Summary
Multiple sclerosis (MS) is an autoimmune disease in which myelin is progressively degraded. Because degraded myelin may both initiate and accelerate disease progression, clearing degraded myelin from extracellular spaces may be critical. In this study, we prepared myelin vesicles (MV) from rat brains as a model of degraded myelin. Murine embryonic fibroblasts (MEFs) rapidly internalized MVs, which accumulated in lysosomes only when these cells expressed low-density lipoprotein receptor-related protein (LRP1). Receptor-associated protein (RAP), which binds LRP1 and inhibits interaction with other ligands, blocked MV uptake by LRP1-expressing MEFs. As a complementary approach, we prepared primary cultures of rat astrocytes, microglia and oligodendrocytes. All three cell types expressed LRP1 and mediated MV uptake, which was inhibited by RAP. LRP1 gene-silencing in oligodendrocytes also blocked MV uptake. Myelin basic protein (MBP), which was expressed as a recombinant protein, bound directly to LRP1. MBP-specific antibody inhibited MV uptake by oligodendrocytes. In experimental autoimmune encephalomyelitis in mice, LRP1 protein expression was substantially increased in the cerebellum and spinal cord. LRP1 colocalized with multiple CNS cell types. These studies establish LRP1 as a major receptor for phagocytosis of degraded myelin, which may function alone or in concert with co-receptors previously implicated in myelin phagocytosis.
Keywords: Myelin, Multiple sclerosis, Low-density lipoprotein receptor-related protein, Phagocytosis, Myelin basic protein, Degeneration
Introduction
Multiple sclerosis (MS) is a chronic debilitating autoimmune disease, usually characterized by remissions and relapses, in which myelin in the CNS is destroyed and survival of myelin-producing oligodendrocytes is compromised (Lucchinetti et al., 1999). The pathogenesis of MS is incompletely understood; both genetic and environmental factors have been implicated. Peripheral activation of CD4+ T cells that recognize myelin-associated proteins probably represents an early event in the development of autoimmunity. The responsible immunogen may be degraded myelin fragments that cross the blood-brain barrier (BBB). Once T cells are activated, they rapidly transfer back across the BBB into the brain (Kermode et al., 1990). At this point, progression of MS involves multiple mediators and cell types (Lucchinetti et al., 2000; Lucchinetti et al., 2004), including microglia and recruited macrophages (Rinner et al., 1995). Progressive destruction of myelin and degradation of its component proteins may further fuel the autoimmune response (Stinissen et al., 1997).
Oligodendrocyte apoptosis may represent an initiating or early event in MS, preceding onset of autoimmunity (Barnett and Prineas, 2004). Apoptotic oligodendrocytes have been identified in areas of the brain that are devoid of lymphocytes, typically in the periphery of well developed lesions (Barnett and Prineas, 2004). The cause of oligodendrocyte cell death is unclear; however, viral infections, cytokine dysregulation and defects in glutamate homeostasis have been suggested (Antony et al., 2004; Matute et al., 2001). Release of degraded myelin from oligodendrocytes undergoing cell death may increase the likelihood of myelin leakage across the BBB. Furthermore, degraded myelin may reinforce the inflammatory response locally. Receptors that have been implicated in myelin phagocytosis and clearance from extracellular spaces include FC receptors, complement receptor-3 (also known as MAC-1), and the scavenger-receptor-AI/II (Smith, 2001).
Low density lipoprotein receptor-related protein 1 (LRP1) is a 600 kDa, type-1 transmembrane receptor, which functions in the endocytosis of over 40 structurally and functionally distinct ligands (Strickland et al., 2002). LRP1-associated ligands typically dissociate in acidified endosomes and are delivered to lysosomes, while LRP1 recycles back to the cell surface. In addition to soluble proteins, LRP1 recognizes ligands that are cell-associated, including C1q and calreticulin (Gardai et al., 2005). By this mechanism, LRP1 promotes phagocytosis of apoptotic cells (Vandivier et al., 2002). LRP1 gene deletion in mice is embryonic lethal (Herz et al., 1992). The reason for this is unclear; however, conditional deletion of LRP1 in different cell types causes various forms of pathophysiology (Lillis et al., 2008).
LRP1 is expressed by multiple cell types in the human brain, including neurons and astrocytes (Wolf et al., 1992). Rat microglia also express LRP1, at least in vitro (Marzolo et al., 2000). The goal of the present study was to determine whether LRP1 functions as a significant receptor in the phagocytosis of degraded myelin. To model degraded myelin, we prepared myelin vesicles (MV) from adult rat brains. Our studies show, for the first time, that LRP1 is essential for MV phagocytosis by fibroblasts, astrocytes, microglia and oligodendrocytes. The function of LRP1 in MV phagocytosis reflects, at least in part, a specific interaction with myelin basic protein (MBP), which is thus, a newly discovered LRP1 ligand. LRP1 expression is substantially increased in both the cerebellum and spinal cord in experimental autoimmune encephalomyelitis (EAE), which is a frequently studied animal model of MS. By mediating cellular internalization of degraded myelin, LRP1 may function as an important regulator of MS progression.
Results
LRP1 is a receptor for MVs
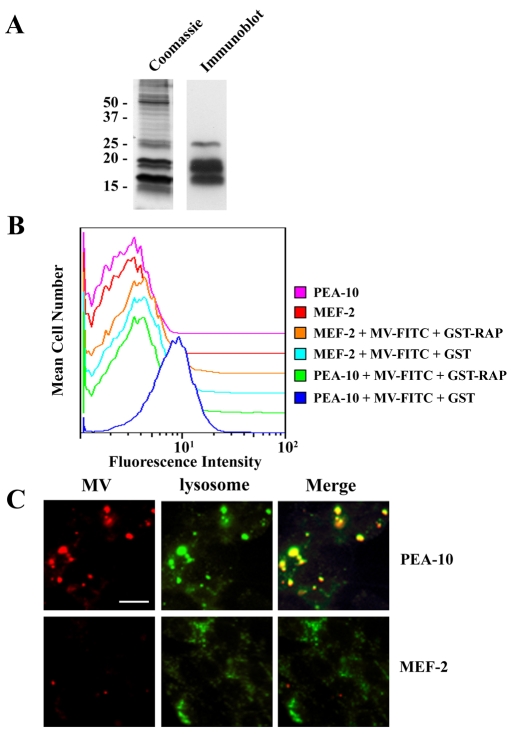
MVs were prepared from adult rat brain as a model for degraded myelin (Norton and Poduslo, 1973). MBP was clearly identified in the MVs by immunoblot analysis and migrated similarly to the most prominent bands observed in Coomassie-Blue-stained gels (Fig. 1A). Others have shown that MBP represents 30% of the total protein content in CNS myelin (Boggs, 2006). To determine whether LRP1 functions in degraded myelin phagocytosis, murine embryonic fibroblasts (MEFs) that were LRP1-negative (MEF-2 cells) or LRP1-positive (PEA-10 cells) and derived from the same culture (Willnow and Herz, 1994) were incubated with fluorescein isothiocyanate (FITC)-labeled MVs for 30 minutes at 37°C. After washing and treatment with Pronase A to remove surface-associated MVs, cells were analyzed by flow cytometry. As shown in Fig. 1B, only the LRP1-positive PEA-10 cells internalized significant amounts of FITC-labeled MVs. When the PEA-10 cells were pre-treated with glutathione-S-transferase receptor-associated protein (GST-RAP), which binds to LRP1 and inhibits its interaction with other known ligands (Herz et al., 1991), MV internalization was blocked. GST, which was added as a control, had no effect on MV internalization. In further control studies, PEA-10 cells were incubated with MVs at 4°C, to preclude MV internalization, and then treated with Pronase A. Cell-associated fluorescence was completely absent, confirming that our method reports internalized MVs (results not shown).
