Summary
Integrins of the β1 subfamily are highly expressed in the early mouse embryo and are essential for the formation of primitive germ layers from the inner cell mass (ICM). We investigated the mechanisms by which αβ1 integrins regulate ICM morphogenesis by using the embryonic-stem-cell-derived embryoid body (EB), a model for peri-implantation development. Ablation of integrin β1 in EBs resulted in endoderm detachment and in maturation defects, which were manifested by the mislocalization of GATA4 in the cytoplasm and the markedly reduced synthesis of basement membrane (BM) components and the lineage marker disabled homolog 2. The mutant endoderm cells failed to spread on BM substrates, but could spread on vitronectin, which induced upregulation of αvβ3 integrin and integrin-dependent GATA4 nuclear translocation. Forced expression of integrin β3 in the mutant EBs completely rescued endoderm morphogenesis, suggesting that integrin β3 can substitute for integrin β1 in the endoderm. Furthermore, the mitogen-activated protein kinases (MAPKs) ERK1 and ERK2 (ERK1/2) and p38 were activated in endoderm in an integrin-dependent fashion. Pharmacological inhibition of ERK1/2 or p38 MAPK blocked vitronectin-induced GATA4 nuclear translocation and endoderm maturation, whereas expression of a constitutively active ERK kinase (MEK1) or p38 MAPK in the mutant cells rescued endoderm maturation in integrin-β1-null endoderm cells. Collectively, these results suggest that integrins are required for both the stable adhesion and maturation of visceral endoderm, the latter being mediated through the activation of ERK1/2 and p38 MAPK.
Keywords: Integrin, Epithelial morphogenesis, Adhesion, GATA4, MAP kinase
Introduction
Integrins are extracellular matrix (ECM) receptors composed of two noncovalently linked subunits, α and β. They have been implicated in the regulation of many cellular processes, such as adhesion, migration, proliferation and survival. In mammals, 24 different integrins have been identified, which can be categorized into three subfamilies: β1-, β2- and αv-based integrins (Hynes, 2002). αβ1 and αvβ integrins are detected throughout embryonic development and are ubiquitously expressed in adult tissues, whereas αβ2 integrins are restricted to white blood cells. Targeted deletion of the integrin-β1 gene in mice was accomplished more than a decade ago; the mutant embryo was found to die shortly after implantation, indicating that αβ1 integrins are essential for early embryogenesis (Fassler and Meyer, 1995; Stephens et al., 1995). The inner cell mass (ICM) of the mutant embryo fails to differentiate into organized endoderm and epiblast. However, what has remained a mystery is how integrins regulate the formation of these primitive germ layers.
During mouse peri-implantation development, the primitive endoderm is the first differentiated cell type to arise from the ICM (Li et al., 2003; Rossant, 2004). Endoderm cells secrete laminin, type IV collagen and other basement membrane (BM) components, which assemble into an underlying BM. The primitive endoderm then differentiates to become the visceral and parietal endoderm. After implantation, the visceral-endoderm-covered epiblast cells adjacent to the BM polarize to form a pseudostratified columnar epithelium, which is the precursor of three definitive germ layers. The cells that do not contact with the BM die by apoptosis, creating the proamniotic cavity. Ablation of the laminin γ1 chain in mice abolishes BM formation and also arrests the development at the peri-implantation stage with a phenotype similar to that of integrin-β1-null mice, suggesting that αβ1 integrins are the primary receptors that interact with the BM and that this interaction is essential for early embryogenesis (Smyth et al., 1999).
Because the embryo at this developmental stage is difficult to access and is almost impossible to be utilized for biochemical and molecular biology studies, embryoid bodies (EBs) cultured from genetically modified embryonic stem (ES) cells are the most useful model to elucidate the mechanisms of early embryonic tissue morphogenesis (Coucouvanis and Martin, 1995; Li and Yurchenco, 2006). We and others have used integrin-β1-null ES cells to study the role αβ1 integrins in BM assembly (Aumailley et al., 2000; Li et al., 2002). Western analysis and immunofluorescence microscopy showed that laminin synthesis is significantly reduced in integrin-β1-null EBs. Given that laminin is mainly produced by endoderm, the result suggests that αβ1 integrins might be required for endoderm differentiation, but this has not been confirmed with endoderm lineage-specific markers.
EBs are usually formed by culturing ES-cell aggregates in suspension in non-adhesive bacteriological Petri dishes. Unlike their wild-type counterparts, integrin-β1-null EBs attach strongly to the plastic surface, resulting in a progressive loss of EBs during the culturing process. In addition, the attached EBs grow much faster than those in suspension, which rapidly acidifies the culture medium. These problems have rendered the analysis of integrin-β1-null EBs difficult. In this study, we created a non-adhesive surface for culturing integrin-β1-null EBs by coating bacteriological Petri dishes with polyhydroxyethylmethacrylate (poly-HEMA). By using various endoderm lineage markers, we show that ablation of integrin β1 in differentiating EBs results in endoderm detachment and in maturation defects. The detached endoderm cells neither adhere and/or spread on BM substrates nor form focal adhesions (FAs), suggesting that integrin-dependent stable adhesion is required for endoderm attachment. The loss of integrin β1 in endoderm cells selectively reduces the expression of BM proteins and the lineage marker disabled homolog 2 (Dab2; also known as Doc2), probably because the endoderm-specific transcription factor GATA4 fails to undergo nuclear translocation. We further show that αvβ3 integrin can substitute for αβ1 integrins in the endoderm to mediate GATA4 nuclear translocation, cellular maturation and morphogenesis. Lastly, we demonstrate that the mitogen-activated protein kinases (MAPKs) ERK1 and ERK2 (ERK1/2) and p38 mediate integrin-dependent GATA4 nuclear translocation and endoderm maturation. These results provide new insights into the mechanisms by which integrins control visceral endoderm morphogenesis.
Results
α5β1 and α6β1 are the major integrin dimers expressed in endoderm and epiblast, respectively
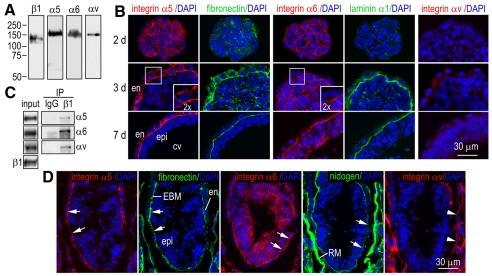
Gene-targeting experiments have demonstrated that integrin β1 is essential for early embryogenesis (Fassler and Meyer, 1995; Stephens et al., 1995). To determine the integrin-α subunits that pair with integrin β1 to mediate germ-layer formation, we performed western blot analysis with integrin-subunit-specific antibodies and showed that integrins α5, α6, αv and β1 were expressed in differentiated (5-7 day) EBs (Fig. 1A). We could not detect the expression of integrins α1, α2, α3, β3, β4 or β5 (data not shown). To gain insight into the spatial and temporal expression of these integrin subunits during EB differentiation, we immunostained normal EBs cultured to the stages before endoderm formation (day 2), with endoderm and BM but before epiblast polarization (day 3) or with fully polarized epiblast and a central cavity (day 7). Prior to endoderm and BM formation, the integrin α5, α6 and β1 subunits were distributed to cell-cell junctions, where they colocalized with the cell-cell adhesion receptor E-cadherin (Fig. 1B and data not shown). Following endoderm formation, integrin α5 was segregated from integrin α6 and targeted to the endoderm, whereas integrin α6 was mainly expressed in the epiblast. Integrins α5, α6 and β1 were condensed at the cell-BM adhesion site, where they colocalized with fibronectin and laminin in the BM (Fig. 1B and data not shown). To verify that fibronectin in the BM is deposited by endoderm and/or epiblast cells but not from serum in the culture medium, we analyzed EBs differentiated from fibronectin-null ES cells. Immunostaining demonstrated that fibronectin was completely absent from the BM in fibronectin-null EBs, indicating that fibronectin is a bona fide component of the embryonic BM (data not shown). Altogether, these data suggest that integrin α5β1 and integrin α6β1 are the main integrin receptors that mediate the adhesion of endoderm and epiblast to the BM, respectively. By contrast, the integrin-αv subunit remained in the cytoplasm at all stages of EB differentiation, although it formed a complex with integrin β1 as assessed by co-immunoprecipitation (Fig. 1C).
