Abstract
Objective
To measure the relative risks of Barrett’s esophagus (BE) associated with demographic factors, measures of adiposity and smoking among patients with gastroesophageal reflux disease (GERD).
Methods
Patients newly diagnosed with specialized intestinal metaplasia (SIM) (n=197) were compared to patients with GERD (n= 418) in a community clinic-based case-control study. Case sub-groups included those with any visible columnar epithelium (VBE) (n=97), and those with a long segment (=2cm) of columnar epithelium (LSBE) (n=54).
Results
Risks increased with older age (adjusted odds ratio (aOR) per decade for SIM=1.3, 95% confidence interval (CI)= 1.1–1.5; VBE aOR=1.4, CI=1.1–1.6; LSBE aOR=1.5, CI=1.2–1.9), male gender (SIM aOR=1.5, CI=1.1–2.2; VBE aOR=2.7, CI=1.6–4.5; LSBE aOR=3.9, CI=1.9–8.1) and possibly Asian race. Increased risk of BE in particular was observed with high waist-to-hip ratio (WHR, male high: =0.9, female high: =0.8) (SIM aOR=1.3, CI=0.9–2.1; VBE aOR=1.9, CI=1.0–3.5; LSBE aOR=4.1, CI=1.5–11.4). These associations were independent of body mass index (BMI) for the VBE and LSBE case groups but not for SIM which was the only case group in which BMI was a significant risk factor. Ever smoking cigarettes increased risk similarly for all case groups (SIM aOR=1.8, CI=1.2–2.6; VBE aOR=1.6, CI=1.0–2.6; LSBE aOR=2.6, CI=1.3–4.9), although dose response relationship was not detected for duration or intensity of smoking.
Conclusions
Older age, male gender and history of smoking increased risk of SIM and BE among GERD patients independent of other risk factors for BE. Central adiposity was most strongly related to risk of VBE and LSBE. These results may be useful in development of risk profiles for screening GERD patients.
Keywords: Barrett’s esophagus, body mass index (BMI), gastroesophageal reflux disease (GERD), epidemiology, obesity
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a major health problem in North America and Europe, with a high prevalence and considerable consequences for those affected. The prevalence of GERD, defined as at least weekly heartburn and/or acid regurgitation, is estimated to be 10–20% in the Western world (1). Treatments are expensive (2;3), costing the US approximately $9 billion annually (4), and those with frequent GERD symptoms experience a decrease in the heath-related quality of life (5;6). Furthermore, GERD is a strong risk factor for esophageal adenocarcinoma (EA) (7;8) and its precursor, Barrett’s esophagus (BE) (9–12). As the incidence of EA has increased in the US more rapidly than any other cancer over the last 30 years (13–16), it is particularly important to identify factors which may influence the conditions that lead to its development: GERD and BE.
Among persons with long-standing GERD symptoms, only about 10 – 15% actually develop BE in their lifetimes; among those who do, most do not progress to EA. Therefore, there must be other cofactors modulating the reflux-related chronic inflammatory effects on the esophageal epithelium. Case-control studies comparing BE cases to general population controls have identified abdominal obesity and cigarette smoking as possible risk factors for BE independent of GERD symptoms (17–19); however much less is known about factors that place GERD patients at higher risk for BE. Identification of such risk factors could help identify which GERD patients to endoscope, and could add to the prediction models being developed for this purpose (20). In this report, we explore the association between BE and demographic factors, various anthropometric measures, and smoking history in a community clinic-based case-control study among patients with chronic GERD symptoms.
PATIENTS AND METHODS
Study Participants
Cases were selected from among western Washington residents aged 20–80 years without previously-diagnosed BE who underwent an upper endoscopy for the investigation of chronic GERD symptoms (e.g. heartburn, acid regurgitation, atypical chest pain, and/or belching), at one of five community gastroenterology clinics between October 1, 1997 and September 30, 2000, as described previously (18). Briefly, potential participants were recruited in conjunction with their endoscopy visit. Cases were defined as those with specialized metaplastic epithelium (SIM) on at least one of four standard four-quadrant biopsies taken just distal to the squamocolumnar junction for the purposes of this study. These were evaluated by one of three University-based pathologists masked to endoscopy findings. Of the 1185 persons who consented to provide biopsies, SIM was found in 208 (17.6%). Of these patients, 193 (92.8%) were successfully interviewed. During the endoscopy, physicians recorded the presence and length of any visible columnar epithelium. Cases were subsequently classified into one to three of the following progressively exclusive groups: 1. SIM cases (i.e., all cases), 2. SIM and visible evidence of columnar epithelium (VBE), and 3. SIM and visible column epithelium greater than 2 cm (long segment BE, LBSE). The second two subgroups adhere to the case definition of BE as described by the American College of Gastroenterology (21), while the most inclusive group, SIM, is consistent with the concept of “ultra-short segment BE” (22–24). Eighteen cases (9.3%) were simultaneously diagnosed with adenocarcinoma (n=2) and/or dysplasia (n=0 high grade; n=16 low grade); these were included in all analyses.
A random sample of approximately 50% of the patients undergoing endoscopy for reflux symptoms but who were biopsy-proven negative for SIM, were chosen to be used as GERD controls. These were frequency-matched to the distribution of cases on the month of biopsy and clinic. Of the 463 patients selected to be GERD controls, 419 (90.8%) were successfully interviewed.
This study was approved by the institutional review board of the Fred Hutchinson Cancer Research Center, Seattle WA.
Data collection
Cases and GERD controls underwent structured interviews by trained staff in their homes or another requested location approximately 1–2 months after endoscopy. The interview, which took about 45 minutes to complete, covered demographic characteristics, smoking and alcohol use history, diet, and relevant health and medication history. Interviewers measured participants’ height and weight, as well as waist, hip and thigh circumference using an established protocol (25). All but two cases completed the portion of the interview with anthropometric measurements. Of the GERD controls, five participants had partial measurements done and three refused.
Antibody levels to Helicobacter pylori were available for the first 50 cases and 97 GERD controls using a total antibody latex agglutination assay kit (Orion Diagnostica, Espoo, Finland)(26).
Statistical Analyses
Multivariate unconditional logistic regression with robust standard errors was used to calculate adjusted odds ratios (OR) and 95% confidence intervals (CI) using Stata/SE (version 9.1) (27).