Fig. 1.
LRP1 is a receptor for myelin vesicles. (A) MVs were analyzed by SDS-PAGE with Coomassie staining and by immunoblot analysis using MBP-specific antibody. (B) PEA-10 and MEF-2 cells were incubated with FITC-labeled MVs for 30 minutes in the presence of GST or GST-RAP. After washing and protease treatment, cells were subjected to flow cytometry analysis. (C) PEA-10 and MEF-2 cells were incubated with Rhodamine-labeled MVs for 30 minutes. After washing, the cells were incubated with Lysotracker for 30 minutes and analyzed by fluorescence microscopy. Scale bar: 50 μm.
As a second approach for testing whether LRP1 is responsible for MV internalization by MEFs, we conducted fluorescence microscopy colocalization studies, using the lysosomal marker, Lysotracker™. MEF-2 and PEA-10 cells were incubated with Rhodamine-labeled MVs for 30 minutes at 37°C. The cells were then washed and cultured for an additional 30 minutes in the presence of Lysotracker. Only the PEA-10 cells internalized substantial amounts of labeled MVs (Fig. 1C), which colocalized with Lysotracker. These results confirm that in MEFs, LRP1 functions as an essential receptor for the phagocytosis of MVs.
CNS cells in primary culture express LRP1
To further study the role of LRP1 in MV internalization, we established primary cultures of oligodendrocytes, astrocytes and microglia from 1-day-old rat pup brains. All three cell types expressed LRP1 mRNA, as shown by real-time qPCR (Fig. 2A). LRP1 expression by oligodendrocytes has not been reported before; however, interestingly, in cell culture, the level of LRP1 mRNA was highest in these cells.
Fig. 2.
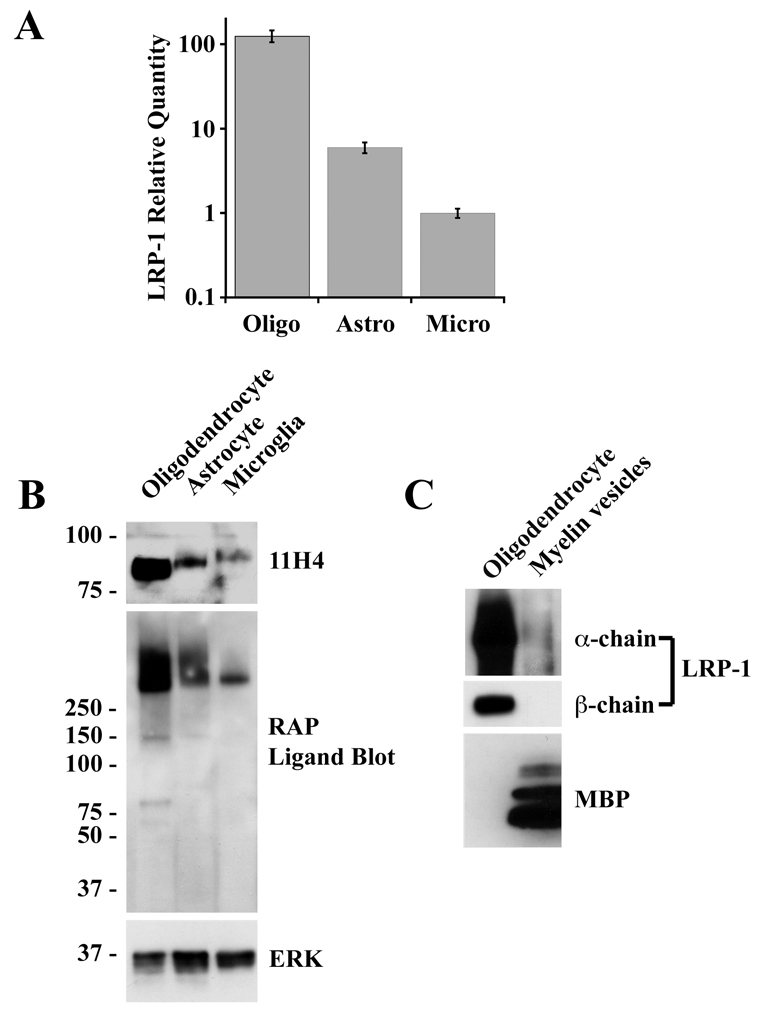
CNS glia express LRP1. (A) RNA was isolated from oligodendrocytes (Oligo), astrocytes (Astro) and microglia (Micro). LRP1 mRNA expression was determined by qPCR (data are means ± s.d.). (B) Protein extracts from oligodendrocytes, astrocytes and microglia were analyzed by immunoblotting with LRP1 β-chain-specific antibody 11H4 and with antibody specific for ERK/MAP kinase as a control for loading. The same samples were also analyzed by RAP-ligand blotting to detect LRP1 α-chain and possibly other LRP family members. (C) MVs were analyzed by immunoblot analysis using specific antibodies that detect MBP or LRP1 β-chain and by RAP ligand blotting. Oligodendrocyte extracts were assessed in the same experiments.
To detect LRP1 at the protein level, we performed immunoblot analysis using monoclonal antibody 11H4, which detects the 85 kDa transmembrane β-chain of LRP1. All three cell types were immunopositive. The highest level of LRP1 β-chain was detected in oligodendrocytes (Fig. 2B), consistent with the results of our qPCR studies. Slight differences in the mobility of the β-chain were observed when the different cell types were compared. Although this result has not yet been explained, the ectodomain of the LRP1 β-chain contains candidate N- and O-glycosylation sites. Intracellular β-chain tyrosine phosphorylation also may explain the differences in mobility (Barnes et al., 2003).
As a second method to detect LRP1 protein expression, we performed ligand-blotting experiments with GST-RAP, which binds to the 515-kDa LRP1 α-chain. This method is less specific because multiple members of the LDL receptor family may be detected, some of which have molecular masses exceeding 250 kDa, including LRP1B and LRP2 (also known as megalin) (Pastrana et al., 2005; Saito et al., 2007). In all three cell types, GST-RAP bound principally to species with apparent masses greater than 250 kDa, consistent with the known mass of LRP1. Again, oligodendrocytes yielded the most intense signal.
Fig. 2C shows that neither the β-chain of LRP1 (immunoblot analysis) nor the α-chain (RAP ligand blotting) was detected at significant levels in MVs prepared from rat brain. The oligodendrocytes in primary culture were immunonegative for MBP. This is an anticipated result for cultured cells (Kamholz, 1996).
