Summary
The arginine-glycine-aspartate (RGD)-binding integrins αvβ6 and αvβ8 activate latent TGFβ1 and TGFβ3 in vivo, but it is uncertain whether other RGD-binding integrins such as integrins αvβ5 and αvβ3 activate these TGFβ isoforms. To define the combined role of αvβ6- and αvβ8-integrin in TGFβ activation, we analyzed mice lacking function of both integrins by means of gene deletion and/or pharmacologic inhibition. Most Itgb6–/–;Itgb8–/– embryos die at mid-gestation; those that survive develop cleft palate–as observed in Tgfb3–/– mice. Itgb8–/– mice treated with an anti-αvβ6-integrin antibody develop severe autoimmunity and lack Langerhans cells–similar to Tgfb1-null mice. These results support a model in which TGFβ3-mediated palate fusion and TGFβ1-mediated suppression of autoimmunity and generation of Langerhans cells require integrins αvβ6 and αvβ8 but not other RGD-binding integrins as TGFβ activators.
Keywords: Autoimmunity, Integrins, Langerhans cells, Palate fusion, TGFb
Introduction
The TGFβ cytokines (TGFβ1, TGFβ2 and TGFβ3) have multiple effects on a wide range of cell types. Knockout of the gene encoding TGFβ1 (Tgfb1–/–) causes incompletely penetrant defects in embryonic vascular development and a lethal autoimmune syndrome driven by activated CD4+ T cells (Shull et al., 1992; Kulkarni et al., 1993). Tgfb2–/– mice have developmental defects in the skeletal, cardiac and genitourinary systems, and Tgfb3–/– mice have cleft palate and variable abnormal lung development (Sanford et al., 1997; Kaartinen et al., 1995; Proetzel et al., 1995).
Processing of the TGFβ pro-protein produces a noncovalent complex between the TGFβ pro-peptide (called latency-associated peptide, LAP) and TGFβ (Annes et al., 2003). The association of LAP with TGFβ has to be altered or eliminated before TGFβ can engage TGFβ receptors. TGFβ activators include proteases that degrade LAP and molecules that nonproteolytically disrupt the latent complex, such as thrombospondin-1 and integrins αvβ6 and αvβ8 (Kojima et al., 1993; Yu and Stamenkovic, 2000; Annes et al., 2003; Mu et al., 2002; Munger et al., 1999).
Integrins αvβ6 and αvβ8 are two of eight integrins that recognize the tri-peptide sequence arginine-glycine-aspartate (RGD) (Hynes, 2002). Both activate TGFβ1 and TGFβ3 through interaction with an RGD sequence present in TGFβ1-LAP and TGFβ3-LAP; the propeptide of TGFβ2 does not have an RGD sequence (Munger et al., 1999; Annes et al., 2002; Mu et al., 2002; Araya et al., 2006). Mice that lack αvβ6 integrin (Itgb6–/–) have inflammatory infiltrates in skin and lungs, emphysema due to upregulated MMP12 expression in alveolar macrophages, reduced numbers of Langerhans cells (LCs) and altered lung surfactant metabolism. All of these abnormalities are indicative of reduced TGFβ signaling (Huang et al., 1996; Morris et al., 2003; Yang et al., 2007; Koth et al., 2007). Whereas Itgb6–/– embryos are normal, some Itgb8–/– embryos die at about embryonic day 10 (E10) because of vasculogenesis failure in the yolk sac, similar to Tgfb1–/– mice (Zhu et al., 2002). Of the surviving Itgb8–/– embryos, ∼10% have cleft palate. Itgb8–/– embryos that escape early embryonic death have abnormal brain vascular morphogenesis (Zhu et al., 2002), and mice with homozygous loss-of-function mutations in both Tgfb1 and Tgfb3 have similar brain vascular defects (Mu et al., 2008).
Mice with a knock-in mutation of Tgfb1 that inactivates the integrin-binding site in TGFβ1-LAP (RGD changed to RGE) duplicate the phenotype of Tgfb1–/– mice (Yang et al., 2007). Thus, RGD-binding integrins are required for TGFβ1 activation in early life. In addition to integrins αvβ6 and αvβ8, the RGD-binding integrins αvβ1, αvβ3, αvβ5 and α8β1 bind TGFβ1-LAP and, in the case of integrin αvβ3, also TGFβ3-LAP (Sheppard, 2005; Munger et al., 1999; Lu et al., 2002; Ludbrook et al., 2003; Munger et al., 1998). Evidence suggests that, under certain circumstances, integrins αvβ3 and αvβ5 activate latent TGFβ1 (Asano et al., 2005a; Asano et al., 2005b; Wipff et al., 2008). Therefore, it is not clear which RGD-binding integrins are relevant to the phenotype of Tgfb1RGE/RGE mice. To better define the RGD-binding integrins required for TGFβ1 and TGFβ3 activation, we analyzed mice that lack the function of the two best-characterized TGFβ-activating integrins, αvβ6 and αvβ8. We did this by generating mice homozygous for null mutations in both Itgb6 and Itgb8, and by pharmacologically inhibiting integrin αvβ6 in Itgb8–/– mice.
