Abstract
Background
To evaluate the sensitivity, specificity and predictive values of spirometry for the diagnosis of chronic obstructive pulmonary disease (COPD) and asthma in patients suspected of suffering from an obstructive airway disease (OAD) in primary care.
Methods
Cross sectional diagnostic study of 219 adult patients attending 10 general practices for the first time with complaints suspicious for OAD. All patients underwent spirometry and structured medical histories were documented. All patients received whole-body plethysmography (WBP) in a lung function laboratory. The reference standard was the Tiffeneau ratio (FEV1/VC) received by the spirometric maneuver during examination with WBP. In the event of inconclusive results, bronchial provocation was performed to determine bronchial hyper-responsiveness (BHR). Asthma was defined as a PC20 fall after inhaling methacholine concentration ≤ 16 mg/ml.
Results
90 (41.1%) patients suffered from asthma, 50 (22.8%) suffered from COPD, 79 (36.1%) had no OAD. The sensitivity for diagnosing airway obstruction in COPD was 92% (95%CI 80–97); specificity was 84% (95%CI 77–89). The positive predictive value (PPV) was 63% (95%CI 51–73); negative predictive value (NPV) was 97% (95%CI 93–99). The sensitivity for diagnosing airway obstruction in asthma was 29% (95%CI 21–39); specificity was 90% (95%CI 81–95). PPV was 77% (95%CI 60–88); NPV was 53% (95%CI 45–61).
Conclusion
COPD can be estimated with high diagnostic accuracy using spirometry. It is also possible to rule in asthma with spirometry. However, asthma can not be ruled out only using spirometry. This diagnostic uncertainty leads to an overestimation of asthma presence. Patients with inconclusive spirometric results should be referred for nitric oxide (NO) – measurement and/or bronchial provocation if possible to guarantee accurate diagnosis.
Background
Asthma is a common chronic disease with a high prevalence of approx. 5% in industrialized nations. It is characterized by a chronic inflammation process which induces bronchial hyper-responsiveness and in most cases, reversible airway obstruction [1]. Another common pulmonary disease is chronic obstructive pulmonary disease (COPD) which shows irreversible airway obstruction, and which is mostly caused by inhaling tobacco smoke [2]. The prevalence of COPD is estimated to be around 10% and expected to be the fourth most important cause of death in 2020 [3]. Due to this high morbidity, general practitioners play a key role in detecting the disease as they see patients during the earlier stages of disease. Spirometric investigation is seen as a gold standard for diagnosing airway obstruction. Therefore, office spirometry is increasingly seen as a quality standard in general practice [4,5].
The efficacy of spirometry in diagnosing COPD was demonstrated by a specialist team, which received referrals for performing spirometry and bronchodilator reversibility testing in patients suspected of having COPD [6]. The DIDASCO Study revealed the difficulty of diagnosing COPD with screening questionnaires only and concluded that spirometry is essential for early diagnosis [7]. These investigations focused on COPD only, which is marked by irreversible airway obstruction. The diagnostic value of spirometry for diagnosing asthma marked by reversible airway obstruction remains unclear. This is of importance, as asthma needs to be diagnosed by bronchial provocation testing when spirometry shows no airway obstruction [8]. One diagnostic study in primary care used spirometry and bronchial provocation testing for identifying patients with asthma and COPD [9]. However, this was only carried out in patients complaining of suffering from a cough; and spirometry was performed by a single specialist. Spirometry and bronchial provocation testing were also used in the DIMCA study [10]. Indeed this was a screening study performed in a specialist center to detect patients in early stadiums of asthma or COPD.
Due to the design of these asthma and COPD trials, there is no evidence of the diagnostic accuracy of spirometry itself. Therefore, the true degree of the associated diagnostic uncertainty for patients with complaints suspected of having an airway obstruction remains unclear. The need for closing this gap of knowledge has been pointed out several times [11,12]. The difficulty is that the pretest probability of a disease and its severity in primary care is lower when compared to a hospital setting, thus hampering the predictive values of diagnostic tests [13,14]. Therefore, test results evaluated in hospital settings can not easily be transferred into general practice [15]. The aim of this study was to investigate the sensitivity, specificity and predictive values of spirometry for diagnosing airway obstruction in asthma and COPD in general practice.
