Summary
Duox NADPH oxidases generate hydrogen peroxide at the air-liquid interface of the respiratory tract and at apical membranes of thyroid follicular cells. Inactivating mutations of Duox2 have been linked to congenital hypothyroidism, and epigenetic silencing of Duox is frequently observed in lung cancer. To study Duox regulation by maturation factors in detail, its association with these factors, differential use of subunits and localization was analyzed in a lung cancer cell line and undifferentiated or polarized lung epithelial cells. We show here that Duox proteins form functional heterodimers with their respective DuoxA subunits, in close analogy to the phagocyte NADPH oxidase. Characterization of novel DuoxA1 isoforms and mispaired Duox-DuoxA complexes revealed that heterodimerization is a prerequisite for reactive oxygen species production. Functional Duox1 and Duox2 localize to the leading edge of migrating cells, augmenting motility and wound healing. DuoxA subunits are responsible for targeting functional oxidases to distinct cellular compartments in lung epithelial cells, including Duox2 expression in ciliated cells in an ex vivo differentiated lung epithelium. As these locations probably define signaling specificity of Duox1 versus Duox2, these findings will facilitate monitoring Duox isoform expression in lung disease, a first step for early screening procedures and rational drug development.
Keywords: NADPH oxidase, Duox, ROS, Lung epithelial cells, Migration
Introduction
Regulated generation of reactive oxygen species (ROS) has been the hallmark of phagocytes and a prerequisite for an efficient microbicidal host response. The responsible enzyme is a multimeric NADPH oxidase, which consists of the integral transmembrane flavocytochrome b558, composed of gp91phox (Nox2) and p22phox, and several cytosolic regulatory proteins (Sumimoto et al., 2005). The occurrence of chronic granulomatous disease (CGD), an inherited immunodeficiency characterized by genetic defects in oxidase components, is an example of the importance of this defense system in bacterial and fungal infections. The discovery of several functionally distinct Nox2 homologs and associated regulatory proteins together with their unique tissue expression patterns suggests that NADPH oxidases fulfill distinct biological functions in various cell types (Bedard and Krause, 2007; Lambeth et al., 2007).
Two calcium-activated, structurally similar NADPH oxidases, Duox1 and Duox2, are primarily expressed in the lung epithelium, mucosal membranes and the thyroid (Caillou et al., 2001; De Deken et al., 2000; Forteza et al., 2005; Geiszt et al., 2003). The physiological role of Duox2 as an H2O2 source for thyroid hormone biosynthesis is supported by the observation that missense and nonsense mutations in Duox2 cause mild to severe hypothyroidism (Grasberger et al., 2007; Moreno et al., 2002). Duox1, the predominantly expressed Duox isoform in the lung, has been linked to lung development, differentiation, mucus production and host defense (Forteza et al., 2005; van der Vliet, 2008). Functional Duox1 cannot compensate for inactivated Duox2 in the thyroid, which is indicative of distinct compartmentalization of Duox enzymes or the presence of diverse activation mechanisms in tissues. DUOX1 and DUOX2 are both located on chromosome 15q15-21 at a complex locus which contains bidirectional arrangement of DUOX1 and DUOX2 with their respective partners DUOXA1 and DUOXA2 (Grasberger and Refetoff, 2006). DuoxA1 and DuoxA2 are integral transmembrane proteins, which were discovered as ER-resident maturation factors required for ER-Golgi transition and maturation of Duox (Grasberger and Refetoff, 2006). Here, we report that DuoxA proteins exit the ER when a heterodimeric complex with Duox has been formed. The characterization of DuoxA1 splice variants, which have not been described previously, reveals their differential ability to contribute to Duox1 activity. One of the DuoxA1 splice variants is capable of functional association with the Duox2 paralog. The translocation of Duox-DuoxA heterodimers to particular membrane compartments, and thus the specific intracellular location of the active oxidase seem to be determined by the associated DuoxA subunit. Both Duox and DuoxA enzymes are enriched at the leading edge of migrating cells, although their distinct distribution on the apical side of differentiated lung epithelium suggests divergent roles in physiological and pathophysiological conditions.
Results
Distinct localization of Duox-DuoxA heterodimers
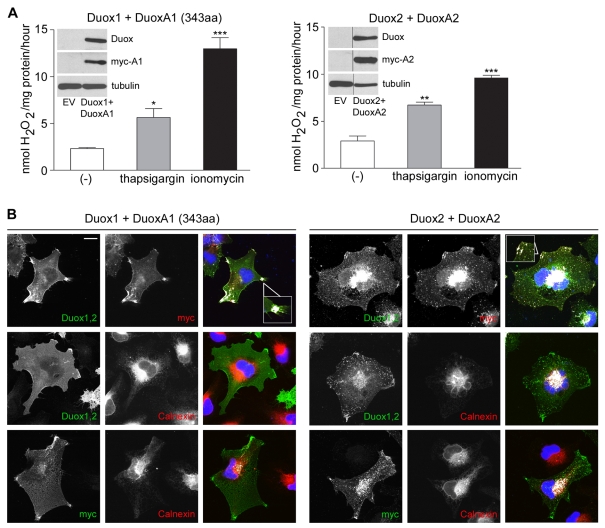
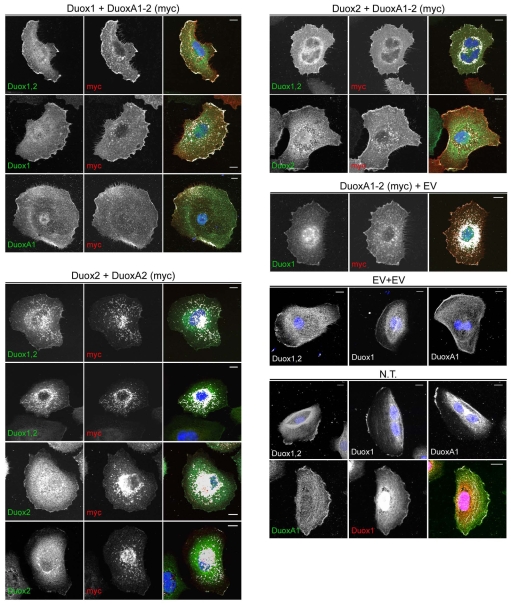
Expression of functional Duox NADPH oxidases requires coexpression of the maturation factors DuoxA1 and DuoxA2, which are essential for Duox-dependent H2O2 production in vitro (Grasberger and Refetoff, 2006). Duox-DuoxA expression is frequently silenced by promoter hypermethylation in lung cancer cell lines, permitting reconstitution of the oxidase in a physiologically relevant background (Luxen et al., 2008). Thus, functional Duox was re-introduced into Duox-DuoxA-deficient NCI-H661 cells by coexpression of Duox1 and DuoxA1 (343aa), or Duox2 and DuoxA2. Increasing intracellular Ca2+ levels by ionomycin or thapsigargin treatment significantly elevated H2O2 generation (Fig. 1A). Since both Duox enzymes responded with comparable ROS production, their subcellular localization was probed. Confocal analysis of NCI-H661 cells expressing functional Duox1 or Duox2 revealed distinct localization of the oxidases (Fig. 1B). Duox1 was almost exclusively present at the edges of plasma membrane protrusions, colocalizing with DuoxA1 (343aa). Duox2, however, colocalized with DuoxA2 in several locations including the ER, intracellular structures reminiscent of trafficking vesicles, and infrequently at the plasma membrane. In contrast to a previous report (Grasberger and Refetoff, 2006), DuoxA1 and DuoxA2 escaped the ER, as confirmed by calnexin staining, and translocated to particular Duox1- or Duox2-containing membrane compartments. Although Duox2 and DuoxA2 were partially retained in the ER, they were clearly present in vesicular and plasma membrane-associated compartments. This result suggested that DuoxA proteins may fulfill additional functions besides solely facilitating the maturation of Duox proteins in the ER.