Glial cells internalize MVs by an LRP1-dependent mechanism
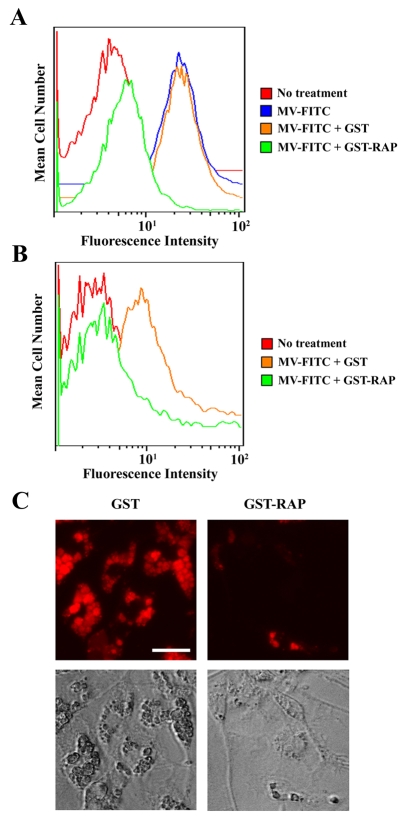
FITC-labeled MVs were incubated with oligodendrocytes for 30 minutes at 37°C, in the presence of GST-RAP, to inhibit the endocytic activity of LRP1, or in the presence of GST, as a control. MV internalization was determined by the flow cytometry method described above for our experiments with MEFs. As shown in Fig. 3A, substantial MV internalization was observed in the presence of GST; however, when the incubations were conducted in the presence of GST-RAP, MV internalization was almost entirely blocked. As a control, we incubated MVs with oligodendrocytes in the absence of GST-RAP and GST. Internalization was unchanged compared with that observed when GST was present (results not shown).
Fig. 3.
MV phagocytosis by CNS glia is inhibited by RAP. Oligodendrocytes (A) and astrocytes (B) were incubated with FITC-labeled MVs for 30 minutes in the presence of GST or GST-RAP. After washing and protease treatment to dissociate surface-associated MVs, the cells were subjected to flow cytometry analysis. (C) Microglia were incubated with Rhodamine-labeled MVs for 30 minutes in the presence of GST or GST-RAP. After washing, the cells were analyzed by fluorescence microscopy and by phase contrast microscopy. Scale bar: 50 μm.
Astrocytes in primary culture internalized fluorescently labeled MVs, as determined by flow cytometry (Fig. 3B). Once again, GST-RAP substantially inhibited MV uptake, whereas GST had no effect. Uptake of MVs by microglia was studied by fluorescence and phase-contrast microscopy. The fluorescence microscopy studies showed substantial internalization of MVs by microglia, which was largely inhibited by GST-RAP. By phase contrast microscopy, microglia that were incubated with MVs showed prominent, distended intracytoplasmic vesicles, again suggesting uptake of substantial amounts of myelin. Vesicular engorgement with myelin was largely inhibited by GST-RAP. These studies demonstrate that LRP1 or another RAP-binding member of the LDL receptor gene family plays an essential role in MV uptake by oligodendrocytes, astocytes and microglia.
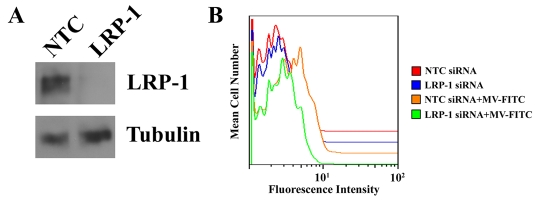
To more specifically test the role of LRP1 in MV phagocytosis by oligodendrocytes, we applied a gene-silencing strategy. Cells were transfected with the previously described rat LRP1-specific siRNA, L2 (Campana et al., 2006), or with non-targeting control (NTC) siRNA. Fig. 4A shows that silencing of LRP1 expression at the protein level was essentially complete as determined by immunoblot analysis. To test the specificity of siRNA L2, we examined its effects on expression of Lrp2 and Lrp1b. Oligodendrocytes expressed low levels of these mRNAs, as determined by qPCR; however, the mRNA levels were not affected by siRNA L2 (results not shown). In additional control studies, we demonstrated that siRNA L2 does not affect LRP-2 mRNA expression in PC12 pheochromocytoma cells, which express higher levels of this protein.
Fig. 4.
LRP1 gene-silencing blocks MV uptake by oligodendrocytes. Oligodendrocytes were transiently transfected with NTC or LRP1-specific siRNA. (A) LRP1 expression at the protein level was assessed 36 hours after silencing by immunoblot analysis with LRP1-specific antibody, 11H4. Blots were also probed for tubulin as a control for load. (B) Oligodendrocytes were incubated with FITC-labeled MVs for 30 minutes. After washing and protease treatment, internalized MVs were detected by flow cytometry.
Fig. 4B shows that MV internalization by oligodendrocytes in which Lrp1 was silenced was substantially decreased compared with cells that were transfected with NTC siRNA. These results confirm that LRP1 mediates MV internalization in oligodendrocytes.
MBP is involved in the LRP1-MV interaction
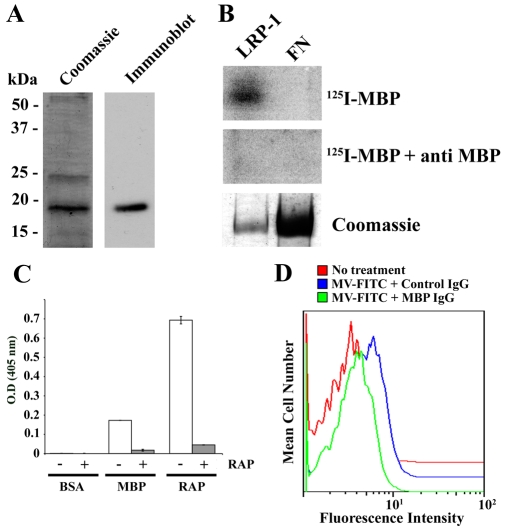
MBP is a major component of myelin purified from the CNS (Boggs, 2006). To determine whether MBP binds to LRP1 and may be responsible for the interaction of LRP1 with MVs, we expressed MBP as a His-tagged fusion protein in bacteria. By SDS-PAGE, recombinant MBP (rMBP) migrated with an apparent mass of 20 kDa, as anticipated (Fig. 5A). In ligand blotting experiments, 125I-labeled rMBP bound to the α-chain of purified rat liver LRP1, which had been subjected to SDS-PAGE (non-reducing conditions) and electro-transferred to polyvinylidene fluoride (PVDF) membranes (Fig. 5B). 125I-rMBP failed to bind to purified fibronectin on the same membranes, providing a negative control.
Fig. 5.
MBP binds to LRP1 and mediates MV uptake. (A) rMBP was expressed as a His-tagged fusion protein in bacteria and subjected to SDS-PAGE with Coomassie staining and immunoblot analysis. (B) Purified rat LRP1 and fibronectin were subjected to SDS-PAGE and electrotransferred to PVDF membranes. The membranes were probed with 125I-labeled rMBP in the presence or absence of MBP-specific antibody. (C) BSA, rMBP and GST-RAP were adsorbed onto plastic wells and incubated with shed LRP1 in the presence of GST-RAP or GST. LRP1 binding to the immobilized phase was detected in an ELISA format, using LRP1 α-chain-specific antibody 8G1. (D) FITC-labeled MVs were incubated with oligodendrocytes in the presence of MBP-specific IgG or non-immune IgG. MV internalization was determined by flow cytometry.