Methods
Design and sample
This cross-sectional study was performed between January 2006 and December 2007 with fourteen general practitioners (GPs) working in ten general practices. 219 patients visiting their GP for the first time with complaints suggestive of obstructive airway disease (OAD) were consecutively included in each practice. Patients visited their GPs with symptoms such as dyspnea, coughing or expectoration. Their medical history was taken with a structured questionnaire. The patients had not been diagnosed previously for OAD and they had not received any previous anti-obstructive medicine. Other exclusion criteria related to well known contra-indications for bronchodilator reversibility testing or bronchial provocation, namely untreated hyperthyreosis, unstable coronary artery disease, and cardiac arrhythmia. Pregnancy also led to exclusion. The study was approved by the Medical Ethics Committee of the University of Heidelberg. Patients gave written informed consent.
On the basis of the pilot study [16] we estimated the pre-test probability of asthma as being 45% and of COPD as 16%. We estimated the sensitivity for diagnosing asthma to be 30% and the specificity to be 90%. The sensitivity and specificity for finding COPD was each estimated to be 90%. Power calculations showed that we had to include at least 208 patients to determine the sensitivities and specificities with a 95% confidence interval of ± 10% [17]. The diagnostic values of spirometry in general practice were calculated separately for each asthma and COPD group to avoid confusion. The diagnostic value for diagnosing asthma under optimal conditions was investigated by pooling all patients and determining the sensitivity, specificity and predictive values of spirometric maneuvers of the lung function laboratory.
Index test: Spirometry in general practice
Ten general practices were equipped with the same electronic spirometer (Medikro SpiroStar USB®) and associated spirometry software. The spirometer was a hand-held instrument for lung function testing that has to be connected via USB device to a computer. Spirometric data, flow-volume and volume-time graphs are displayed in real-time on the personal computer as the patient performs the spirometry test. A calibration file saves the calibration data for internal quality assurance. Instrument performance is regularly monitored and performance deviations are identified by the software. The software also compares the measured values with reference tables. The best of three consecutive spirometry recordings was used in accordance with the guidelines of the European Respiratory Society [18]. The maximal inspiratory and expiratory flow volume curves were generated by forced deep inspiration and expiration with short intervening periods of tidal breathing; patients used a nose clip. The maneuver was performed in a sitting position. Patients with a FEV1 (forced expiratory volume in one second) < 80% of predicted received a bronchodilation test with an additional performance of spirometry 20 minutes after inhaling salbutamol. Obstructive airway disease was diagnosed if FEV1/VC ≤ 70% and/or FEV1 < 80% [4,5]. Obstruction was considered to be reversible on salbutamol (which indicates a diagnosis of asthma) if the bronchodilation response Δ FEV1 was ≥12% of the baseline and ≥200 ml [4] and norm values were reached. GPs were asked to make their diagnoses based on the test results.
All of the GPs were appropriately trained in the key aspects of the diagnosis and management of asthma and COPD, as well as in performing and interpreting spirometry during two educational meetings. The practice assistants completed an intensive 6-hour course and were trained in performing and interpreting spirometry. Two outreach visits were also performed with repeated individual education at a direct, practical level, until the optimal performance of the spirometry was secured.
Reference test: Bodyplethysmography and bronchial provocation
After diagnosis by their GP, all patients were referred to the lung function laboratory of the University Medical Hospital at once for investigation with whole-body plethysmography (WBP). If therapy was necessary due to asthma or COPD, it was initiated by the GP. However, patients were instructed not to use any bronchodilator or inhaled steroid twelve hours before visiting the lung function laboratory. Spirometry is normally the routine method for measuring the lung volume required to diagnose airflow obstruction – i.e. (forced) vital capacity ((F)VC) or FEV1. However, spirometry is not capable of providing information about intrathoracic residual volume or total airway resistance. A WBP is required to measure residual volume (RV), functional residual capacity (FRC), and total lung capacity (TLC). Therefore, the advantage of WBP over spirometry is that it is able to distinguish between restrictive and obstructive processes. Additionally, the resistance to airflow can be evaluated and the response of airway resistance, airway conductance and thoracic gas volume can be determined in response to bronchodilator reversibility testing and bronchial provocation. In particular circumstances, measurement of these lung volumes are strictly necessary for a correct physiological diagnosis [19,20]. However, as WBP is only common in highly developed health care systems and the added value on top of spirometry remains unclear, it is only recommended in a few guidelines [21-23].