Fig. 1.
Expression of integrin subunits in EBs and early mouse embryos. (A) Cell lysates of 5-day-old normal EBs were subjected to western blot analysis for integrin subunits β1, α1, α2, α3, α5, α6, αv, β3, β4 and β5. β1, α5, α6 and αv were expressed in differentiated EBs. (B) Normal EBs at different developing stages (days 2-7) were immunostained for integrins α5, α6 and αv, and for the BM components fibronectin and laminin. The insets are 2× magnifications of the boxed regions. en, endoderm; epi, epiblast; cv, cavitation. (C) Co-immunoprecipitation showed that integrin β1 formed a complex with the integrin α5, α6 and αv subunits. (D) Immunofluorescence analysis of E6.5 mouse embryos showed that integrin α5 was mainly expressed in the endoderm, whereas α6 was mainly expressed in the epiblast, and both were recruited to the embryonic BM (EBM) zone (arrows), as revealed by fibronectin and nidogen immunostaining. Integrin αv was not detected at the cell-EBM adhesion but at the trophoblast–Reichert's-membrane (RM) interface (arrowheads).
To correlate the above findings with in vivo embryonic development, we examined the expression of the integrin subunits in embryonic day 6.5 (E6.5) mouse embryos by immunofluorescence microscopy. Similar to differentiated EBs, the integrin-α5 subunit was mainly expressed in the endoderm, whereas integrin α6 was mainly expressed in the epiblast (Fig. 1D). Integrin αv was not detected in the embryo proper but was present at the trophoblast–Reichert's-membrane adhesion site and colocalized with integrin β3 (Fig. 1D and data not shown). We also detected integrins α2 and α3 at trophoblast–Reichert's-membrane adhesions (data not shown). Altogether, our data suggest that α5β1 and α6β1 integrins are spatially segregated to endoderm and epiblast, respectively, whereas αvβ3, α2β1 and α3β1 integrins might mediate trophoblast function.
Ablation of integrin β1 causes detachment and defective maturation of visceral endoderm
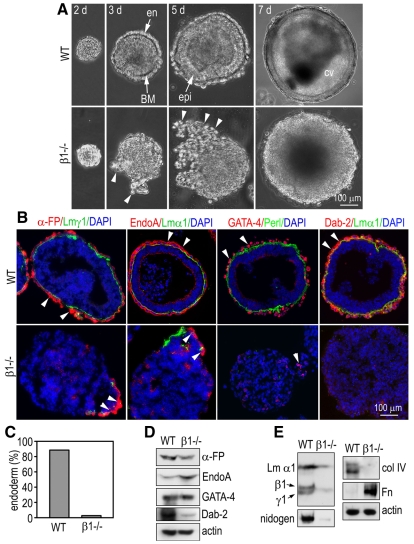
To elucidate the mechanisms whereby αβ1 integrins regulate primitive germ-layer formation, we analyzed integrin-β1-null EBs differentiated in bacteriological Petri dishes coated with poly-HEMA. When cultured in suspension for 2 days, both wild-type and integrin-β1-null ES-cell aggregates were compacted into spherules with a smooth surface (Fig. 2A). On the next day, a distinct outer layer of endoderm developed in normal EBs that was separated from the centrally located apolar cells by a BM. As the EBs further differentiated, the epiblast cells adjacent to the BM polarized to form a pseudostratified columnar epithelium, whereas the cells not in contact with the BM died by apoptosis, creating a central proamniotic-like cavity. In sharp contrast to this normal differentiation process, most of the integrin-β1-null EBs failed to form a continuous endoderm layer. Clusters of loosely associated round cells could be observed on the EB surface as well as in the conditioned medium (Fig. 2A). Immunostaining for the endoderm markers revealed that, in normal EBs, α-fetoprotein (α-FP), cytokeratin Endo A and Dab2 were present in the cytoplasm of endoderm cells, whereas GATA4 was detected predominantly in the nucleus, which reflects its role as an endoderm-specific transcription factor in peri-implantation embryos (Soudais et al., 1995). By contrast, in the mutant EBs, we only observed segments or clusters of endoderm cells positive for α-FP and cytokeratin Endo A in ∼30% of the EBs examined (Fig. 2B). We did not observe Dab2 immunofluorescence in these EBs. After 7 days, endoderm cells were largely absent from the surface of mutant EBs, which developed neither epiblast nor a central cavity. Western blot analysis of the EBs and the detached endoderm cells showed that the expression of α-FP, Endo A and GATA4 was comparable between wild-type and the mutant EBs, whereas Dab2 expression was markedly reduced in the mutants, confirming our immunostaining results (Fig. 2D). Altogether, these data suggest that endoderm cells differentiate inadequately and fail to attach on the EB surface in the absence of αβ1 integrins. As a consequence, BM formation and epiblast polarization are blocked.
Fig. 2.
Ablation of integrin β1 in embryoid bodies (EBs) disrupts endoderm morphogenesis. Wild-type (WT) and integrin-β1-null (β1–/–) EBs were cultured in suspension for 2-7 days. (A) Live phase micrographs show endoderm (en) formation on day 3, and epiblast (epi) polarization and cavitation (cv) on days 5 and 7, respectively, in wild-type EBs (arrows). By contrast, endoderm cells detached from or loosely adhered to the EB surface (arrowheads) in most of the mutant EBs. BM, basement membrane. (B) 5-day-old EBs were immunostained for the endoderm markers α-fetoprotein (α-FP), cytokeratin EndoA, GATA4 and disabled-2 (Dab2). BM is identified by laminin α1/γ1 chain (Lm α1/γ1) or perlecan (perl) immunofluorescence. Arrowheads point to positively stained endoderm cells. (C) EBs that had developed a continuous α-FP-positive endoderm layer were counted and plotted as a percentage of total EBs examined. More than 85% of the WT EBs developed a continuous endoderm layer, whereas less than 4% of the mutant EBs had nearly normal endoderm. (D) Western analysis was performed for the expression of endoderm markers on 5-day-old normal and mutant EBs collected together with detached endoderm cells. Actin serves as loading control. (E) Normal endoderm cells were harvested by brief trypsinization and mechanical dissociation. The detached integrin-β1-null endoderm cells were collected from the conditioned medium. The expression of the BM proteins laminin-111, nidogen, collagen IV (Col IV) and fibronectin (Fn) was analyzed by western blotting.
We and Aumailley et al. previously reported that integrin-β1-null EBs, when the whole EB extracts were analyzed by western blotting, exhibit markedly reduced synthesis of laminin and nidogen (Aumailley et al., 2000; Li et al., 2002). To test whether this reduced synthesis of BM components is caused by a blockade of endoderm lineage commitment or a defect in endoderm cell maturation, we collected normal endoderm cells by brief trypsinization and mechanical dissociation, and compared them with detached mutant endoderm cells positively stained for α-FP (Fig. 3A). As shown in Fig. 2E, western blot analysis demonstrated an 80-90% reduction in the synthesis of laminin-111, nidogen and collagen IV by the mutant cells. Fibronectin expression, however, was markedly increased. The enhanced fibronectin synthesis by mutant endoderm cells might compensate for the reduced cell adhesion to this substrate. Given that a major function of the endoderm is to synthesize and secrete BM components, these results indicate that αβ1 integrins regulate the functional maturation of endoderm cells rather than their lineage commitment.
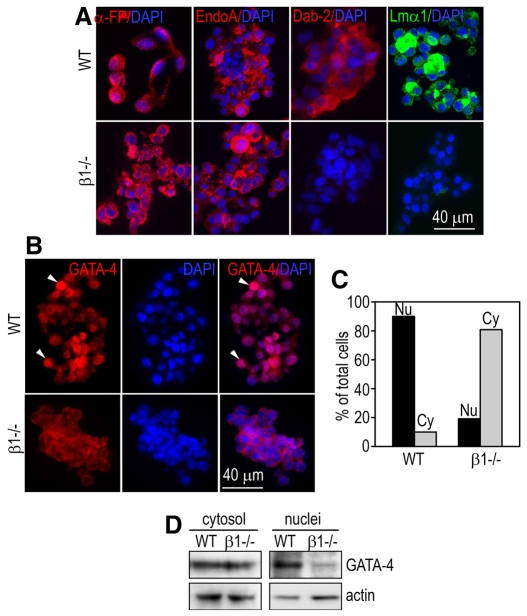
Fig. 3.