Results and Discussion
The originally described Itgb8–/– mice (C57BL/6J + 129/Sv genetic background) died at or before birth (Zhu et al., 2002). After being backcrossed to the ICR background, however, Itgb8–/– mice had a median survival of ∼2 months despite being born with brain hemorrhage, making it possible to assess the effect of complete loss of integrin αvβ8 in adult mice. At birth, these Itgb8–/– mice were present at the expected Mendelian ratio (data not shown). The external appearance of hemorrhage disappeared after the first week, and brains of 2-week-old mice that were examined histologically showed normal vasculature and no hemorrhage. Itgb8–/– mice are ∼60% smaller than control littermates (Fig. 1A). Cleft palate was observed once among ∼100 newborn knockout mice.
Fig. 1.
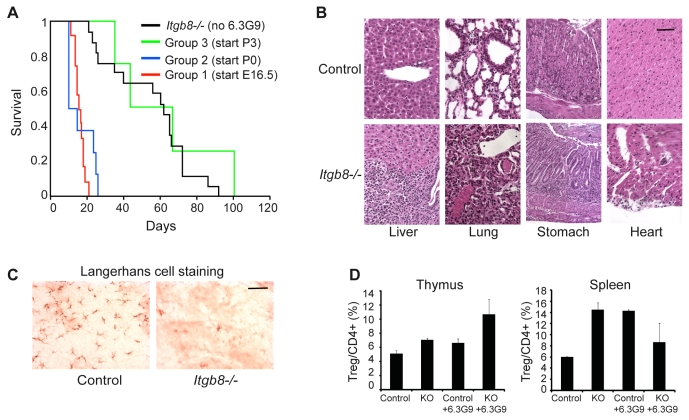
Phenotype of adult Itgb8–/– mice. (A) Itgb8–/– mice are smaller than control mice. White bars show control weights (n=13), black bars show Itgb8–/– weights (n=14). Error bars represent the s.e.m. (B) Representative hematoxylin- and eosin-stained sections of liver and colon of Itgb8–/– mice (9 weeks of age, original magnification 400×) demonstrate lack of inflammation. Scale bar: 100 μm.
We observed 17 adult Itgb8–/– mice until death occurred naturally or until their morbidity required sacrifice (see Table 1). In most of mice, weakness (particularly in the hindlimbs) developed at 7-10 weeks of age, and the mice became moribund as they lost mobility. This is similar to mice with a conditional deletion of Itgav in the central nervous system; those mice are born with brain hemorrhage but survive and develop axonal deterioration in the spinal cord and cerebellum, leading to ataxia and loss of hindlimb coordination (McCarty et al., 2005). Two Itgb8–/– mice developed rectal prolapse, and two Itgb8–/– mice developed localized infections (pyometra and periorbital abscess in one mouse each); occasional abscesses were also reported in Smad3-null mice (Yang et al., 1999). Histological examination of adult Itgb8–/– mice (lung, heart, liver, spleen, kidney, bladder, brain, thymus, pancreas, stomach, colon) showed no abnormalities aside from the above sporadic abnormalities. Mice with a conditional deletion of Itgb8 in hematopoietic cells or, specifically, in dendritic cells develop a late-onset autoimmune syndrome, characterized by splenomegaly, hepatitis and colitis with death between 4 and 10 months of age (Travis et al., 2007). However, we found no splenomegaly, hepatitis or colitis in adult Itgb8–/– mice, which all died before 4 months of age (Fig. 1B).
Table 1.