Measurement technique of whole-body plethysmography and bronchial provocation
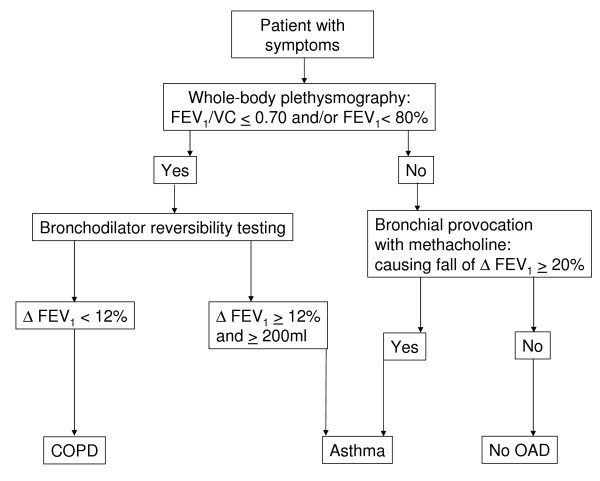
During WBP, the patient sits inside an airtight chamber and makes respiratory efforts against the closed shutter, causing chest volume to expand and decompressing the air in the lungs. The increase in chest volume reduces the box's volume, thus increasing the pressure in the box. The procedures were performed according to standard protocols [21]. Lung function reference values that had been adjusted for sex, age, and height were used [24]. Patients with FEV1 < 80% of predicted received a bronchodilation test with an additional performance of WBP 20 minutes after inhaling salbutamol. An obstructive airway disease was diagnosed if FEV1 < 80% and/or FEV1/VC ≤ 0.70. The obstruction was classified as reversible on a salbutamol (indicating asthma) when Δ FEV1 was ≥12% and ≥200 ml from the baseline value [4] and norm values were reached. In all other cases, the obstruction was classified as not reversible (Figure 1). If there was no bronchial obstruction, bronchial provocation was performed to determine bronchial hyper-responsiveness (BHR). Bronchial provocation is considered to be the best method for diagnosing asthma [25], although there is conflicting evidence [26], probably arising from variations in the population studied, as the diagnostic value increases with pre-test probability of the disease [27]. Trained lung function technicians measured bronchial hyper-responsiveness to methacholine according to the ATS guideline [8]. A diagnosis of 'asthma' was made if there was a 20% fall in FEV1 (PC20) from the baseline value after inhaling methacholine stepwise until the maximum concentration (16 mg/ml). The pneumologist was blinded against the diagnosis of the GP.
Figure 1.
Diagnostic decision making with the reference standard (whole-body plethysmography and bronchial provocation) in the lung function laboratory (COPD = Chronic obstructive pulmonary disease; OAD = obstructive airway disease).
Data analysis
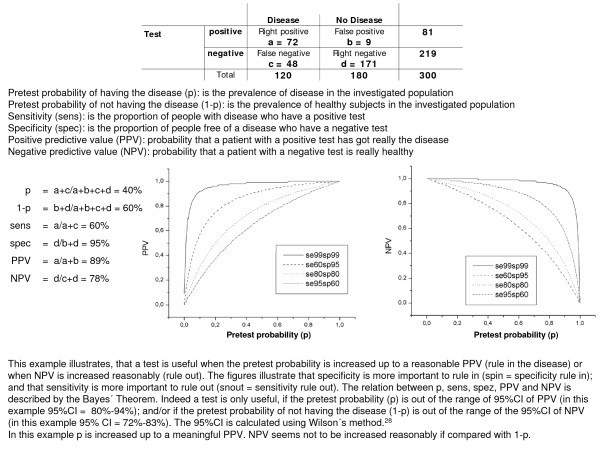
The baseline data were presented descriptively. The sensitivity, specificity, and predictive values of the spirometric investigation (FEV1 and/or FEV1/VC) in general practice were calculated with two-by-two contingency tables with the diagnosis of the pneumologist (WBP and bronchial provocation) as 'gold standard'. The data were analyzed with SPSS 15.0 for Windows. 95% confidence intervals were calculated using Wilson's method [28] with the statistical package CIA (Confidence Interval Analysis) [29]. An explanation of how to interpret PPV and NPV is provided in figure 2.
