the extent of unbending and hybrid-domain swing-out, which might be both integrin- and agonist-specific. We propose that the information discussed in this Commentary is integrated into a five-component model that provides a scenario that takes into account much of the current biochemical and structural data, and also highlights a possible conformational distinction between inside-out and outside-in signalling (Fig. 2). The dynamic equilibrium that exists between active and inactive integrin challenges our ability to investigate the validity of proposed intermediate forms, but these problems are gradually being overcome to provide new insights into integrin structure-function relationships. In the future, it will be of great importance to obtain crystal structures of additional integrin conformations and of integrins that are bound to macromolecular ligands.
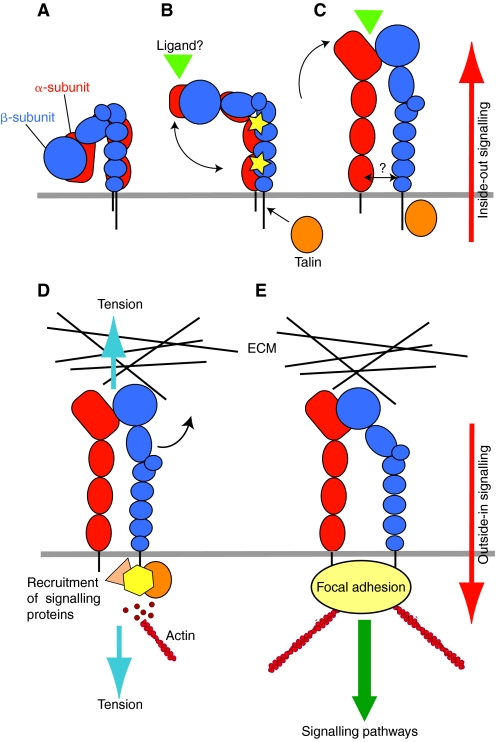
Fig. 2.
Integrin conformation-function relationships: a model. A five-component model illustrating conformational changes that are associated with inside-out and outside-in integrin signalling. The α-subunit is in red and the β-subunit in blue. The figure shows the three major conformational states that have been identified so far: inactive (A), primed (B) and ligand bound (C) (ligand is represented by a green triangle), together with possible intermediate conformers. Panels A-C represent conformations that mediate inside-out signalling, and panels D and E, outside-in signalling (the direction is indicated by red arrows). (A) Inactive integrin adopts a compact, most probably bent conformation in which the α- and β-subunit leg, transmembrane and cytoplasmic domains are closely associated. (B) The inherent flexibility of the knees allows for a degree of movement or `breathing' in this structure. Intracellular signals, culminating in the binding of talin (orange oval) to the β-subunit tail, causes relaxation of the leg restraints, allowing some further unbending that is sufficient to expose the epitopes of stimulatory antibodies in the leg regions (represented by yellow stars). A concomitant small outward movement of the hybrid domain primes the ligand-binding pocket to achieve a high-affinity conformation that is ready to accept ligand. The point at which a high-affinity conformation is reached may be integrin- and agonist-specific, and might take place before the receptor is fully extended. (C) The primed integrin binds ligand, which represents the end-point of inside-out signalling. At this stage the integrin is probably in an extended conformation, but the hybrid domain might remain in its primed position and, although some destabilisation and rearrangement of the legs has occurred, their degree of separation is not known. (D,E) The binding of talin and ligand initiate focal contact formation. As the cytoskeleton matures, tension (D, blue arrows) is generated on the integrin receptor across the cell membrane. (E) The force applied to the integrin headpiece triggers further outward movement of the hybrid domain, strengthening receptor-ligand binding and allowing the formation of stable focal adhesions and the initiation of intracellular signalling cascades (green arrow), the end-point of outside-in signalling.
The work in the authors' laboratory that contributed to this Commentary was supported by grants from the Wellcome Trust (to M.J.H.). Deposited in PMC for release after 6 months.