Findings in 17 Itgb8–/– adult mice
| Age at death (days) | Observations | Cause of death |
|---|---|---|
| 21 | Impaired mobility | D |
| 25 | Impaired mobility | D |
| 26 | Impaired mobility | D |
| 35 | Impaired mobility | D |
| 40 | Rectal prolapse, periorbital abscess | S |
| 56 | Weakness in hind limbs | S |
| 60 | Impaired mobility | D |
| 62 | Impaired mobility | D |
| 65 | Rectal prolapse | S |
| 65 | Weakness in hind limbs | S |
| 66 | Sparse hair | D |
| 72 | Weakness in hind limbs | S |
| 72 | Impaired mobility | D |
| 72 | Genital prolapse | S |
| 72 | Immobile | S |
| 86 | Weakness in hind limbs, pyometra | S |
| 92 | Impaired mobility | D |
| 74 | Weakness in hind limbs | S |
Itgb8–/– mice were observed until death or until sacrifice was required because of morbidity (which was most often due to poor mobility and inability to feed)
D, natural death; S; sacrificed
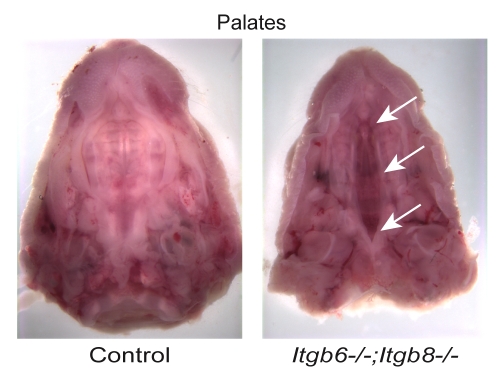
We next crossed Itgb8+/– mice with mice bearing a null allele for Itgb6 and identified Itgb8+/–;Itgb6+/– mice for breeding. Embryonic lethality for the Itgb8–/–;Itgb6–/– genotype was high. Of 327 neonates from Itgb8+/–;Itgb6+/– parents, only three (1%) were Itgb8–/–;Itgb6–/–, indicating that ∼85% of Itgb8–/–;Itgb6–/– embryos died before birth (see supplementary material Table S1). Two of the three Itgb8–/–;Itgb6–/– neonates had cleft palate without other craniofacial abnormalities, as occurs in Tgfb3–/– and Itgav–/– mice (Fig. 2). Cleft palates occurred in one of 14 Itgb8–/–;Itgb6+/– mice, but in none of the 16 Itgb8–/–;Itgb6+/+ neonates or in any other neonates in these litters. Thus, integrins αvβ6 and αvβ8 appear to be crucial for palate formation but, because the pentrance of cleft palate was not 100% in this small sample, there might be a role for other TGFβ3 activators in this process. Histologic findings in Itgb8–/–;Itgb6–/– neonates were otherwise unremarkable, with the exception of the lungs, which appeared to have a slight delay in alveolar development. However, because these changes might be due to respiratory distress (Proetzel et al., 1995), and because of the small number of samples, we cannot conclude that lung development is abnormal in these mice. However, lung development is abnormal in some Tgfb3–/– mice (Kaartinen et al., 1995; Proetzel et al., 1995) but is normal in Itgav-null mice (Bader et al., 1998), which lack αvβ6-, αvβ8- and three other αv-integrins.
Fig. 2.
Cleft palate in Itgb6–/–;Itgb8–/– neonates. The control sample shows normally fused palatal shelves, whereas the Itgb6–/–;Itgb8–/– sample demonstrates a cleft of the secondary palate. Arrows indicate cleft palate.
To assess the effect of the lack of integrins αvβ6 and αvβ8 on immune function in older mice, we treated Itgb8–/– mice with murine mAb 6.3G9, a specific inhibitor of integrin αvβ6 (Weinreb et al., 2004). To avoid interfering with palate fusion, we started treatment after palate fusion, which occurs at E15, and protocols differed regarding the time the treatments were started (see Table 2). All injections were 10 mg/kg, a dose that produces an essentially complete inhibition of integrin αvβ6 (Horan et al., 2007; Puthawala et al., 2008).
Table 2.
Phamacolocical blockage of αvβ6 integrin in mice
| Mice group | Genotype | Antibody | n | Result |
|---|---|---|---|---|
| 1 (treatments started at E16.5) | Itgb8–/– | 6.3G9 | 12 | Death at days 11-22; multi-organ inflammation in all |
| Non-KO | 6.3G9 | 12 | Littermates sacrificed in parallel; lung inflammation only | |
| Itgb8–/– | Control (1E6) | 2 | Sacrificed at weeks 6.5 and 9 – no inflammation | |
| Non-KO | Control (1E6) | 2 | Sacrificed at weeks 6.5 and 9 – no inflammation | |
| 2 (treatments started at P0) | Itgb8–/– | 6.3G9 | 8 | Death at days 11-26; multi-organ inflammation in all |
| Non-KO | 6.3G9 | 8 | Littermates sacrificed in parallel; lung inflammation only | |
| Itgb8–/– | Control (1E6) | 3 | One mouse death at day 25, two mice sacrificed at day 25; no inflammation | |
| Non-KO | Control (1E6) | 3 | Sacrificed at day 25; no inflammation | |
| 3 (treatments started at P3) | Itgb8–/– | 6.3G9 | 4 | Death at days 35-101; variable inflammation |
| Non-KO | 6.3G9 | 4 | Littermates sacrificed in parallel; lung inflammation only |
Mice groups 1, 2 and 3, three protocols used to treat mice with either mAb 6.3G9 against αvβ6 integrin or mAb 1E6 (control). Group 1 mice were treated prenatally by injecting pregnant females with 10 mg/kg of 6.3G9 (or control antibody) on E16.5 and E18.5, and then postnatally beginning on P3 and continuing weekly. Group 2 mice were first injected on the day of birth (P0) and then weekly. Group 3 mice were first injected on P3 and then weekly
When integrin αvβ6 inhibition was initiated before birth (E16.5) or at birth [referred to as postnatal day 0 (P0)] (see Table 2, groups 1 or 2, respectively), mice became moribund or died between days 11 and 26 (Fig. 3A). In all cases, littermates that were non-knockout (non-KO), i.e. Itgb8+/– or Itgb8+/+, and had been treated with the same antibody regimen appeared healthy at the time of the demise of the 6.3G9-treated Itgb8–/– mice. All 6.3G9-treated Itgb8–/– mice had extensive mononuclear cell infiltrates in heart, lung, liver, stomach and pancreas (Fig. 3B). Similarly to Tgfb1–/– and Tgfb1RGE/RGE mice, they also lacked LCs, a subtype of dendritic cell in the epidermis (Fig. 3C) (Borkowski et al., 1996; Yang et al., 2007). By contrast, non-KO littermates that had been treated with 6.3G9 and were sacrificed in parallel had epidermal LCs and showed no signs of inflammation except in the lungs of half the treated animals (consistent with loss of integrin αvβ6 function). The survival of these 6.3G9-treated Itgb8–/– mice (Fig. 3A) was slightly shorter than that reported for Tgfb1–/– and Tgfb1RGE/RGE mice (Kulkarni et al., 1993; Yang et al., 2007), most of which die between 3 and 4 weeks of age.