Figure 2.
Calculation example for the relation between pretest probability, sensitivitiy, specificity, positive predictive value (PPV) and negative predictive value (NPV).
Results
Study population
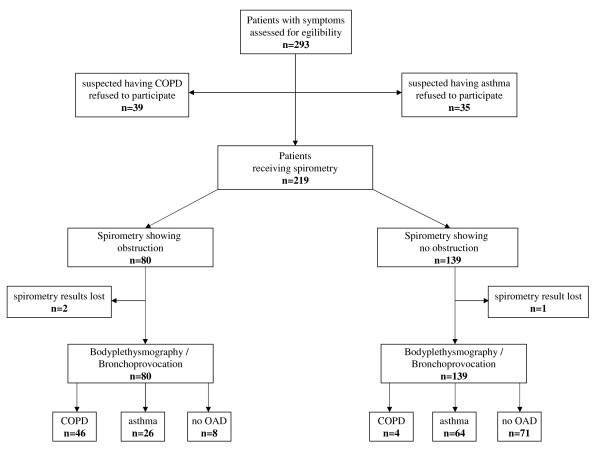
A total of 293 patients were assessed for eligibility (Figure 3). 74 patients received spirometry but did not want to receive whole body plethysmography and eventually bronchial provocation. Therefore, altogether 219 patients participated in the study (127 [57.7%] were female) (Table 1). The average age was 43.8 years. The average body mass index (BMI) was 25.3 (SD 4.4). Of the participating patients, 78 (35.6%) showed airway obstruction in general practice and 138 (63.0%) no abnormal findings in spirometry (Figure 3). Three spirometric results were lost to follow-up. According to the diagnostic decision making in the lung function laboratory, 90 (41.1%) patients had asthma, 50 (22.8%) of the participating patients had irreversible airway obstruction (COPD), and 79 (36.1%) showed no abnormal findings. A diagnosis of asthma was made in 76 of the cases with bronchial provocation, with only 14 patients identified solely on the basis of bronchodilator reversibility testing. The decision that the bronchial provocation was positive was made in 74 cases by 20% fall of FEV1 and in two cases by extreme increase of airway resistance accompanied by development of clinical symptoms of asthma during bronchial provocation. There were no significant differences in sex (p = 0.719) or obesity (BMI ≥ 30) (p = 0.272) between the diagnoses (chi-square test).
Figure 3.
Flow chart of inclusion and diagnostic work up (COPD = Chronic obstructive pulmonary disease; OAD = obstructive airway disease; OAD = obstructive airway disease).
Table 1.
Characteristics of the study population. Values are number (proportion) or mean (SD); OAD = obstructive airway disease; COPD = Chronic obstructive airway disease (n = 219)
|
Overall n (%) |
Asthma n (%) |
COPD n (%) |
No OAD n (%) |
|
| n | 219 (100) | 90 (100) | 50 (100) | 79 (100) |
| Female | 127 (57.7) | 55 (61.1) | 26 (54.1) | 46 (58.2) |
| Obesity | 30 (13.7) | 8 (8.9) | 10 (20.0) | 12 (15.2) |
| Age (mean in years [sd]) | 43.8 [15.6] | 37.9 [14.4] | 56.9 [11.5] | 42.1 [14.4] |
| Do you sometimes suffer from shortness of breath? (yes) | 135 (61.4) | 55 (61.1) | 39 (76.3) | 41 (51.9) |
| Have you suffered from wheezing in your chest? (yes) | 108 (49.1) | 47 (52.2) | 30 (63.2) | 30 (38.0) |
| Do you often suffer from a cough? (yes) | 126 (57.3) | 39 (43.3) | 32 (65.8) | 55 (69.6) |
| Do you often suffer from expectoration? (yes) | 74 (33.6) | 22 (24.4) | 20 (36.8) | 32 (40.5) |
| Have you been woken up with a feeling of tightness in your chest? (yes) | 49 (22.3) | 27 (30.0) | 9 (10.5) | 13 (16.5) |
| Have you been woken up by an attack of shortness of breath? (yes) | 48 (21.8) | 24 (26.7) | 10 (18.4) | 14 (17.7) |
| Have you suffered an asthma attack? (yes) | 14 (6.4) | 11 (12.2) | 2 (2.6) | 1 (1.3) |
| Do you suffer from any nasal allergies? (yes) | 92 (41.8) | 44 (48.9) | 14 (31.6) | 34 (43.0) |
| Do you often suffer from a common cold? (yes) | 73 (33.2) | 18 (20.0) | 18 (26.3) | 37 (46.8) |
| Do you smoke or did you smoke? (yes) | 118 (53.4) | 35 (38.9) | 43 (86.8) | 39 (49.4) |
| How much do/did you smoke? (mean in pack year [SD]) | 11.6 [17.7] | 6.6 [12.9] | 28.8 [21.7] | 6.4 [11.9] |
Performance of spirometry in general practice
Spirometry was performed with full adherence to ERS guidelines in 86 (39.8%) cases (Table 2). There was moderate adherence to ERS in 82 (38.0%) cases. In 48 (22.2%) cases the ERS criteria were not fulfilled. E.g., the flow-volume curves were deformed or not exactly reproduced. Altogether, 78 (36.1%) spirometric maneuvers showed airway obstruction. However, a bronchial reversibility test was only performed in 37 (47.4%) of these 78 cases.