Fig. 1.
ER exit of Duox1-DuoxA1 (343aa) and Duox2-DuoxA2. Analysis of NCI-H661 cells transiently expressing Duox1-DuoxA1 (343aa) or Duox2-DuoxA2. (A) Duox-dependent H2O2 production was determined with and without addition of ionomycin or thapsigargin. n=3 each; *P<0.05; **P<0.01; ***P<0.001 versus unstimulated. Graphs show mean ± s.e.m. Immunoblots were probed for Duox (α-Duox1,2), DuoxA (α-Myc) and α-tubulin. (B) Colocalization of Duox1-DuoxA1-Myc and Duox2-DuoxA2-Myc. Inserts depict particular locations of Duox1-DuoxA1 and Duox2-DuoxA2 at higher magnification. Colocalization is shown in white in the merged images in the right column. Calnexin served as ER marker. Scale bar, 10 μm.
Heterodimerization of Duox-DuoxA is heme dependent
The colocalization of Duox1 with DuoxA1, and Duox2 with DuoxA2 suggested complex formation of these proteins. To address this question, coimmunoprecipitation experiments of Duox proteins with the corresponding DuoxA proteins were performed in transiently transfected H661 cells (Fig. 2A). Indeed, Duox1 and Duox2 were detected after precipitation of DuoxA1 and DuoxA2, respectively, indicating close interaction of DuoxA with Duox. This interaction was abolished after treatment of H661 cells stably expressing Duox1-DuoxA1 or Duox2-DuoxA2 with the heme synthesis inhibitor succinyl acetone (SA; Fig. 2B). Thus, acquisition of heme by Duox is essential for association with DuoxA, a scenario reminiscent of Nox2-p22phox heterodimer or iNOS homodimer formation (DeLeo et al., 2000; Panda et al., 2005; Yu et al., 1997). In accord, heme incorporation was essential for H2O2 generation by Duox enzymes (Fig. 2C). These results indicate that DuoxA1 and DuoxA2 are not only maturation factors for Duox1 and Duox2, but are rather an integral part of the functional oxidase complex.
Fig. 2.
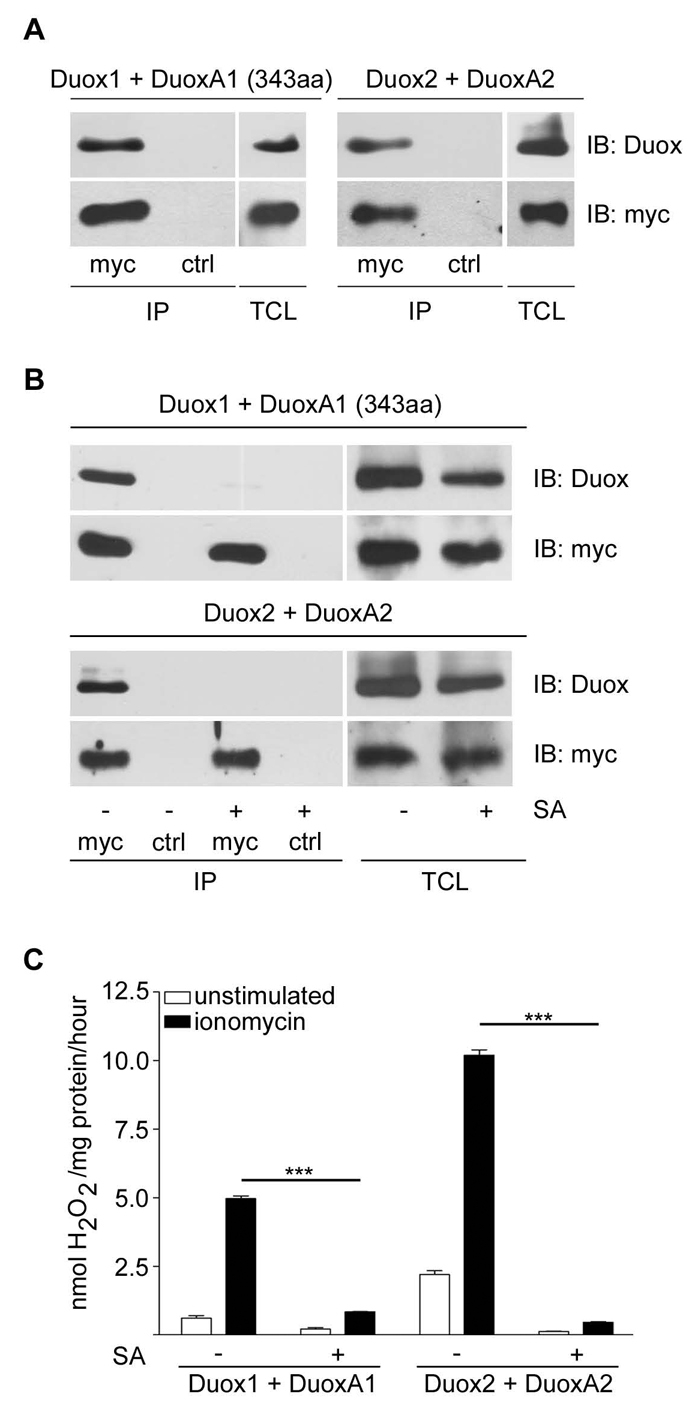
Heme-dependent heterodimer formation of Duox-DuoxA. (A) Coimmunoprecipitation of DuoxA (α-Myc IP) with the respective Duox isoforms in transiently transfected H661 cells. Control IP (ctrl) was done with α-GST. Immunoblotting (IB) of IPs and total cell lysates (TCL) was performed with α-Duox1,2 or α-Myc antibodies. (B,C) H661 cells stably expressing Duox1-DuoxA1 (343aa) or Duox2-DuoxA2 were cultured for 4 days in the presence or absence of 10 μg/ml SA prior to (B) α-DuoxA (Myc IP) or α-GST (ctrl IP) immunoprecipitation, followed by immunoblotting (IB) as indicated, or (C) H2O2 production determined with or without addition of ionomycin. n=3 each; ***P<0.001 versus untreated. Graphs show mean ± s.e.m.