Demographic factors and measurements of adiposity and smoking were divided into potential risk categories, with the low risk category used as the referent group. Demographic factors examined included age assessed continuously and categorically (<50 years old/ 50+), gender, race (White/Black/Asian American/Other) and education (high school or less/technical school/college or more). Measures of adiposity examined included BMI (kg/m2), waist circumference (cm) (WC), WHR and weight to thigh ratio (WTR). The categories used for BMI (< 25 kg/m2/ 25–29.99/ 30 +), as well as the gender-specific categories used for WC (Male Low/Medium/High: <93 cm/94–101.99/102+; Female Low/Medium/High: <79cm/79–87.99/88+) and WTR (Male Low/Medium/High: 0.97–1.66/1.66–1.80/1.80–2.72; Female Low/Medium/High: 1.02–1.42/1.42–1.61/1.62–2.4) were those used and discussed in a recent report from this study (18). In that report, the WHR high-risk category was 0.9+ for males and 0.85+ for females. However, when examining the risk of BE by 0.05-increments for this investigation, substantial risk of BE was found in lower values of WHR for females, though not in males. Thus, in this investigation the high-risk categories for the binary categorization of WHR are 0.9+ for males and 0.8+ for females. We also present the relative risks by 0.05-increments of WHR.
History of cigarette use was analyzed in four different forms: ever/never binary, current/ever/never categorical, total pack-years in three categories as defined by the median of the entire study population (never/<13.5/ 13.5+), and years since cessation. Alcohol consumption was evaluated for beer, wine and liquor separately based on a lifetime-averaged frequency of drinks (weekly/<weekly-daily/daily+). Heartburn and acid regurgitation frequency were categorized similarly.
The potential confounding effects of age, gender, race and education level as well as design variables, clinic and time period of endoscopy (1998–1999/2000–2002), were assessed, and factors that made more than a 10% difference in the ORs for an appreciable number of associations were included in adjusted models, along with measures of obesity and cigarette smoking. Trend tests were based on using continuous measures in the logistic regression models for age, BMI, WC, WHR, WTR, pack-years and years since smoking cessation, with all but age considered in natural logarithm form in addition to untransformed continuous. Stratified ORs presented are based on logistic regression models that included interaction coefficients between the subgroup classification of interest and WHR.
RESULTS
Table 1 presents selected socio-demographic characteristics of cases and GERD controls. Of 197 cases with SIM, 97 were VBE cases and, of those, 54 were cases of LSBE. All participants reported at least one GERD symptom. The most common symptom was heartburn (92% of both cases and GERD controls), with 77% GERD controls and 76% of cases reporting a frequency of at least once per week.
Table 1.
Socio-demographic characteristics of Barrett’s esophagus cases and GERD controls
| Cases (n=193) | GERD Controls (n=418) | ||
|---|---|---|---|
| Characteristic | No. (%) | No. (%) | |
| Age | |||
| 20–39 | 27(14.0) | 97(23.2) | |
| 40–49 | 49(25.4) | 118(28.2) | |
| 50–59 | 57(29.5) | 105(25.1) | |
| 60–80 | 60 (31.1) | 98(23.4) | |
| Sex | |||
| Male | 118(61.1) | 214(51.2) | |
| Female | 75 (38.9) | 204(48.8) | |
| Race | |||
| White | 172 (89.1) | 374(89.5) | |
| Black or African | 3 (1.6) | 18(4.3) | |
| Asian Americans | 9 (4.7) | 6(1.4) | |
| American Indian/Eskimo | 3 (1.6) | 4(1.0) | |
| Other/Unknown | 6 (3.1) | 16(3.8) | |
| Hispanic | |||
| Yes | 9(4.7) | 12(2.9) | |
| No | 184 (95.3) | 405(96.9) | |
| Unknown | 0(0.0) | 3(0.5) | |
| Education Level | |||
| High School or less | 49(25.4) | 103(24.6) | |
| Technical School | 9(4.7) | 20(4.8) | |
| College or more | 135(70.0) | 293(70.1) | |
| Unknown | 0(0.0) | 2(0.5) | |
| Income Level | |||
| Under 45,000 | 57(29.5) | 146(35.0) | |
| 45,000–74,999 | 56(29.0) | 116(27.8) | |
| 75,000+ | 61(31.6) | 128(30.6) | |
| Unknown | 19(9.8) | 28(6.7) | |
Older age and male gender were associated with increased risk for each of the case groups (Table 2). Relative risks associated with older age were quite similar across case groups (age per decade adjusted odds ratio (aOR) for SIM=1.3, 95% confidence interval (CI)= 1.1–1.5; VBE aOR=1.4, CI=1.1–1.6; LSBE aOR=1.5, CI=1.2–1.9). On the other hand, the strength of the association with male gender increased from SIM to LSBE. Compared to White participants, Asians were at increased risk for all three case groups, whereas those of African or other ancestry were at reduced risk. Similar results for race and age were found for SIM-only cases, (i.e., the 96 participants with SIM who had no visible columnar epithelium); however, in this group there was no association seen with gender.
Table 2.
