Summary
All cellular processes are determined by adhesive interactions between cells and their local microenvironment. Integrins, which constitute one class of cell-adhesion receptor, are multifunctional proteins that link cells to the extracellular matrix and organise integrin adhesion complexes at the cell periphery. Integrin-based adhesions provide anchor points for assembling and organising the cytoskeleton and cell shape, and for orchestrating migration. Integrins also control the fate and function of cells by influencing their proliferation, apoptosis and differentiation. Moreover, new literature demonstrates that integrins control the cell-division axis at mitosis. This extends the influence of integrins over cell-fate decisions, as daughter cells are frequently located in new microenvironments that determine their behaviour following cell division. In this Commentary, I describe how integrins influence cell-fate determination, placing particular emphasis on their role in influencing the direction of cell division and the orientation of the mitotic spindle.
Keywords: Integrin signalling, Adhesion complex, Adhesome, Growth factor receptors, Proliferation, Cell cycle, Apoptosis, Anoikis, Differentiation, Stem cells, Mitosis, Metaphase, Mitotic spindle, Cell fate, Adhesion
Introduction
The formation of complex tissues, including the different types of cells within them and their spatial organisation, depends upon a sequential series of cell-fate decisions that begins with the fertilisation of a single cell. Although growth factors1 (GFs) are commonly regarded as being the main extracellular ligands that control these decisions, the extracellular matrix (ECM) and intercellular adhesions provide equally important instructions for controlling gene expression and the fates of all cells. Indeed, the precise integration of the intracellular signalling networks that result from each of these microenvironmental extracellular cues controls all of the different types of fate decision that cells make, including whether they proliferate or exit the cell cycle, survive or undergo apoptosis, express tissue-specific genes, or undertake the appropriate morphogenesis and movements for the cell and tissue type.
Integrins, which constitute a class of cell-matrix receptor, are central in all these aspects of cell-fate determination. They signal to the cell interior through adhesion complexes, which are plasma-membrane-associated platforms that are assembled around the cytoplasmic face of clustered, ECM-associated integrins. Integrin adhesions comprise several types of specialised sites of cell interaction with the ECM, including focal complexes, focal adhesions, fibrillar adhesions and podosomes (Zaidel-Bar et al., 2007). Integrin adhesions are sites of cytoskeletal assembly and, together with cell-cell contact points, control the internal architecture of the cell. They also recruit both adaptors [such as paxillin, p130Cas, integrin-linked kinase (ILK) and guanine-nucleotide exchange factors (GEFs)] and enzymes (such as the protein kinases focal-adhesion kinase (FAK), Src and Jnk, Rho-family GTPases and lipid kinases), which trigger distal signalling pathways that control cell-fate decisions such as survival, growth, migration and differentiation (Giancotti and Tarone, 2003; Lo, 2006).
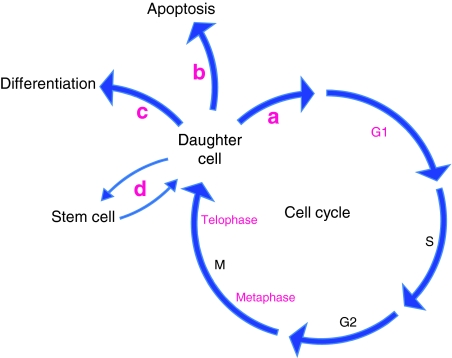
We have recently reviewed the mechanisms by which integrins cooperate with soluble factors to control cell phenotype (Streuli and Akhtar, 2009). Readers are also referred to articles that examine how integrins control cell fate in specific cell- and tissue-types such as endothelia, oligodendrocytes, breast epithelia and epidermis (Avraamides et al., 2008; Baron et al., 2005; Katz and Streuli, 2007; Watt, 2002). Here, I will briefly mention how integrins are involved in proliferation, apoptosis and differentiation, and will then focus on one of the more exciting developments in the integrin field, which is their role in the post-G1 or -S stages of the cell cycle, at metaphase and telophase. By influencing the axis of division and cytokinesis, integrins determine the positioning and, therefore, the microenvironment of daughter cells. In turn, this guides cell-fate decisions. It is now evident that integrins have more extensive control over aspects of cell behaviour than has previously been recognised (Fig. 1).
Fig. 1.
Multiple points in the control of cell cycle and fate by integrins. The points in the cell cycle at which integrins have an essential role are shown in pink, and include G1, metaphase and telophase. The fates of daughter cells are also integrin-dependent. They become committed to (a) re-enter the cell cycle, (b) survive or apoptose or (c) express tissue-specific genes and differentiate. (d) In the case of stem cells, one daughter cell will enter the cell cycle and then undergo one of the three fates shown in (a-c), whereas the other will remain a stem cell.
The integrin `adhesion checkpoint' controls proliferation, apoptosis and differentiation
Proliferation
All adherent cells require adhesion to the ECM to progress through the cell cycle. The activation of growth factor receptors (GFRs) is not sufficient for proliferation, and integrin signalling is needed as well. For example, integrin-mediated adhesion to the ECM is necessary for the activation of mitogen activated protein (MAP) kinase kinase (MEK1) downstream of Raf, the translocation of the extracellular signal-regulated protein kinases 1 and 2 (Erk1/2) into the nucleus and the phosphorylation of the transcription factor Elk1, transcription of the epidermal growth factor (EGF)-responsive gene Erg1, degradation of the cell-cycle inhibitor p27 through the ubiquitin ligase Skp2, expression of cyclin D1 and progression through the G1-S stage of the cell cycle (Pu and Streuli, 2002; Schwartz and Ginsberg, 2002). So, multiple stages in the pathway that links GFs to early-response genes are regulated by integrins and, in their absence, the cell cycle is unable to proceed through G1. This `adhesion checkpoint' operates in addition to the conventional G1-S restriction point, and specifies whether cells are situated in the right microenvironmental location to progress through the cell cycle. Good evidence that integrins and their associated adhesion proteins are required for cell-cycle control comes from genetic studies using mice, in which cell-type-specific knockouts perturb proliferation (Grashoff et al., 2003; Li et al., 2005; McLean et al., 2004).