GATA4 is mislocalized to the cytoplasm in integrin-β1-null endoderm cells. (A,B) Endoderm cells collected from normal (WT) and integrin-β1-null (β1–/–) EBs were immunostained for α-FP, cytokeratin EndoA, Dab2, laminin α1 and GATA4. Nuclei were counterstained with DAPI. Dab2 and laminin are not detected in the mutant cells. GATA4 is mainly localized to the nucleus in normal endoderm cells but is present in the cytoplasm in the mutants (arrowheads). (C) Endodermal cells with cytosolic (Cy) or nuclear (Nu) GATA4 staining were counted by fluorescence microscopy and plotted as a percentage of total cells examined. (D) Cytosolic or nuclear fractions of cell lysates were analyzed by western blotting for GATA4 expression. Actin serves as loading control.
GATA4 is mislocalized to cytoplasm in integrin-β1-null endoderm cells
To confirm that integrin-β1-null endoderm cells have committed to the visceral endoderm lineage but are functionally immature, we immunostained endoderm cells that were detached from EBs. Both normal and mutant endoderm cells were positive for α-FP and cytokeratin Endo A (Fig. 3A). However, Dab2 and laminin were only detected in wild-type but not in mutant cells. These results demonstrate for the first time the existence of two separated steps of visceral endodermal differentiation, lineage commitment and maturation; the latter is controlled by αβ1 integrins. Furthermore, although the expression of GATA4 at the protein level was comparable between normal and mutant EBs (Fig. 2D), immunofluorescence microscopy revealed that GATA4 was localized to distinct cellular compartments. In normal endoderm cells, GATA4 was predominantly targeted to the nucleus, whereas in the mutant cells it was mainly present in the cytoplasm (Fig. 3B). Western blot analysis of the cytosolic and nuclear extracts of endoderm cells supports our immunofluorescence observation, demonstrating a significant reduction of GATA4 in the nuclear fraction of the mutant endoderm cells (Fig. 3C). These results suggest that αβ1 integrins are required for GATA4 nuclear localization. Given that GATA4 and GATA6 (GATA4/6) have been shown to regulate the transcription of laminin and Dab2, it is likely that the reduced GATA4 levels in the nucleus account for the decreased expression of laminin and Dab2 in the mutant endoderm cells (Futaki et al., 2004; Morrisey et al., 2000).
Integrin-β1-null endoderm cells fail to form focal adhesions on laminin and fibronectin
To elucidate the ECM ligands that might interact with integrins and mediate adhesion of endoderm cells to the BM, we isolated endoderm cells from 5-day normal EBs and cultured them on fibronectin, laminin-111 or type IV collagen. The endoderm cells spread nicely on fibronectin and formed typical FAs, as revealed by paxillin immunofluorescence. α5β1 but not αvβ3 integrin was present in the FAs (supplementary material Fig. S1 and data not shown). The cells spread equally well on laminin-111 substrate, although they formed smaller and more-linear FAs that contained paxillin and α6β1 integrin. By contrast, most of the cells failed to attach on type IV collagen, and a few cells that did attach were unable to spread and form FAs. The endoderm cells could also spread on vitronectin and assemble FAs but with reduced efficiency. Integrins αv and β1 colocalized with paxillin at the FAs. However, it is unlikely that the binding of αvβ1 integrin to vitronectin plays a role in the endoderm adhesion because vitronectin is not present in the BM (data not shown). The adhesion of endoderm cells on fibronectin and vitronectin was selectively blocked by the peptide RGDS but not RGES, whereas the adhesion on laminin-111 was not affected by RGDS (data not shown). These data suggest that the interaction of α5β1 and α6β1 integrins with their corresponding ligands, fibronectin and laminin, probably mediates adhesion of endoderm cells to the BM in an RGD-dependent and -independent fashion, respectively. These new results might also explain why the visceral endoderm develops normally in the absence of either integrin α5 or α6 (Georges-Labouesse et al., 1996; Yang et al., 1993).
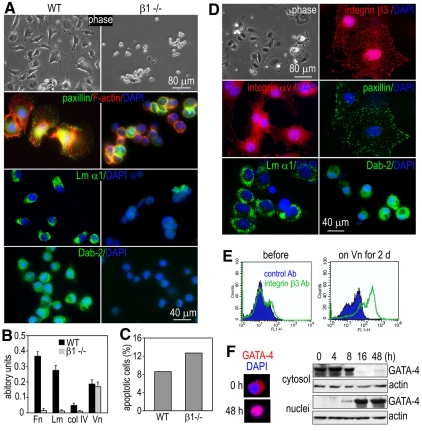
To test whether the detachment of integrin-β1-null endoderm cells from the EB surface is due to reduced cell adhesion, we collected detached mutant endoderm cells from the conditioned medium and cultured them on BM substrates for 2 days. Over 90% of wild-type endoderm cells attached and spread on fibronectin with well-developed FAs and actin stress fibers, whereas less than 20% of the mutant cells attached but remained rounded (Fig. 4A). Neither FAs nor actin stress fibers developed in the adherent mutant cells. Of note, strong immunofluorescence of laminin and Dab2 was observed in all normal endoderm cells examined. By contrast, less than 20% of the mutant endoderm cells were weakly positive for laminin and none expressed Dab2. Mutant endoderm cells were unable to attach on laminin-111. A short-term (1 hour) adhesion assay also revealed defective adhesions of the mutant cells (Fig. 4B). To test whether apoptosis might also contribute to the detachment of endoderm cells, we immunostained the normal and mutant cells for the apoptosis marker cleaved caspase-3. There was a slightly higher percentage of apoptotic cells in the mutant EBs (Fig. 4C). This increased level of apoptosis might occur after cell detachment because of loss of anchorage. Nonetheless, it is unlikely that apoptosis is the main cause of detachment of endoderm cells in the mutant EBs. Altogether, these data indicate that integrin β1 is essential for the stable adhesion and functional maturation of endoderm cells.
Fig. 4.
Integrin-β1-null endoderm cells fail to adhere and/or spread on BM substrates but can spread on vitronectin; vitronectin upregulates integrin β3 and induces GATA4 nuclear translocation. (A) Live phase micrographs show normal (WT) and integrin-β1-null (β1–/–) endoderm cells cultured on fibronectin for 16 hours. The cells were fixed and immunostained for paxillin, laminin α1 chain (Lm α1) and Dab2. F-actin was stained with rhodamine-phalloidin. (B) Endoderm cells were plated on various ECM substrates for 1 hour and adherent cells were quantified by crystal-violet staining and spectrophotometry. n=4 for each group. (C) Endoderm cells collected from the conditioned medium were fixed and immunostained for the apoptosis marker cleaved caspase-3. Positive cells were counted and plotted as a percentage of total cells examined. [n=326 for the wild-type (WT) and n=308 for integrin-β1-null (β1–/–) cells]. (D) Integrin-β1-null endoderm cells were grown on vitronectin (Vn)-coated coverslips for 2 days and immunostained for integrin β3, integrin αv, paxillin, laminin α1 and Dab2. (E) The expression of integrin β3 was analyzed by FACS in integrin-β1-null endoderm cells before and after 2 days of culturing on vitronectin. (F) Integrin-β1-null endoderm cells were cultured on vitronectin, and GATA4 levels in cytosolic and nuclear fractions were assayed by western blotting at designated time points. The intracellular distribution of GATA4 was also analyzed by immunostaining. Vitronectin induced GATA4 nuclear translocation.
Because the cell-adhesion assay demonstrated that the mutant endoderm cells could adhere on vitronectin to the same extent as the normal control, we tested whether other αvβ integrin(s) can compensate for the loss of αvβ1 integrin. Indeed, immunostaining showed that integrin β3 was expressed and colocalized with αv and paxillin at FAs (Fig. 4D). FACS analysis confirmed significant upregulation of integrin β3 in integrin-β1-null endoderm cells cultured on vitronectin (Fig. 4E). Interestingly, αvβ3-integrin-mediated adhesion of the mutant cells on vitronectin was coupled with enhanced laminin-111 and Dab2 expression. Furthermore, GATA4 was translocated from the cytoplasm to the nucleus in parallel with integrin αvβ3 induction as assessed by immunostaining and western blot analysis of cytosolic and nuclear extracts (Fig. 4F). These results suggest that the upregulation of αvβ3 integrin can compensate for the loss of αβ1 integrins to promote the adhesion and maturation of the endoderm cells. Moreover, this vitronectin-induced rescue provides a tractable system to dissect the role of integrin signaling in endoderm morphogenesis.