Table 2.
Performance of spirometry in general practice (n = 216)
| Interpretation of flow-volume curve | n (%) | Bronchodilation test | n (%) |
| Full adherence to ERS | 86 (39.8) | Was not necessary | 138 (36.1) |
| Adherence to ERS but only two flow-volume curves | 69 (31.9) | Was necessary and performed | 37 (17.1) |
| No adherence to ERS but first flow-volume curve perfect and showing no pathological signs | 13 (6.0) | Was necessary but not performed | 41 (19.0) |
| No adherence to ERS showing no obstruction | 15 (6.9) | ||
| No adherence to ERS indicating airway obstruction | 33 (15.2) |
Estimates of diagnostic accuracy of spirometry in general practice
In relation to the COPD diagnosis, 26 patients were diagnosed false positive (Table 3). 12 of these spirometric maneuvers showed full/moderate adherence, and 14 were not according to guidelines. Four patients were diagnosed as false negative as the forced maneuvers in spirometry were performed weakly, thus resulting in a virtually normal Tiffeneau ratio. In these cases was FEV1 > 80% of predicted and FEV1/VC < 0.70 in the WBP as reference standard. Sensitivity was 92% and specificity 84%. Thus the pretest probability could be enhanced reasonably from 23% to a posttest probability (PPV) of 63%; and COPD could be ruled out with high certainty (NPV 97%).
Table 3.
2 × 2 table of spirometry for diagnosing airway obstruction in patients with COPD (n = 208; asthma patients with FEV1 <80% of predicted in general practice and in lung function laboratory excluded)
| COPD | No COPD | ||
| Spirometry + | 44 | 26 | |
| Spirometry - | 4 | 134 | |
| 208 | |||
| Pretest probability of having COPD 23% | |||
| Pretest probability of not having COPD 77% | |||
| Sensitivity | 92% (95%CI 80–97) | ||
| Specificity | 84% (95%CI 77–89) | ||
| PPV | 63% (95%CI 51–73) | ||
| NPV | 97% (95%CI 93–99) | ||
63 patients with asthma were diagnosed false negative as they showed no abnormal findings in spirometry (Table 4). It was only possible to identify them through bronchial provocation. Eight patients were diagnosed false positive; two of these spirometric maneuvers showed good adherence, and six were not according to guidelines. The pretest probability was enhanced from 41% up to 77%. However, asthma could not be ruled out, since NPV (53%) was similar to the pretest probability of 'not having asthma' (1-p = 59%); and 1-p was within the confidence interval of NPV (95%CI 45–61). The spirometric results as a part of the WBP investigation in the lung function laboratory are given in Table 5. Only 14 patients were identified by airway obstruction FEV1 < 80% of predicted and positive bronchial reversibility testing. In addition to this, under these optimal conditions with optimal differentiation between asthma and COPD, the sensitivity for diagnosing asthma solely on basis of spirometric maneuvers was only 16%. Again, NPV was similar to the pretest probability of 'not having asthma'.
Table 4.