Odds ratios (OR) and trend tests for Barrett's esophagus associated with demographic characteristics and measures of adiposity by case group
| SIM |
VBE |
LSBE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. of GERD Controls | No. of Cases | OR (95% CI) | P7 | No. of Cases | OR (95% CI) | p | No. of Cases | OR (95% CI) | p |
| Age1 | <0.001 | 0.001 | 0.001 | |||||||
| <50 | 215 | 76 | 1 | 35 | 1 | 20 | 1 | |||
| =50 | 203 | 117 | 1.6(1.1–2.4) | 62 | 1.8(1.1–3.0) | 34 | 1.8(0.9–3.3) | |||
| Gender2 | 0.020 | <0.001 | <0.001 | |||||||
| Female | 204 | 75 | 1 | 26 | 1 | 11 | 1 | |||
| Male | 214 | 118 | 1.5(1.1–2.2) | 71 | 2.7(1.6–4.5) | 43 | 3.9(1.9–8.1) | |||
| Race2 | 0.069 | 0.116 | 0.612 | |||||||
| White | 374 | 172 | 1 | 91 | 1 | 53 | 1 | |||
| Black | 18 | 3 | 0.5(0.1–1.9) | 0 | - | 0 | - | |||
| Asian | 6 | 9 | 3.9(1.3–11.6) | 4 | 4.3(0.9–20.1) | 1 | 2.0(0.1–31.0) | |||
| Other | 10 | 5 | 0.8(0.2–3.3) | 1 | 0.3 (0.03–3.6) | 0 | - | |||
| BMI3 (kg/m2) | 0.011 | 0.294 | 0.057 | |||||||
| <25 | 114 | 37 | 1 | 25 | 1 | 11 | 1 | |||
| 25–29.99 | 153 | 75 | 1.3(0.8–2.2) | 35 | 0.9(0.5–1.6) | 21 | 1.2(0.5–2.6) | |||
| =30 | 146 | 79 | 1.7(1.1–2.7) | 37 | 1.1(0.6–2.1) | 22 | 1.5(0.7–3.4) | |||
| Waist Circumference3,4 | 0.067 | 0.307 | 0.071 | |||||||
| Low | 114 | 36 | 1 | 19 | 1 | 9 | 1 | |||
| Medium | 115 | 59 | 1.5(0.9–2.6) | 30 | 1.6(0.8–3.1) | 15 | 1.7(0.7–4.2) | |||
| High | 186 | 96 | 1.6(1.0–2.6) | 48 | 1.7(0.9–3.1) | 30 | 2.3(1.1–5.2) | |||
| Waist-to-hip ratio3,5 | ||||||||||
| Low | 121 | 39 | 1 | 0.556 | 14 | 1 | 0.281 | 4 | 1 | 0.015 |
| High | 294 | 152 | 1.3(0.9–2.1) | 83 | 1.9(1.0–3.5) | 50 | 4.1(1.5–11.4) | |||
| Waist-to-thigh ratio3,6 | 0.075 | 0.070 | 0.117 | |||||||
| Low | 141 | 39 | 1 | 16 | 1 | 7 | 1 | |||
| Medium | 147 | 69 | 1.6(1.0–2.5) | 39 | 2.2(1.1–4.1) | 23 | 2.8(1.1–7.1) | |||
| High | 127 | 83 | 1.9(1.2–3.1) | 42 | 2.2(1.1–4.3) | 24 | 2.5(1.0–6.3) | |||
Abbreviations: SIM: Specialized intestinal metaplasia; VBE: Visible Barrett’s Esophagus; LSBE: Long-segmented Barrett’s esophagus; BMI: Body Mass Index
Adjusted for gender, WHR, cigarette use (Ever/Never) and clinic
Adjusted for age, WHR, cigarette use (Ever/Never) and clinic
Adjusted for age, gender, cigarette use (Ever/Never) and clinic
Male Low/Medium/High: <94 cm/94–101.99cm/=102; Female Low/Medium/High: <79cm/79–87.99cm/=88
Male Low/High: <0.9/=0.9; Female Low/High: <0.8/=0.8
By tertiles, Male Low/Medium/High: 0.97–1.66/1.66–1.80/1.80–2.72; Female Low/Medium/High: 1.02–1.42/1.42–1.61/1.62–2.4
P-values corresponding to a trend test with the characteristic modeled as a log continuous variable for BMI, WC, WHR and WTR, a trend test with age modeled as a continuous variable and an overall Wald test for gender and race
Among the measures of adiposity, WHR and WTR were the strongest predictors of risk for both the VBE and LSBE case groups (Table 2). The associations with BMI and WC were not as strong and generally lacked statistical significance. An exception was that among all SIM cases there was a significant positive trend for BMI. This could be wholly attributed to a moderately strong association with BMI in the SIM-only case group (BMI 25–29.99 aOR= 2.3, CI= 1.1–4.5; BM =30 aOR= 2.7, CI=1.4–5.4.)
When WHR and BMI were modeled simultaneously, the association between WHR and all SIM cases lost strength (aOR=1.1, CI = 0.7–1.8) and BMI estimates were relatively unaffected. In contrast, WHR’s association with VBE (aOR= 2.0, CI = 1.0–3.9) and LSBE (aOR= 4.0, CI = 1.4 – 11.6) remained strong and the estimates for BMI’s association with these case groups were reduced to near unity. Similar results were seen when WTR was used in place of WHR. None of the associations presented in the Table 2 were appreciably affected when adjusted for race, education, time period of endoscopy, frequency of heartburn, acid regurgitation, or alcohol consumption.
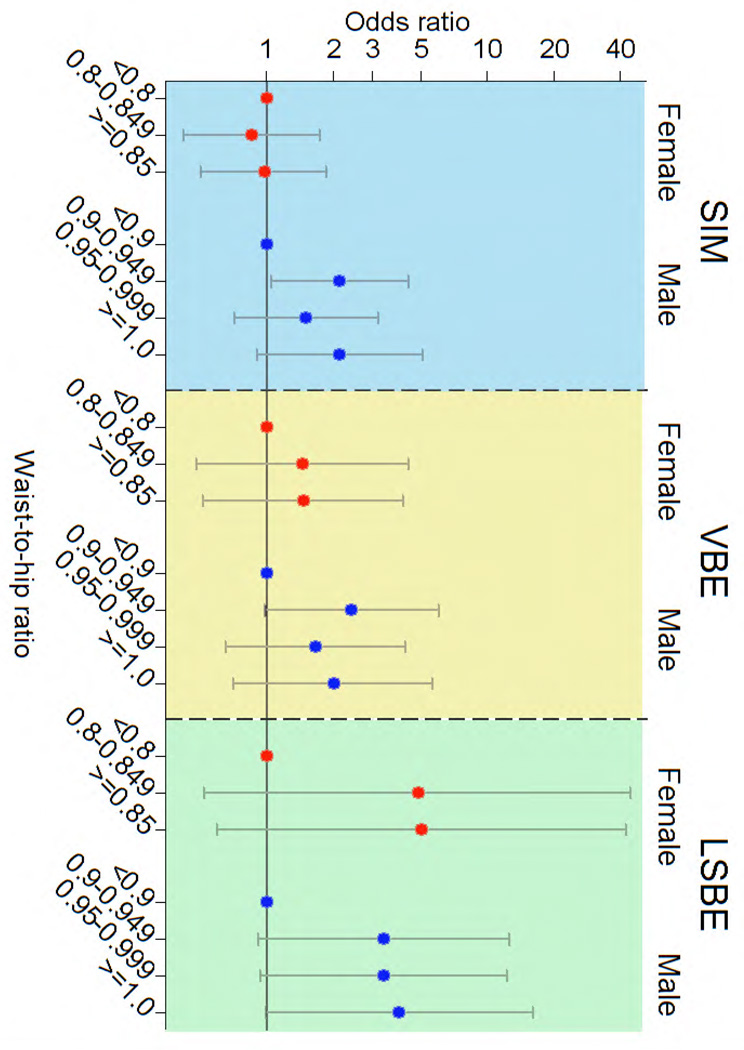
Figure 1 illustrates that for most gender-specific case groups, after the initial increase, there was not a substantial further increase in the ORs for each of the 0.05-increment WHR. When modeled continuously either as natural log continuous (Table 2), untransformed continuous or in 0.05-increments, the trend tests were not statistically significant. A similar threshold by gender was seen when WTR was modeled.