Shed LRP1, which was purified from human plasma and contains the intact α-chain (Quinn et al., 1999) bound to rMBP, which was immobilized on microtiter plates (Fig. 5C). In control studies, shed LRP1 also bound to immobilized GST-RAP but did not bind to immobilized bovine serum albumin (BSA). To prove that the interaction of shed LRP1 with rMBP was specific, shed LRP1 was added to wells with immobilized rMBP in the presence of GST-RAP. Binding was almost entirely blocked. These results indicate that the structure of MBP, when released from the constraints of a membrane, includes regions that bind directly to LRP1. We hypothesized that MBP may play a role in the phagocytosis of MVs by LRP1.
To test the hypothesis that MBP binding to LRP1 is involved in MV internalization, FITC-labeled MVs were incubated with oligodendrocytes in the presence of MBP-specific antibody or non-specific IgG. MV internalization was determined by flow cytometry. Fig. 5D shows that MBP-specific antibody substantially inhibited MV uptake by the cells.
LRP1 expression is increased in mice with EAE
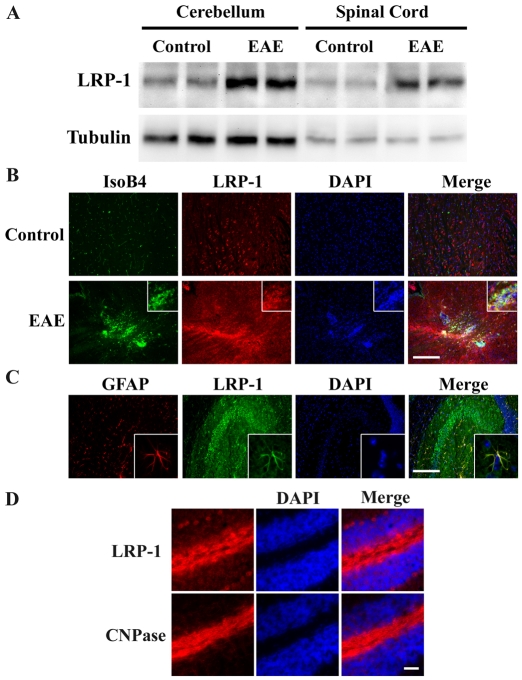
Because EAE is a frequently studied animal model of MS, we assessed LRP1 expression in the CNS of mice with EAE. Immunoblot analysis was used to compare LRP1 levels in extracts of cerebellum and spinal cord from mice, 16 days after immunization with proteolipid protein peptide (PLP), which induces EAE, and from control mice that were injected with vehicle. At day 16, significant inflammation is present in the CNS and clinical symptoms are evident, as previously described (Adams et al., 2007). Fig. 6A shows that LRP1 protein expression was substantially increased in both spinal cord and cerebellum extracts of PLP-immunized mice.
Fig. 6.
LRP1 expression in EAE. (A) Protein extracts of the cerebellum and spinal cord of mice that were treated to induce EAE, and from control mice, were subjected to immunoblot analysis to detect LRP1, using the LRP1 β-chain-specific antibody, 11H4. The same blots were also probed to detect tubulin as a control for loading. (B) Sagittal brain section from mice with EAE, and control mice, were stained to detect the microglia/macrophage marker, IsoB4 (green) and LRP1 (red). Equivalent regions of the brains of control and EAE mice are shown. The insets show a macrophage/microglial cell infiltrate at higher magnification. Scale bar, 200 μm. (C) Sagittal brain section were immunostained to detect astrocytes (GFAP, red) and LRP1 (green). Images of the hippocampus are shown. The insets show astrocytes that are immmunopositive for both GFAP and LRP1. Scale bar, 200 μm. (D) Sequential sagittal brain sections were immunostained for oligodendrocytes (CNPase) and LRP1. Scale bar: 50 μm.
To further assess LRP1 expression in EAE, immunofluorescence microscopy studies were performed. Sagittal sections of brain from control and PLP-treated animals were immunostained for LRP1 and for binding of Griffonia simplicifolia isolectin B4 (IsoB4), a specific marker for microglia and macrophages. As shown in Fig. 6B, in control brain, LRP1 was widely expressed, possibly by neurons, as previously described (Ishiguro et al., 1995; Wolf et al., 1992). Resting microglia did not stain robustly for LRP1. By contrast, in EAE, clusters of IsoB4-positive cells that were also strongly immunopositive for LRP1 were evident.
In separate studies, we immunostained brain sections to detect the astrocyte marker, glial fibrillary acidic protein (GFAP), and LRP1. Fig. 6C shows that in normal brain, GFAP-positive cells were also LRP1-positive, as previously described (Ishiguro et al., 1995). Astrocytes were also LRP1-positive in PLP-treated mice. Finally, white matter tracks in the cerebellum, which were robustly immunopositive for the oligodendrocyte marker, 2′, 3′-cyclic nucleotide 3′-phosphodiesterase (CNPase), were also strongly LRP1-immunopositive in both control and PLP-treated mice (Fig. 6D). Because of the intimate relationship between oligodendrocytes and axons in these tracks, we could not discern whether the LRP1 was expressed by neurons or oligodendrocytes. We conclude that the increase in LRP1 expression in EAE is attributable, at least in part, to microglia and infiltrating macrophages; however, other cell types, including neurons and oligodendrocytes may be involved also.
Discussion
The structure of the LRP1 α-chain includes four clusters of complement-like repeats (Herz and Strickland, 2001). The second and fourth clusters mediate binding of most LRP1 ligands. Although simple binary complexes of LRP1 with ligands are internalized by cells, there is also evidence for formation of LRP1-containing multi-protein complexes, in which ligands bridge LRP1 to other receptors or form LRP1 homodimers (Makarova et al., 2008). When multicomponent complexes are formed, endocytosis still occurs and the ligands are delivered to lysosomes (Gonias et al., 2004). Receptors that are bridged to LRP1, such as uPAR and tissue factor, may be cleared from the cell surface or recycle back to the plasma membrane (Gonias et al., 2004; Nykjaer et al., 1997). In concert with other proteins, such as C1q and calreticulin, LRP1 is converted from an endocytic receptor into one that mediates the phagocytosis of apoptotic cells (Gardai et al., 2005). Although this process remains incompletely understood, it is probable that multiple copies of cell-surface LRP1 are recruited to allow large particle phagocytosis.
In this study, we identified LRP1 as an essential receptor involved in phagocytosis of MVs. Our experiments were performed with MEFs, oligodendrocytes, microglia and astrocytes. In all four cell types, the function of LRP1 in MV phagocytosis was substantial, suggesting that this LRP1 activity is not cell-type-specific. In studies with MEFs and oligodendrocytes, we obtained equivalent results when GST-RAP was added to neutralize the ligand-binding activity of LRP1 or when LRP1 was deficient. In both cases, MV uptake was blocked. These studies suggest that the ability of RAP to block MV internalization by MEFs and oligodendrocytes represents antagonism of LRP1 activity. In microglia and astrocytes, we studied the activity of LRP1 only by adding GST-RAP. Thus, in these cells, we cannot rule out the function of other LDL receptor homologues that bind RAP, such as LRP2 or the VLDL receptor (Bu, 2001), in addition to LRP1. The essential role of LRP1 in MV internalization demonstrated here also does not preclude cooperation with other receptors implicated in MV internalization, such as complement receptor-3/MAC-1 (Smith, 2001). Of note, LRP1 and complement receptor-3/MAC-1 have been reported to colocalize on the surfaces of macrophages and may cooperate in regulating macrophage cell migration (Cao et al., 2006).