The mechanism by which integrins control proliferation involves both a direct crosstalk between integrins and GFRs, and GF-independent signalling from integrins themselves. In some cells, for example, Erk signalling is induced directly by integrin adhesion, whereas the Akt pathway (which also promotes proliferation) can be activated downstream of integrins through mechanisms separate to those of GFRs (Velling et al., 2008). The Rho GTPase Rac1 is also required for proliferation, and its activity depends both on enzymes that bind to the β1-integrin tail and on its integrin-mediated recruitment to membrane microdomains (Del Pozo et al., 2004; Hirsch et al., 2002).
In addition to GF-independent signalling, integrins also send intracellular proliferation signals by collaborating with GFRs. This occurs both through the formation of integrin-GFR complexes, which causes ligand-independent activation of GFRs, and through joint control of signalling output. In epithelial cells, for example, integrin adhesion causes rapid tyrosine phosphorylation and kinase activation of the EGF receptor (EGFR) in the absence of EGF. A fraction of EGFR associates with β1 integrins in a complex with Src and p130Cas, which are required for EGFR phosphorylation, and this contact with integrins causes EGFR to recruit a variety of adaptors that stimulate the Erk and Akt pathways, cyclin D1 synthesis, and phosphorylation of the cell-cycle-regulatory proteins Cdk4 and Rb (Bill et al., 2004; Moro et al., 2002). Importantly, integrin activation leads to phosphorylation of a distinct subset of tyrosine residues of EGFR, leaving the major EGF-responsive residue, Y1148, unphosphorylated. So, although GFs signal through specific residues within the GFR tail, integrins are also essential for optimal signalling because they directly affect the tyrosine phosphorylation of different amino acids, thereby influencing the overall signalling output. Thus, an essential part of cell-cycle control is the joint requirement for ECM adhesion and GFs for sustained, synergistic signalling.
The ability of GFRs to recruit signalling adaptors is highly dependent on integrins in several other cases, illustrating that cells frequently use integrins to fine-tune GFR-induced cellular responses. For example, integrin adhesion to the ECM component fibronectin promotes the association of the protein tyrosine phosphatase SHP2 with platelet-derived growth factor receptor β (PDGFRβ), leading to the dephosphorylation of the tyrosine residue that would otherwise bind to the GTPase-activating protein RasGAP – with the consequence that Ras and Erk1 activation are sustained, and more cells enter the cell cycle (DeMali et al., 1999). In endothelial cells, α5β1 integrin stably interacts with Tie2 (also known as TEK), a receptor for the angiopoietin Ang-1, thereby amplifying both the strength and duration of angiogenic signals sufficiently for blood-vessel formation to occur (Cascone et al., 2005). In this case, it may be that optimal receptor output occurs when integrins recruit FAK and the GFR binds to the p85 subunit of phosphoinositide 3-kinase (PI3K), providing synergistic activation of efficient downstream signals such as the Akt pathway. In further examples, αvβ3 integrin and vascular endothelial growth factor receptor 2 (VEGFR2) co-regulate angiogenesis through the activation of shared downstream transducers such as Src (Mahabeleshwar et al., 2007), β1 integrins associate with the IL3β receptor and directly activate Jak2-Stat5 signalling, which is necessary for cell-cycle progression (Defilippi et al., 2005), and α5β1 synergises with GFRs within lipid rafts to control cyclin D1 and the cell cycle via the joint activation of FAK signalling to PI3K and Shc signalling to Rac1 (Del Pozo and Schwartz, 2007; Mettouchi et al., 2001).
The conclusion from this section is that integrins and GFRs are more than just individual receptors that bind to their respective ligands. Rather, they are a joint sensing apparatus that enables cells to detect where they are and how they should respond to instructive messages from other cells – together, they specify whether cells proliferate. A similar argument applies to the cell-fate decisions of survival and differentiation, which are similarly influenced by the local microenvironment through integrins and by soluble factors.
Apoptosis
Integrins are essential determinants of cell survival and, in many cases, prevention or alteration of integrin adhesion triggers a form of apoptosis that is known as anoikis (Gilmore, 2005). Anoikis is particularly relevant when cells become located in ECM environments in which they are not developmentally programmed to reside. For example, mammary epithelial cells are normally situated on a laminin-rich basement membrane but, if they are displaced to a stromal ECM of collagen I, they undergo anoikis (Pullan et al., 1996). This dependency on a specific ECM composition works through two mechanisms. In one mechanism, which is similar to the integrin-GFR collaboration for proliferation (see above), ECM adhesion and GFs [insulin-like growth factor 1 (IGF1) in the case of mammary cells] are both required for maximal Akt-dependent survival signalling (Farrelly et al., 1999). The second mechanism involves an intriguing function for integrins, which is the regulation of protein trafficking between cytosol and mitochondria. In this mechanism, integrins maintain the pro-apoptotic protein Bax in the cytosol, whereas altered cell adhesion leads to mitochondrial translocation of Bax (together with a conformational change) and activation of the intrinsic apoptosis pathway (Gilmore et al., 2000). Interestingly, the survival signalling mechanisms that are activated at integrin adhesions are distinct in cells from different embryonic lineages, because fibroblasts depend on the association of FAK with p130Cas, whereas epithelial cells require FAK signalling through alternate pathways that involve paxillin (Almeida et al., 2000; Zouq et al., 2009). Together, these studies in the mammary-cell system demonstrate that integrin adhesions form an essential adhesion checkpoint that is employed by cells to determine whether they apoptose or survive.