Figure 1.
Odds ratios1 for associated with waist-to-hip ratio by case group and gender.
1Adjusted for age, cigarette smoking history and clinic
The associations between WHR and risk of all three case types tended to be stronger among those with a BMI of 25 or greater and those with at least weekly GERD symptoms (Table 3). In contrast, there was no apparent pattern in variation of ORs in strata defined by age, gender, smoking history or race (race data not shown). None of the variations in OR described in Table 3 was statistically significant.
Table 3.
| Subgroup | GERD Controls |
SIM |
VBE |
LSBE |
|||
|---|---|---|---|---|---|---|---|
| No. High/Low | No. High/Low | OR (95% CI) | No. High/Low | OR (95% CI) | No. High/Low | OR (95% CI) | |
| Males | 157/56 | 102/15 | 1.9(1.0–3.7) | 63/8 | 2.2(1.0–4.9) | 40/3 | 3.6(1.1–12.2) |
| Females | 137/65 | 50/24 | 1.0(0.5–1.7) | 20/6 | 1.5(0.6–4.1) | 10/1 | 5.4(0.7–44.8) |
| Less than 50 years old | 131/83 | 55/20 | 1.6(0.9–2.9) | 27/8 | 1.9(0.8–4.5) | 16/4 | 2.3(0.7–7.0) |
| 50+ years old | 163/38 | 97/19 | 1.1(0.6–2.1) | 56/6 | 1.8(0.7–4.6) | 34/0 | - |
| Nonsmoker | 145/66 | 50/17 | 1.1(0.6–2.1) | 28/8 | 1.2(0.5–2.9) | 14/1 | 4.7(0.6–36.8) |
| Ever Smoked | 149/55 | 102/22 | 1.5(0.9–2.7) | 55/6 | 2.8(1.1–7.0) | 36/3 | 3.9(1.1–13.3) |
| BMI <25 | 65/49 | 19/18 | 1.0(0.5–2.2) | 15/10 | 1.4(0.6–3.6) | 9/2 | 4.0(0.8–19.7) |
| BMI 25+ | 243/56 | 133/21 | 1.3(0.7–2.3) | 68/4 | 3.1(1.1–9.2) | 41/2 | 4.1(0.9–18.8) |
| Heartburn <weekly | 65/31 | 35/11 | 1.1(0.5–2.4) | 18/6 | 0.8(0.3–2.4) | 10/2 | 1.0(0.2–5.5) |
| Heartburn weekly+ | 226/89 | 117/28 | 1.4(0.9–2.4) | 65/8 | 2.7(1.2–6.1) | 40/2 | 7.4(1.8–30.1) |
| Acid regurgitation <weekly | 132/97 | 77/34 | 1.1(0.7–2.0) | 51/15 | 1.4(0.7–2.8) | 30/5 | 3.4(1.0–12.0) |
| Acid regurgitation weekly+ | 100/83 | 54/25 | 1.7(0.9–3.4) | 24/7 | 5.1(1.2–22.3) | 16/3 | 6.4(1.0–42.4) |
Abbreviations: SIM: Specialized intestinal metaplasia; VBE: Visible Barrett’s Esophagus; LSBE: Long-segmented Barrett’s esophagus; BMI: Body Mass Index
Adjusted for clinic, as well as gender, age and cigarette smoking history
High WHR defined as =0.9 for males and =0.8 for females
A history of ever smoking cigarettes was significantly associated with each of the case groups when adjusted for age, gender, WHR and clinic (Table 4), with little evidence of a dose-response relationship when smoking was measured in pack-years (all p-values of trend tests = 0.05). Smoking cessation was not observed to decrease risk. These estimates were not appreciably different when further adjusted for race, education, time period of endoscopy or frequencies of heartburn, acid regurgitation or alcohol consumption. In contrast, in the SIM-only case group, there was weak evidence of a dose-response relationship: relative to nonsmokers, current smokers were at higher risk (aOR=2.3, CI= 1.2–4.5) than former smokers (adjusted OR = 1.9, CI = 1.1–3.2) and the trend test for pack-years was statistically significant (p<.0.01).
Table 4.