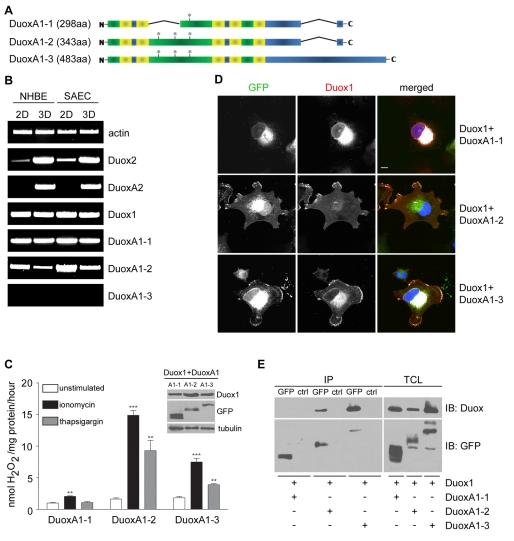
Characterization of novel DuoxA1 variants
A database search for human DuoxA1 identified three different DuoxA1 splice variants with respective sizes of 298aa (DuoxA1-1), 343aa (DuoxA1-2) and 483aa (DuoxA1-3; Fig. 3A). We analyzed expression of all three variants in human primary bronchial epithelial (NHBE) cells and small airway epithelial cells (SAEC), comparing typical cell culture conditions (2D) with fully differentiated epithelial layers (3D; Fig. 3B). DUOXA1-1 and DUOXA1-2 mRNA was present, whereas transcripts for DUOXA1-3 were not detected. Differentiation of NHBE cells or SAEC in air-liquid (ALI) culture increased DUOX2 and DUOXA2 transcripts considerably.
Fig. 3.
Differential expression and localization of DuoxA1 splice variants. (A) Schematic representation of DuoxA1-(1-3). Variants share the putative five transmembrane (TM) structure. DuoxA1-1 (298aa) lacks two of three putative N-glycosylation sites. DuoxA1-3 contains an extended C terminus. Green, extracellular regions; yellow, TM domains; blue, intracellular regions; *, N-glycosylation sites. (B) RT-PCR using undifferentiated (2D) und ALI-differentiated SAEC and NHBE cells (14 days; 3D). (C-E) Analysis of NCI-H661 cells transiently transfected with Duox1 and EGFP-fused DuoxA1-1, DuoxA1-2 and DuoxA1-3. (C) H2O2 production was measured after stimulation with ionomycin or thapsigargin. **P<0.01; ***P<0.001 versus unstimulated. Data are presented as mean values ± s.e.m.; n=3 each. Immunoblot detection of Duox1 (α-Duox1,2), DuoxA1 (α-GFP) and α-tubulin in TCL. (D) Localization of Duox1 (α-Duox1,2) and DuoxA1 (GFP) variants by immunofluorescence. Colocalization is shown in white. Scale bar, 10 μm. (E) Immunoblotting (IB) for Duox1 (α-Duox1,2) and DuoxA1 (α-GFP) was performed after immunoprecipitation (IP α-GFP). Control IP (ctrl) was done with α-GST. TCL was probed for Duox1 and DuoxA1 variant expression as indicated.
Next, we investigated if all three DuoxA1 splice variants contribute to Duox1 function (Fig. 3C). The shortest form, DuoxA1-1, which lacks two potential glycosylation sites in a putative extracellular loop, supported Duox1-dependent H2O2 production only minimally. In comparison to the Duox1-DuoxA1-2 dimer, the Duox1-DuoxA1-3 complex was moderately active. Confocal analysis revealed that the presence of a particular DuoxA1 splice variant determined the localization of Duox1 (Fig. 3D). DuoxA1-1 remained together with Duox1 exclusively in the ER, colocalizing with calnexin (calnexin staining not shown). When DuoxA1-3 and Duox1 were coexpressed, both proteins were found in the ER as well as at the plasma membrane. Only expression of DuoxA1-2 permitted complete ER exit of Duox1 and cell surface targeting of the heterodimer. These results correlate well with ROS generation, as functional Duox1 seems to be localized mainly at the plasma membrane. Heterodimer formation of DuoxA1-2 or DuoxA1-3 with Duox1 was confirmed by coimmunoprecipitation of these proteins (Fig. 3E). Although both, Duox1 and DuoxA1-1, are highly expressed in the ER, Duox1 did not coimmunoprecipitate with DuoxA1-1 (Fig. 3E).
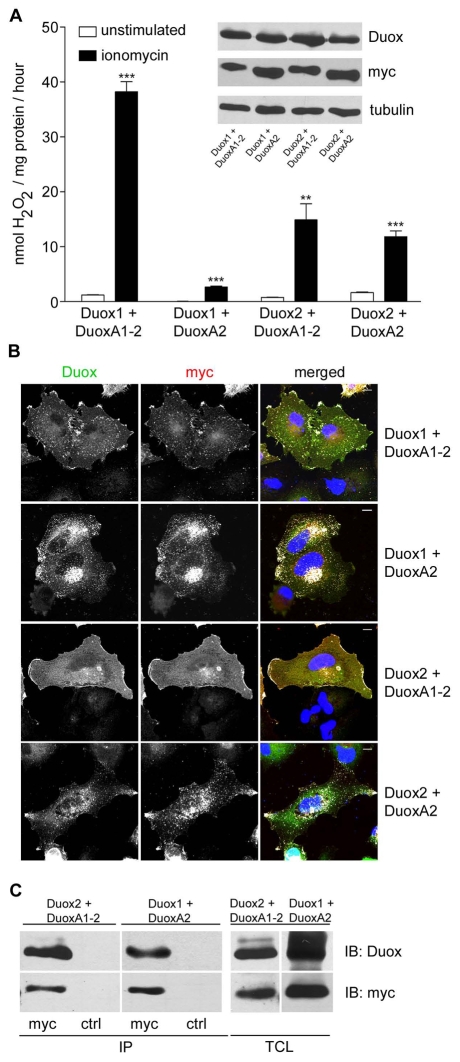
Mispaired DuoxA1-2-Duox2 heterodimers are functional
DUOX1-DUOXA1 and DUOX2-DUOXA2 are predicted to form a tightly linked transcriptional unit. Assuming distinct roles for Duox1 versus Duox2 in lung and thyroid physiology, mutational inactivation of DUOXA will probably trigger disease (Zamproni et al., 2008). In these cases, compensation by the related DuoxA protein might be advantageous. Mismatch experiments were performed to test the activity of putative Duox1-DuoxA2 and Duox2-DuoxA1-2 complexes when coexpressed in NCI-H661 lung cancer cells. Although pairing Duox1 with DuoxA2 caused a 90% reduction in Duox1-mediated H2O2 production (Fig. 4A), combining Duox2 with DuoxA1-2 generated H2O2 at levels similar to those observed in Duox2-DuoxA2 reconstituted cells (Fig. 4A). By contrast, coexpression of Duox2 with DuoxA1-1 or DuoxA1-3 did not lead to substantial functional recovery of Duox2 (data not shown). Immunostaining of NCI-H661 cells expressing Duox1-DuoxA1-2, Duox1-DuoxA2, Duox2-DuoxA1-2 or Duox2-DuoxA2 revealed that the DuoxA subunit is responsible for targeting the oxidase heterodimer to distinct compartments in H661 cells. Whereas Duox1-DuoxA1-2 and Duox2-DuoxA1-2 complexes localized to protrusions and the plasma membrane, Duox1-DuoxA2 and Duox2-DuoxA2 heterodimers were predominantly located in the ER or at vesicular structures (Fig. 4B). Mispaired Duox2-DuoxA1-2 as well as Duox1-DuoxA2 heterodimer formation was also confirmed by coimmunoprecipitation (Fig. 4C). Thus, when expressed in H661 lung cancer cells, both DuoxA proteins can form ROS-generating Duox2 complexes at membranes, whereas the modestly functional Duox1-DuoxA2 dimer seems to be trapped in the ER.