MBP is a major component of CNS myelin (Boggs, 2006) and one of three major myelin-associated proteins that serve as a target in EAE (Wekerle et al., 1994). MBP also is a major auto-antigen in MS (Ota et al., 1990). Proper expression of MBP is essential for myelin development, compaction and maintenance, as evidenced by abnormalities observed in the Shiverer mouse (Molineaux et al., 1986). The structure of MBP is highly variable as a result of alternative mRNA splicing and post-translational modifications, which are diverse and extensive (Boggs, 2006). Post-translational modification may regulate penetration of MBP from within the membrane bilayer and localized availability to proteases, which contribute to degradation (Musse et al., 2006). We demonstrated that recombinant MBP, which is not membrane-associated, binds to LRP1 by a RAP-inhibited mechanism. MBP-specific antibody inhibits the interaction of MVs with LRP1. These results suggest that MBP is an LRP1 ligand and that this interaction is at least partially responsible for MV phagocytosis by LRP1. However, our data do not indicate an interaction of LRP1 with MBP in intact myelin. Instead, we hypothesize that MBP binds to LRP1 only when MBP is presented in the context of degraded myelin. Further work will be necessary to confirm that the LRP1 recognition site in MBP is available in MVs. If not, an alternative explanation for the results of our antibody study is the possibility that large amounts of MBP in MVs allow the antibody to block interactions with other essential LRP1-binding myelin proteins.
Although LRP1 was detected in all three CNS glia in primary culture, oligodendrocytes expressed LRP1 at considerably higher levels than astrocytes and microglia. This result was not anticipated because in our previous immunohistochemistry studies of adult human brain, oligodendrocytes were negative for LRP1 (Lopes et al., 1994). However, Ishiguro et al. (Ishiguro et al., 1995) showed that LRP1 mRNA expression varies in rat brain during development, both prenatally and postnatally. These same investigators also identified LRP1 mRNA in glial cells that were probably oligodendrocytes. Our primary cultures were established using 1-day-old rat pups. Thus, it is possible that the age of the rodents contributed to the high level of LRP1 detected in vitro. It is interesting to note that oligodendrogliomas are LRP1 positive (Lopes et al., 1994).
When EAE was induced in mice, LRP1 expression was substantially increased in vivo in the cerebellum and spinal cord. Infiltrating macrophages and/or activated microglia were at least partially responsible for the increase in total LRP1; however, other CNS cell types may also have been involved. Because LRP1 is expressed in EAE, it is reasonable to propose that LRP1 may function in the phagocytosis of degraded myelin in the CNS in EAE and MS. However, LRP1 is also expressed by cells outside the CNS (Moestrup et al., 1992). Thus, the significance of our results, identifying LRP1 as a major myelin receptor, remains to be determined. For example, LRP1 that is expressed in the liver functions to clear the blood of diverse proteins (Gonias et al., 1982). Thus, hepatic LRP1 may inhibit development of an immune response to myelin or oppose progression of MS by eliminating degraded myelin products from the bloodstream. However, LRP1 has also been implicated in antigen presentation (Hart et al., 2004). This activity involves extracellular heat shock proteins and glucose-regulated protein, which are ligands for LRP1 (Basu et al., 2001; Calderwood et al., 2007). When LRP1, which is expressed by antigen-presenting cells, internalizes candidate immunogens in complex with heat shock proteins or glucose-regulated protein, antigen presentation on MHC1 is facilitated (Arnold-Schild et al., 1999; Singh-Jasuja et al., 2000). Thus, binding of myelin-associated proteins such as MBP to LRP1 may result in T cell activation and autoimmune disease initiation. We hypothesize that the effects of LRP1 on MS pathophysiology, resulting from its activity as a receptor for degraded myelin, probably depend on the cell type that expresses the LRP1.
LRP1 demonstrates other activities that may be important in MS. In neurons (May et al., 2004) and Schwann cells (Campana et al., 2006), LRP1 has been described as a pro-survival receptor. In Schwann cells, LRP1 regulates survival by its effects on the phosphatidyl inositol 3-kinase–Akt pathway (Campana et al., 2006). LRP1 also regulates inflammation. As a membrane-anchored receptor, LRP1 may control cell-surface expression of tumor necrosis factor receptor 1 and cell signaling to NF-κB (Gaultier et al., 2008b). Furthermore, a soluble form of LRP1 may be released from the cell surface, which inhibits cell signaling in response to TNF-α (Gaultier et al., 2008a). LRP1 is an important regulator of the BBB and thus may control leakage of degenerated myelin and/or MBP out of the brain in early stages of MS (An et al., 2008; Yepes et al., 2003). Thus, the activity of LRP1 in MS progression may reflect the integrated effects of diverse pathways controlled by this receptor.
Materials and Methods
Reagents
NHS-fluorescein and Rhodamine were from Pierce. Purified human fibronectin, tubulin-specific antibody and IsoB4-FITC were from Sigma. GFAP-specific antibody was from Zymed. MBP-specific antibody and CNPase-specific antibody were from Abcam. LRP1-specific monoclonal antibody 11H4, which recognizes the β-chain of the rat protein, and 8G1, which recognizes the α-chain of the human protein were purified from conditioned medium of hybridoma cells obtained from the ATCC. Antibody specific for ERK/MAP kinase was from Zymed. GST-RAP was expressed in bacteria and purified as previously described (Gaultier et al., 2008a). As a control, we also expressed GST in bacteria transformed with the empty vector, pGEX-2T. qPCR reagents, including primers and probes were from Applied BioSystems.
Purification of LRP1
GST-RAP was coupled to Sepharose to form an affinity resin, as previously described (Gaultier et al., 2008a). Shed LRP1 was purified from outdated human plasma. The purified protein includes the intact α-chain and the ectodomain region of the β-chain (Quinn et al., 1999). Full-length LRP1 was purified from adult rat livers, which were extracted by homogenization in 20 mM Tris-HCl, 150 mM NaCl, pH 7.4 with 1% Triton X-100, 1 mM CaCl2 and protease inhibitor cocktail. After centrifugation to clear the extract, affinity chromatography was performed using GST-RAP-Sepharose (Gaultier et al., 2008a). Purified proteins were analyzed by SDS-PAGE and immunoblot analysis with antibody 8G1 (human LRP1) or 11H4 (rat LRP1) and by RAP ligand blotting (Gaultier et al., 2008a).
Cloning, expression and purification of rMBP
Specific primers were designed to hybridize to the 5′ and 3′ termini of the rat MBP open reading frame and allow cloning into pET-30a(+) (Novagen). The forward and reverse primer sequences were: 5′-gaattcatggcatcacagaagagacc-3′ and 5′-aagctttcagcgtcttgccatgggag-3′, respectively. Cloning was facilitated by including EcoRI and HindIII restriction sites at the 5′ and 3′ termini, respectively. PCR was performed using cDNA, generated from rat brain mRNA. A single major amplicon with the anticipated mass was obtained and cloned into the vector. The sequence of the construct was verified and the plasmid was transformed into BL-21 E. coli for protein expression (Stratagene). rMBP was purified by affinity chromatography using the Profinia Native IMAC purification kit and chromatography system (Bio-Rad).