Odds ratios1 and trend tests for Barrett’s esophagus associated with measures of cigarette smoking
| SIM |
VBE |
LSBE |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | No. of GERD Controls | No. of Cases | OR (95% CI) | No. of Cases | OR (95% CI) | No. of Cases | OR (95% CI) | |
| Smoking Categories | ||||||||
| Never | 212 | 68 | 1 | 36 | 1 | 15 | 1 | |
| Ever | 206 | 125 | 1.8(1.2–2.6) | 61 | 1.6(1.0–2.6) | 39 | 2.6(1.3–4.9) | |
| Nonsmoker | 212 | 68 | 1 | 36 | 1 | 15 | 1 | |
| Former | 150 | 96 | 1.8(1.2–2.6) | 50 | 1.8(1.1–2.9) | 32 | 2.8(1.4–5.7) | |
| Current | 56 | 29 | 1.8(1.0–3.0) | 11 | 1.3(0.6–2.7) | 7 | 1.8(0.7–4.9) | |
| Pack-years | ||||||||
| Non-smoker | 212 | 68 | 1 | 36 | 1 | 15 | 1 | |
| <13.5 | 114 | 62 | 1.7(1.1–2.6) | 31 | 1.7(1.0–2.8) | 18 | 2.6(1.2–5.5) | |
| =13.5 | 92 | 63 | 1.9(1.2–2.9) | 30 | 1.6(0.9–2.9) | 21 | 2.5(1.2–5.5) | |
| Cessation2 | ||||||||
| Current smoker | 56 | 29 | 1 | 11 | 1 | 7 | 1 | |
| 1–10 years | 55 | 30 | 1.0(0.5–2.0) | 15 | 1.3(0.5–3.4) | 8 | 1.2(0.4–4.0) | |
| >10 years | 95 | 66 | 1.1(0.6–2.0) | 35 | 1.5(0.6–3.6) | 24 | 2.2(0.8–5.6) | |
Abbreviations: SIM: Specialized intestinal metaplasia; VBE: Visible Barrett’s Esophagus; LSBE: Long-segmented Barrett’s esophagus
Adjusted for age (<50/50+), gender, waist-to-hip ratio (low/high) and clinic
Among former or current smokers only
There was no association between any of the case groups and Hispanic origin, education or any type of alcohol consumption when adjusted for age, gender, cigarette smoking, WHR and clinic (data not shown). In an unadjusted analysis, drinking beer daily or more frequently was associated with all case groups; the strongest association was in the LSBE group (crude OR= 2.7, CI=1.3 – 5.7). However, adjustment substantially reduced the strength and statistically significance of these associations (e.g. LSBE adjusted OR= 1.2, CI=0.5–3.1), with gender and cigarette smoking history as the major confounding factors.
In the small number of cases (SIM n=50; VBE n=19, LSBE n=10) and GERD controls (n=97) in whom antibody levels had been measured, WHR and male gender were associated with risk among those who were H. pylori negative (WHR OR=2.1, CI=0.9–5.6; Male OR=1.6, CI=0.7–4.1), but not those who were H. pylori positive (WHR OR=1.1, CI=0.4–3.5; Male OR= 0.9, CI=0.3–2.6) in unadjusted analyses. In contrast, the association between SIM and cigarette smoking history (ever/never) or age (<50/50+ or by decade) were similar in the two groups.
DISCUSSION
In this community clinic-based case-control study we observed a number of factors that increased risk for SIM and BE among patients with chronic GERD symptoms. Older age, male gender and possibly Asian race were associated with increased risk independent of other proposed risk factors for BE. Measures of central adiposity, WHR and WTR, were also associated with case status, with relative risk increasing from SIM to VBE and from VBE to LSBE. Risk did not increase appreciably once a gender-specific threshold was reached. The association with WHR was independent of BMI for VBE and LSBE cases, whereas in the SIM case group the relationship lost strength when adjusted for BMI. The relationship between BE and WHR was stronger in patients with more frequent GERD symptom and those with BMI’s greater than 25 kg/m2. We observed a moderately increased risk of BE with a history of cigarette smoking, but little evidence of a dose-response relationship with intensity or duration of smoking, and no evidence of decreased risk with cessation.
BE is traditionally thought of as a disease of middle-aged and older, white men (28;29) and most of our results support this demographic profile. We are unaware of other studies that performed a multivariate analysis to investigate these demographic factors among patients with chronic GERD symptoms, though the increasing risk of BE associated with increasing age we observed is consistent with three recent studies that compared BE cases to patients undergoing endoscopy for any indication (30–32). Furthermore, two of these studies appear to support our finding that the relationship with age may not change by case group, with one study in Sweden reporting statistically significant adjusted ORs for age of 1.05 per year (approximately 1.6 per decade) for patients in case groups with similar definitions to our SIM and VBE groups (31) and another in the United Kingdom (30) reporting an statistically significant adjusted OR of 1.03 (approximately 1.3 per decade) in an LSBE case group.
Male gender was identified as a strong risk factor in the United Kingdom study (30), as well as in a multi-center investigation in the US (32), which also used LSBE as its case definition. In contrast, no significant relationship between gender and risk of SIM and VBE was observed in the Swedish study (31). Male gender as a risk factor for BE is also supported by the predominance of males among BE cases reported in numerous studies, regardless of whether BE is defined by SIM or columnar epithelium-line esophagus (33). The increase in strength of the association with male sex from SIM to VBE and from VBE to LSBE in our study was similar to that observed in data presented by Hirota et al (34), in which there was an increase in the male/female sex ratio from short-segmented BE cases to LSBE. In addition, reports have noted increased presence and severity of erosive esophagitis among males, suggesting that males may experience increased severity of reflux or susceptibility to its immediate complications (35; 36).
Although our study population was mainly White, consistent with BE studies among patients undergoing endoscopy (32; 37), we detected lower risk for Blacks for SIM and no Black case participants with VBE. That participants of Asian origin appeared to be at higher risk for BE than Whites was unexpected. Despite the small number of Asian participants in our study, this finding was statistically significant in the overall (SIM) group and increased risk was found in all three case groups. This contrasts with two other studies done in US populations in which Asian Americans were at lower risk of BE (32; 38). It is possible that the findings in the SIM group are due to its heterogeneity, as it likely includes a substantial proportion of cardia metaplasias, for which Asians are likely at higher risk due to for H. pylori pangastritis. We note that the findings in the VBE and LSBE groups are particularly limited by small sample size, and suggest that further investigation is needed into risk of SIM and BE associated with Asian race.
Central adiposity was a risk factor for SIM and BE among our study population with chronic GERD symptoms. The strength of the associations between either WHR or WTR and the BE case groups were not as strong as we found in our study comparing these BE cases to population controls (18), which is consistent with Corley et al’s (17) results comparing VBE cases to GERD and population controls using WC and WTR as measures of central adiposity. This suggests that central adiposity plays a role in at least two steps of EA disease progression: from healthy to chronic GERD, and from GERD to BE. Comparing VBE cases to GERD controls, Corley et al., also found evidence of a trend of increasing risk with increasing WC, with no evidence of a threshold effect. Interestingly, this threshold effect was observed for both WC and WTR in the analysis with population controls as the comparison group.