Fig. 4.
DuoxA1-2 can substitute for DuoxA2 in functional Duox2-DuoxA heterodimerization. Transient expression of Duox1, Duox2, DuoxA1-2 and DuoxA2 in NCI-H661 cells. (A) H2O2 production was measured in response to stimulation with ionomycin. **P<0.01; ***P<0.001 versus unstimulated. Shown are mean values ± s.e.m.; n=3 each. Expression of Duox1, Duox2, DuoxA1-2 (α-Myc), DuoxA2 (α-Myc) and α-tubulin was determined by immunoblotting. (B) Localization of Duox1-DuoxA1-2-Myc, Duox1-DuoxA2-Myc, Duox2-DuoxA1-2-Myc and Duox2-DuoxA2-Myc was determined by immunofluorescence in NCI-H661 cells. Colocalization of Duox (α-Duox) and DuoxA (α-Myc) is shown in white. Scale bar, 10 μm. (C) Detection of Duox2 or Duox1 after α-Myc IP of DuoxA1-2 and DuoxA2, respectively. α-GST IP (ctrl) served as control. IPs and TCL of NCI-H661 cells were probed for Duox (α-Duox) and DuoxA (α-Myc) expression.
Expression of endogenous Duox1 and Duox2 in differentiated lung epithelial cells
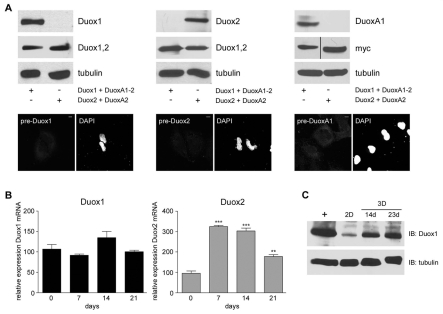
To visualize endogenous Duox and DuoxA proteins, antibodies specific for Duox1, Duox2 and DuoxA1-(1-3) were generated and evaluated by immunoblot using Duox1-DuoxA1-2 and Duox2-DuoxA2 overexpressing cell lysates. Probing with anti-Duox1,2 (Luxen et al., 2008), which recognizes both Duox isoforms, and anti-Myc antibodies served as expression control (Fig. 5A, upper panel). Preimmune sera of the Duox1, Duox2 and DuoxA1 antibodies did not detect Duox or DuoxA when overexpressed in H661 cells (Fig. 5A, lower panel). Real-time PCR of SAEC differentiated for 21 days, showed no change in DUOX1 transcript levels, whereas DUOX2 mRNA increased significantly (Fig. 5B). On the protein level, Duox1 protein increased, which might be due to enhanced protein stability in differentiated cells (Fig. 5C). Although DUOX2 was upregulated during differentiation (Fig. 3B, Fig. 5B), Duox2 protein was not detected in SAEC 3D lysates (data not shown). Exogenously expressed Duox2, however, was readily visualized, indicating that endogenous Duox2 protein levels were below the detection limit of the anti-Duox2 antibody in immunoblotting.
Fig. 5.
Expression analysis of endogenous Duox in differentiated lung epithelial cells. (A) Upper panel: validation of α-Duox1, α-Duox2 and α-DuoxA1 antibodies. TCL of cells expressing Duox1-DuoxA1-2-Myc or Duox2-DuoxA2-Myc were probed with isoform-specific antibodies as indicated. α-tubulin served as loading control. α-Duox1,2 and α-Myc blotting served as expression control. Lower panel: cells expressing Duox1-DuoxA1-2 or Duox2-DuoxA2 were stained with DAPI and preimmune sera derived from rabbits used for Duox1, Duox2 or DuoxA1 antibody generation as indicated. Images in both channels are depicted in white. Scale bar, 10 μm. (B) Real-time PCR of DUOX1 and DUOX2 in SAEC, either undifferentiated (2D; 0 days) or differentiated in ALI-culture for 7, 14 and 21 days (3D). Columns show the means ± s.e.m. and represent three different samples, which were individually normalized to actin. **P<0.01; ***P<0.001 versus transcript levels in 2D samples; n=3. (C) Upregulation of Duox1 protein during differentiation. Immunoblot analysis of endogenous Duox1 in undifferentiated (2D) and differentiated [14 and 23 days (d); 3D] SAEC. Lysates of NCI-H661 cells transfected with Duox1-DuoxA1-2 were used as control (+).
Visualization of endogenous Duox-DuoxA in primary airway epithelial cells
Initial Duox-DuoxA localization experiments were performed in the NCI-H661 lung cancer cell line. To validate these findings in a primary cell context, SAEC were transduced with lentivirus encoding for Duox1, Duox2, DuoxA1-2 and DuoxA2. Co-staining with anti-Duox1,2 (Luxen et al., 2008) and with anti-Myc for detection of the DuoxA Myc epitope tag, showed that localization of Duox heterodimers in SAEC was consistent with our observations in NCI-H661 cells. Duox1-DuoxA1-2 and Duox2-DuoxA1-2 complexes were seen lining the plasma membrane, whereas Duox2-DuoxA2 localized mainly to intracellular membrane structures and the ER (Fig. 6). The same pattern was observed when Duox1-, Duox2- and DuoxA1-specific antibodies were used. To probe for endogenous proteins, SAEC were either transduced with lentiviral particles expressing vector (EV) in order to subject cells to a similar treatment as used for Duox overexpression, or were left untreated (N.T.). Again, Duox1 and DuoxA1 were detected at the plasma membrane. However, both proteins were also localized on internal membranes, which may represent immature forms of these proteins. Endogenous Duox2 could not be visualized, as functional Duox2-DuoxA2 complexes cannot be formed in SAEC 2D (see Fig. 3B). To determine if endogenous Duox1 and DuoxA1 colocalize in SAEC, Cy3-labelled anti-Duox1 was used for co-staining, as Duox1 and DuoxA1 antibodies were raised in rabbits. The heterodimer was clearly lining the plasma membrane, although labeling with the anti-Duox1 antibody caused unspecific nuclear staining.
Fig. 6.
Detection of endogenous Duox1 and DuoxA1 protein in primary human lung epithelial cells (2D). Lentivirally transduced SAEC expressing the indicated combinations were used for localization studies. SAEC transduced with vector (EV) or not transduced (N.T.) served as control. Comparison of α-Duox1,2 antibody staining with α-Duox1 or with α-Duox2 antibodies (all green); and of α-Myc (red) staining with α-DuoxA1 (green) antibody. Endogenous coexpression of DuoxA1 and Duox1 was visualized by co-staining of DuoxA1 (green) with Cy3-labeled Duox1 antibody (red). Colocalization in merged pictures is shown in white. Separate staining of endogenous Duox1 and DuoxA1 in right panels 4 and 5 is depicted in white with nuclei in blue (DAPI). Scale bars, 10 μm.