Forced expression of integrin β3 in integrin-β1-null EBs rescues endoderm development
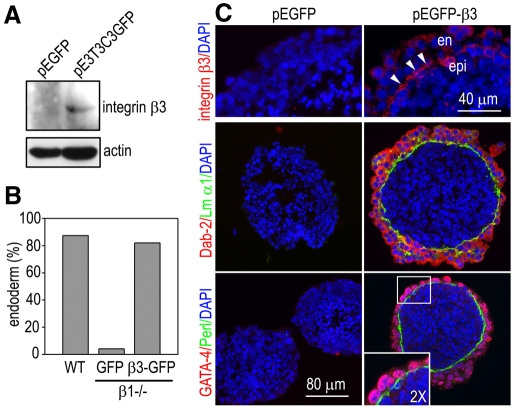
To test whether integrin β3 can substitute for integrin β1 to mediate endoderm morphogenesis, we cultured EBs using integrin-β1-null ES cells stably expressing GFP-tagged full-length integrin β3 (pGFPE3T3C31-47) (Foletti et al., 2005). Phase-contrast microscopy demonstrated that expression of integrin β3 in integrin-β1-null EBs completely rescued endoderm morphogenesis (Fig. 5). Immunostaining for the endoderm markers α-FP, cytokeratin Endo A and Dab2 showed a continuous endoderm layer on the surface of the integrin-β3-rescued EBs but not on that of GFP-transduced controls. GATA4 was localized to the nucleus of the β3-transduced endoderm cells. A linear BM was deposited under the endoderm and integrin β3 was condensed at the BM zone. Despite the restoration of endoderm and BM, integrin-β3 transduction failed to induce epiblast polarization and cavitation in integrin-β1-null EBs (Fig. 5 and data not shown). These results indicate that integrin β3 can functionally substitute for integrin β1 to mediate endoderm development and BM assembly but not epiblast polarization and cavitation.
Fig. 5.
Forced expression of integrin β3 in integrin-β1-null EBs rescues endoderm morphogenesis. (A) EBs were cultured for 5 days using integrin-β1-null ES cells stably transfected with integrin-β3–GFP (β3-GFP) or GFP. The expression of integrin β3 was analyzed by western blotting. (B) Wild-type and integrin-β1-null EBs expressing integrin-β3–GFP or GFP were cultured for 5 days and endoderm formation was examined by live phase microscopy. The percentage of EBs with endoderm formation was plotted. n=344 for the wild-type, n=328 for the GFP and n=311 for the β3-GFP group. (C) The β3-GFP or GFP EBs were cultured for 5 days and immunostained for integrin β3 and the endoderm markers Dab2 and GATA4. BM was stained for laminin α1 or perlecan. Integrin-β3–GFP is recruited to the BM zone (arrowheads). The inset is a 2× magnification of the boxed region to show GATA4 nuclear staining. en, endoderm; epi, epiblast.
Knockdown of integrin αv inhibits vitronectin-induced maturation of integrin-β1-null endoderm cells
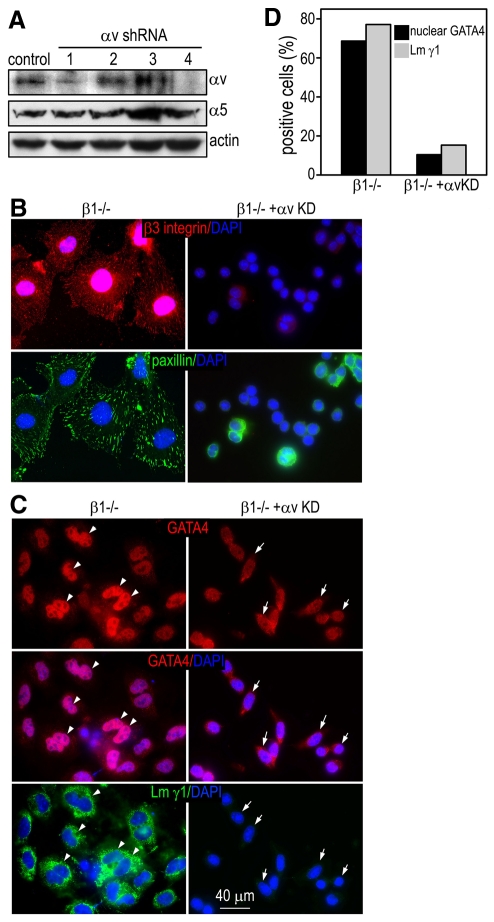
To confirm that the upregulation of αvβ3 integrin accounts for vitronectin-induced GATA4 nuclear translocation and the expression of Dab2 and laminin in integrin-β1-null endoderm cells, we chose to knockdown the expression of integrin αv, which dimerizes not only with integrin β3 but also with integrins β5, β6 and β8, thus eliminating possible contributions from these integrins. To this end, integrin-β1-null ES cells were infected with retrovirus expressing shRNAs targeting mouse integrin αv. A total of 24 stable clones expressing four different shRNAs were generated and western blot analysis showed that shRNA4 almost completely knocked down integrin αv expression without affecting that of integrin α5 (Fig. 6A). We collected the endoderm cells that detached from the integrin-β1-null EBs that were transduced with the control vector, pSM2, or pSM2-αv shRNA4. When cultured on vitronectin for 2 days, the control cells spread and formed FAs that contained integrin αvβ3 and paxillin (Fig. 6B and data not shown). GATA4 was primarily located in the nucleus and laminin in the peri-nuclear region, possibly associated with the endoplasmic reticulum and/or Golgi complex. By contrast, the mutant cells with integrin-αv knockdown neither spread nor formed FAs. GATA4 resided largely in the cytoplasm and laminin was barely detectable. There is close correlation between GATA4 nuclear staining and laminin expression (Fig. 6C,D). These results demonstrate that the vitronectin-induced GATA4 nuclear translocation and the maturation of endoderm cells are mediated by the upregulation of αvβ3 integrin.
Fig. 6.
Knockdown of integrin αv in integrin-β1-null endoderm cells blocks vitronectin-induced FA formation, GATA4 nuclear translocation and laminin synthesis. (A) Integrin-β1-null ES cells were infected with retrovirus expressing short hairpin RNAs (shRNAs) targeting mouse integrin αv. Stable cell lines were established by puromycin selection. Western blots show that transduction of shRNA1 and shRNA4 inhibits the expression of integrin αv but not that of integrin α5. (B,C) Integrin-β1-null endoderm cells stably expressing integrin-αv shRNA or control vector pSM2 were cultured on vitronectin for 2 days. The cells were fixed and immunostained for integrin β3, paxillin, GATA4 and laminin γ1. Nuclei were counterstained with DAPI. GATA4 (red) is mainly localized to the nucleus (purple when merged with DAPI staining) in control cells (arrowheads). By contrast, it is distributed primarily in the cytoplasm in integrin-αv knockdown (αv KD) cells (arrows). The GATA4 nuclear staining closely correlates with laminin expression (arrows and arrowheads). (D) Cells with positive laminin γ1 and nuclear GATA4 staining were counted and plotted.