Fig. 3.
(A) Kaplan-Meier survival curves for Itgb8–/– mice (n=17), Itgb8–/– mice with 6.3G9 treatment starting at P3 (n=4), Itgb8–/– mice with 6.3G9 treatment starting at P0 (n=8), and Itgb8–/– mice with 6.3G9 treatment starting at E16.5 (n=12). (B) Hematoxylin and eosin staining of liver, lung, stomach and heart of representative Itgb8–/– and littermate non-KO control mice treated with 6.3G9 starting at E16.5 and sacrificed at 19 days. (C) Itgb8–/– mice treated with 6.3G9 starting at E16.5 lack Langerhans cells (LCs). Representative examples of epidermal sheets stained with an antibody that recognizes LCs. (D) Tregs isolated from thymus and spleen were identified by staining for FOXP3. Cells are from control mice, Itgb8–/– mice, and control and Itgb8–/– mice treated with 6.3G9 starting at E16.5. (n=3-4 for thymus, n=3 for spleen). Error bars represent the s.e.m. Scale bar: 100 μm.
In four additional Itgb8–/– mice, 6.3G9 treatment was started on P3 (Table 1, group 3). The survival of these mice was indistinguishable from that of untreated Itgb8–/– mice (Fig. 3A). Inflammatory changes were less marked: whereas all four mice had lung inflammation, consistent with inhibition αvβ6 integrin, only two of four had gastric inflammation or inflammation of the liver, and none had inflammation of the pancreas or heart.
Mice that lack Foxp3, a transcription factor that specifies regulatory T cell lineage (Treg), develop autoimmunity similar to that in Tgfb1–/– mice, which suggests a role for TGFβ1 in Treg development and/or function (Fontenot et al., 2003). Thymus-derived Tregs are generated in a TGFβ1-independent manner because they are present in Tgfb1–/– mice (Marie et al., 2005) and in mice whose type 2 TGFβ receptor (TGFβR2) is conditionally deleted in CD4+ cells (Marie et al., 2006). By contrast, TGFβ1 deficiency causes increased proliferation and decreased survival of peripheral or `adaptive' Tregs (Sakaguchi, 2004; Wan and Flavell, 2007; Marie et al., 2006; Rubtsov and Rudensky, 2007).
We quantified Tregs in 6.3G9-treated Itgb8–/– mice and in non-KO control mice at P8, when significant numbers of thymic Tregs are found in wild-type mice (Fontenot et al., 2005). Inhibition of αvβ6 integrin was initiated before birth, as in Group 1 (see Table 2). Thymic and splenic Tregs in 6.3G9-treated Itgb8–/– mice were not reduced, as also observed in Tgfb1RGE/RGE mice (Fig. 3D) (Yang et al., 2007). The 6.3G9-treated Itgb8–/– mice did not show histological evidence of inflammation at P8 (data not shown). P17 mice were evaluated in studies that demonstrated reduced numbers of splenic Tregs in TGFβ1-deficient mice (Marie et al., 2006; Rubtsov and Rudensky, 2007), which might explain the different result in our study.