Duox translocation during cell migration
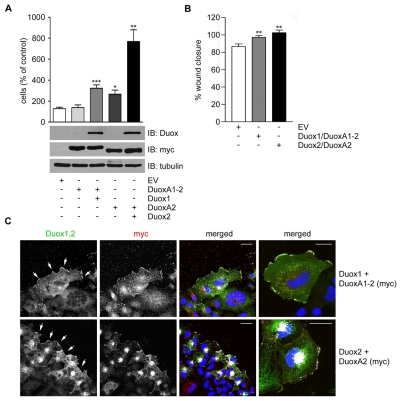
Duox1 has been linked to motility by increasing cellular migration rates in cell culture conditions (Koff et al., 2006; Luxen et al., 2008; Wesley et al., 2007). However, functional Duox2 is not expressed in these conditions in primary airway cells (SAEC, NHBE). To compare migration rates, NCI-H661 cells transduced with Duox1-DuoxA1-2 or with Duox2-DuoxA2 were analyzed in Transwell chambers. Although cells expressing Duox1-DuoxA1-2 migrated faster than control cells expressing empty vector (EV) or DuoxA1-2 alone, reconstitution of Duox2-DuoxA2 increased chemotaxis substantially above those values (Fig. 7A). Expression of DuoxA2 alone, but not DuoxA1, enhanced cell motility in this cell type, which may indicate that DuoxA2 might possess an additional, yet unknown function. Directional migration was also examined by creating a wound in a confluent monolayer of NCI-H661 cells and analyzing the wound closure. Cells expressing functional Duox1 or Duox2 were able to migrate into the wounded area faster than cells expressing EV (Fig. 7B). Again, cells reconstituted with Duox2-DuoxA2 showed more efficient wound closure than cells expressing Duox1-DuoxA1-2 at consecutive time points. The increased speed of wound closure using the Duox2-DuoxA2 complex versus the Duox1-DuoxA1 complex reached significance at the 8 hours observation time point (not shown).
Fig. 7.
Reintroduction of functional Duox1 or Duox2 augments cellular migration. NCI-H661 cells stably expressing empty vector (EV), DuoxA1-2, Duox1-DuoxA1-2, DuoxA2 or Duox2-DuoxA2. (A) Cells were seeded on top of Transwell inserts and migration was initiated by addition of FCS to the bottom of the chamber. Cells underneath the filter were counted after 5.5 hours. *P<0.05; **P<0.01; ***P<0.001 versus EV. Data are shown as mean values ± s.e.m.; n=3 each. (B) Confluent monolayers of cells expressing EV, Duox1-DuoxA1-2 or Duox2-DuoxA2 were wounded and wound closure was determined after 4.5 hours. Data were collected from 12 random fields and are expressed as the percentage of wounds closed. **P<0.01 compared to EV. (C) Cells expressing Duox1-DuoxA1-2 or Duox2-DuoxA2 were grown to confluency and localization of Duox-DuoxA was determined 1 hour after wounding of the monolayer by immunostaining for Duox (Duox1,2; green) and DuoxA (Myc; red). Arrows indicate plasma membrane localization of Duox1 and Duox2, respectively. The images on the far right show single migrating cells. Colocalization is indicated in white. Scale bar, 30 μm.
As the Duox1-DuoxA1 complex was concentrated on the leading edge of randomly migrating cells (see Fig. 6), the localization of Duox1-DuoxA1 and Duox2-DuoxA2 heterodimers was analyzed during directional migration. Confluent monolayers of NCI-H661 cells stably expressing Duox1-DuoxA1 or Duox2-DuoxA2 were wounded. Confocal images of directionally migrating cells were taken to assess the localization of Duox-DuoxA proteins. Both Duox isoforms and their DuoxA partners localized to the leading edge of migrating cells. The Duox2 complex appeared more concentrated at protrusions in approximately 50% of the migrating cells, although some of the complex remained intracellular (Fig. 7C). Single cell analysis of motile cells confirmed the localization pattern observed at the wound edges (Fig. 7C, right panel, merged). In summary, our data suggest that despite their initially distinct intracellular localization, both Duox1 and Duox2 concentrate at the leading edge after wounding and may provide ROS for accelerated cell migration.
Differentiation alters the localization of endogenous Duox1 and Duox2
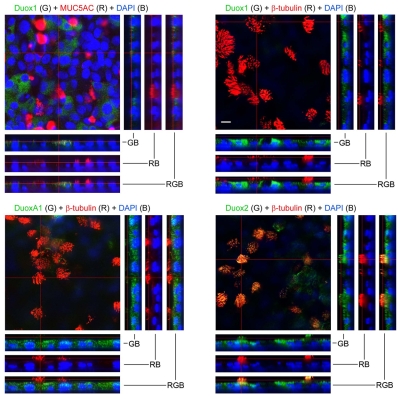
The unique contribution of Duox2 mutations to the development of congenital hypothyroidism or the changes in Duox isoform expression profiles by cytokine stimulation, suggest distinct biological functions for Duox1 and Duox2 (Harper et al., 2005; Moreno et al., 2002; Vigone et al., 2005). The development of antibodies that are capable of distinguishing between Duox1 and Duox2 now permits the analysis of their presence and localization in healthy and diseased lung tissues. As a first step in this direction, differentiated SAEC were stained for Duox1, DuoxA1 and Duox2 in combination with the mucin marker MUC5AC or the ciliary marker β-tubulin IV (Fig. 8). Minimal background staining was detected when using preimmune sera. Confocal z series revealed Duox1 and DuoxA1 primarily on the apical side of non-ciliated cells, but not in MUC5AC-expressing goblet cells. Ciliated and non-ciliated polarized SAEC showed Duox2-specific surface staining with particular enrichment in β-tubulin-containing cilia. Three-dimensional reconstruction of the differentiated SAEC layer confirmed localization of Duox2 in ciliary structures (see Movie 1 in supplementary material).
Fig. 8.
Distinct localization of endogenous Duox1, DuoxA1 and Duox2 in differentiated airway epithelial cells (3D). ALI-differentiated SAEC (14 days) were stained for mucin (MUC5AC; red, R), cilia (β-tubulin IV; red, R) and Duox1, Duox2 or DuoxA1 (all green, G) using isoform-specific antibodies. Nuclear staining was with DAPI (blue, B). Per insert, several Z-sections at 0.3 μm intervals were taken throughout the entire depth of multiple cell layers. Orthogonal views correspond to the crosslines in the rectangular overview and show two color overlays of green and blue (GB), red and blue (RB) and three color merge (red, green, blue; RGB), with colocalization indicated in yellow. Scale bar, 10 μm.
Discussion
Oxidant generation and oxidative stress are common aspects of many respiratory diseases. In the past, migrating immune cells were considered the main culprit, causing lung tissue injury and inflammation. This scenario has changed since the discovery of Duox NADPH oxidases present at the apical side of the lung epithelium. It seems probable that Duox follows the phagocyte oxidase paradigm, being beneficial or destructive depending on the context. As long as our understanding of basic regulatory mechanisms of these novel oxidases is so rudimentary, intervention strategies cannot be developed. To this end, we investigated the spatial expression and regulation of Duox isoforms in the lung. Our results reveal that Duox1 and Duox2 form functional heterodimeric complexes with their respective DuoxA maturation factors. The formation of the Duox1-DuoxA1 or Duox2-DuoxA2 heterodimer is dependent on heme incorporation into the Nox-homology domain, indicating that Duox enzymes follow the cytochrome b558 paradigm of heterodimerization with an associated subunit (DeLeo et al., 2000; Yu et al., 1998; Yu et al., 1997). Complex formation is essential for translocation of the dimer to particular subcellular locations and constitutes the initial form of Duox regulation.