2 × 2 table of spirometry for diagnosing airway obstruction in patients with asthma in general practice (n = 168; patients with COPD excluded)
| asthma | no asthma | ||
| Spirometry + | 26 | 8 | |
| Spirometry - | 63 | 71 | |
| 168 | |||
| Pretest probability of having asthma 41% | |||
| Pretest probability of not having asthma 59% | |||
| Sensitivity | 29% (95%CI 21–39) | ||
| Specificity | 90% (95%CI 81–95) | ||
| PPV | 77% (95%CI 60–88) | ||
| NPV | 53% (95%CI 45–61) | ||
Table 5.
2 × 2 table of spirometry for diagnosing airway obstruction in patients with asthma in lung function laboratory (all patients included with differentiation between asthma and COPD)
| asthma | no asthma | ||
| Spirometry + | 14 | 0 | |
| Spirometry - | 76 | 129 | |
| 219 | |||
| Pretest probability of having asthma 41% | |||
| Pretest probability of not having asthma 59% | |||
| Sensitivity | 16% (95%CI 10–24) | ||
| Specificity | 100% (95%CI 97–100) | ||
| PPV | 100% (95%CI 79–100) | ||
| NPV | 63% (95%CI 56–69) | ||
Diagnostic decision making by the GPs
The comparison of the diagnoses by the general practitioners with the diagnoses of the pneumologists demonstrated a reasonable agreement with respect to COPD (Table 6). Additionally, the GPs suspected asthma correctly in 76.7% of asthma cases despite the diagnostic uncertainty using spirometry. Indeed the prevalence of asthma was overestimated with 58.2% of healthy subjects suspected of having asthma; and 7.8% of patients with asthma were considered to be healthy.
Table 6.
Agreement between pneumologists' and general practitioners' diagnoses
| Pneumlogist\GP | Asthma | COPD | No OAD | Restrictive lung disease |
| n (%) | n (%) | n (%) | n (%) | |
| Asthma (n = 90) | 69 (76.7) | 14 (15.5) | 7 (7.8) | 0 (0) |
| COPD (n = 50) | 7 (16.2) | 41 (82.0) | 1 (2.7) | 1 (0) |
| No OAD (n = 79) | 46 (58.2) | 8 (10.1) | 25 (31.6) | 0 (0) |
Discussion
To our knowledge, this is the first study evaluating the diagnostic accuracy of spirometry for diagnosing airflow obstruction in patients with asthma or COPD in primary care. We found that the use of spirometry is feasible within general practice after training GPs and practice nurses. Under these conditions, the presence or absence of COPD can be estimated with a comparatively high diagnostic accuracy. It is also possible to rule in asthma. However, it was impossible to rule out asthma as the sensitivity was too low.
The prevalence of COPD is increasing in nearly all countries of the world and a high diagnostic accuracy is a prerequisite of optimal therapeutic management. The important role of spirometry for diagnosing airway obstruction has already been demonstrated [6,7,30,31]. However, the diagnostic accuracy of spirometry for diagnosing COPD has been unknown up to now, thus leading to diagnostic uncertainty in suspected cases of COPD. Our results demonstrate that the pretest-probability of 22% of patients presenting themselves with complaints suggestive of airway obstruction can be increased up to a post-test probability of 63% for having COPD. This comparatively low PPV might be surprising, as the sensitivity was 84% and specificity was 92%. However, this is explainable by the low pretest probability. Another reason might be due to sub-maximal maneuvers, leading to false positive results by underestimation of FEV1 [11]. As a consequence, more efforts in terms of continuous education would be necessary for an improvement of performance and an interpretation of spirometry. Nevertheless, COPD can be definitively excluded (NPV 97%) when spirometry is performed optimally. For these reasons, spirometry should be used regularly for diagnosing and managing COPD in primary care.