Variations on this theme of ECM control of survival have recently emerged. For instance, certain ECM molecules [such as cysteine-rich protein 61 (Cyr61, also known as CCN1) and elastin microfibril interfacer 2 (EMILIN2)] actively promote apoptosis (Marastoni et al., 2008). Reduced integrin signalling induces autophagy in some cases, allowing cells to survive for a period of time until they can engage with the ECM again (Lock and Debnath, 2008). Loss of ECM adhesion can result in a novel form of cell death called entosis, in which one live cell invades another, after which the live internalised cell becomes degraded (Overholtzer et al., 2007). Finally, the degree of mechanical tension on the cell, which is mediated through integrins, can determine whether cells apoptose or survive (Hsieh and Nguyen, 2005).
Differentiation
Cell differentiation, which is the result of defined combinations of transcription factors that cause the cell-type-specific patterns of gene expression that characterise individual cells, arises through the lineage-determination events of development. For some cell types, the involvement of integrins during their developmental programming to become fully mature, differentiated cells has been extensively characterised. As an illustration, in oligodendrocyte development in the CNS, progenitor cells differentiate into oligodendrocytes. These cells send out processes to contact axons (only those whose integrins make connections with axonal laminin-2 survive), whereupon they elaborate massive myelin sheets to ensheathe the target neurons. Each of these stages involves specific combinations of GFs (for developmental timing) and integrins (to provide spatial cues to ensure that each stage of the programme only occurs in the right place). Oligodendrocyte differentiation is a particularly neat example, because two integrin-GFR switches occur. In the first switch, PDGFαR collaborates with the vitronectin receptor αvβ3 integrin to promote proliferation of oligodendrocytes but, upon contact between cell processes and laminin-2, the same GFR (now in lipid rafts) works with α6β1 integrin to send survival signals. In the second switch, the EGF-family protein neuregulin sends survival and proliferation signals in oligodendrocyte precursors but, upon contact with laminin-2, the neuregulin receptors ErbB2 and ErbB4 provide signals that promote oligodendrocyte differentiation instead (Baron et al., 2005).
The coordination of spatial and temporal cues for specifying differentiation is likely to be important for all tissues. In the skin, the epithelial cells that contact the basement membrane (at the interface with dermis) are stem cells and contain the proliferating population, whereas those in suprabasal layers exit the cell cycle, and differentiate by keratinising and producing the stratum corneum. This transition is controlled directly by β1 integrins, which promote survival and proliferation of basal cells, whereas loss of integrin adhesion (when cells move to the cutaneous layers of epidermis) permits differentiation to occur (Watt, 2002).
More is known about the intracellular mechanism of β1-integrin signalling in the differentiation of epithelial cells of the mammary gland. During pregnancy, this tissue develops in a temporally and spatially regulated manner, so that epithelial cells only produce their differentiation products (milk) at the right time and place – that is, during lactation and in epithelial cells within acini (Naylor and Streuli, 2006). The spatial signals that control this cell-fate process are provided by β1-integrin interactions with the basement-membrane protein laminin 1, whereas the temporal signals are supplied by the hormone prolactin (a soluble endocrine factor that signals through transmembrane cytokine receptors). Active integrin signalling is essential for driving differentiation, because genetic ablation of β1 integrin either in vivo or in a primary 3D-tissue-culture model inhibits the ability of prolactin to activate its signalling pathway, which involves Jak2 and Stat5 (the transcription factor for milk proteins) (Naylor et al., 2005). Genetic knockout approaches have been used to identify the proximal integrin-adhesion proteins that transmit signals, one of which is integrin-linked kinase (ILK) (Akhtar et al., 2009). Further on in the pathway, Rac1 serves as a nodal point to integrate signals between integrins and cytokine receptors, as expression of a constitutively active form of Rac1 in either β1-integrin- or ILK-null cells rescues the lactational defect (Akhtar and Streuli, 2006). The link between ILK and Rac1 is not understood, but presumably involves an integrin-adhesion-recruited GEF that can activate Rac1 in a spatially restricted manner. Integrin adhesions might, therefore, provide a platform for localised GEF activation, which in turn might lead to the transmission of highly localised signals (in this case, prolactin signalling in mammary cells).
Integrins control the cell-division axis
Integrins collaborate with growth, survival and differentiation factors to specify what cells do (as discussed above), so it follows that placing cells in different surroundings will have profound effects on their fate decisions. The ECM environment of a cell is determined in several ways. First, cells can synthesise and deposit their own matrix, which causes them to express tissue-specific genes (Streuli and Bissell, 1990). Second, cell migration (which relies on integrin signalling) provides a natural means for cells to move into a new environment and take on a different role. Finally, integrins can modify the orientation of cytokinesis so that two daughter cells of a mitotic division are located in different microenvironments. Choosing the axis of cell division is of central importance both in lineage determination, when stem-cell progeny exit the niche that their parent occupied, and during the normal development and maintenance of tissues, and here I discuss the cell-division axis in the context of integrin function.