The presence of several DuoxA1 isoforms could be an additional facet of post-transcriptional regulation in respect to Duox1 function. The shortest form, DuoxA1-1, features a deletion of two putative N-glycosylation sites in the extracellular loop between transmembrane domains 2 and 3. Thus, correct processing of DuoxA1-1 in the ER might not take place, preventing the formation of a functional Duox1-DuoxA1-1 heterodimer. The DuoxA1-3 splice variant contains additional C-terminal amino acids. This DuoxA1 form was initially identified in RNA derived from a mixture of brain, lung and testis tissues, and might not be expressed in lung epithelium per se. It is also possible that alveolar type II cells, which are not present in the ex-vivo-differentiated lung epithelium, express DuoxA1-3 (Fischer et al., 2007; Nagai et al., 2008) or that DuoxA1-3 is predominantly expressed during development. DuoxA1-3 is capable of forming a functional complex with Duox1 when coexpressed in lung cancer cells. Even though the extent of H2O2 production observed after stimulation with ionomycin varies between experiments, we consistently observed a similar pattern with Duox1-DuoxA1-2 being the most efficient source of H2O2, whereas Duox2-DuoxA2, Duox2-DuoxA1-2 and Duox1-DuoxA1-3 were less efficient. These differences might be due to slightly varying expression levels, and not to localization of the Duox complexes, as diffusible H2O2 was measured.
The overall structural arrangement of DuoxA1 and DuoxA2 is very similar and mutational inactivation of DuoxA, as observed in mild hypothyroidism (Zamproni et al., 2008), poses the question of whether DuoxA isoforms might be capable of compensating for each other. DuoxA2 only minimally supported Duox1 function, whereas the Duox2-DuoxA1-2 combination produced levels of H2O2 similar to the Duox2-DuoxA2 pair. Although association of both mismatched pairs occurred, the majority of the inactive Duox1-DuoxA2 complex remained in the ER. Our data show that, at least theoretically, Duox2 could compensate for Duox1 and DuoxA2 inactivation by utilizing DuoxA1-2. Co-expression of Duox2 with DuoxA1-2 in HeLa cells led to minor rescue of unstimulated H2O2 generation (Zamproni et al., 2008). When repeating these experiments, very modest basal and ionomycin-stimulated H2O2 production was detected in HeLa cells (not shown).
Confocal analysis revealed that functional Duox1 and Duox2 localize to different compartments in lung cancer and lung epithelial cells. The fact that Duox1-DuoxA1 is targeted to the plasma membrane in resting cells, while Duox2-DuoxA2 remained mostly in distinct intracellular compartments, may suggest diverse binding partners and biological functions for both proteins. Duox1 may generate H2O2 on-site to promote cell migration, a hypothesis supported by other studies, which have linked Duox1 to cell migration (Koff et al., 2006; Luxen et al., 2008), probably via a ROS-TACE pro ligand EGF receptor cascade (Wesley et al., 2007). In addition, Duox1 was linked to airway surface liquid acidification and epithelial mucin production (Schwarzer et al., 2004; Shao and Nadel, 2005). Duox2 localized to intracellular structures, which may indicate that this oxidase will be activated by a different set of stimuli. Despite the distinct localization in resting cells, a substantial fraction of Duox2 translocated to the leading edge of directionally migrating cells upon wounding and polarization. In fact, exogenous Duox2 was more efficient in promoting cell migration than Duox1, although both Duox proteins were expressed similarly.
In contrast to the Duox2-DuoxA2 complex, the mispaired Duox2-DuoxA1-2 heterodimer localized to the plasma membrane in a pattern reminiscent of Duox1-DuoxA1-2. Hence, the location of the Duox2-DuoxA1-2 complex indicates that DuoxA subunits contain information for intracellular targeting of the oxidase complex. However, our data were generated in lung cancer cells or in primary lung epithelial cells. Expression of mispaired Duox2-DuoxA1-2 in HeLa cells did not lead to efficient Duox2 expression at the plasma membrane (not shown). It seems that these cells, which are derived from the cervix, a tissue deficient in endogenous Duox, lack the processing features that enable formation of a functional Duox2-DuoxA1-2 heterodimer. It must be noted that in contrast to previously published data, even coexpression of the Duox2-DuoxA2 pair in HeLa cells did not result in substantial Duox2 localization along the plasma membrane (not shown). However, these differences might be due to the use of non-permeabilized HeLa cells overexpressing epitope-tagged Duox2, for imaging in these studies (Grasberger et al., 2007; Grasberger and Refetoff, 2006). When HeLa cells were permeabilized prior to staining, the majority of Duox2 remained intracellular, similar to our observations.
In ex-vivo-differentiated lung epithelial layers both Duox proteins reside at the apical surface with Duox2 selectively enriched in cilia. This finding, if confirmed in more detail, could shed some light on Duox function in the lung. A recent report showed that the high molecular mass polymer hyaluronan (HA) is synthesized at the apical pole of airway epithelial cells (Manzanares et al., 2007). ROS led to depolymerization of HA into low molecular mass fragments, which stimulated ciliary beat frequency (CBF). It is conceivable that Duox2-mediated ROS is produced in cilia, thus contributing to CBF during mucociliary clearance.
In summary, our data demonstrate that Duox1 and Duox2 localize distinctly in lung epithelial cells as well as in ex-vivo-differentiated lung epithelia. The localization of functional Duox-DuoxA heterodimers seems to be controlled by the associated DuoxA subunit, thus providing another level of regulation. Whether one or both Duox isoforms are required for lung epithelial repair is still unresolved and needs to be addressed in a more physiological context. Further work is required to assign discrete functions to specific Duox heterodimers in lung epithelium maintenance, and to probe how these processes might be altered in lung disease.
Materials and Methods
Cell culture and cell transfection
Primary cells, NHBE and SAEC (Lonza, Walkersville, MD), were maintained in small airway basal medium (SABM) plus small airway growth medium (SAGM) singlequots (Lonza). For re-differentiation and formation of a three-dimensional (3D) polarized epithelium, cells were grown at the air-liquid interphase (ALI) according to the method of Radyuk et al. (Radyuk et al., 2003). Briefly, cells were seeded on collagen-coated (type IV, 15 μg/cm2; Sigma, St Louis, MO) semi-permeable membranes (0.4 μm pore; Corning Costar Transwell Clear), in serum-free culture medium, consisting of 50% DMEM high glucose (Invitrogen, Carlsbad, CA) and 50% bronchial epithelial cell basal medium (BEBM; Lonza), supplemented with growth factors (BEGM singlequots; Lonza) and 50 nM retinoic acid (Sigma). After 2 days the apical medium was removed and cells cultured for up to 3 weeks. Lung cancer cells (NCI-H661) were a gift from F.J. Piedrafita (Sidney Kimmel Cancer Center, La Jolla, CA). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen) containing 10% FCS and 10 mmol/l Hepes (Invitrogen). DuoxA1-1, DuoxA1-2 and DuoxA2 cDNAs were cloned from SAEC, whereas DuoxA1-3 cDNA was obtained from Open Biosystems (Huntsville, AL, USA). Human Duox1, Duox2 (kindly provided by F. Miot), DuoxA1-1 (298aa, BC020841.1), DuoxA1-2 (343aa, DQ489735.1), DuoxA1-3 (483aa, BC029819.1) and DuoxA2 were cloned into pcDNA3. Epitope tags were introduced C terminal to DuoxA2 (Myc), DuoxA1-1 (EGFP), DuoxA1-2 (Myc, EGFP) and DuoxA1-3 (EGFP). For transient overexpression, H661 cells (1.5×105) were seeded in six-well plates and were transfected with a total of 600 ng DNA using Lipofectamine-Plus (Invitrogen) according to the manufacturer's instructions. Transfection efficiency was approximately 30-40%. Succinyl acetone (SA; Sigma) was dissolved in PBS and treatment was for 4 days in RPMI, containing Hepes and 1% NuSerum™ V (BD Biosciences, San Jose, CA) in the presence or absence of 10 μg/ml SA.