ERK1/2 and p38 MAPK mediate integrin-dependent GATA4 nuclear translocation and endoderm-cell maturation
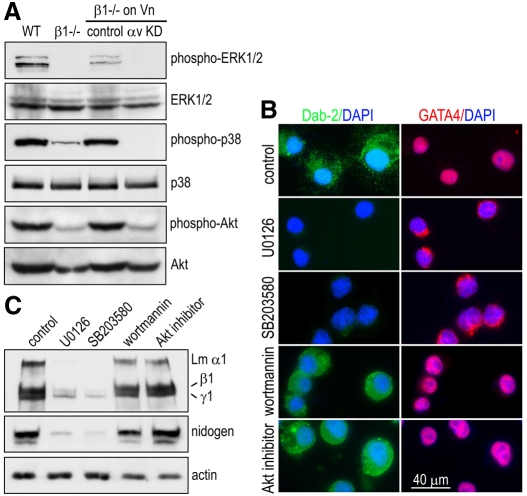
We next sought to determine the mechanism by which integrins regulate the maturation of endoderm cells. It has been suggested that mitogen-activated protein kinase (MAPK) and phosphoinositol 3-kinase (PI3K)-Akt pathways are implicated in endoderm development (Chen et al., 2000; Hamazaki et al., 2006; Verheijen et al., 1999). Furthermore, integrin signaling has been shown to be essential for the activation of ERK1/2 and the PI3K-Akt pathway in adherent cells (Pankov et al., 2003; Schwartz and Ginsberg, 2002). We therefore tested whether integrins are required for MAPK and Akt activation in endoderm cells and whether the activation of these kinases leads to integrin-dependent endoderm maturation. Western blot analysis of isolated endoderm cells showed that integrin-β1 ablation completely abolished ERK1/2 activation and markedly suppressed p38 MAPK and Akt activation, as assessed using the phosphorylation-specific antibodies that detect the activated kinases (Fig. 7A). When the mutant endoderm cells were grown on vitronectin for 2 days to induce integrin-β3 expression (Fig. 4D,E), the activation of ERK1/2, p38 MAPK and Akt was restored. However, knockdown of integrin αv abrogated the vitronectin-induced rescue. These findings suggest that integrins are required for the activation of MAPK and PI3K-Akt pathways in endoderm cells. Next, we tested the effect of inhibition of these kinases on GATA4 nuclear translocation and endoderm maturation using specific inhibitors. Treatment of integrin-β1-null endoderm cells with the MEK1/2 (MAPK kinase) inhibitor U0126 or the MEK1 inhibitor PD98059 blocked GATA4 nuclear translocation and notably reduced the expression of Dab2, laminin and nidogen (Fig. 7B,C and data now shown). The p38 MAPK inhibitor SB203580 produced a similar effect. By contrast, treatment with the PI3K inhibitor wortmannin or the Akt inhibitor had minimum or no effect on GATA4 nuclear translocation or the expression of Dab2, laminin and nidogen. Thus, the inhibition of MEK-ERK and p38 MAPK pathways selectively blocks GATA4 nuclear translocation and endoderm maturation. We also tested whether these inhibitory effects might result from a blockade of integrin-β3 upregulation or a reduction in cell adhesion on vitronectin. Immunofluorescence microscopy revealed that integrin β3 was readily detected at FAs in mutant endoderm cells in all experimental groups, although the MEK1/2 inhibitor U0128 slightly reduced cell spreading (supplementary material Fig. S2). Altogether, these results suggest that the integrin-dependent activation of MAPK pathways probably mediates GATA4 nuclear translocation and endoderm maturation.
Fig. 7.
ERK1/2 and p38 MAPK activation is suppressed in integrin-β1-null endoderm cells, and their inhibition abrogates vitronectin-induced GATA4 nuclear translocation and cellular maturation. (A) Endoderm cells isolated from 5-day EBs were either analyzed directly or cultured on vitronectin (Vn) for 2 days and then analyzed by western blotting. The activation of ERK1/2, p38 MAPK and Akt was detected using kinase phosphorylation-specific antibodies. (B) Integrin-β1-null endoderm cells were cultured on vitronectin and treated with the MEK1/2 inhibitor U0126 (10 μM), the p38 MAPK inhibitor SB203580 (10 μM), the PI3K inhibitor wortmannin (1 μM) or Akt1/2 inhibitor (10 μM). After 2 days, the cells were fixed and stained for Dab2 and GATA4. (C) The cells treated with the above kinase inhibitors were subject to western blot analysis for laminin-111 and nidogen.
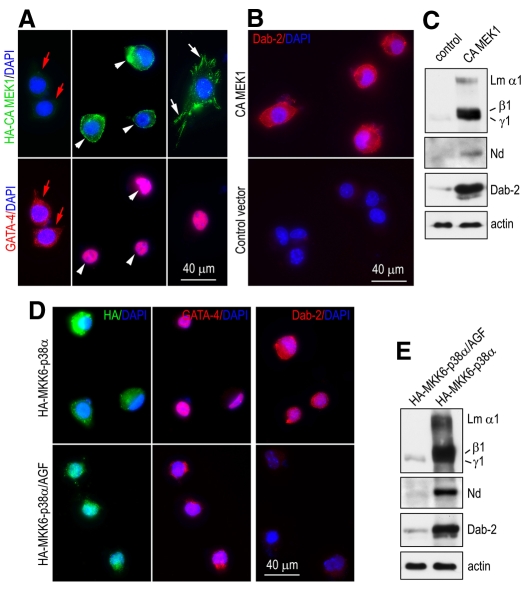
If the MAPKs are downstream effectors of integrins that mediate GATA4 nuclear translocation and endoderm maturation, expression of constitutively active (CA) MAPKs should bypass the requirement of integrins and rescue the defects of the mutant endoderm cells. To test this hypothesis, we transfected the mutant cells with CA MEK1, H-Ras or p38 MAPK, and cultured them on fibronectin, which does not support the spreading of the mutant cells. Immunofluorescence microscopy showed that the HA-tagged CA MEK1 was mainly localized to the plasma membrane in transfected cells. Approximately 10% of transfected cells spread on fibronectin. In these cells, HA-tagged CA MEK1 was accumulated at FAs, suggesting that the activation of the MEK-ERK pathway promotes endoderm-cell spreading in the absence of α5β1 integrin (Fig. 8A). In all the cells expressing HA-tagged CA MEK1, GATA4 was detected in the nucleus and Dab2 in the cytoplasm (Fig. 8A,B). In non-transfected cells or cells transfected with the control vector, GATA4 remained in the cytoplasm and Dab2 was not detected. Western blot analysis confirmed our immunostaining results, demonstrating markedly increased Dab2 expression in the mutant cells transfected with HA-tagged CA MEK1 (Fig. 8C). In addition, the expression of the BM components laminin-111 and nidogen was also dramatically upregulated. Transfection of the mutant cells with CA p38 MAPK or CA H-Ras also led to GATA4 nuclear translocation and increased expression of Dab2 and the BM proteins (Fig. 8D,E; supplementary material Fig. S3) although the cells remained rounded on fibronectin. These data suggest that activation of the Ras-MEK-ERK and p38 MAPK pathways induces GATA4 nuclear translocation and endoderm-cell maturation independently on cell spreading.
Fig. 8.
Expression of constitutively active MEK1 or p38 MAPK induces GATA4 nuclear translocation and cellular maturation in integrin-β1-null endoderm cells. (A,B) Integrin-β1-null endoderm cells were transfected with HA-tagged constitutively active (CA) MEK1 or control vector pcDNA3. The transfected cells were grown on fibronectin for 2 days and stained for HA-tag, GATA4 and Dab2. In untransfected cells, GATA4 was found mainly in the cytoplasm (red arrows). HA–CA-MEK1 expression led to GATA4 nuclear localization and increased Dab2 expression (white arrowheads). Some of the transfected cells spread on fibronectin and formed FAs containing HA–CA-MEK1 (white arrows). (C) Western blots show increased expression of laminin, nidogen and Dab2 in HA–CA-MEK1-transfected cells. (D) Integrin-β1-null endoderm cells were transfected with CA HA-tagged MKK6-p38α or the non-phosphorylatable mutant MKK6-p38α/AGF. The transfected cells were stained for HA-tag, GATA4 and Dab2. (E) Western blots show increased expression of laminin, nidogen and Dab2 in MKK6-p38α-transfected cells.
Discussion
In this study, we investigated the mechanisms through which integrins promote visceral endoderm formation by analyzing integrin-β1-null EBs and demonstrate that: (1) αβ1 integrins are essential for the adhesion of endoderm cells to the BM; (2) integrins are specifically required for endoderm maturation but not for lineage commitment; (3) integrin β3 can substitute for integrin β1 to mediate endoderm morphogenesis; (4) integrins facilitate the nuclear translocation of the endoderm-specific transcription factor GATA4; and (5) ERK1/2 and p38 MAPK mediate integrin-dependent GATA4 nuclear translocation and endoderm maturation.