A particularly interesting example of the influence of the microenvironment relates to stem cells. These cells rely on the ECM and other local signals to retain them in a specialised niche, which allows self-renewal but prevents differentiation (Scadden, 2006). Epidermal stem cells, for example, have high levels of integrins in comparison to their non-stem-cell neighbours (integrin ligation inhibits differentiation), and follicular stem cells in the Drosophila ovary secrete laminin A, which creates a niche for αPS1βPS integrins to anchor the cells and control proliferation (Jones and Watt, 1993; O'Reilly et al., 2008). Daughter cells that exit the niche take on fates that are determined by integrin interactions with different ECMs.
Orientation of the mitotic spindle – studies in 2D cultures
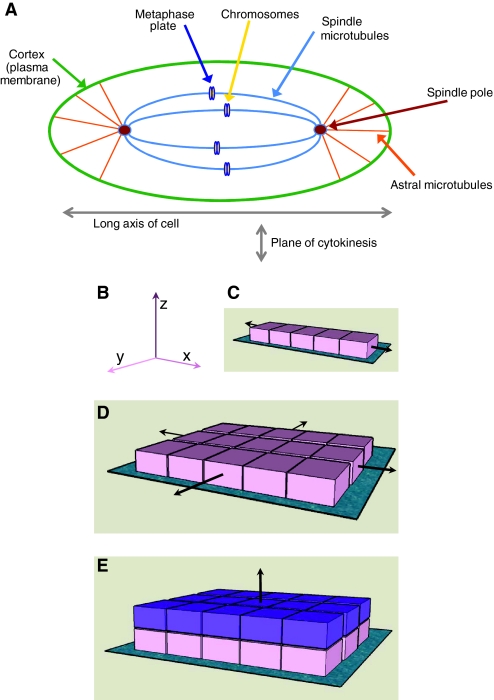
It has been known for some time that cell shape controls the orientation of the mitotic spindle, which aligns along the long axis of the cell (O'Connell and Wang, 2000) (Fig. 2A). More recently, several experimental approaches have demonstrated that integrin-mediated adhesion to the ECM controls the mitotic-spindle axis (Toyoshima and Nishida, 2007b). In these studies, which were conducted in conventional 2D tissue culture, cells divide in the plane of the dish to which they adhere, requiring alignment of the mitotic spindle parallel to the substratum (in the xy direction) (Fig. 2B-D).
Fig. 2.
Integrins orient the cell-division axis. (A) The mitotic spindle aligns along the long axis of the cell, which lies at right angles to the plane of cytokinesis. A mitotic cell is shown, and the microtubule connections between the cell edge (cortex, plasma membrane) and spindle poles, and between the spindle poles and chromosomes (which accumulate at the metaphase plate) are highlighted. (B) Cells adhere to the ECM through integrins. They can either divide along the plane of the ECM (turquoise in panels C-E) to which they adhere (x- or y-axes), or away from it (z-axis). (C,D) Tension provided along the x-axis causes unilateral extension (C), which becomes bilateral if tension is also provided in the y-axis (D), forming a sheet of cells. (E) Orientation of the plane of division along the z-axis causes cells to become displaced away from the initial ECM. These new (purple) cells have a microenvironment that is different to the parental (pink) cells. Thus, following cell division, daughter cells become located either in a similar niche to their parents, or in a new microenvironment.
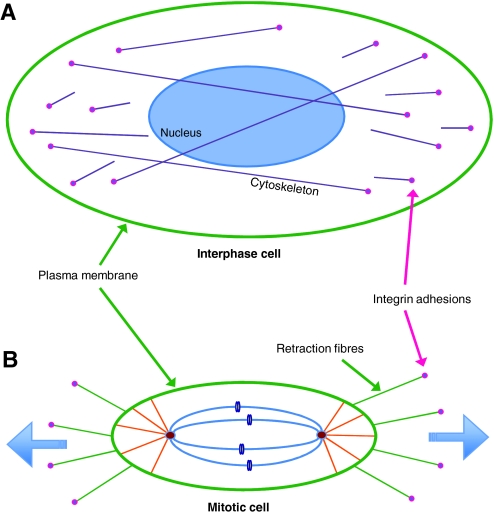
Using micro-patterned ECM substrata that allow single cells to adhere with specific topologies, it has been shown that the tension exerted by the ECM (through integrin-containing adhesion) generates defined actin-cytoskeleton force fields within cells during interphase (Thery et al., 2005). During metaphase, cells round up to undergo mitosis, but retraction fibres transfer the polarity of tension that was established in interphase to the astral microtubules (which link the spindle poles with the cell cortex and, therefore, the plasma membrane), thereby aligning the mitotic spindle (Fig. 3) (Thery and Bornens, 2006). Retraction fibres are bound to the substratum by integrins, so cell-matrix adhesion determines the internal architecture of cells, which subsequently defines the spindle position and, therefore, the division axis at mitosis. Indeed, β1 integrins are necessary for accurate assembly of spindle poles during metaphase, and altering the NPxY sequence within the β1-integrin cytoplasmic tail (which binds to integrin-adhesion-adaptor proteins such as talin) causes aberrant cytokinesis (Reverte et al., 2006).
Fig. 3.
Retraction fibres provide resistive forces to orient the mitotic spindle. (A) Interphase cell, showing sites of attachment to the ECM via integrin adhesions and the intracellular cytoskeleton. This is the view that is seen when looking down on cells in 2D culture. (B) Mitotic cell that has rounded up in preparation for cytokinesis. The cell remains attached to the substratum through retraction fibres, which provide resistance so that tension can build up within microtubules and thereby orient the spindle.