Cell culture
MEFs that are genetically deficient in LRP1 (MEF-2 cells) and control LRP1-positive MEFs (PEA-10 cells) were obtained from the ATCC. PEA-10 and MEF-2 cells were cloned from the same culture, heterozygous for LRP1 gene disruption, and selected with the LRP1-selective toxin, Pseudomonas exotoxin A (Willnow and Herz, 1994). MEFs were cultured in DMEM (Hyclone) with 10% FBS and penicillin and streptomycin.
Cultures of glial cells were prepared according to the method of McCarthy and De Vellis (McCarthy and de Vellis, 1980). Briefly, the cerebral cortex was isolated from P1 Sprague-Dawley rat pups, minced, and digested for 30 minutes at 37°C in Hanks' balanced salt solution (HBSS) containing 0.25% trypsin (Invitrogen) and 0.1% of pancreatin (EMD Bioscience). The cells from each rat were plated in separate 75 cm2 tissue culture flasks coated with 10 μg/ml of poly-D-lysine (Sigma). Cultures were maintained in DMEM with 10% FBS and penicillin/streptomycin for 10 days. Flasks were agitated by rotation (180 rpm) for 30 minutes at 37°C to detach microglia. Oligodendrocytes were released by the equivalent method over 18 hours. Finally, the astrocytes were collected by trypsin treatment. All three glial cell types were plated in tissue culture dishes coated with 10 μg/ml of poly-D-lysine and cultured in DMEM with 10% FBS and penicillin and streptomycin. To assess purity of the primary cultures, immunostaining was performed using cell-type-specific antibodies targeting CNPase (oligodendrocytes), IsoB4 (microglia) and GFAP (astrocytes).
To silence LRP1 in oligodendrocytes, cells were transfected with siRNA L2, targeting Lrp1 (2.0 μg) using nucleofector technology as described previously (Campana et al., 2006). Control cells were transfected with NTC siRNA (Dharmacon). Lrp1 siRNA L2 specificity was tested by determining mRNA levels for the related receptors, LRP1b and LRP2, which have similar molecular masses to LRP1.
Myelin vesicle purification
MVs were purified as described by Norton et al. (Norton and Poduslo, 1973). In brief, adult female Sprague-Dawley rat brains were homogenized in 0.32 M sucrose, first by using a polytron and then, a Dounce homogenizer. Myelin was recovered by sucrose gradient centrifugation. MVs were washed extensively in H2O. The pellet was resuspended in 0.32 M sucrose, layered over 0.85 M sucrose and subjected to centrifugation at 75,000 g for 30 minutes. The MVs were recovered and resuspended in 20 mM sodium phosphate, 150 mM NaCl, pH 7.4 (PBS). The purity of preparation was analyzed by Coomassie Blue staining and immunoblot analysis for MBP.
SDS-PAGE and immunoblot analysis
Proteins from cultured cells were extracted in 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS in PBS with 2 mM PMSF, 2 mM EDTA and 2 mM sodium orthovanadate (RIPA buffer). Extracts from cerebellum and spinal cord were obtained by Dounce homogenization in 0.5% Triton X-100, 250 mM Hepes, pH. 7,4, 1 mM EDTA. Equal amounts of cellular protein were subjected to SDS-PAGE and electrotransferred to PVDF membranes (Bio-Rad). Proteins were visualized using 0.2% Ponceau-S in 3% trichloroacetic acid prior to immunoblot analysis. Membranes were then blocked with 5% nonfat dry milk in TBS, 0.1% Tween 20. Purified primary antibodies and horseradish peroxidase-conjugated secondary antibodies (GE Healthcare) were diluted in the same buffer. Detection was performed using Western Lightning horseradish peroxidase chemiluminescence (Perkin-Elmer, Boston, MA) and Hyblot CL Films (Denville, South Plainfield, NJ).
Ligand blotting
Purified rMBP (100 μg) was labeled with Na125I using Iodo-Beads (Pierce), according to manufacturer's instructions. Rat liver LRP1 and fibronectin (5 μg) were subjected to SDS-PAGE and electro-transferred to PVDF membranes. Membranes were blocked for 1 hour in PBS, 0.1% Tween 20, 5% dry milk and then incubated with 10 nM 125I-labeled MBP in the same buffer for 12 hours at 4°C. Membranes were washed extensively with PBS, 0.1% Tween 20 and imaged using a Phosphorimager (Bio-Rad).
In RAP ligand-blotting experiments, proteins electrotransferred to PVDF membranes were probed first with GST-RAP (50 nM) and then with GST-specific antibody. Bound antibody was imaged with Western Lightning horseradish peroxidase chemiluminescence.
Immobilized ligand-binding studies
Purified rMBP, GST-RAP and BSA (0.1 mg/ml) were diluted in PBS containing 0.5 mM Ca2+ and Mg2+ (PBS Ca/Mg) and absorbed in ELISA plates for 18 hours at 4°C. Wells were blocked with PBS Ca/Mg containing 3% BSA for 1 hour. Human shed LRP1 (200 nM) was pre-incubated with a 10-fold molar excess (2 μM) of GST, GST-RAP or vehicle for 15 minutes at 20°C in PBS Ca/Mg containing 0.3% BSA and then added to the wells for 1 hour at 20°C. The wells were washed. Retained LRP1 was detected with antibody 8G1, in an ELISA format using 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) as a colorimetric substrate.
MV internalization assays
MVs were labeled with NHS-fluorescein or Rhodamine according to the manufacturer's instructions. The MVs were then incubated with cells for 30 minutes at 37°C. In some studies, the cells were pre-treated with GST-RAP or GST (0.25 μM) for 1 hour before adding the labeled MV. Other cells were pre-treated for 20 minutes with 20 μg/ml of MBP-specific antibody or non immune IgG. The GST-RAP, GST or antibody was maintained during the MV incubation period. At the end of an incubation, cells were washed with ice-cold PBS containing 0.5% BSA and treated with 0.25% (w/v) Pronase A (Roche) for 15 minutes at 4°C to release cell-associated MVs that were not internalized. After three additional washes, the cells were subjected to flow cytometry analysis using a FACSCanto Instrument (BD Biosciences). Data were analyzed using FlowJo software (Treestar, Ashland, OR).
MV internalization was also assessed by fluorescence microscopy using a Leica DMRE inverted microscope. In these experiments, Rhodamine-labeled MVs were incubated with cells for 30 minutes. The cells were then washed and cultured for an additional 30 minutes at 37°C. In some studies, Lysotracker (Invitrogen) was added during the final 30 minutes incubation, prior to imaging.
Quantitative PCR
Total RNA was extracted from cultures, using the NucleoSpin kit (Macherey-Nagel, Bethlehem, PA). cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR (qPCR) was performed using a System 7300 instrument (Applied BioSystems) and a one-step program: 95°C, 10 minutes; 95°C, 30 seconds, 60°C, 1 minute, for 40 cycles. HPRT gene expression was measured as a normalizer for each sample. Results were analyzed by the relative quantity (ΔΔCt) method, as previously described (Thellin et al., 1999). All experiments were performed in triplicate with internal triplicate determinations.