A major function of integrins is to mediate cell adhesion to the ECM, which is crucial for tissue morphogenesis and maintenance. Besides mediating cell-matrix adhesions, integrins can also transmit ECM signals across the plasma membrane through FA proteins to regulate many cellular processes, such as migration, proliferation, differentiation and apoptosis (Miranti and Brugge, 2002). In Drosophila, integrin is crucial for muscle attachment to the epidermis (Brown et al., 2002). It has also been shown to control gene expression in the midgut epithelium independently of ligand-binding capacity (Martin-Bermudo and Brown, 1999). These results suggest a role for integrins in regulating cell adhesion and gene expression in vivo. It is yet largely unknown how integrins regulate tissue formation during mammalian embryonic development. In this study, we investigated the mechanisms of integrin-dependent endoderm differentiation using ES-cell-derived EBs. We observed that integrin-β1-null endoderm cells detached from the EB surface and could not form a continuous endoderm layer. Immunostaining of the mutant endoderm cells for the apoptosis marker cleaved caspase-3 only revealed a slight increase of apoptosis compared with normal controls. However, the detached mutant cells fail to adhere and/or spread on the BM proteins laminin and fibronectin. These results suggest that the loss of integrin-dependent adhesion rather than increased apoptosis accounts for the endoderm detachment in integrin-β1-null EBs. Interestingly, the mutant endoderm cells are able to adhere and spread on vitronectin through the upregulation of integrin β3. Although vitronectin is not a component of the embryonic BM, this finding prompted us to test the hypothesis that integrin β3 might be able to substitute for integrin β1 to mediate endoderm morphogenesis. Indeed, forced expression of integrin β3 in integrin-β1-null EBs completely rescued endoderm development and BM assembly. Surprisingly, the BM formation was not followed by epiblast polarization and cavitation even though integrin β3 was recruited to the cell-BM adhesion site. These data suggest that integrin β3 can substitute for integrin β1 to induce endoderm formation, whereas integrin β1 is specifically required for epiblast polarization and cavitation. Such a germ-layer-specific requirement of integrins has not been reported in mammalian development. In Drosophila, the RGD-binding integrin αPS2βPS (similar to αvβ integrins) is able to replace the function of the laminin-binding integrin αPS1βPS (similar to α6β1 integrin) in the eye but not in the embryo (Martin-Bermudo et al., 1997; Roote and Zusman, 1996). However, swapping the cytoplasmic domains between the two β-subunits does not alter their function, suggesting that the ligand-binding ability of the extracellular domains determines their functional specificity. These data, together with our findings that αvβ3 integrin shares a subset of ligands in the BM with α5β1 integrin (the major integrin expressed in endoderm) and that it is recruited to the BM zone in the rescued EBs, provide an explanation for the compensation of αvβ3 integrin for the loss of αβ1 integrins in mediating endoderm development.
We have previously reported that addition of laminin-111 to culture media can rescue endoderm, BM and the epiblast epithelium in a small fraction of integrin-β1-null EBs (Li et al., 2002). Immunoblotting and immunostaining experiments revealed that integrin β3 but not β4 is upregulated after laminin treatment. Interestingly, forced expression of integrin β3 in integrin-β1-null EBs rescued endoderm and BM but not epiblast and cavitation. Because the expression level of integrin β3 in the laminin-treated EBs is comparable to that of β3 transfection (data not shown), we speculate that treatment of integrin-β1-null EBs with laminin upregulates additional integrins or other laminin receptors that are responsible for laminin-induced rescue.
A major function of endoderm is to synthesize and secrete BM proteins, which assemble into an underlying BM (Li et al., 2004; Li et al., 2003). The BM acts both as a substratum for germ-layer attachment and as an inductive signal for epiblast polarization. We and others have shown that integrin-β1-null EBs or teratomas have a reduced ability to synthesize laminin (Aumailley et al., 2000; Li et al., 2002; Sasaki et al., 1998). In this study, we further show that, in integrin-β1-null EBs, endoderm differentiation is initiated – as evidenced by the expression of the lineage markers α-FP, cytokeratin Endo A and GATA4. However, the mutant endoderm cells display significantly reduced expression of laminin, nidogen and type IV collagen, as well as Dab2, an adaptor protein that is involved in endocytosis and is essential for endoderm formation during early embryogenesis (Morris et al., 2002; Yang et al., 2002). Altogether, these results suggest that integrins are required for the functional maturation of endoderm cells.
The expression of Dab2 and laminin has been shown to be controlled by the endoderm-specific transcription factors GATA4 and GATA6, which are interdependent in the regulation of endoderm differentiation (Futaki et al., 2004; Morrisey et al., 2000). Ablation of GATA4 in ES cells blocks endoderm formation during EB differentiation (Soudais et al., 1995). By contrast, overexpression of GATA4/6 is sufficient to drive ES cells down to the endoderm lineage, possibly by turning on the expression of BM proteins and Dab2 (Fujikura et al., 2002). In this study, we show that GATA4 was mislocalized to the cytoplasm instead of the nucleus in integrin-β1-null endoderm cells, although the level of GATA4 protein expression was not altered. We further show that culturing of the mutant cell on vitronectin upregulated αvβ3 integrin expression and induced GATA4 nuclear translocation, which was prevented by the knockdown of integrin αv. These results suggest that the reduced expression of BM proteins and Dab2 by the mutant endoderm cells is probably caused by failure of GATA4 nuclear translocation in the absence of integrins.
Recently, it has been shown that endoderm differentiation is regulated by the Grb2-MAPK (ERK1/2) pathway in both mouse embryos and ES cells (Chazaud et al., 2006; Hamazaki et al., 2006). Previous work from Lonai's group suggests that the PI3K-Akt pathway is also involved (Chen et al., 2000; Li et al., 2001). Here, we provide three lines of evidence that integrins regulate endoderm-cell maturation through the activation of ERK1/2 and p38 MAPK. First, ablation of integrin β1 abolished and/or inhibited ERK1/2 and p38 MAPK activation, which was restored by vitronectin-induced upregulation of αvβ3 integrin. Conversely, knockdown of integrin αv completely abrogated this rescue effect. These data suggest that ERK1/2 and p38 MAPK activation depend on integrins. Second, the MEK1/2 inhibitor U0126 and the p38 MAPK inhibitor SB203580 selectively blocked vitronectin-induced, integrin-dependent GATA4 nuclear translocation and the expression of Dab2 and BM proteins. Finally, transfection of the mutant endoderm cells with CA MEK1 or p38 MAPK promoted endoderm maturation. This is the first time that p38 MAPK has been shown to regulate endoderm differentiation. Although Akt activation also depends on integrins, we were unable to show that inhibition of PI3K or Akt alters vitronectin-induced endoderm maturation.
Questions that arise from these results are: how do integrins activate the MAPKs and how do the activated MAPKs control endoderm maturation? Integrin-mediated cell adhesion has been shown to be required for growth-factor-induced activation of the Ras-Raf-MEK-ERK MAPK pathway (Renshaw et al., 1999; Renshaw et al., 1997). Studies in NIH3T3 cells demonstrated that ERK2 activation caused by the expression of constitutively active Ras or Raf was sensitive to cell detachment, whereas that by CA MEK1 was not, suggesting that the control step of adhesion-dependent MAPK activation lies between Raf and MEK (Renshaw et al., 1997). Here, we show that both constitutively active MEK1 and Ras promoted GATA4 nuclear translocation and endoderm maturation in the absence of integrin-mediated cell spreading. Our results indicate that MAPK regulation by integrins is likely to be at the level of growth-factor receptors. In this regard, epidermal growth factor (EGF) and fibroblast growth factor (FGF) receptors are possible candidates because they have been implicated in endoderm regulation (Szuts et al., 1998; Wu and Adamson, 1996). p38 MAPK can be activated by the small GTPases Cdc42 and Rac1. During EB differentiation, these GTPases are activated in a BM- and integrin-dependent fashion (data not shown). Thus, they might mediate integrin-dependent p38 MAPK activation (Zhang et al., 1995). It is also possible that integrins regulate the ERK and p38 MAPK pathways indirectly by modulating the production of growth factors.
The mechanisms by which MAPKs regulate endoderm differentiation are largely unknown. A recent study has suggested that activation of MAPK and/or inhibition of tyrosine phosphatases induce endoderm by suppressing Nanog, a transcription factor that maintains ES cells in a pluripotent state (Hamazaki et al., 2006). Now we show that both ERK1/2 and p38 MAPK regulate the nuclear translocation of GATA4, a transcription factor that controls the expression of Dab2 and laminins. Thus, our findings suggest a novel mechanism whereby integrins regulate MAPK activation, which promotes endoderm maturation by inducing GATA4 nuclear translocation.