The concept that integrin-mediated tension controls the orientation of the mitotic spindle extends to the level of whole organs, in which forces that are imposed upon cells at the integrin-ECM interface specify the polarity of division, ultimately shaping tissues and determining the fate of cells within them. At the molecular level, the position of the mitotic spindle is controlled by a variety of signalling proteins at the junction between astral microtubules and retraction fibres. These include complexes that contain cytoskeletal adaptors, such as EB1, APC and mDia, G proteins and their regulators, which generate tension on microtubules, and NuMA, which stabilises microtubules at spindle poles (Du and Macara, 2004; Merdes et al., 2000). Some of these proteins, including EB1, have been directly shown to be involved with (indirectly) linking integrins to the spindle pole (Toyoshima and Nishida, 2007a).
Aligning the mitotic spindle parallel to the substratum also requires integrins. If β1 integrins are depleted or inhibited, the spindle becomes misaligned so that one pole is higher than the other (in the z direction) (Toyoshima and Nishida, 2007a). Through a mechanism involving PI3K, integrin localises the lipid P13K phosphatidylinositol (3,4,5)-triphosphate [PtdIns(3,4,5)P3] in the equatorial part of the plasma membrane opposite to where the spindle forms (i.e. midway up the z-axis) in metaphase cells. PtdIns(3,4,5)P3 then (indirectly) recruits dynactin to a similar location, allowing a cortical dynein-dynactin motor complex to pull on the astral microtubules and, thereby, to orient the spindle correctly (Toyoshima et al., 2007).
Direction of cell division in a 3D-tissue context
In tissues, where cells are organised in three dimensions rather than the two dimensions that are associated with conventional tissue culture, epithelial cells either divide laterally (with daughter cells retaining the same ECM context and adhesion plane that their parent had), or distally from their original contact points (so that future generations of cells become situated in a new cellular microenvironment) (Fig. 2E). Integrins have a role in this aspect of cell division because they can influence the orientation of the cleavage plane. An example of this is the embryonic skin, which until recently was thought to stratify by a combination of lateral divisions of stem cells and transit-amplifying cells, followed by migration of differentiating cells into the suprabasal layer. It has now been shown, however, that an additional mechanism is also involved. The basal cells of skin align their mitotic spindles either parallel with, or perpendicular to, the basement membrane – with a high percentage aligning in the perpendicular direction (such that cells divide away from the basement membrane; Fig. 2E) at the time in embryonic development when the epidermis begins to stratify and the suprabasal cells start to differentiate (Lechler and Fuchs, 2005). In this example, spindle orientation depends upon cell adhesion to both the ECM (through β1 integrin) and to other cells (through α-catenin). These adhesions establish apical polarity via PKCζ and a Par3-LGN-mInsc-NuMA-dynactin complex (respectively) – spindles become oriented randomly following deletions of either integrin or cell-cell adhesion genes. So, in epidermal cells, integrins influence final fate decisions through multiple mechanisms, because they determine whether cells divide laterally or perpendicularly to the basement membrane, as well as directly controlling their ability to differentiate and proliferate (Adams and Watt, 1989; Fuchs, 2008; Lopez-Rovira et al., 2005; Watt, 2002).
Similar to their function in epithelial cells, integrins have a role in defining the axis of cell division in other cell types, a finding that has been revealed by integrin-gene-deletion studies. In the mammary gland, β1 integrins maintain the location of basal epithelial cells adjacent to the basement membrane and, in their absence, stem-cell progeny become mislocated to the luminal epithelial compartment (Taddei et al., 2008). Likewise, in the Drosophila egg chamber, βPS integrins maintain the positioning of follicular epithelial cells (Fernandez-Minan et al., 2007). In myospheroid mutants (which have a defective βPS-integrin-encoding gene), multi-layering of the follicle cells occurs because the orientation of the mitotic spindle becomes randomised. Interestingly, this is not because apical-basal polarity is disrupted but is possibly the result of perturbed integrin signalling and consequent disorganisation of the actin and microtubule cytoskeletons, and mispositioning of the spindle poles. β1 integrins also control several aspects of the cell cycle in chondrocytes, one of which is the direction of cell division – directionality is disrupted following deletion of the gene encoding β1 integrin, and many of the cells become bi- and multi-nucleate, which results in chondrodysplasia (Aszodi et al., 2003).
Integrin-adhesion proteins at spindle poles
It has emerged recently that several proteins involved with integrin-based adhesions during interphase have key roles at spindle poles during mitosis. This adds a new dimension to understanding the function of integrins, because some proteins that normally serve adaptor functions at sites of cell adhesion with the ECM have now been discovered to have additional roles and locations during mitosis. For instance, myosin-X (but not myosin-II) is located at the tips of filopodia (cytoplasmic projections at the leading edge of cells) and in focal complexes at the periphery of cells (Zhang et al., 2004). Myosin-X is also required for accurate spindle orientation (in mitosis and meiosis), and altered myosin-X expression leads to spindle pole mis-orientation and fragmentation (Toyoshima and Nishida, 2007a; Weber et al., 2004; Woolner et al., 2008). Interestingly, this protein not only binds to microtubules (through its FERM domain) at the spindle pole itself, but also to the cytoplasmic domain of integrins (again through the FERM domain). Whether myosin-X is involved in the control of spindle orientation by integrin, or whether it has separate roles in interphase vs prophase and metaphase cells, is not known.