References
- Adair, B. D. and Yeager, M. (2002). Three-dimensional model of the human platelet integrin αIIbβ3 based on electron cryomicroscopy and x-ray crystallography. Proc. Natl. Acad. Sci. USA 99, 14059-14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adair, B. D., Xiong, J. P., Maddock, C., Goodman, S. L., Arnaout, M. A. and Yeager, M. (2005). Three-dimensional EM structure of the ectodomain of integrin αVβ3 in a complex with fibronectin. J. Cell Biol. 168, 1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout, M. A., Goodman, S. L. and Xiong, J. P. (2007). Structure and mechanics of integrin-based cell adhesion. Curr. Opin. Cell Biol. 19, 495-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrof, N. S., Salas, A., Shimaoka, M., Chen, J. and Springer, T. A. (2006). Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry 45, 15020-15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni, G., Ma, L., Blue, M. L. and Hemler, M. E. (1998). Divalent cations and ligands induce conformational changes that are highly divergent among β1 integrins. J. Biol. Chem. 273, 6670-6678. [DOI] [PubMed] [Google Scholar]
- Beglova, N., Blacklow, S. C., Takagi, J. and Springer, T. A. (2002). Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nat. Struct. Biol. 9, 282-287. [DOI] [PubMed] [Google Scholar]
- Calzada, M. J., Alvarez, M. V. and Gonzalez-Rodriguez, J. (2002). Agonist-specific structural rearrangements of integrin αIIbβ3: confirmation of the bent conformation in platelets at rest and after activation. J. Biol. Chem. 277, 39899-39908. [DOI] [PubMed] [Google Scholar]
- Cheng, M., Foo, S. Y., Shi, M. L., Tang, R. H., Kong, L. S., Law, S. K. and Tan, S. M. (2007). Mutation of a conserved asparagine in the I-like domain promotes constitutively active integrins αLβ2 and αIIbβ3. J. Biol. Chem. 282, 18225-18232. [DOI] [PubMed] [Google Scholar]
- Chigaev, A., Buranda, T., Dwyer, D. C., Prossnitz, E. R. and Sklar, L. A. (2003). FRET detection of cellular α4-integrin conformational activation. Biophys. J. 85, 3951-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chigaev, A., Waller, A., Zwartz, G. J., Buranda, T. and Sklar, L. A. (2007). Regulation of cell adhesion by affinity and conformational unbending of α4β1 integrin. J. Immunol. 178, 6828-6839. [DOI] [PubMed] [Google Scholar]
- Coutinho, A., Garcia, C., Gonzalez-Rodriguez, J. and Lillo, M. P. (2007). Conformational changes in human integrin αIIbβ3 after platelet activation, monitored by FRET. Biophys. Chem. 130, 76-87. [DOI] [PubMed] [Google Scholar]
- Frelinger, A. L., 3rd, Cohen, I., Plow, E. F., Smith, M. A., Roberts, J., Lam, S. C. and Ginsberg, M. H. (1990). Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J. Biol. Chem. 265, 6346-6352. [PubMed] [Google Scholar]
- Garcia-Alvarez, B., de Pereda, J. M., Calderwood, D. A., Ulmer, T. S., Critchley, D., Campbell, I. D., Ginsberg, M. H. and Liddington, R. C. (2003). Structural determinants of integrin recognition by talin. Mol. Cell 11, 49-58. [DOI] [PubMed] [Google Scholar]
- Goksoy, E., Ma, Y. Q., Wang, X., Kong, X., Perera, D., Plow, E. F. and Qin, J. (2008). Structural basis for the autoinhibition of talin in regulating integrin activation. Mol. Cell 31, 124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J., Lim, C. J., Watanabe, N., Soriani, A., Ratnikov, B., Calderwood, D. A., Puzon-McLaughlin, W., Boussiotis, V. A., Shattil, S. J. and Ginsberg, M. H. (2006). Reconstructing and deconstructing agonist-induced activation of integrin αIIbβ3. Curr. Biol. 18, 1796-1806. [DOI] [PubMed] [Google Scholar]
- Hato, T., Yamanouchi, J., Yakushijin, Y., Sakai, I. and Yasukawa, M. (2006). Identification of critical residues for regulation of integrin activation in the β6-α7 loop of the integrin β3 I-like domain. J. Thromb. Haemost. 4, 2278-2280. [DOI] [PubMed] [Google Scholar]
- Hughes, P. E., Diaz-Gonzalez, F., Leong, L., Wu, C., McDonald, J. A., Shattil, S. J. and Ginsberg, M. H. (1996). Breaking the integrin hinge: a defined structural constraint regulates integrin signaling. J. Biol. Chem. 271, 6571-6574. [DOI] [PubMed] [Google Scholar]