Travis and colleagues have shown that conditional loss of αvβ8 integrin in all leukocytes or, specifically, in dendritic cells causes autoimmunity (Travis et al., 2007). These experiments were performed by crossing mice with a floxed Itgb8 allele with mice that expressed Cre recombinase under the control of either the Vav1 or CD11c (Itgax) promoter. In both cases, a wasting syndrome with inflammation in the colon and the portal triads of the liver began at ∼4-5 months of age. Death occurred naturally or when their morbidity required sacrifice, in most cases after 6 months of age. In unpublished work, this group also generated Itgb6–/–;(Vav1-cre)Itgb8fl/fl mice. These mice had a similar wasting and autoimmune syndrome, but with an accelerated course, with death occurring by 23 weeks (personal communication, Dean Sheppard, UCSF, San Francisco, CA, and Mark Travis, University of Manchester, UK). Neither of these inflammatory phenotypes is as severe as in TGFβ1-deficient or 6.3G9-treated Itgb8–/– mice. Together, these observations and our findings suggest that expression of αvβ8 integrin by both dendritic cells and non-immune cells, in combination with αvβ6 integrin, is needed to prevent excessive lymphocyte activation. αvβ8 integrin is widely expressed on many non-immune cell types, such as neurons, fibroblasts and epithelial cells (Zhu et al., 2002; Araya et al., 2007; Nishimura et al., 1998; Proctor et al., 2005).
6.3G9-treated Itgb8–/– mice have a shorter survival time than Tgfb1–/– and Tgfb1RGE/RGE mice. The reasons for this are not known. The genetic background might be responsible. In addition, the mice might be physiologically impaired because of brain hemorrhage and low body weight. It is also possible that TGFβ3, which is activated by αvβ6- and αvβ8-integrins, subtly ameliorates the immune phenotype of TGFβ1-deficient mice. If so, this rescue effect might be absent in 6.3G9-treated Itgb8–/– mice, because αvβ6 and αvβ8 can activate TGFβ3. In regard to this hypothesis, it is of interest to consider mice with a T-cell-specific deletion of the type II TGFβ receptor, because in these mice T cells would be unresponsive to all three TGFβ isoforms. In one of two reports on such mice, (CD4-Cre)Tgfbr2fl/fl mice appear to have a slightly more aggressive inflammatory process than do Tgfb1-null mice (Li et al., 2006).
The finding that the inhibition of integrin αvβ6 in Itgb8–/– mice initiated at P3 results in a much milder inflammatory syndrome than when inhibition is initiated at P0 or before birth remains unexplained, but might relate to dynamics of Treg development in the first few days of life. Classic experiments have shown that thymectomy on the third day of life (P2) leads to autoimmunity, whereas thymectomy on the first or seventh day (P0 or P6, respectively) does not, suggesting suppressor cells are released from the thymus later than autoreactive cells (reviewed by Shevach et al., 2001). Rudensky's group has demonstrated a rapid increase in thymic Tregs in the first few days of life, with the biggest increase occurring at days 3-4 (Fontenot et al., 2005). Previous experiments have indicated that thymic Treg-cell development is largely independent of TGFβ (Marie et al., 2005; Marie et al., 2006). However, a recent report that describes mice in which TGFβ signaling is conditionally ablated in T cells by means of floxed Tgfbr1 alleles and Cre recombinase expressed under control of the CD4 promoter, has demonstrated that thymic Treg development is dramatically blocked at days P2-P4; by 1 week, thymic Treg-cell levels had normalized owing to expansion of Tregs in an IL2-dependent manner (Liu et al., 2008). CD4+CD25+Foxp3+ Tregs from these mice showed an activated phenotype and were defective in in-vitro suppressor assays. Thus, our results might indicate that sufficient numbers of normally functioning Tregs are released by P3 to suppress inflammation, but if TGFβ signaling is eliminated earlier, normal Tregs are not released and severe inflammation ensues.
The present findings complement our recent report on the phenotype of mice that lack integrin-responsive TGFβ1 and totally lack TGFβ3 (Tgfb1RGE/RGE;Tgfb3–/–). These mice showed the expected abnormalities seen in Tgfb1RGE/RGE or Tgfb3–/– mice, as well as one new abnormality not present in any TGFβ mutant mouse: abnormal brain vascular morphogenesis that appears to be identical to that in mice that lack αvβ8 integrin (Zhu et al., 2002; Bader et al., 1998). Here, we show that mice that lack the function of both αvβ6- and αvβ8-integrin display abnormalities not seen in either of the two single-integrin-knockout mice that recapitulate the major abnormalities in Tgfb3-null mice (cleft palate) and Tgfb1-null mice (severe inflammation and lack of LCs). Taken together, these findings reveal a system in which two TGFβ isoforms and two TGFβ activators act cooperatively in several developmental processes.
Materials and Methods
Mice
Itgb8–/– mice were from Louis Reichardt (UCSF, San Francisco, CA) and had been backcrossed for at least six generations onto the ICR background (Charles River Laboratories, Boston, MA). Itgb6–/– mice on a C57BL/6J background were obtained from Dean Sheppard (University of California, San Francisco, CA) and were backcrossed three generations onto the ICR background before crossing with Itgb8–/– mice.