Lentivirus production and cell transduction
For lentiviral transduction, human Duox1, Duox2, DuoxA1-2 and DuoxA2 were cloned into CGW lentiviral expression vectors (Duox1 and Duox2 into bicistronic vector coexpressing mCherry) (Luxen et al., 2008). Virus particles were generated as described elsewhere (Swan et al., 2006). SAEC and NCI-H661 cells were incubated with viral supernatants for 3 days in the presence of polybrene (4 μg/ml; Sigma). Infected NCI-H661 cells were sorted for mCherry (stable cell line, 100% transduction), whereas primary SAEC remained unsorted (50-60% transduction efficiency).
Nucleic acid extraction and PCR
Total RNA was isolated with the RNeasy kit (Qiagen, Valencia, CA). 1 μg of RNA was reverse transcribed using a High Capacity cDNA RT kit (Applied Biosystems, Foster City, CA). PCR was done with GoTaq Flexi DNA Polymerase (Promega, Madison, WI) for 35 cycles, except actin, which had 18 cycles. Primer sequences were: actin (fwd: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′, rev: 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′); Duox1 (fwd: 5′-GCAGGACATCAACCCTGCACTCTC-3′, rev: 5′-CTGCCATCTACCACACGGATCTGC-3′); Duox2 (fwd: 5′-CCGGCAATCATCTATGAGGT-3′, rev: 5′-TTGGATGATGTCAGCCAGCC-3′), DuoxA1-1 (fwd: 5′-GAGCTTCAGTTCCCAAATTTGCTAC-3′, rev: 5′-GACAGCCTAGTACACTCTCCGC-3′), DuoxA1-2 (fwd: 5′-GTCAGCACCAACACATCATACAAGG-3′, rev: 5′-GGGTGTGCCTCTTTACAGTATGC-3′); DuoxA1-3 (fwd: 5′-GTCAGCACCAACACATCATACAAGG-3′, rev: 5′-CTGGACCAAAGCGCCAAGGAC-3′); DuoxA2 (fwd: 5′-CTTCCTGCTCATCTTGCCGG-3′, Rev: 5′-GAGAGCAGCACGTTGGAGAGG-3′). Real-time PCR for the detection of DUOX1 and DUOX2 transcripts was as described previously (Luxen et al., 2008).
Immunoblotting and immunoprecipitation
Total cell lysates (TCL; 30 μg) were electrophoresed and blotted as described previously (Martyn et al., 2006). Primary antibodies were anti-Myc (Cell Signaling, Danvers, MA), anti-GFP (Molecular Probes, Eugene, OR) and anti-α-tubulin (DM1a; Sigma). Antibodies to human Duox1, Duox2 and DuoxA1 were raised in rabbits to residues 988-1011 Duox1, 634-648 Duox2 and 280-299 DuoxA1-2 (Abgent Inc., San Diego, CA; Open Biosystems). DuoxA1 antibody recognizes DuoxA1-(1-3); Duox antibody (α-Duox or α-Duox1,2) detects Duox1 and Duox2 as shown previously (Luxen et al., 2008; Pacquelet et al., 2008). Immunoprecipitation was performed as described elsewhere (Grasberger et al., 2007), using anti-Myc (9E10, Babco, Richmond, CA), anti-GST (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-GFP (GeneTex, San Antonio, TX).
Immunofluorescence
Cells were grown on collagen-coated glass coverslips or differentiated on Transwell inserts, fixed with 4% PFA and permeabilized with 0.5% Triton X-100. Primary antibodies were Myc (9E10), β-tubulin IV (Sigma), MUC5AC (45M1, Labvision, Fremont, CA), calnexin (Chemicon, Billerica, MA); nuclei were stained with DAPI (Sigma). For some experiments the Duox1-specific antibody was labeled with Cy3 according to the manufacturer's protocol (GE Healthcare UK Ltd., Little Chalfont, Buckinghamshire, UK). Secondary antibodies conjugated to Alexa Fluor 568, 488 or 568 (Invitrogen) were used. Confocal laser-scanning microscopy images were taken on a Bio-Rad Rainbow Radiance 2100 microscope using Zeiss-Bio-Rad LaserSharp 2000 software (Nikon 60× oil, Plan-Apo-1.4 NA; Nikon 100× oil Plan-Apo-1.4 NA). Colocalization between two fluorescently labeled proteins was quantified using Image J (NIH Imaging, version 1.34) and Zeiss LSM ExaminerBio-Rad LaserSharp 2000 (version 6.0), by obtaining the threshold range of real over-background signal. The average real threshold range was used to calculate the M values (correlation coefficients) for a minimum of 20 cells in at least two independent experiments (Cornell et al., 2006). Metamorph 6.2 software (Molecular Devices Corporation, Sunnyvale, CA) was used to illustrate orthogonal planes and Imaris-Autoquant (Bitplane Inc., Saint Paul, MN) was used for three-dimensional reconstruction.
ROS production
H2O2 generation was determined in adherent cells by HVA assay in the presence or absence of 2 μM ionomycin or 1 μM thapsigargin (Invitrogen) as described previously (Martyn et al., 2006; Pacquelet et al., 2008).
Transwell migration assay
Transwell migration experiments were performed on semipermeable membranes in 24-well plate inserts (8 μm; Corning Costar Transwell Clear, Corning, NY), which were precoated with 0.10 μg/ml bovine fibronectin (Sigma). In brief, transduced NCI-H661 cells were suspended in 0.5% fetal calf serum (FCS)-containing medium. Cells (1×105) were seeded in triplicate on top of the inserts and were allowed to attach for 30 minutes. Directional migration was induced through addition of 10% FBS-containing medium to the bottom of the chamber and cells were allowed to migrate for 5.5 hours at 37°C. Remaining cells on top of the membrane were removed with a cotton swab and cells adherent to the bottom of the membrane were fixed with 4% PFA for 10 minutes, stained with 0.1% Crystal Violet for 30 minutes, and counted in at least eight random fields.
Wounding assay
Transduced NCI-H661 cells were grown to confluency on photoetched coverslips (Bellco Glas, Inc., Vineland, NJ), which were precoated with 10 μg/ml bovine fibronectin. Pictures of 12 different fields were taken using a phase-contrast microscope immediately after wounding and for up to 4.5 hours. Wound areas were measured using Metamorph 6.2 software. For localization analysis during directed migration, cells were seeded on collagen-coated coverslips before a linear wound was introduced. Cells were fixed 1 hour post-wounding and stained as indicated.