- Iwasaki, K., Mitsuoka, K., Fujiyoshi, Y., Fujisawa, Y., Kikuchi, M., Sekiguchi, K. and Yamada, T. (2005). Electron tomography reveals diverse conformations of integrin αIIbβ3 in the active state. J. Struct. Biol. 150, 259-267. [DOI] [PubMed] [Google Scholar]
- Jin, M., Andricioaei, I. and Springer, T. A. (2004). Conversion between three conformational states of integrin I domains with a C-terminal pull spring studied with molecular dynamics. Structure 12, 2137-2147. [DOI] [PubMed] [Google Scholar]
- Kamata, T., Handa, M., Sato, Y., Ikeda, Y. and Aiso, S. (2005). Membrane-proximal α/β stalk interactions differentially regulate integrin activation. J. Biol. Chem. 280, 24775-24783. [DOI] [PubMed] [Google Scholar]
- Kim, M., Carman, C. V. and Springer, T. A. (2003). Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301, 1720-1725. [DOI] [PubMed] [Google Scholar]
- Lau, T. L., Dua, V. and Ulmer, T. S. (2008a). Structure of the integrin αIIb transmembrane segment. J. Biol. Chem. 283, 16162-16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, T. L., Partridge, A. W., Ginsberg, M. H. and Ulmer, T. S. (2008b). Structure of the integrin β3 transmembrane segment in phospholipid bicelles and detergent micelles. Biochemistry 47, 4008-4016. [DOI] [PubMed] [Google Scholar]
- Lu, C., Ferzly, M., Takagi, J. and Springer, T. A. (2001). Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. J. Immunol. 166, 5629-5637. [DOI] [PubMed] [Google Scholar]
- Luo, B. H. and Springer, T. A. (2006). Integrin structures and conformational signaling. Curr. Opin. Cell Biol. 18, 579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, B. H., Springer, T. A. and Takagi, J. (2004). A specific interface between integrin transmembrane helices and affinity for ligand. PLoS Biol. 2, e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleverty, C. J., Lin, D. C. and Liddington, R. C. (2007). Structure of the PTB domain of tensin 1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 16 1223-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould, A. P. (1996). Getting integrins into shape: recent insights into how integrin activity is regulated by conformational changes. J. Cell Sci. 109 2613-2618. [DOI] [PubMed] [Google Scholar]
- Mould, A. P. and Humphries, M. J. (2004). Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Curr. Opin. Cell Biol. 16, 544-551. [DOI] [PubMed] [Google Scholar]
- Mould, A. P., Barton, S. J., Askari, J. A., McEwan, P. A., Buckley, P. A., Craig, S. E. and Humphries, M. J. (2003a). Conformational changes in the integrin β A domain provide a mechanism for signal transduction via hybrid domain movement. J. Biol. Chem. 278, 17028-17035. [DOI] [PubMed] [Google Scholar]
- Mould, A. P., Symonds, E. J., Buckley, P. A., Grossmann, J. G., McEwan, P. A., Barton, S. J., Askari, J. A., Craig, S. E., Bella, J. and Humphries, M. J. (2003b). Structure of an integrin-ligand complex deduced from solution x-ray scattering and site-directed mutagenesis. J. Biol. Chem. 278, 39993-39999. [DOI] [PubMed] [Google Scholar]
- Nishida, N., Xie, C., Shimaoka, M., Cheng, Y., Walz, T. and Springer, T. A. (2006). Activation of leukocyte β2 integrins by conversion from bent to extended conformations. Immunity 25, 583-594. [DOI] [PubMed] [Google Scholar]
- Oxley, C. L., Anthis, N. J., Lowe, E. D., Vakonakis, I., Campbell, I. D. and Wegener, K. L. (2008). An integrin phosphorylation switch: the effect of β3 integrin tail phosphorylation on Dok1 and talin binding. J. Biol. Chem. 283, 5420-5426. [DOI] [PubMed] [Google Scholar]