In contrast to these promising results is the limited value of spirometry in excluding asthma. This might be explained by the reversibility of airway obstruction in asthma. It proved possible to speculate that patients with mild or moderate asthma show no airway obstruction when spirometry is performed. In these cases, it was necessary for the GP to estimate the presence or absence of asthma on the basis of the patient history and inconclusive spirometry. This was misleading in 53 (24.2%) of cases (46 patients false positive and 7 patients false negative). Therefore, alternative methods need to be found for diagnosing asthma in primary care. Guidelines recommend using the measurement of peak-flow-variability to diagnose asthma in case of inconclusive spirometry. However, the low diagnostic value of peak-flow-variability in primary care has already been demonstrated [32]. The SAPALDIA study, which used an epidemiologic approach, has also shown a poor diagnostic value [33]. The measurement of exhaled nitric oxide (NO) which is elevated in eosinophilic airway inflammation [34] has been shown to be more promising [35], although the technology is expensive. Therefore, patients suspected of having asthma might be tested with NO measurement or should be referred for bronchial provocation if possible to guarantee accurate diagnosis. Nevertheless, spirometry should be used in diagnosing asthma, as the positive predictive value has been comparatively high in general practice.
One important limitation was that 22% of the spirometric maneuvers were not performed correctly in general practice. However, with the analysis of the spirometric maneuvers as part of the WBP investigation in the lung function laboratory, we received accurate diagnostic values of spirometry. Our results revealed that the predictive values of general practice were slightly lower than in the lung function laboratory. In addition to this, it was not possible to include all patients consecutively, as some patients were not willing to travel to the lung function laboratory of the Medical Hospital. This might have led to an overestimation of the diagnostic accuracy of spirometry [36]. However, that would also emphasize the impossibility of excluding asthma solely with spirometry. Another limitation is due to the choice of the cut-off points. Our use of the ratio FEV1/VC ≤ 0.70 as is still recommended by GOLD [5] may have led to some overestimation of airway obstruction in older patients [37] and underestimation in younger patients [38]. The ATS/ERS guideline therefore suggests using lower limits of normal, which is statistically defined by the 5th lower percentile of a reference population, to provide more accurate diagnoses [19]. This diagnostic algorithm was not integrated in the spirometric software at the time of our study. Moreover, we are aware of the limitations of a one-off lung function test to determine a final diagnosis, as a negative bronchodilator response can occur due to fixed airway obstruction in asthma. A trial of steroids might have been necessary to differentiate between asthma and COPD in some patients. Nevertheless, these limitations do not hamper our finding that asthma cannot be excluded solely with spirometry. The WBP showed little added value on top of spirometry. We used it as a reference standard to distinguish between overlapping diseases, COPD and restrictive lung disorder. However, we only experienced two changes in making the diagnosis with the added information of WBP. In two patients suffering from dyspnea attacks, the airway resistance was very high during bronchial provocation, but FEV1 remained normal. Moreover, we found no patient with restrictive lung disorder, which indicates a low prevalence in primary care settings. Therefore, the added value of WBP for primary care is limited and it should be reserved for patients who are difficult to diagnose and show persistent complaints.
It was not possible to specify the alternate diagnosis of the patients with no OAD, which is a typical problem of diagnostic studies in primary care. It was impossible to perform every investigation (e.g. gastroscopy to determine gastro-oesophageal reflux; x-ray) until a definite diagnosis could be made. This would not have been allowed by the Ethics Committee. However, this limitation does not alter the results of spirometric investigation. Finally, the participating GPs and practice assistants were highly motivated and received intensive training. Nevertheless, 22% of the spirometric maneuvers showed no guideline adherence. In particular bronchodilation testing was not performed regularly which might be due to organisational reasons and time constraints in general practice. The GPs estimated fourteen patients to suffer from COPD. However, the final pneumologists' diagnosis of these patients was asthma due to positive bronchodilator testing. Therefore, this lack of performance led the GPs to over-estimate COPD and under-estimate asthma in patients with airway obstruction. This is of importance as patients with asthma need to be treated preferably with inhaled steroids. However, our results are better than demonstrated by Miravitlles et al. [39], which might be due to the repeated education of the whole practice team. Nevertheless, these results are not satisfying enough. Further efforts are necessary to improve the performance of spirometry, as this could enhance the diagnostic accuracy. It has already been established that GPs are able to perform and interpret spirometry after educational meetings [40] and that performing spirometry has a positive impact on medical decision making [6,30,41]. It therefore seems reasonable and valuable to implement high quality spirometry in primary care.