Genotyping
PCR was carried out using primers specific for Itgb8 wild-type and targeted alleles (mutant primers 5′-AGAGGCCACTTGTGTAGCGCCAAG-3′ and 5′-GGAGGCATACAGTCTAAATTGT-3′ and wild-type primers 5′-ATTATCTGGTTGATGTGTCAGC-3′ and 5′-AGAGAGGAACAAATATCCTTCCC-3′). The PCR protocol was 94°C for 4 minutes, 35 cycles of 94°C for 40 seconds, 56°C for 30 seconds and 72°C for 30 seconds, and 72°C for 7 minutes. For Itgb6 wild-type and targeted alleles, PCR was carried out using primers flanking the insertion site of the neomycin-resistance gene (5′-TGTTAATGGCAAAATGTGCT-3′ and 5′-CAGTTCTGACATTGTTCAG-3′) and flanking Itgb6 exon 4 (5′-GTGAGCAGACTCTGCAAGTGC-3′ and 5′-CTGCAAGGGTTGGTGATTTCC-3′). PCR protocols were, for Itgb6 wild-type allele, 94°C for 4 minutes, 32 cycles of 94°C for 30 seconds, 58°C for 30 seconds and 72°C for 50 seconds, and 72°C for 10 minutes and, for Itgb6 KO allele, 94°C for 4 minutes, 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds and 72°C for 20 seconds, and 72°C for 10 minutes.
Treatments
Group 1 mice were treated at E16.5 and E18.5 by injecting pregnant females with 10 mg/kg 6.3G9 (or the control IgG1 monoclonal antibody 1E6) (Puthawala et al., 2008; Hahm et al., 2007; Weinreb et al., 2004). At birth, Itgb8–/– mice were identified by the presence of brain hemorrhage (confirmed by genotyping). Injections of 10 mg/kg into Itgb8–/– mice and littermate controls continued at P3 and then once a week. Group 2 mice were first injected on P0 and then once a week. Group 3 mice were first injected on P3 and then once a week. Mice were observed daily and sacrificed if moribund. Tissues were collected at death for histology. Two types of control mice were used for this experiment: control non-KO littermates that received the same injections of 6.3G9 as the Itgb8–/– mice, and Itgb8–/– mice treated with control IgG1 mAb. Non-KO mice receiving 6.3G9 were sacrificed when their 6.3G9-treated KO littermates died; no non-KO mice that had received 6.3G9 died before similarly treated KO littermates. The NYU School of Medicine animal care committee approved all procedures, which conformed to NIH guidelines.
LC immunostaining
LCs in epidermal sheets were detected as described previously (Thomas et al., 2001; Yang et al., 2007). LC staining was performed on epidermal sheets isolated from backs of 6.3G9-treated Itgb8–/– mice at time of death and compared with samples from littermate non-KO control mice sacrificed at the same time. Epidermal sheets from seven Itgb8–/– and eight non-KO mice treated with 6.3G9 were stained. 6.3G9 treatment was started at E16.5.
Flow cytometric analysis
Single-cell suspensions from spleen or thymus (after RBC lysis) were incubated with anti-CD4-FITC antibody with Mouse BD Fc Block (BD Biosciences), washed, incubated in permeabilization/fixation buffer, re-incubated with Fc Block and anti-Foxp3-PE antibody (eBioscience), washed, and analyzed using a FACscan flow cytometer (Becton Dickinson).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/2/????/DC1
This work was funded by NIH grant R01 HL063786 and an Irma T. Hirschl Scholar Award from the Irma T. Hirschl/Monique Weill-Caulier Trusts (both to J.S.M.). We thank Paola Mita for help with Langerhans cell staining and Ezra Dweck for helpful discussions and technical assistance.