In terms of BMI, evidence from this investigation, and others including a recent meta-analysis by Cook et al (17;31;33;39), strongly suggests that BMI is not a risk factor for BE among those undergoing endoscopy. However, the trend seen for BMI in the SIM-only case group, independent of WHR, suggests that BMI plays a role in early in the development of BE. Alternatively, as discussed above, this association may result from the heterogeneity of the SIM group, as distinguishing metaplasia of the gastric cardia from metaplasia of the esophagus and gastroesophageal junction using clinical or histopathological criteria can be problematic. Thus, if measures of central adiposity are causally related to development of BE among those with chronic GERD, but not to gastric cardia metaplasia, then one would expect the strongest associations to be found in the most specific case group: LSBE, as is evident in these data.
Another approach to addressing the heterogeneity of the overall case group was our exploratory analysis focused on the H. pylori negative SIM cases, under the assumption that H. pylori positive cases were more likely to represent H. pylori-induced gastric cardia metaplasia. The results were consistent with this line of reasoning: among H. pylori negative cases, the association between risk of SIM and WHR was strong, whereas there was no association in the H. pylori positive group. It is also possible that the effect of central adiposity on the risk of SIM is less among persons with H. pylori gastritis due to less acidic refluxate (40). This difference by H. pylori status was also seen to a lesser extent in the association between SIM and gender, but not the association with age or cigarette smoking. Regarding gender, these observations are consistent with the lack of gender association with gastric cardia metaplasia as reported by El-Serag, et al., (41). These observations should be interpreted cautiously, though, as they are based on a small number of participants with antibody measurements and were analyzed in the SIM group, which is the least specific case group.
The stronger association seen between WHR and BE in the subgroup of participants with higher BMI (Table 4) suggests that central adiposity and obesity may act together in increasing risk of BE development. Thus, those chronic GERD patients with both a high BMI and significant central adiposity may be a potential targets for screening and prevention efforts. Another subgroup that may be important is those with high WHR and frequent reflux symptoms. The potential interaction between the two among GERD patients is consistent with studies comparing either BE or EA cases to population controls (18;19).
One possible biologic explanation for the relationship between BE and central adiposity is that the adipocyte's extensive metabolic activity may play a role in promoting BE and EA. A number of bioactive substances (adipokines) are produced in excess of normal in visceral fat, including free fatty acids, leptin and inflammatory cytokines (42–44). One consequence of this metabolic activity, particularly the production of fatty acids, is the development of insulin resistance (45;46) and resulting hyperinsulinemia, which can have direct effects on tumor development by promoting proliferation and inhibiting apoptosis and increasing IGF bioavailability (47). Adiponectin is found in decreased concentrations among overweight persons, and it has been negatively correlated with a number of obesity related cancers (48), perhaps due to the absence of its anti-inflammatory effects (49;50). Pro-inflammatory cytokines and receptors, such as IL-6, TNF-alpha and leptin, are produced in significant amounts by adipocytes, particularly those in the visceral compartment (51–56). Leptin can also promote cancer via mitogenic and angiogenic mechanisms (57). Comparing BE cases to population controls, Kendall et al (58) observed an association between leptin and BE independent of BMI in males but not females. In a smaller pilot analysis, they found no relationship between BE and adiponectin for either gender. Given that there is evidence of a relationship between central adiposity and BE risk in women, it seems that more research is needed to investigate the apparently discrepant results.
Our results regarding smoking, including the lack of dose-response relationship with increasing cumulative exposure and strength of estimates, are consistent with recent case-control studies based on population controls (18;19). Although, cigarette smoking was found to be significantly associated with each of the case groups that included those with visible evidence of columnar epithelium, it did not show a pattern of increasing risk by case group, as was found by Johnasson et al., (31). This and the dose-response seen in the SIM-only case group suggests that it is a risk factor for metaplasia of the gastric cardia (59) in addition to BE.
Strengths to our study include its design, breadth and depth of risk factors measured, standardized case classification and clinic-matched controls that were quite similar to cases in reporting of chronic GERD symptoms. This study is one of the first to examine the relative risk of BE among individuals with chronic GERD and multiple measures of overweight and central adiposity, including BMI, WC, WHR and WTR. Furthermore, we were able to classify cases by their endoscopic appearance, as well as histologically through a standardized protocol, which included four quadrant biopsies interpreted by a small number of university-based pathologists.
There are potential limitations as well. It is possible that GERD patients receiving an endoscopy and agreeing to go undergo our initial screening process, may not be representative of all GERD patients. Although our participation rate was high (>90%) among cases and GERD controls that underwent the initial screening process, we were unable to determine the rate of refusal among all endoscopy patients at the participating clinics and whether those not included differed in some way to the cases studied. While it was not always feasible to blind the interviewers as to case status, they were not told of the specific hypotheses that would be examined, and it seems unlikely that their knowledge of case status biased the interview or physical measurements in important ways. Also, we had limited sample size for some of our analyses, particularly those regarding Asian race and results stratified by H. pylori status.
We conclude that central adiposity is a risk factor for BE among GERD patients, suggesting that it may used as a factor in screening for endoscopy, perhaps more effectively in patients that also have more frequent GERD symptoms and/or have higher BMIs. We also conclude that at least some of the effects of older age and male gender on risk of esophageal adenocarcinoma manifest themselves in the transition from GERD to BE, possibly via increased severity of reflux or increased susceptibility to its effects. A history of cigarette smoking also predicted risk of BE, although we found no evidence of increased risk with increasing cumulative exposure, and no evidence of decreased risk with smoking cessation. These observations underscore the need for additional research focused on developing risk profiles for screening GERD patients that include age, sex, smoking and central adiposity, as well as continued research in identification of the mechanisms underlying obesity's role in promoting BE and EA.
STUDY HIGHLIGHTS
-
What is current knowledge?
Barrett’s esophagus (BE) markedly increases risk of esophageal adenocarcinoma
Reflux is a key risk factor, but only a small percentage develop BE
Obesity and smoking act independently of reflux in increasing BE risk in the general population
Little is known about predictors of BE among those with reflux
-
What is new here?