Other integrin-adhesion proteins are found at centrosomes even at interphase. For example ILK and NEDD9 (also known as hEF1 or CASL) – an adaptor protein related to p130Cas – are located at both integrin adhesions and centrosomes. ILK binds to two spindle-pole proteins, CKAP5 and RUVB1, and disrupting either the kinase activity or expression of ILK prevents the interaction between these proteins and the mitotic kinase aurora A (which is necessary for microtubule polymerisation and spindle orientation), leading to the formation of supernumerary spindle poles (Fielding et al., 2008). One of the major ILK-binding proteins at integrin adhesions is α-parvin (PARVA), which is not seen at centrosomes. This confirms that there are distinct modes of ILK recruitment and/or function at these two discrete subcellular locales. NEDD9 also has a fascinating role at mitosis, and might coordinate the exit of cells from interphase with the formation of the mitotic spindle. NEDD9 regulates the stability of the primary cilium (which is present in many non-cycling cells but is disassembled at mitotic entry) by activating aurora A at cilia, which leads to phosphorylation of the tubulin deacetylase HDAC6 and cilial resorption (Pugacheva et al., 2007). The poles of the mitotic spindle share several components with primary cilia, such as the centriole; they also recruit NEDD9, which activates aurora A at spindle poles and is also necessary for several steps in mitosis, including the recruitment of microtubules (Pugacheva and Golemis, 2005). NEDD9, therefore, has essential roles both dissolving cilia and in forming mitotic spindles. Zyxin (which is involved with F-actin polymerisation) is another integrin-adhesion protein that associates with spindle poles during mitosis, where it becomes phosphorylated and associates with the mitotic serine/threonine kinases LATS1 and LATS2 (Hirota et al., 2000).
By and large, the proteins mentioned in this section are multifunctional adaptors. It is possible that they are utilised by cells to organise the cytoskeleton in different ways at interphase and metaphase, thus providing a rationale for their appearance in both integrin adhesions and centrosomes. An alternate and intriguing idea is that they are messengers that link integrin adhesions with the early stages of mitosis. Thus, the reduction of cell adhesion at prophase might be coordinated with centrosome duplication and mitotic-spindle formation, through the movement of specific integrin-adhesion components to the centrosome. This process might have deleterious consequences if the levels of integrin adhesion proteins are modified by genetic or epigenetic means. ILK and Cas family members, for example, are elevated in cancer, and an attractive hypothesis is that their deregulation causes the disease by inducing aberrant numbers of spindle poles and aneuploidy.
Summary
Although much of the discussion above has established a central role for integrin-mediated adhesion in the orientation of the mitotic spindle and, therefore, the location and fate of daughter cells, understanding the precise mechanisms involved is still a new science. ECM-generated tension and accurate positioning of cortical motors would seem to be of importance, but we do not yet fully understand the role of individual integrin-associated proteins. Nevertheless, these new studies demonstrate the breadth of involvement of integrins with cell-cycle regulation, and place them as lynchpins in the division of cells and the resulting cell fates that ensue. This involvement has been extended recently by the discovery of integrins at the cleavage furrow in mid- to late telophase, which concentrate at the furrow through endocytic trafficking that is regulated by the small GTPase Rab21 (Pellinen et al., 2008). An important question that now needs to be addressed is the degree to which the control of the cell-division axis by integrins determines the outcome of stem-cell divisions in specific tissues and, by extension, the influence of aberrant integrin signalling in cancer stem cells. The role of integrins in cancer stem cells is discussed in detail in an accompanying article (Pontier and Muller, 2008).
Conclusions
The multicellular nature of metazoans means that all cellular processes need to be tuned by adhesive interactions between cells and their local microenvironment. The spatial organisation of cells within tissues requires sophisticated networks of extracellular signals to control their survival and proliferation, movements and positioning, and differentiated function. These cellular characteristics are mediated by multiple inputs, and integrin adhesion now shares centre stage with the well-known soluble developmental signals for controlling all fate decisions.
C.H.S.'s research is supported by the Wellcome Trust and Breast Cancer Campaign. Deposited in PMC for release after 6 months.
Footnotes
Included with the term `growth factors' are other soluble factors such as survival and differentiation factors, hormones and cytokines.
References
- Adams, J. C. and Watt, F. M. (1989). Fibronectin inhibits the terminal differentiation of human keratinocytes. Nature 340, 307-309. [DOI] [PubMed] [Google Scholar]
- Akhtar, N. and Streuli, C. H. (2006). Rac1 links integrin-mediated adhesion to the control of lactational differentiation in mammary epithelia. J. Cell Biol. 173, 781-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar, N., Marlow, R., Lambert, E., Schatzmann, F., Lowe, E. T., Cheung, J., Katz, E., Li, W., Wu, C., Dedhar, S. et al. (2009). Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development (in press). [DOI] [PMC free article] [PubMed]
- Almeida, E. A., Ilic, D., Han, Q., Hauck, C. R., Jin, F., Kawakatsu, H., Schlaepfer, D. D. and Damsky, C. H. (2000). Matrix survival signaling: from fibronectin via focal adhesion kinase to c-Jun NH(2)-terminal kinase. J. Cell Biol. 149, 741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aszodi, A., Hunziker, E. B., Brakebusch, C. and Fassler, R. (2003). Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraamides, C. J., Garmy-Susini, B. and Varner, J. A. (2008). Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8, 604-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, W., Colognato, H. and ffrench-Constant, C. (2005). Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia 49, 467-479. [DOI] [PubMed] [Google Scholar]