References
- Annes, J. P., Rifkin, D. B. and Munger, J. S. (2002). The integrin αVβ6 binds and activates latent TGFβ3. FEBS Lett. 511, 65-68. [DOI] [PubMed] [Google Scholar]
- Annes, J. P., Munger, J. S. and Rifkin, D. B. (2003). Making sense of latent TGFβ activation. J. Cell Sci. 116, 217-224. [DOI] [PubMed] [Google Scholar]
- Araya, J., Cambier, S., Morris, A., Finkbeiner, W. and Nishimura, S. L. (2006). Integrin-mediated transforming growth factor β activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am. J. Pathol. 169, 405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya, J., Cambier, S., Markovics, J. A., Wolters, P., Jablons, D., Hill, A., Finkbeiner, W., Jones, K., Broaddus, V. C., Sheppard, D. et al. (2007). Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J. Clin. Invest. 117, 3551-3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano, Y., Ihn, H., Yamane, K., Jinnin, M., Mimura, Y. and Tamaki, K. (2005a). Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J. Immunol. 175, 7708-7718. [DOI] [PubMed] [Google Scholar]
- Asano, Y., Ihn, H., Yamane, K., Jinnin, M., Mimura, Y. and Tamaki, K. (2005b). Involvement of αvβ5 integrin-mediated activation of latent transforming growth factor β1 in autocrine transforming growth factor β signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 52, 2897-2905. [DOI] [PubMed] [Google Scholar]
- Bader, B. L., Rayburn, H., Crowley, D. and Hynes, R. O. (1998). Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell 95, 507-519. [DOI] [PubMed] [Google Scholar]
- Borkowski, T. A., Letterio, J. J., Farr, A. G. and Udey, M. C. (1996). A role for endogenous transforming growth factor β1 in langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal langerhans cells. J. Exp. Med. 184, 2417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot, J. D., Gavin, M. A. and Rudensky, A. Y. (2003). Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330-336. [DOI] [PubMed] [Google Scholar]
- Fontenot, J. D., Dooley, J. L., Farr, A. G. and Rudensky, A. Y. (2005). Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202, 901-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm, K., Lukashev, M. E., Luo, Y., Yang, W. J., Dolinski, B. M., Weinreb, P. H., Simon, K. J., Chun Wang, L., Leone, D. R., Lobb, R. R. et al. (2007). αvβ6 integrin regulates renal fibrosis and inflammation in alport mouse. Am. J. Pathol. 170, 110-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan, G. S., Wood, S., Ona, V., Li, D. J., Lukashev, M. E., Weinreb, P. H., Simon, K. J., Hahm, K., Allaire, N. E., Rinaldi, N. J. et al. (2007). Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 177, 56-65. [DOI] [PubMed] [Google Scholar]
- Huang, X. Z., Wu, J. F., Cass, D., Erle, D. J., Corry, D., Young, S. G., Farese, R. V. and Sheppard, D. (1996). Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J. Cell Biol. 133, 921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. [DOI] [PubMed] [Google Scholar]
- Kaartinen, V., Voncken, J. W., Shuler, C., Warburton, D., Bu, D., Heisterkamp, N. and Groffen, J. (1995). Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 11, 415-421. [DOI] [PubMed] [Google Scholar]
- Kojima, S., Nara, K. and Rifkin, D. B. (1993). Requirement for transglutaminase in the activation of latent transforming growth factor-β in bovine endothelial cells. J. Cell Biol. 121, 439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koth, L. L., Alex, B. T., Hawgood, S., Nead, M. A., Sheppard, D., Erle, D. J. and Morris, D. G. (2007). Integrin β6 mediates phospholipid and collectin homeostasis by activation of latent TGFβ1. Am. J. Respir. Cell Mol. Biol. 37, 651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, A. B., Huh, C. G., Becker, D., Geiser, A., Lyght, M., Flanders, K. C., Roberts, A. B., Sporn, M. B., Ward, J. M. and Karlsson, S. (1993). Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 90, 770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. O., Sanjabi, S. and Flavell, R. A. (2006). Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455-471. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Zhang, P., Li, J., Kulkarni, A. B., Perruche, S. and Chen, W. (2008). A critical function for TGFβ signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632-640. [DOI] [PubMed] [Google Scholar]
- Lu, M., Munger, J. S., Steadele, M., Busald, C., Tellier, M. and Schnapp, L. M. (2002). Integrin α8β1 mediates adhesion to LAP-TGFβ1. J. Cell Sci. 115, 4641-4648. [DOI] [PubMed] [Google Scholar]
- Ludbrook, S. B., Barry, S. T., Delves, C. J. and Horgan, C. M. (2003). The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem. J. 369, 311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie, J. C., Letterio, J. J., Gavin, M. and Rudensky, A. Y. (2005). TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 1061-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie, J. C., Liggitt, D. and Rudensky, A. Y. (2006). Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25, 441-454. [DOI] [PubMed] [Google Scholar]
- McCarty, J. H., Lacy-Hulbert, A., Charest, A., Bronson, R. T., Crowley, D., Housman, D., Savill, J., Roes, J. and Hynes, R. O. (2005). Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132, 165-176. [DOI] [PubMed] [Google Scholar]
- Morris, D. G., Huang, X., Kaminski, N., Wang, Y., Shapiro, S. D., Dolganov, G., Glick, A. and Sheppard, D. (2003). Loss of integrin αvβ6-mediated TGF-β activation causes Mmp12-dependent emphysema. Nature 422, 169-173. [DOI] [PubMed] [Google Scholar]