- Phan, U. T., Waldron, T. T. and Springer, T. A. (2006). Remodeling of the lectin-EGF-like domain interface in P- and L-selectin increases adhesiveness and shear resistance under hydrodynamic force. Nat. Immunol. 7, 883-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin-Faucher, E., Gao, M., Schulten, K. and Vogel, V. (2006). How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J. Cell Biol. 175, 349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco, M., Rosano, C., Weisel, J. W., Horita, D. A. and Hantgan, R. R. (2008). Integrin conformational regulation: uncoupling extension/tail separation from changes in the head region by a multiresolution approach. Structure 16, 954-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, M., Foo, S. Y., Tan, S. M., Mitchell, E. P., Law, S. K. and Lescar, J. (2007a). A structural hypothesis for the transition between bent and extended conformations of the leukocyte β2 integrins. J. Biol. Chem. 282, 30198-30206. [DOI] [PubMed] [Google Scholar]
- Shi, X., Ma, Y. Q., Tu, Y., Chen, K., Wu, S., Fukuda, K., Qin, J., Plow, E. F. and Wu, C. (2007b). The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J. Biol. Chem. 282, 20455-20466. [DOI] [PubMed] [Google Scholar]
- Shimaoka, M., Takagi, J. and Springer, T. A. (2002). Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485-516. [DOI] [PubMed] [Google Scholar]
- Shimaoka, M., Xiao, T., Liu, J. H., Yang, Y., Dong, Y., Jun, C. D., McCormack, A., Zhang, R., Joachimiak, A., Takagi, J. et al. (2003). Structures of the α L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112, 99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., de Pereda, J. M., Ginsberg, M. H. and Calderwood, D. A. (2003). Talin binding to integrin β tails: a final common step in integrin activation. Science 302, 103-106. [DOI] [PubMed] [Google Scholar]
- Takagi, J. and Springer, T. A. (2002). Integrin activation and structural rearrangement. Immunol. Rev. 186, 141-163. [DOI] [PubMed] [Google Scholar]
- Takagi, J., Erickson, H. P. and Springer, T. A. (2001). C-terminal opening mimics `inside-out' activation of integrin α5β1. Nat. Struct. Biol. 8, 412-416. [DOI] [PubMed] [Google Scholar]
- Takagi, J., Petre, B. M., Walz, T. and Springer, T. A. (2002). Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110, 599-611. [DOI] [PubMed] [Google Scholar]
- Takagi, J., Strokovich, K., Springer, T. A. and Walz, T. (2003). Structure of integrin α5β1 in complex with fibronectin. EMBO J. 22, 4607-4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, W. (2006). For catch bonds, it all hinges on the interdomain region. J. Cell Biol. 174, 911-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, W. E., Vogel, V. and Sokurenko, E. (2008). Biophysics of catch bonds. Annu. Rev. Biophys. 37, 399-416. [DOI] [PubMed] [Google Scholar]
- Vinogradova, O., Velyvis, A., Velyviene, A., Hu, B., Haas, T., Plow, E. and Qin, J. (2002). A structural mechanism of integrin αIIbβ3 “inside-out” activation as regulated by its cytoplasmic face. Cell 110, 587-597. [DOI] [PubMed] [Google Scholar]
- Wegener, K. L., Partridge, A. W., Han, J., Pickford, A. R., Liddington, R. C., Ginsberg, M. H. and Campbell, I. D. (2007). Structural basis of integrin activation by talin. Cell 128, 171-182. [DOI] [PubMed] [Google Scholar]
- Xiao, T., Takagi, J., Coller, B. S., Wang, J. H. and Springer, T. A. (2004). Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432, 59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, J. P., Stehle, T., Diefenbach, B., Zhang, R., Dunker, R., Scott, D. L., Joachimiak, A., Goodman, S. L. and Arnaout, M. A. (2001). Crystal structure of the extracellular segment of integrin αVβ3. Science 294, 339-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, J. P., Stehlt, T., Zhang, R., Joachimiak, A., Goodman, S. L. and Arnaout, M. A. (2002). Crystal structure of the extracellular segment of integrin αVβ3 in complex with and Arg-Gly-Asp ligand. Science 296, 151-155. [DOI] [PubMed] [Google Scholar]
- Yang, W., Shimaoka, M., Salas, A., Takagi, J. and Springer, T. A. (2004). Intersubunit signal transmission in integrins by a receptor-like interaction with a pull spring. Proc. Natl. Acad. Sci. USA 101, 2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, F., Liu, J., Winkler, H. and Taylor, K. A. (2008). Integrin αIIbβ3 in a membrane environment remains the same height after Mn2+ activation when observed by cryoelectron tomography. J. Mol. Biol. 378, 976-986. [DOI] [PMC free article] [PubMed] [Google Scholar]