- Bill, H. M., Knudsen, B., Moores, S. L., Muthuswamy, S. K., Rao, V. R., Brugge, J. S. and Miranti, C. K. (2004). Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol. Cell. Biol. 24, 8586-8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone, I., Napione, L., Maniero, F., Serini, G. and Bussolino, F. (2005). Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J. Cell Biol. 170, 993-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi, P., Rosso, A., Dentelli, P., Calvi, C., Garbarino, G., Tarone, G., Pegoraro, L. and Brizzi, M. F. (2005). {beta}1 Integrin and IL-3R coordinately regulate STAT5 activation and anchorage-dependent proliferation. J. Cell Biol. 168, 1099-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pozo, M. A. and Schwartz, M. A. (2007). Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends Cell Biol. 17, 246-250. [DOI] [PubMed] [Google Scholar]
- Del Pozo, M. A., Alderson, N. B., Kiosses, W. B., Chiang, H. H., Anderson, R. G. and Schwartz, M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839-842. [DOI] [PubMed] [Google Scholar]
- DeMali, K. A., Balciunaite, E. and Kazlauskas, A. (1999). Integrins enhance platelet-derived growth factor (PDGF)-dependent responses by altering the signal relay enzymes that are recruited to the PDGF beta receptor. J. Biol. Chem. 274, 19551-19558. [DOI] [PubMed] [Google Scholar]
- Du, Q. and Macara, I. G. (2004). Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503-516. [DOI] [PubMed] [Google Scholar]
- Farrelly, N., Lee, Y. J., Oliver, J., Dive, C. and Streuli, C. H. (1999). Extracellular matrix regulates apoptosis in mammary epithelium through a control on insulin signaling. J. Cell Biol. 144, 1337-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Minan, A., Martin-Bermudo, M. D. and Gonzalez-Reyes, A. (2007). Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 17, 683-688. [DOI] [PubMed] [Google Scholar]
- Fielding, A. B., Dobreva, I., McDonald, P. C., Foster, L. J. and Dedhar, S. (2008). Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J. Cell Biol. 180, 681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, E. (2008). Skin stem cells: rising to the surface. J. Cell Biol. 180, 273-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti, F. G. and Tarone, G. (2003). Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19, 173-206. [DOI] [PubMed] [Google Scholar]
- Gilmore, A. P. (2005). Anoikis. Cell Death Differ. 12 Suppl. 2, 1473-1477. [DOI] [PubMed] [Google Scholar]
- Gilmore, A. P., Metcalfe, A. D., Romer, L. H. and Streuli, C. H. (2000). Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J. Cell Biol. 149, 431-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff, C., Aszodi, A., Sakai, T., Hunziker, E. B. and Fassler, R. (2003). Integrin-linked kinase regulates chondrocyte shape and proliferation. EMBO Rep. 4, 432-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota, T., Morisaki, T., Nishiyama, Y., Marumoto, T., Tada, K., Hara, T., Masuko, N., Inagaki, M., Hatakeyama, K. and Saya, H. (2000). Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J. Cell Biol. 149, 1073-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, E., Barberis, L., Brancaccio, M., Azzolino, O., Xu, D., Kyriakis, J. M., Silengo, L., Giancotti, F. G., Tarone, G., Fassler, R. et al. (2002). Defective Rac-mediated proliferation and survival after targeted mutation of the beta1 integrin cytodomain. J. Cell Biol. 157, 481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, M. H. and Nguyen, H. T. (2005). Molecular mechanism of apoptosis induced by mechanical forces. Int. Rev. Cytol. 245, 45-90. [DOI] [PubMed] [Google Scholar]
- Jones, P. H. and Watt, F. M. (1993). Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713-724. [DOI] [PubMed] [Google Scholar]
- Katz, E. and Streuli, C. H. (2007). The extracellular matrix as an adhesion checkpoint for mammary epithelial function. Int. J. Biochem. Cell Biol. 39, 715-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T. and Fuchs, E. (2005). Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N., Zhang, Y., Naylor, M. J., Schatzmann, F., Maurer, F., Wintermantel, T., Schuetz, G., Mueller, U., Streuli, C. H. and Hynes, N. E. (2005). Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 24, 1942-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo, S. H. (2006). Focal adhesions: what's new inside. Dev. Biol. 294, 280-291. [DOI] [PubMed] [Google Scholar]
- Lock, R. and Debnath, J. (2008). Extracellular matrix regulation of autophagy. Curr. Opin. Cell Biol. 20, 583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rovira, T., Silva-Vargas, V. and Watt, F. M. (2005). Different consequences of beta1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J. Invest. Dermatol. 125, 1215-1227. [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar, G. H., Feng, W., Reddy, K., Plow, E. F. and Byzova, T. V. (2007). Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ. Res. 101, 570-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marastoni, S., Ligresti, G., Lorenzon, E., Colombatti, A. and Mongiat, M. (2008). Extracellular matrix: a matter of life and death. Connect. Tissue Res. 49, 203-206. [DOI] [PubMed] [Google Scholar]
- McLean, G. W., Komiyama, N. H., Serrels, B., Asano, H., Reynolds, L., Conti, F., Hodivala-Dilke, K., Metzger, D., Chambon, P., Grant, S. G. et al. (2004). Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18, 2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes, A., Heald, R., Samejima, K., Earnshaw, W. C. and Cleveland, D. W. (2000). Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J. Cell Biol. 149, 851-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi, A., Klein, S., Guo, W., Lopez-Lago, M., Lemichez, E., Westwick, J. K. and Giancotti, F. G. (2001). Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol. Cell 8, 115-127. [DOI] [PubMed] [Google Scholar]
- Moro, L., Dolce, L., Cabodi, S., Bergatto, E., Boeri Erba, E., Smeriglio, M., Turco, E., Retta, S. F., Giuffrida, M. G., Venturino, M. et al. (2002). Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 277, 9405-9414. [DOI] [PubMed] [Google Scholar]
- Naylor, M. J. and Streuli, C. H. (2006). Integrin regulation of mammary gland development. In Integrins and Development (ed. E. Danen), pp. 176-185. Georgetown, TX: Landes Bioscience.