Induction of experimental autoimmune encephalomyelitis (EAE)
All experiments were approved by the University of California, San Diego, Institutional Animal Care and Use Committee. EAE was induced in 6-week-old female SJL mice by subcutaneous immunization with 150 μg of PLP as previously described (Adams et al., 2007). Animals were euthanized 16 days later.
Immunofluorescence microscopy
Sagittal sections of mouse brain were prepared as previously described (Akassoglou et al., 2003). The sections were fixed with 4% formaldehyde for 30 minutes at 20°C, permeabilized with 0.1% Triton X-100 for 30 minutes, and then blocked with MOM mouse IgG blocking solution for 30 minutes (Vector). Sections were incubated with primary antibodies targeting GFAP (1/500), LRP1 (1 μg/ml) and CNPase (1/100) or with FITC-labeled IsoB4 diluted in the same buffer for 12 hours at 4°C. Following incubation with the other antibodies, secondary antibodies (Invitrogen) were introduced for 1 hour at 20°C (1 μg/ml). The sections were mounted in Prolong gold with DAPI (Invitrogen). Cells were imaged with a Leica DMRE inverted microscope, equipped with a Hamamatsu digital camera.
The authors thank Christina Sigurdson for critical reading of this manuscript. This work was supported by NIH grants R01 NS-054671, NS-057456, HL-60551, NS-052189, and National Multiple Sclerosis Society pilot grant PP1341. Deposited in PMC for release after 12 months.
References
- Adams, R. A., Bauer, J., Flick, M. J., Sikorski, S. L., Nuriel, T., Lassmann, H., Degen, J. L. and Akassoglou, K. (2007). The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 204, 571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou, K., Douni, E., Bauer, J., Lassmann, H., Kollias, G. and Probert, L. (2003). Exclusive tumor necrosis factor (TNF) signaling by the p75TNF receptor triggers inflammatory ischemia in the CNS of transgenic mice. Proc. Natl. Acad. Sci. USA 100, 709-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, J., Zhang, C., Polavarapu, R., Zhang, X., Zhang, X. and Yepes, M. (2008). Tissue-type plasminogen activator and the low density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain. Blood 112, 2787-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony, J. M., van Marle, G., Opii, W., Butterfield, D. A., Mallet, F., Yong, V. W., Wallace, J. L., Deacon, R. M., Warren, K. and Power, C. (2004). Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 7, 1088-1095. [DOI] [PubMed] [Google Scholar]
- Arnold-Schild, D., Hanau, D., Spehner, D., Schmid, C., Rammensee, H. G., de la Salle, H. and Schild, H. (1999). Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 162, 3757-3760. [PubMed] [Google Scholar]
- Barnes, H., Ackermann, E. J. and van der Geer, P. (2003). v-Src induces Shc binding to tyrosine 63 in the cytoplasmic domain of the LDL receptor-related protein 1. Oncogene 22, 3589-3597. [DOI] [PubMed] [Google Scholar]
- Barnett, M. H. and Prineas, J. W. (2004). Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55, 458-468. [DOI] [PubMed] [Google Scholar]
- Basu, S., Binder, R. J., Ramalingam, T. and Srivastava, P. K. (2001). CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14, 303-313. [DOI] [PubMed] [Google Scholar]
- Boggs, J. M. (2006). Myelin basic protein: a multifunctional protein. Cell Mol. Life Sci. 63, 1945-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu, G. (2001). The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int. Rev. Cytol. 209, 79-116. [DOI] [PubMed] [Google Scholar]
- Calderwood, S. K., Mambula, S. S., Gray, P. J., Jr and Theriault, J. R. (2007). Extracellular heat shock proteins in cell signaling. FEBS Lett. 581, 3689-3694. [DOI] [PubMed] [Google Scholar]
- Campana, W. M., Li, X., Dragojlovic, N., Janes, J., Gaultier, A. and Gonias, S. L. (2006). The low-density lipoprotein receptor-related protein is a pro-survival receptor in Schwann cells: possible implications in peripheral nerve injury. J. Neurosci. 26, 11197-11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, C., Lawrence, D. A., Li, Y., Von Arnim, C. A., Herz, J., Su, E. J., Makarova, A., Hyman, B. T., Strickland, D. K. and Zhang, L. (2006). Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. EMBO J. 25, 1860-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai, S. J., McPhillips, K. A., Frasch, S. C., Janssen, W. J., Starefeldt, A., Murphy-Ullrich, J. E., Bratton, D. L., Oldenborg, P. A., Michalak, M. and Henson, P. M. (2005). Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123, 321-334. [DOI] [PubMed] [Google Scholar]
- Gaultier, A., Arandjelovic, S., Li, X., Janes, J., Dragojlovic, N., Zhou, G. P., Dolkas, J., Myers, R. R., Gonias, S. L. and Campana, W. M. (2008a). A shed form of low density lipoprotein receptor-related protein regulates peripheral nerve injury and neuropathic pain. J. Clin. Invest. 118, 161-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier, A., Arandjelovic, S., Niessen, S., Overton, C. D., Linton, M. F., Fazio, S., Campana, W. M., Cravatt, B. F., 3rd and Gonias, S. L. (2008b). Regulation of tumor necrosis factor receptor-1 and the IKK-NF-kappaB pathway by LDL receptor-related protein explains the antiinflammatory activity of this receptor. Blood 111, 5316-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonias, S. L., Fuchs, H. E. and Pizzo, S. V. (1982). A unique pathway for the plasma elimination of alpha 2-antiplasmin-protease complexes in mice. Thromb. Haemost. 48, 208-210. [PubMed] [Google Scholar]
- Gonias, S. L., Wu, L. and Salicioni, A. M. (2004). Low density lipoprotein receptor-related protein: regulation of the plasma membrane proteome. Thromb. Haemost. 91, 1056-1064. [DOI] [PubMed] [Google Scholar]
- Hart, J. P., Gunn, M. D. and Pizzo, S. V. (2004). A CD91-positive subset of CD11c+ blood dendritic cells: characterization of the APC that functions to enhance adaptive immune responses against CD91-targeted antigens. J. Immunol. 172, 70-78. [DOI] [PubMed] [Google Scholar]
- Herz, J. and Strickland, D. K. (2001). LRP: a multifunctional scavenger and signaling receptor. J. Clin. Invest. 108, 779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz, J., Goldstein, J. L., Strickland, D. K., Ho, Y. K. and Brown, M. S. (1991). 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J. Biol. Chem. 266, 21232-21238. [PubMed] [Google Scholar]
- Herz, J., Clouthier, D. E. and Hammer, R. E. (1992). LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell 71, 411-421. [DOI] [PubMed] [Google Scholar]
- Ishiguro, M., Imai, Y. and Kohsaka, S. (1995). Expression and distribution of low density lipoprotein receptor-related protein mRNA in the rat central nervous system. Brain Res. Mol. Brain Res. 33, 37-46. [DOI] [PubMed] [Google Scholar]
- Kamholz, J. A. (1996). Regulation of myelin development. Mult. Scler. 2, 236-240. [DOI] [PubMed] [Google Scholar]