Statistical analysis
Data are presented as mean ± s.e.m. of triplicates from at least three independent experiments. Differences in H2O2 production (mg of protein per hour), transcript levels and migration rates were analyzed using a two-tailed Student's t-test. P<0.05 was considered as statistically significant. Significance levels were *P<0.05; **P<0.01; ***P<0.001.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/8/1238/DC1
We thank M. Lehmann and B. Kiosses for technical assistance and K. Schreiber for graphical support. This work was supported by NIH grants AI77042, AI24838 and CDC grant CI000095 (U.G.K.) and NIH grants HL91219 and AI49165 (B.E.T.). Deposited in PMC for release after 12 months.
References
- Bedard, K. and Krause, K. H. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245-313. [DOI] [PubMed] [Google Scholar]
- Caillou, B., Dupuy, C., Lacroix, L., Nocera, M., Talbot, M., Ohayon, R., Deme, D., Bidart, J. M., Schlumberger, M. and Virion, A. (2001). Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J. Clin. Endocrinol. Metab. 86, 3351-3358. [DOI] [PubMed] [Google Scholar]
- Cornell, C. T., Kiosses, W. B., Harkins, S. and Whitton, J. L. (2006). Inhibition of protein trafficking by coxsackievirus b3: multiple viral proteins target a single organelle. J. Virol. 80, 6637-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deken, X., Wang, D., Many, M. C., Costagliola, S., Libert, F., Vassart, G., Dumont, J. E. and Miot, F. (2000). Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 275, 23227-23233. [DOI] [PubMed] [Google Scholar]
- DeLeo, F. R., Burritt, J. B., Yu, L., Jesaitis, A. J., Dinauer, M. C. and Nauseef, W. M. (2000). Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 275, 13986-13993. [DOI] [PubMed] [Google Scholar]
- Fischer, H., Gonzales, L. K., Kolla, V., Schwarzer, C., Miot, F., Illek, B. and Ballard, P. L. (2007). Developmental regulation of DUOX1 expression and function in human fetal lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 292, L1506-L1514. [DOI] [PubMed] [Google Scholar]
- Forteza, R., Salathe, M., Miot, F., Forteza, R. and Conner, G. E. (2005). Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 32, 462-469. [DOI] [PubMed] [Google Scholar]
- Geiszt, M., Witta, J., Baffi, J., Lekstrom, K. and Leto, T. L. (2003). Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 17, 1502-1504. [DOI] [PubMed] [Google Scholar]
- Grasberger, H. and Refetoff, S. (2006). Identification of the maturation factor for dual oxidase: evolution of an eukaryotic operon equivalent. J. Biol. Chem. 281, 18269-18272. [DOI] [PubMed] [Google Scholar]
- Grasberger, H., De Deken, X., Miot, F., Pohlenz, J. and Refetoff, S. (2007). Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol. Endocrinol. 21, 1408-1421. [DOI] [PubMed] [Google Scholar]
- Harper, R. W., Xu, C., Eiserich, J. P., Chen, Y., Kao, C. Y., Thai, P., Setiadi, H. and Wu, R. (2005). Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett. 579, 4911-4917. [DOI] [PubMed] [Google Scholar]
- Koff, J. L., Shao, M. X., Kim, S., Ueki, I. F. and Nadel, J. A. (2006). Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. J. Immunol. 177, 8693-8700. [DOI] [PubMed] [Google Scholar]
- Lambeth, J. D., Kawahara, T. and Diebold, B. (2007). Regulation of Nox and Duox enzymatic activity and expression. Free Radic. Biol. Med. 43, 319-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxen, S., Belinsky, S. A. and Knaus, U. G. (2008). Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res. 68, 1037-1045. [DOI] [PubMed] [Google Scholar]
- Manzanares, D., Monzon, M. E., Savani, R. C. and Salathe, M. (2007). Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am. J. Respir. Cell Mol. Biol. 37, 160-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn, K. D., Frederick, L. M., von Loehneysen, K., Dinauer, M. C. and Knaus, U. G. (2006). Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 18, 69-82. [DOI] [PubMed] [Google Scholar]
- Moreno, J. C., Bikker, H., Kempers, M. J., van Trotsenburg, A. S., Baas, F., de Vijlder, J. J., Vulsma, T. and Ris-Stalpers, C. (2002). Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N. Engl. J. Med. 347, 95-102. [DOI] [PubMed] [Google Scholar]
- Nagai, K., Betsuyaku, T., Suzuki, M., Nasuhara, Y., Kaga, K., Kondo, S. and Nishimura, M. (2008). Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid. Redox Signal. 10, 705-714. [DOI] [PubMed] [Google Scholar]
- Pacquelet, S., Lehmann, M., Luxen, S., Regazzoni, K., Frausto, M., Noack, D. and Knaus, U. G. (2008). Inhibitory action of NoxA1 on dual oxidase activity in airway cells. J. Biol. Chem. 283, 24649-24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda, K., Chawla-Sarkar, M., Santos, C., Koeck, T., Erzurum, S. C., Parkinson, J. F. and Stuehr, D. J. (2005). Visualizing inducible nitric-oxide synthase in living cells with a heme-binding fluorescent inhibitor. Proc. Natl. Acad. Sci. USA 102, 10117-10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyuk, S. N., Mericko, P. A., Popova, T. G., Grene, E. and Alibek, K. (2003). In vitro-generated respiratory mucosa: a new tool to study inhalational anthrax. Biochem. Biophys. Res. Commun. 305, 624-632. [DOI] [PubMed] [Google Scholar]
- Schwarzer, C., Machen, T. E., Illek, B. and Fischer, H. (2004). NADPH oxidase-dependent acid production in airway epithelial cells. J. Biol. Chem. 279, 36454-36461. [DOI] [PubMed] [Google Scholar]
- Shao, M. X. and Nadel, J. A. (2005). Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. USA 102, 767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto, H., Miyano, K. and Takeya, R. (2005). Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 338, 677-686. [DOI] [PubMed] [Google Scholar]
- Swan, C. H., Buhler, B., Steinberger, P., Tschan, M. P., Barbas, C. F., 3rd and Torbett, B. E. (2006). T-cell protection and enrichment through lentiviral CCR5 intrabody gene delivery. Gene Ther. 13, 1480-1492. [DOI] [PubMed] [Google Scholar]
- van der Vliet, A. (2008). NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic. Biol. Med. 44, 938-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigone, M. C., Fugazzola, L., Zamproni, I., Passoni, A., Di Candia, S., Chiumello, G., Persani, L. and Weber, G. (2005). Persistent mild hypothyroidism associated with novel sequence variants of the DUOX2 gene in two siblings. Hum. Mutat. 26, 395. [DOI] [PubMed] [Google Scholar]
- Wesley, U. V., Bove, P. F., Hristova, M., McCarthy, S. and van der Vliet, A. (2007). Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J. Biol. Chem. 282, 3213-3220. [DOI] [PubMed] [Google Scholar]
- Yu, L., Zhen, L. and Dinauer, M. C. (1997). Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J. Biol. Chem. 272, 27288-27294. [DOI] [PubMed] [Google Scholar]
- Yu, L., Quinn, M. T., Cross, A. R. and Dinauer, M. C. (1998). Gp91(phox) is the heme binding subunit of the superoxide-generating NADPH oxidase. Proc. Natl. Acad. Sci. USA 95, 7993-7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamproni, I., Grasberger, H., Cortinovis, F., Vigone, M. C., Chiumello, G., Mora, S., Onigata, K., Fugazzola, L., Refetoff, S., Persani, L. et al. (2008). Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J. Clin. Endocrinol. Metab. 93, 605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.