- Mu, D., Cambier, S., Fjellbirkeland, L., Baron, J. L., Munger, J. S., Kawakatsu, H., Sheppard, D., Broaddus, V. C. and Nishimura, S. L. (2002). The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157, 493-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, Z., Yang, Z., Yu, D., Zhao, Z. and Munger, J. S. (2008). TGFβ1 and TGFβ3 are partially redundant effectors in brain vascular morphogenesis. Mech. Dev. 125, 508-516. [DOI] [PubMed] [Google Scholar]
- Munger, J. S., Harpel, J. G., Giancotti, F. G. and Rifkin, D. B. (1998). Interactions between growth factors and integrins: latent forms of transforming growth factor-β are ligands for the integrin αvβ1. Mol. Biol. Cell 9, 2627-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger, J. S., Huang, X., Kawakatsu, H., Griffiths, M. J., Dalton, S. L., Wu, J., Pittet, J. F., Kaminski, N., Garat, C., Matthay, M. A. et al. (1999). The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319-328. [DOI] [PubMed] [Google Scholar]
- Nishimura, S. L., Boylen, K. P., Einheber, S., Milner, T. A., Ramos, D. M. and Pytela, R. (1998). Synaptic and glial localization of the integrin αvβ8 in mouse and rat brain. Brain Res. 791, 271-282. [DOI] [PubMed] [Google Scholar]
- Proctor, J. M., Zang, K., Wang, D., Wang, R. and Reichardt, L. F. (2005). Vascular development of the brain requires β8 integrin expression in the neuroepithelium. J. Neurosci. 25, 9940-9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel, G., Pawlowski, S. A., Wiles, M. V., Yin, M., Boivin, G. P., Howles, P. N., Ding, J., Ferguson, M. W. and Doetschman, T. (1995). Transforming growth factor-β3 is required for secondary palate fusion. Nat. Genet. 11, 409-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthawala, K., Hadjiangelis, N., Jacoby, S. C., Bayongan, E., Zhao, Z., Yang, Z., Devitt, M. L., Horan, G. S., Weinreb, P. H., Lukashev, M. E. et al. (2008). Inhibition of integrin αvβ6, an activator of latent transforming growth factor-β, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 177, 82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov, Y. P. and Rudensky, A. Y. (2007). TGFβ signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 7, 443-453. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, S. (2004). Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531-562. [DOI] [PubMed] [Google Scholar]
- Sanford, L. P., Ormsby, I., Gittenberger-de Groot, A. C., Sariola, H., Friedman, R., Boivin, G. P., Cardell, E. L. and Doetschman, T. (1997). TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development 124, 2659-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard, D. (2005). Integrin-mediated activation of latent transforming growth factor β. Cancer Metastasis Rev. 24, 395-402. [DOI] [PubMed] [Google Scholar]
- Shevach, E. M., McHugh, R. S., Piccirillo, C. A. and Thornton, A. M. (2001). Control of T cell activation by CD4+CD25+ suppressor T cells. Immunol Rev. 182, 58-67. [DOI] [PubMed] [Google Scholar]
- Shull, M. M., Ormsby, I., Kier, A. B., Pawlowski, S., Diebold, R. J., Yin, M., Allen, R., Sidman, C., Proetzel, G. and Calvin, D. (1992). Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 359, 693-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R. M., Belsito, D. V., Huang, C., Chen, L. Z., Ormsby, I., Simmons, W. J., Cowin, P., Shaw, J., Doetschman, T. and Thorbecke, G. J. (2001). Appearance of langerhans cells in the epidermis of Tgfβ1(–/–) SCID mice: paracrine and autocrine effects of transforming growth factor-β1 and -β2(1). J. Invest. Dermatol. 117, 1574-1580. [DOI] [PubMed] [Google Scholar]
- Travis, M. A., Reizis, B., Melton, A. C., Masteller, E., Tang, Q., Proctor, J. M., Wang, Y., Bernstein, X., Huang, X., Reichardt, L. F. et al. (2007). Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. Y. and Flavell, R. A. (2007). `Yin-yang' functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol. Rev. 220, 199-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb, P. H., Simon, K. J., Rayhorn, P., Yang, W. J., Leone, D. R., Dolinski, B. M., Pearse, B. R., Yokota, Y., Kawakatsu, H., Atakilit, A. et al. (2004). Function-blocking integrin αvβ6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J. Biol. Chem. 279, 17875-17887. [DOI] [PubMed] [Google Scholar]
- Wipff, P. J., Rifkin, D. B., Meister, J. J. and Hinz, B. (2008). Myofibroblast contraction activates latent TGFβ1 from the extracellular matrix. J. Cell Biol. 179, 1311-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Letterio, J. J., Lechleider, R. J., Chen, L., Hayman, R., Gu, H., Roberts, A. B. and Deng, C. (1999). Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J. 18, 1280-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., Mu, Z., Dabovic, B., Jurukovski, V., Yu, D., Sung, J., Xiong, X. and Munger, J. S. (2007). Absence of integrin-mediated TGFβ1 activation in vivo recapitulates the phenotype of TGFβ1-null mice. J. Cell Biol. 176, 787-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q. and Stamenkovic, I. (2000). Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163-176. [PMC free article] [PubMed] [Google Scholar]
- Zhu, J., Motejlek, K., Wang, D., Zang, K., Schmidt, A. and Reichardt, L. F. (2002). β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129, 2891-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.