Among persons with reflux:- Risk of BE increased by 30–50% per decade of age
- Most of the male excess of BE occurs in the step from reflux to BE
- Central adiposity substantially increases risk of BE
- There is a threshold effect of adiposity rather than a dose-response relationship
- Central adiposity and reflux act synergistically in causing BE
- History of smoking increases risk modestly
ACKNOWLEDGMENTS
We would like to thank the study staff for their hard work and dedication. We also sincerely appreciate the time devoted to this study by the many participating gastroenterologists and their staff, without whom this study could not have been conducted. Finally, we particularly wish to thank the study participants for their generous contributions of time and commitment to research.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Thomas L. Vaughan, M.D., M.P.H.
Specific author contributions: All authors participated meaningfully in this study.
Financial support: National Cancer Institute (R01 CA72866; K05 CA124911)
Potential competing interests: None.
REFERENCES
- 1.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005 May;54(5):710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heikkinen TJ, Haukipuro K, Koivukangas P, Sorasto A, Autio R, Sodervik H, Makela H, Hulkko A. Comparison of costs between laparoscopic and open Nissen fundoplication: a prospective randomized study with a 3-month followup. J Am.Coll.Surg. 1999 Apr;188(4):368–376. doi: 10.1016/s1072-7515(98)00328-7. [DOI] [PubMed] [Google Scholar]
- 3.Myrvold HE, Lundell L, Miettinen P, Pedersen SA, Liedman B, Hatlebakk J, Julkunen R, Levander K, Lamm M, Mattson C, et al. The cost of long term therapy for gastro-oesophageal reflux disease: a randomised trial comparing omeprazole and open antireflux surgery. Gut. 2001 Oct;49(4):488–494. doi: 10.1136/gut.49.4.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002 May;122(5):1500–1511. doi: 10.1053/gast.2002.32978. [DOI] [PubMed] [Google Scholar]
- 5.Eloubeidi MA, Provenzale D. Health-related quality of life and severity of symptoms in patients with Barrett's esophagus and gastroesophageal reflux disease patients without Barrett's esophagus. Am.J Gastroenterol. 2000 Aug;95(8):1881–1887. doi: 10.1111/j.1572-0241.2000.02235.x. [DOI] [PubMed] [Google Scholar]
- 6.Revicki DA, Wood M, Maton PN, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am.J Med. 1998 Mar;104(3):252–258. doi: 10.1016/s0002-9343(97)00354-9. [DOI] [PubMed] [Google Scholar]
- 7.Farrow DC, Vaughan TL, Sweeney C, Gammon MD, Chow WH, Risch HA, Stanford JL, Hansten PD, Mayne ST, Schoenberg JB, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000 Mar;11(3):231–238. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 8.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N.Engl.J Med. 1999 Mar 18;340(11):825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 9.Falk GW. Barrett's esophagus. Gastroenterology. 2002 May;122(6):1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski JA, Harrison RF, Perry I, Balkwill F, Tselepis C. Barrett's metaplasia. Lancet. 2000 Dec 16;356(9247):2079–2085. doi: 10.1016/S0140-6736(00)03411-5. [DOI] [PubMed] [Google Scholar]
- 11.Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett's esophagus. Am J Gastroenterol. 2002 Aug;97(8):1888–1895. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 12.Sampliner RE. Epidemiology, pathophysiology, and treatment of Barrett's esophagus: reducing mortality from esophageal adenocarcinoma. Med Clin.North Am. 2005 Mar;89(2):293–312. doi: 10.1016/j.mcna.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998 Nov 15;83(10):2049–2053. [PubMed] [Google Scholar]
- 14.Jeon J, Luebeck E, Moolgavkar S. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States) Cancer Causes and Control. 2006 Sep;17(7):971–981. doi: 10.1007/s10552-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 15.Holmes RS, Vaughan T. Epidemiology and pathogensis of esphogeal cancer. J Natl.Cancer Inst. Forthcoming [Google Scholar]
- 16.Pohl H, Welch HG. The Role of Overdiagnosis and Reclassification in the Marked Increase of Esophageal Adenocarcinoma Incidence. J Natl Cancer Inst. 2005 Jan 19;97(2):142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 17.Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, Leighton P, Quesenberry C, Rumore GJ, Buffler PA. Abdominal obesity and body mass index as risk factors for Barrett's esophagus. Gastroenterology. 2007 Jul;133(1):34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 18.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett's esophagus. Gastroenterology. 2007 Aug;133(2):403–411. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Smith KJ, O′Brien SM, Smithers BM, Gotley DC, Webb PM, Green AC, Whiteman DC. Interactions among smoking, obesity, and symptoms of acid reflux in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2005 Nov;14(11 Pt 1):2481–2486. doi: 10.1158/1055-9965.EPI-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerkhof M, Steyerberg EW, Kusters JG, Kuipers EJ, Siersema PD. Predicting presence of intestinal metaplasia and dysplasia in columnar-lined esophagus: a multivariate analysis. Endoscopy. 2007 Sep;39(9):772–778. doi: 10.1055/s-2007-966737. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Sampliner R. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett's esophagus. Am J Gastroenterol. 2008 Mar;103(3):788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 22.Mueller J, Werner M, Stolte M. Barrett's esophagus: histopathologic definitions and diagnostic criteria. World J Surg. 2004 Feb;28(2):148–154. doi: 10.1007/s00268-003-7050-4. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P, Sampliner RE. Short segment Barrett's esophagus and intestinal metaplasia of the cardia--it's not all symantics!!! Am J Gastroenterol. 1998 Nov;93(11):2303–2304. doi: 10.1111/j.1572-0241.1998.02303.x. [DOI] [PubMed] [Google Scholar]
- 24.Spechler SJ. Short and ultrashort Barrett's esophagus--what does it mean? Semin.Gastrointest.Dis. 1997 Apr;8(2):59–67. [PubMed] [Google Scholar]
- 25.Lohman T, Roche AF, Martorell M. Chicago, IL: Human Kinetics Books; 1988. Anthropometric standardization reference manual. [Google Scholar]