Materials and Methods
Culturing of ES cells and embryoid bodies
The ES cell lines used for this study were wild-type R1 and D3 ES cells, integrin-β1-null G201 ES cells (Fassler et al., 1995), and fibronectin-null ES cells (kindly provided by Richard Hynes of Howard Hughes Medical Institute, CA and Massachusetts Institute of Technology, MA). Wild-type and fibronectin-null ES cells were cultured on mitomycin-C-treated STO cells, and integrin-β1-null ES cells were directly grown on plastic culture dishes (Li et al., 2002). EB differentiation was initiated from ES-cell aggregates in suspension culture as described before except that they were cultured in poly-HEMA-coated bacteriological Petri dishes (Li and Yurchenco, 2006).
Antibodies
The anti-integrin antibodies used in this study are summarized in supplementary material Table S1. Dab2 (clone 52) monoclonal antibody (mAb) was from BD Biosciences. Laminin-γ1 mAb (clone A5) was from Upstate Biotechnology. Fibronectin polyclonal antibody (pAb) was from Sigma. Laminin-α1 RG70 and nidogen pAbs were gifts from Peter Yurchenco (Robert Wood Johnson Medical School, NJ). Perlecan mAb was from Chemicon. Cytokeratin-EndoA mAb (clone TROMA-1) was from the Developmental Studies Hybridoma Bank (DSHB). α-fetoprotein pAb was from ICN. GATA4 and vitronectin pAbs were from Santa Cruz Biotechnologies. HA-tag mAb was from Cell Signaling. FITC-, Cy3-, Cy5- and horseradish-peroxidase-conjugated secondary antibodies were from Jackson Immunochemicals.
Stable transduction of integrin-β1-null ES cells
For the stable expression of integrin β3, integrin-β1-null ES cells were transfected with pE3T3C3 1-47 vector encoding full-length human integrin β3 tagged with GFP at its C-terminus (kindly provided by Curzio Ruegg, Swiss Institute for Experimental Cancer Research, Switzerland) using Lipofectamine 2000 reagent (Invitrogen) (Foletti et al., 2005). pEGFP-N1 was used as the control vector. Stable ES-cell clones were selected based on GFP fluorescence. For knockdown of integrin αv, four retroviral vectors expressing shRNAs targeting to mouse integrin αv were purchased from Open Biosystems. The sense sequences of the shRNAs are 5′-CCCAGGATGGCTTCAATGATAT-3′, 5′-CGGGCCTATTGTTCAGCACATA-3′, 5′-CCCTGTGCATTTAAGACAGAAA-3′ and 5′-ACCAGACCCGTTGTCACTGTAA-3′. To generate stable integrin-αv-knockdown cell lines, HEK293 retroviral packaging cells were transfected with the pSM2-shRNA constructs or the control vector pSM2 using Lipofectamine 2000 reagent. The conditioned medium was then mixed with equal volumes of fresh medium and added to integrin-β1-null ES cells. After 48 hours, the cells were selected with 1 μg/ml puromycin. Antibiotic-resistant colonies were cloned, expanded and grown in the medium containing 1 μg/ml puromycin.
Transient transfection of integrin-β1-null endoderm cells
Endoderm cells detached from 5-day integrin-β1-null EBs were collected from the conditioned medium by differential settling by gravity. The cells were transfected with the following vectors using Lipofectamine 2000 reagent: pMCL-MKK1-R4F expressing a HA-tagged CA MEK1 (kindly provided by Natalie Ahn, University of Colorado, CO), pCXN2-Ras-IRES-GFP expressing a FLAG-tagged CA H-Ras (H-RasV12, kindly provided by Noaki Mochizuki, National Cardiovascular Center Research Institute, Japan), and pcDNA3-MKK6-p38α expressing a CA HA-tagged MAPK kinase 6 (MKK6)-p38 MAPK fusion protein (kindly provided by Guan Chen, Medical college of Wisconsin, WI) (Mansour et al., 1994; Qi et al., 2007). pcDNA3, pCXN2-IRES-GFP and pcDNA3-MKK6-p38α/AGF (the non-phosphorylatable p38α) were used as control vectors, respectively. After transfection, the cells were grown on fibronectin (10 μg/ml)-coated glass coverslips or 60-mm dishes for 48 hours. The cells were then either fixed with 3% paraformaldehyde for immunostaining or harvested for western blot analysis.
Immunofluorescence
E6.5 mouse embryos and EBs were fixed with 3% paraformaldehyde. The specimens were imbedded in OCT compound and 4-μm-thick cryosections were prepared. The experimental procedures and animal care were approved by the Institutional Animal Care and Use Committee. EB processing and immunostaining were carried out as described (Li et al., 2006). Slides were examined with a Nikon inverted fluorescence microscope (Eclipse TE 300) and digital images were acquired with a cooled CCD CoolSnap camera (Photometrics) controlled by IP Lab software (Scanalytics).
Cell-adhesion assay
Adhesion of endoderm cells to BM substrates was analyzed as described (Plopper et al., 1998). Tissue culture plates were coated with ECM substrates at 10 μg/ml. Cells were incubated at 37°C for 1 hour. Adherent cells were stained with 0.1% crystal violet and assayed by spectrophotometry.
FACS analysis
Integrin-β1-null endoderm cells were fixed with 3% paraformaldehyde and immunostained with a rabbit anti-integrin β3 polyclonal antibody or pre-immune IgG followed by an FITC-labeled secondary antibody. Specific labeling was analyzed by fluorescence-activated cell sorting (FACS; FACSCalibur, BD).
Immunoprecipitation and immunoblotting
EBs were collected by settling by gravity, washed once in phosphate-buffered saline (PBS) and lysed in radio-immunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate) containing protease and phosphatase inhibitor cocktails. Immunoprecipitation and immunoblotting were performed as described (Li et al., 2002).
Cytosolic and nuclear fractionation
Normal endoderm cells were dissociated from 5-day EBs by brief trypsinization followed by repeatedly pipetting and then separated from the rest of the EBs by differential settling by gravity. The latter procedure was repeated twice to remove all the EBs from the endoderm-cell preparation. Integrin-β1-null endoderm cells detached from the mutant EBs were collected from the conditioned medium by differential settling. The cells were suspended in PBS with protease inhibitors and disrupted in three freeze-thaw cycles. Cell lysates were centrifuged at 2800 g at 4°C for 20 minutes and the supernatant containing the cytoplasm proteins was collected as cytosolic preparation. The pellet was extracted on ice with RIPA buffer containing 0.25% SDS for 30 minutes. The debris was removed by centrifugation and the supernatant harvested as nuclear preparation.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/2/233/DC1
This work was supported by NIH grant R01GM081674 and New Jersey Stem Cell Grant 07-2042-014-84 to S.L. Deposited in PMC for release after 12 months.