- Naylor, M. J., Li, N., Cheung, J., Lowe, E., Lambert, E., Marlow, R., Wang, P., Schatzmann, F., Winntermantel, T., Schuetz, G. et al. (2005). Ablation of beta1-integrin in mammary epithelium reveals a key role for integrin in glanular morphogenesis and differentation. J. Cell Biol. 171, 717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell, C. B. and Wang, Y. L. (2000). Mammalian spindle orientation and position respond to changes in cell shape in a dynein-dependent fashion. Mol. Biol. Cell 11, 1765-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly, A. M., Lee, H. H. and Simon, M. A. (2008). Integrins control the positioning and proliferation of follicle stem cells in the Drosophila ovary. J. Cell Biol. 182, 801-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer, M., Mailleux, A. A., Mouneimne, G., Normand, G., Schnitt, S. J., King, R. W., Cibas, E. S. and Brugge, J. S. (2007). A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131, 966-979. [DOI] [PubMed] [Google Scholar]
- Pellinen, T., Tuomi, S., Arjonen, A., Wolf, M., Edgren, H., Meyer, H., Grosse, R., Kitzing, T., Rantala, J. K., Kallioniemi, O. et al. (2008). Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell 15, 371-385. [DOI] [PubMed] [Google Scholar]
- Pontier, S. M. and Muller, W. J. (2008). Integrins in mammary stem-cell biology and breast-cancer progression – a role in cancer stem cells? J. Cell Sci. 122, 207-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu, Q. Q. and Streuli, C. H. (2002). Integrin control of cell cycle: a new role for ubiquitin ligase. BioEssays 24, 17-21. [DOI] [PubMed] [Google Scholar]
- Pugacheva, E. N. and Golemis, E. A. (2005). The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat. Cell Biol. 7, 937-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva, E. N., Jablonski, S. A., Hartman, T. R., Henske, E. P. and Golemis, E. A. (2007). HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan, S., Wilson, J., Metcalfe, A., Edwards, G. M., Goberdhan, N., Tilly, J., Hickman, J. A., Dive, C. and Streuli, C. H. (1996). Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J. Cell Sci. 109, 631-642. [DOI] [PubMed] [Google Scholar]
- Reverte, C. G., Benware, A., Jones, C. W. and LaFlamme, S. E. (2006). Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis. J. Cell Biol. 174, 491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden, D. T. (2006). The stem-cell niche as an entity of action. Nature 441, 1075-1079. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A. and Ginsberg, M. H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65-E68. [DOI] [PubMed] [Google Scholar]
- Streuli, C. H. and Bissell, M. J. (1990). Expression of extracellular matrix components is regulated by substratum. J. Cell Biol. 110, 1405-1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli, C. H. and Akhtar, N. (2009). Signal cooperation between integrins and other receptor systems. Biochem. J. (in press). [DOI] [PubMed]
- Taddei, I., Deugnier, M. A., Faraldo, M. M., Petit, V., Bouvard, D., Medina, D., Fassler, R., Thiery, J. P. and Glukhova, M. A. (2008). Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat. Cell Biol. 10, 716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery, M. and Bornens, M. (2006). Cell shape and cell division. Curr. Opin. Cell Biol. 18, 648-657. [DOI] [PubMed] [Google Scholar]
- Thery, M., Racine, V., Pepin, A., Piel, M., Chen, Y., Sibarita, J. B. and Bornens, M. (2005). The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947-953. [DOI] [PubMed] [Google Scholar]
- Toyoshima, F. and Nishida, E. (2007a). Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 26, 1487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima, F. and Nishida, E. (2007b). Spindle orientation in animal cell mitosis: roles of integrin in the control of spindle axis. J. Cell Physiol. 213, 407-411. [DOI] [PubMed] [Google Scholar]
- Toyoshima, F., Matsumura, S., Morimoto, H., Mitsushima, M. and Nishida, E. (2007). PtdIns(3,4,5)P3 regulates spindle orientation in adherent cells. Dev. Cell 13, 796-811. [DOI] [PubMed] [Google Scholar]
- Velling, T., Stefansson, A. and Johansson, S. (2008). EGFR and beta1 integrins utilize different signaling pathways to activate Akt. Exp. Cell Res. 314, 309-316. [DOI] [PubMed] [Google Scholar]
- Watt, F. M. (2002). Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, K. L., Sokac, A. M., Berg, J. S., Cheney, R. E. and Bement, W. M. (2004). A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature 431, 325-329. [DOI] [PubMed] [Google Scholar]
- Woolner, S., O'Brien, L. L., Wiese, C. and Bement, W. M. (2008). Myosin-10 and actin filaments are essential for mitotic spindle function. J. Cell Biol. 182, 77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar, R., Itzkovitz, S., Ma'ayan, A., Iyengar, R. and Geiger, B. (2007). Functional atlas of the integrin adhesome. Nat. Cell Biol. 9, 858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H., Berg, J. S., Li, Z., Wang, Y., Lang, P., Sousa, A. D., Bhaskar, A., Cheney, R. E. and Stromblad, S. (2004). Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat. Cell Biol. 6, 523-531. [DOI] [PubMed] [Google Scholar]
- Zouq, N. K., Keeble, J. A., Lindsay, J., Valentijn, A. J., Zhang, L., Mills, D., Turner, T. E., Streuli, C. H. and Gilmore, A. P. (2009). FAK engages multiple pathways for maintaining survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J. Cell Sci. (in press). [DOI] [PMC free article] [PubMed]