- 26.Midolo PD, Lambert JR, Russell EG, Lin SK. A practical single sample dry latex agglutination test for Helicobacter pylori antibody detection. J Clin.Pathol. 1995 Oct;48(10):969–971. doi: 10.1136/jcp.48.10.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stata/SE 9.0 for Windows StataCorp. College Station, TX: 2005. [Google Scholar]
- 28.Cameron AJ, Zinsmeister AR, Ballard DJ, Carney JA. Prevalence of columnar-lined (Barrett's) esophagus. Comparison of population-based clinical and autopsy findings. Gastroenterology. 1990 Oct;99(4):918–922. doi: 10.1016/0016-5085(90)90607-3. [DOI] [PubMed] [Google Scholar]
- 29.Falk GW. Barrett's esophagus. Gastroenterology. 2002 May;122(6):1569–1591. doi: 10.1053/gast.2002.33427. [DOI] [PubMed] [Google Scholar]
- 30.Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett's esophagus. Am.J Epidemiol. 2005 Sep 1;162(5):454–460. doi: 10.1093/aje/kwi218. [DOI] [PubMed] [Google Scholar]
- 31.Johansson J, Hakansson HO, Mellblom L, Kempas A, Johansson KE, Granath F, Nyren O. Risk factors for Barrett's oesophagus: a population-based approach. Scand.J Gastroenterol. 2007 Feb;42(2):148–156. doi: 10.1080/00365520600881037. [DOI] [PubMed] [Google Scholar]
- 32.Lieberman D, Fennerty MB, Morris CD, Holub J, Eisen G, Sonnenberg A. Endoscopic evaluation of patients with dyspepsia: results from the national endoscopic data repository. Gastroenterology. 2004 Oct;127(4):1067–1075. doi: 10.1053/j.gastro.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 33.Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am.J Epidemiol. 2005 Dec 1;162(11):1050–1061. doi: 10.1093/aje/kwi325. [DOI] [PubMed] [Google Scholar]
- 34.Hirota WK, Loughney TM, Lazas DJ, Maydonovitch CL, Rholl V, Wong RK. Specialized intestinal metaplasia, dysplasia, and cancer of the esophagus and esophagogastric junction: prevalence and clinical data. Gastroenterology. 1999 Feb;116(2):277–285. doi: 10.1016/s0016-5085(99)70123-x. [DOI] [PubMed] [Google Scholar]
- 35.el-Serag H, Sonnenberg A. Associations between different forms of gastro-oesophageal reflux disease. Gut. 1997 Nov;41(5):594–599. doi: 10.1136/gut.41.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007 Feb;41(2):131–137. doi: 10.1097/01.mcg.0000225631.07039.6d. [DOI] [PubMed] [Google Scholar]
- 37.Abrams JA, Fields S, Lightdale CJ, Neugut AI. Racial and ethnic disparities in the prevalence of Barrett's esophagus among patients who undergo upper endoscopy. Clin.Gastroenterol.Hepatol. 2008 Jan;6(1):30–34. doi: 10.1016/j.cgh.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lam KD, Phan JT, Garcia RT, Trinh H, Nguyen H, Nguyen K, Triadafilopoulos G, Vutien P, Nguyen L, Nguyen MH. Low Proportion of Barrett's Esophagus in Asian Americans. Am.J Gastroenterol. 2008 Jun 16; doi: 10.1111/j.1572-0241.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- 39.El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett's esophagus. Am J Gastroenterol. 2005 Oct;100(10):2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 40.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin.Invest. 2004 Feb;113(3):321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Serag H, Graham D, Rabeneck L, Avid A, Richardson P, Genta R. Prevalence and determinants of histological abnormalities of the gastric cardia in volunteers. Scand J Gastroenterol. 2007 Oct;42(10):1158–1166. doi: 10.1080/00365520701299915. [DOI] [PubMed] [Google Scholar]
- 42.Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines--energy regulation from the human perspective. J Nutr. 2006 Jul;136(7 Suppl):1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- 43.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am.Coll.Cardiol. 2006 Mar 21;47(6):1093–1100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 44.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N.Engl.J Med. 1996 Feb 1;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 45.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr.Rev. 2000 Dec;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 46.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin.Invest. 2003 Dec;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat.Rev.Cancer. 2004 Aug;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 48.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br.J Cancer. 2006 May 8;94(9):1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br.J Cancer. 2006 May 8;94(9):1221–1225. doi: 10.1038/sj.bjc.6603051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol.Chem. 2005 May 6;280(18):18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 51.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin.Endocrinol.Metab. 1998 Mar;83(3):847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 52.Higdon JV, Frei B. Obesity and oxidative stress: a direct link to CVD? Arterioscler.Thromb.Vasc.Biol. 2003 Mar 1;23(3):365–367. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- 53.Otero M, Lago R, Lago F, Casanueva FF, Dieguez C, Gomez-Reino JJ, Gualillo O. Leptin, from fat to inflammation: old questions and new insights. FEBS Lett. 2005 Jan 17;579(2):295–301. doi: 10.1016/j.febslet.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 54.Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines--energy regulation from the human perspective. J Nutr. 2006 Jul;136(7 Suppl):1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- 55.Tsukui S, Kanda T, Nara M, Nishino M, Kondo T, Kobayashi I. Moderate-intensity regular exercise decreases serum tumor necrosis factor-alpha and HbA1c levels in healthy women. Int.J Obes.Relat Metab Disord. 2000 Sep;24(9):1207–1211. doi: 10.1038/sj.ijo.0801373. [DOI] [PubMed] [Google Scholar]
- 56.Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, Zurakowski A. Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int.J Obes.Relat Metab Disord. 2000 Nov;24(11):1392–1395. doi: 10.1038/sj.ijo.0801398. [DOI] [PubMed] [Google Scholar]
- 57.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg.Res. 2004 Feb;116(2):337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Kendall BJ, Macdonald GA, Hayward NK, Prins JB, Brown I, Walker N, Pandeya N, Green AC, Webb PM, Whiteman DC. Leptin and the risk of Barrett's oesophagus. Gut. 2008 Jan 4; doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 59.Malfertheiner P, Peitz U. The interplay between Helicobacter pylori, gastro-oesophageal reflux disease, and intestinal metaplasia. Gut. 2005 Mar;54(Suppl 1):i13–i20. doi: 10.1136/gut.2004.041533. [DOI] [PMC free article] [PubMed] [Google Scholar]