References
- Aumailley, M., Pesch, M., Tunggal, L., Gaill, F. and Fassler, R. (2000). Altered synthesis of laminin 1 and absence of basement membrane component deposition in (beta)1 integrin-deficient embryoid bodies. J. Cell Sci. 113, 259-268. [DOI] [PubMed] [Google Scholar]
- Brown, N. H., Gregory, S. L., Rickoll, W. L., Fessler, L. I., Prout, M., White, R. A. and Fristrom, J. W. (2002). Talin is essential for integrin function in Drosophila. Dev. Cell 3, 569-579. [DOI] [PubMed] [Google Scholar]
- Chazaud, C., Yamanaka, Y., Pawson, T. and Rossant, J. (2006). Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev. Cell 10, 615-624. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Li, X., Eswarakumar, V. P., Seger, R. and Lonai, P. (2000). Fibroblast growth factor (FGF) signaling through PI 3-kinase and Akt/PKB is required for embryoid body differentiation. Oncogene 19, 3750-3756. [DOI] [PubMed] [Google Scholar]
- Coucouvanis, E. and Martin, G. R. (1995). Signals for death and survival: a two-step mechanism for cavitation in the vertebrate embryo. Cell 83, 279-287. [DOI] [PubMed] [Google Scholar]
- Fassler, R. and Meyer, M. (1995). Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 9, 1896-1908. [DOI] [PubMed] [Google Scholar]
- Fassler, R., Pfaff, M., Murphy, J., Noegel, A. A., Johansson, S., Timpl, R. and Albrecht, R. (1995). Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 128, 979-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foletti, A., Alghisi, G. C., Ponsonnet, L. and Ruegg, C. (2005). Isolated integrin beta3 subunit cytoplasmic domains require membrane anchorage and the NPXY motif to recruit to adhesion complexes but do not discriminate between beta1- and beta3-positive complexes. Thromb. Haemost. 94, 155-166. [DOI] [PubMed] [Google Scholar]
- Fujikura, J., Yamato, E., Yonemura, S., Hosoda, K., Masui, S., Nakao, K., Miyazaki Ji, J. and Niwa, H. (2002). Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 16, 784-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki, S., Hayashi, Y., Emoto, T., Weber, C. N. and Sekiguchi, K. (2004). Sox7 plays crucial roles in parietal endoderm differentiation in F9 embryonal carcinoma cells through regulating Gata-4 and Gata-6 expression. Mol. Cell. Biol. 24, 10492-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse, E., Messaddeq, N., Yehia, G., Cadalbert, L., Dierich, A. and Le Meur, M. (1996). Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370-373. [DOI] [PubMed] [Google Scholar]
- Hamazaki, T., Kehoe, S. M., Nakano, T. and Terada, N. (2006). The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol. Cell. Biol. 26, 7539-7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. [DOI] [PubMed] [Google Scholar]
- Li, L., Arman, E., Ekblom, P., Edgar, D., Murray, P. and Lonai, P. (2004). Distinct GATA6- and laminin-dependent mechanisms regulate endodermal and ectodermal embryonic stem cell fates. Development 131, 5277-5286. [DOI] [PubMed] [Google Scholar]
- Li, S. and Yurchenco, P. D. (2006). Matrix assembly, cell polarization, and cell survival: analysis of peri-implantation development with cultured embryonic stem cells. Methods Mol. Biol. 329, 113-125. [DOI] [PubMed] [Google Scholar]
- Li, S., Harrison, D., Carbonetto, S., Fassler, R., Smyth, N., Edgar, D. and Yurchenco, P. D. (2002). Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 157, 1279-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Edgar, D., Fassler, R., Wadsworth, W. and Yurchenco, P. D. (2003). The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell 4, 613-624. [DOI] [PubMed] [Google Scholar]
- Li, X., Talts, U., Talts, J. F., Arman, E., Ekblom, P. and Lonai, P. (2001). Akt/PKB regulates laminin and collagen IV isotypes of the basement membrane. Proc. Natl. Acad. Sci. USA 98, 14416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, S. J., Matten, W. T., Hermann, A. S., Candia, J. M., Rong, S., Fukasawa, K., Vande Woude, G. F. and Ahn, N. G. (1994). Transformation of mammalian cells by constitutively active MAP kinase kinase. Science 265, 966-970. [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo, M. D. and Brown, N. H. (1999). Uncoupling integrin adhesion and signaling: the betaPS cytoplasmic domain is sufficient to regulate gene expression in the Drosophila embryo. Genes Dev. 13, 729-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo, M. D., Dunin-Borkowski, O. M. and Brown, N. H. (1997). Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain. EMBO J. 16, 4184-4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranti, C. K. and Brugge, J. S. (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83-E90. [DOI] [PubMed] [Google Scholar]
- Morris, S. M., Tallquist, M. D., Rock, C. O. and Cooper, J. A. (2002). Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 21, 1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey, E. E., Musco, S., Chen, M. Y., Lu, M. M., Leiden, J. M. and Parmacek, M. S. (2000). The gene encoding the mitogen-responsive phosphoprotein Dab2 is differentially regulated by GATA-6 and GATA-4 in the visceral endoderm. J. Biol. Chem. 275, 19949-19954. [DOI] [PubMed] [Google Scholar]
- Pankov, R., Cukierman, E., Clark, K., Matsumoto, K., Hahn, C., Poulin, B. and Yamada, K. M. (2003). Specific beta1 integrin site selectively regulates Akt/protein kinase B signaling via local activation of protein phosphatase 2A. J. Biol. Chem. 278, 18671-18681. [DOI] [PubMed] [Google Scholar]
- Plopper, G. E., Domanico, S. Z., Cirulli, V., Kiosses, W. B. and Quaranta, V. (1998). Migration of breast epithelial cells on Laminin-5: differential role of integrins in normal and transformed cell types. Breast Cancer Res. Treat. 51, 57-69. [DOI] [PubMed] [Google Scholar]
- Qi, X., Pohl, N. M., Loesch, M., Hou, S., Li, R., Qin, J. Z., Cuenda, A. and Chen, G. (2007). p38alpha antagonizes p38gamma activity through c-Jun-dependent ubiquitin-proteasome pathways in regulating Ras transformation and stress response. J. Biol. Chem. 282, 31398-31408. [DOI] [PubMed] [Google Scholar]
- Renshaw, M. W., Ren, X. D. and Schwartz, M. A. (1997). Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 16, 5592-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw, M. W., Price, L. S. and Schwartz, M. A. (1999). Focal adhesion kinase mediates the integrin signaling requirement for growth factor activation of MAP kinase. J. Cell Biol. 147, 611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roote, C. E. and Zusman, S. (1996). Alternatively spliced forms of the Drosophila alphaPS2 subunit of integrin are sufficient for viability and can replace the function of the alphaPS1 subunit of integrin in the retina. Development 122, 1985-1994. [DOI] [PubMed] [Google Scholar]
- Rossant, J. (2004). Lineage development and polar asymmetries in the peri-implantation mouse blastocyst. Semin. Cell Dev. Biol. 15, 573-581. [DOI] [PubMed] [Google Scholar]
- Sasaki, T., Forsberg, E., Bloch, W., Addicks, K., Fassler, R. and Timpl, R. (1998). Deficiency of beta 1 integrins in teratoma interferes with basement membrane assembly and laminin-1 expression. Exp. Cell Res. 238, 70-81. [DOI] [PubMed] [Google Scholar]
- Schwartz, M. A. and Ginsberg, M. H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65-E68. [DOI] [PubMed] [Google Scholar]
- Smyth, N., Vatansever, H. S., Murray, P., Meyer, M., Frie, C., Paulsson, M. and Edgar, D. (1999). Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144, 151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais, C., Bielinska, M., Heikinheimo, M., MacArthur, C. A., Narita, N., Saffitz, J. E., Simon, M. C., Leiden, J. M. and Wilson, D. B. (1995). Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development 121, 3877-3888. [DOI] [PubMed] [Google Scholar]
- Stephens, L. E., Sutherland, A. E., Klimanskaya, I. V., Andrieux, A., Meneses, J., Pedersen, R. A. and Damsky, C. H. (1995). Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9, 1883-1895. [DOI] [PubMed] [Google Scholar]
- Szuts, D., Eresh, S. and Bienz, M. (1998). Functional intertwining of Dpp and EGFR signaling during Drosophila endoderm induction. Genes Dev. 12, 2022-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijen, M. H., Wolthuis, R. M., Bos, J. L. and Defize, L. H. (1999). The Ras/Erk pathway induces primitive endoderm but prevents parietal endoderm differentiation of F9 embryonal carcinoma cells. J. Biol. Chem. 274, 1487-1494. [DOI] [PubMed] [Google Scholar]
- Wu, J. X. and Adamson, E. D. (1996). Kinase-negative mutant epidermal growth factor receptor (EGFR) expression during embryonal stem cell differentiation favours EGFR-independent lineages. Development 122, 3331-3342. [DOI] [PubMed] [Google Scholar]
- Yang, D. H., Smith, E. R., Roland, I. H., Sheng, Z., He, J., Martin, W. D., Hamilton, T. C., Lambeth, J. D. and Xu, X. X. (2002). Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev. Biol. 251, 27-44. [DOI] [PubMed] [Google Scholar]
- Yang, J. T., Rayburn, H. and Hynes, R. O. (1993). Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development 119, 1093-1105. [DOI] [PubMed] [Google Scholar]
- Zhang, S., Han, J., Sells, M. A., Chernoff, J., Knaus, U. G., Ulevitch, R. J. and Bokoch, G. M. (1995). Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270, 23934-23936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.