- Kermode, A. G., Thompson, A. J., Tofts, P., MacManus, D. G., Kendall, B. E., Kingsley, D. P., Moseley, I. F., Rudge, P. and McDonald, W. I. (1990). Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis: pathogenetic and clinical implications. Brain 113, 1477-1489. [DOI] [PubMed] [Google Scholar]
- Lillis, A. P., Van Duyn, L. B., Murphy-Ullrich, J. E. and Strickland, D. K. (2008). LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 88, 887-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, M. B., Bogaev, C. A., Gonias, S. L. and VandenBerg, S. R. (1994). Expression of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein is increased in reactive and neoplastic glial cells. FEBS Lett. 338, 301-305. [DOI] [PubMed] [Google Scholar]
- Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M. and Lassmann, H. (1999). A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain 122, 2279-2295. [DOI] [PubMed] [Google Scholar]
- Lucchinetti, C., Bruck, W., Parisi, J., Scheithauer, B., Rodriguez, M. and Lassmann, H. (2000). Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47, 707-717. [DOI] [PubMed] [Google Scholar]
- Lucchinetti, C. F., Bruck, W. and Lassmann, H. (2004). Evidence for pathogenic heterogeneity in multiple sclerosis. Ann. Neurol. 56, 308. [DOI] [PubMed] [Google Scholar]
- Makarova, A., Bercury, K. K., Adams, K. W., Joyner, D., Deng, M., Spoelgen, R., Koker, M., Strickland, D. K. and Hyman, B. T. (2008). The LDL receptor-related protein can form homo-dimers in neuronal cells. Neurosci. Lett. 442, 91-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo, M. P., von Bernhardi, R., Bu, G. and Inestrosa, N. C. (2000). Expression of alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J. Neurosci. Res. 60, 401-411. [DOI] [PubMed] [Google Scholar]
- Matute, C., Alberdi, E., Domercq, M., Perez-Cerda, F., Perez-Samartin, A. and Sanchez-Gomez, M. V. (2001). The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 24, 224-230. [DOI] [PubMed] [Google Scholar]
- May, P., Rohlmann, A., Bock, H. H., Zurhove, K., Marth, J. D., Schomburg, E. D., Noebels, J. L., Beffert, U., Sweatt, J. D., Weeber, E. J. et al. (2004). Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. Mol. Cell. Biol. 24, 8872-8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, K. D. and de Vellis, J. (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85, 890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup, S. K., Gliemann, J. and Pallesen, G. (1992). Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 269, 375-382. [DOI] [PubMed] [Google Scholar]
- Molineaux, S. M., Engh, H., de Ferra, F., Hudson, L. and Lazzarini, R. A. (1986). Recombination within the myelin basic protein gene created the dysmyelinating shiverer mouse mutation. Proc. Natl. Acad. Sci. USA 83, 7542-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musse, A. A., Boggs, J. M. and Harauz, G. (2006). Deimination of membrane-bound myelin basic protein in multiple sclerosis exposes an immunodominant epitope. Proc. Natl. Acad. Sci. USA 103, 4422-4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton, W. T. and Poduslo, S. E. (1973). Myelination in rat brain: method of myelin isolation. J. Neurochem. 21, 749-757. [DOI] [PubMed] [Google Scholar]
- Nykjaer, A., Conese, M., Christensen, E. I., Olson, D., Cremona, O., Gliemann, J. and Blasi, F. (1997). Recycling of the urokinase receptor upon internalization of the uPA:serpin complexes. EMBO J. 16, 2610-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota, K., Matsui, M., Milford, E. L., Mackin, G. A., Weiner, H. L. and Hafler, D. A. (1990). T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 346, 183-187. [DOI] [PubMed] [Google Scholar]
- Pastrana, D. V., Hanson, A. J., Knisely, J., Bu, G. and Fitzgerald, D. J. (2005). LRP 1 B functions as a receptor for Pseudomonas exotoxin. Biochim. Biophys. Acta 1741, 234-239. [DOI] [PubMed] [Google Scholar]
- Quinn, K. A., Pye, V. J., Dai, Y. P., Chesterman, C. N. and Owensby, D. A. (1999). Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP). Exp. Cell Res. 251, 433-441. [DOI] [PubMed] [Google Scholar]
- Rinner, W. A., Bauer, J., Schmidts, M., Lassmann, H. and Hickey, W. F. (1995). Resident microglia and hematogenous macrophages as phagocytes in adoptively transferred experimental autoimmune encephalomyelitis: an investigation using rat radiation bone marrow chimeras. Glia 14, 257-266. [DOI] [PubMed] [Google Scholar]
- Saito, A., Iino, N., Takeda, T. and Gejyo, F. (2007). Role of megalin, a proximal tubular endocytic receptor, in calcium and phosphate homeostasis. Ther. Apher. Dial. 11 Suppl. 1, S23-S26. [DOI] [PubMed] [Google Scholar]
- Singh-Jasuja, H., Toes, R. E., Spee, P., Munz, C., Hilf, N., Schoenberger, S. P., Ricciardi-Castagnoli, P., Neefjes, J., Rammensee, H. G., Arnold-Schild, D. et al. (2000). Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J. Exp. Med. 191, 1965-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. E. (2001). Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc. Res. Tech. 54, 81-94. [DOI] [PubMed] [Google Scholar]
- Stinissen, P., Raus, J. and Zhang, J. (1997). Autoimmune pathogenesis of multiple sclerosis: role of autoreactive T lymphocytes and new immunotherapeutic strategies. Crit. Rev. Immunol. 17, 33-75. [DOI] [PubMed] [Google Scholar]
- Strickland, D. K., Gonias, S. L. and Argraves, W. S. (2002). Diverse roles for the LDL receptor family. Trends Endocrinol. Metab. 13, 66-74. [DOI] [PubMed] [Google Scholar]
- Thellin, O., Zorzi, W., Lakaye, B., De Borman, B., Coumans, B., Hennen, G., Grisar, T., Igout, A. and Heinen, E. (1999). Housekeeping genes as internal standards: use and limits. J. Biotechnol. 75, 291-295. [DOI] [PubMed] [Google Scholar]
- Vandivier, R. W., Ogden, C. A., Fadok, V. A., Hoffmann, P. R., Brown, K. K., Botto, M., Walport, M. J., Fisher, J. H., Henson, P. M. and Greene, K. E. (2002). Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J. Immunol. 169, 3978-3986. [DOI] [PubMed] [Google Scholar]
- Wekerle, H., Kojima, K., Lannes-Vieira, J., Lassmann, H. and Linington, C. (1994). Animal models. Ann. Neurol. 36 Suppl., S47-S53. [DOI] [PubMed] [Google Scholar]
- Willnow, T. E. and Herz, J. (1994). Genetic deficiency in low density lipoprotein receptor-related protein confers cellular resistance to Pseudomonas exotoxin A: evidence that this protein is required for uptake and degradation of multiple ligands. J. Cell Sci. 107, 719-726. [PubMed] [Google Scholar]
- Wolf, B. B., Lopes, M. B., VandenBerg, S. R. and Gonias, S. L. (1992). Characterization and immunohistochemical localization of alpha 2-macroglobulin receptor (low-density lipoprotein receptor-related protein) in human brain. Am. J. Pathol. 141, 37-42. [PMC free article] [PubMed] [Google Scholar]
- Yepes, M., Sandkvist, M., Moore, E. G., Bugge, T. H., Strickland, D. K. and Lawrence, D. A. (2003). Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 112, 1533-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]