Conclusion
COPD can be estimated with high diagnostic accuracy using spirometry. It is also possible to rule in asthma with spirometry. However, asthma can not be ruled out only using spirometry. This diagnostic uncertainty leads to an overestimation of asthma presence. Patients with inconclusive spirometric results should be referred for NO – measurement and/or bronchial provocation if possible to guarantee accurate diagnosis.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AS designed the study, performed the analyses and wrote the manuscript. LG trained the practice assistants, managed the data and helped to write the manuscript. LT helped to manage the data and to write the manuscript. TS and GJ helped to interpret the data and with writing. FJM made the final diagnoses as pneumologist and helped to write the manuscript. JS helped to write the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The trial was funded by the Federal Ministry of Education and Research (BMBF), Germany; grant no. 01GK0515. The funding source had no involvement in the design, collection, analysis or interpretation of the data.
Contributor Information
Antonius Schneider, Email: antonius.schneider@lrz.tum.de.
Lena Gindner, Email: lena.gindner@med.uni-heidelberg.de.
Lisa Tilemann, Email: lisa.tilemann@med.uni-heidelberg.de.
Tjard Schermer, Email: T.Schermer@hag.umcn.nl.
Geert-Jan Dinant, Email: GeertJan.Dinant@HAG.unimaas.nl.
Franz Joachim Meyer, Email: joachim.meyer@med.uni-heidelberg.de.
Joachim Szecsenyi, Email: joachim.szecsenyi@med.uni-heidelberg.de.
References
- Tattersfield AE, Knox AJ, Britton JR, Hall IP. Asthma. Lancet. 2002;360:1313–1322. doi: 10.1016/S0140-6736(02)11312-2. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362:1053–1061. doi: 10.1016/S0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- National Institute of Health Global Initiative for Asthma – Global Strategy for Asthma Management and Prevention (GINA) 2008. http://www.ginasthma.com
- National Institute of Health Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2008. http://www.goldcopd.com
- Walker PP, Mitchell P, Diamantea F, Warburton CJ, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006;28:945–952. doi: 10.1183/09031936.06.00019306. [DOI] [PubMed] [Google Scholar]
- Buffels J, Degryse J, Heyrman J, Decramer M. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO Study. Chest. 2004;125:1394–1399. doi: 10.1378/chest.125.4.1394. [DOI] [PubMed] [Google Scholar]
- Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, MacIntyre NR, McKay RT, Wanger JS, Anderson SD, Cockcroft DW, Fish JE, Sterk PJ. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- Thiadens HA, de Bock GH, Dekker FW, Huysman JA, Van Houwelingen JC, Springer MP, Postma DS. Identifying asthma and chronic obstructive pulmonary disease in patients with persistent cough presenting to general practitioners: descriptive study. BMJ. 1998;316:1286–1290. doi: 10.1136/bmj.316.7140.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom G van den, van Schayck CP, van Mollen MP, Tirimanna PR, den Otter JJ, van Grunsven PM, Buitendijk MJ, van Herwaarden CL, van Weel C. Active detection of chronic obstructive pulmonary disease and asthma in the general population. Results and economic consequences of the DIMCA program. Am J Respir Crit Care Med. 1998;158:1730–1738. doi: 10.1164/ajrccm.158.6.9709003. [DOI] [PubMed] [Google Scholar]
- Derom E, van Weel C, Liistro G, Buffels J, Schermer T, Lammers E, Wouters E, Decramer M. Primary care spirometry. Eur Respir J. 2008;31:197–203. doi: 10.1183/09031936.00066607. [DOI] [PubMed] [Google Scholar]
- Poels PJ, olde Hartman TC, Schermer TR, Albers M, van Weel C. Influence of spirometry on patient management in diagnostic studies unknown. Chest. 2006;129:1733–1734. doi: 10.1378/chest.129.6.1733. [DOI] [PubMed] [Google Scholar]
- Ledley RS, Lusted LB. Reasoning foundations of medical diagnosis; symbolic logic, probability, and value theory aid our understanding how physicians reason. Science. 1959;130:9–21. doi: 10.1126/science.130.3366.9. [DOI] [PubMed] [Google Scholar]
- O'Connor GT, Sox HC., Jr Bayesian reasoning in medicine: the contributions of Lee B. Lusted, MD. Med Decis Making. 1991;11:107–111. doi: 10.1177/0272989X9101100206. [DOI] [PubMed] [Google Scholar]
- Knottnerus JA, van Weel C, Muris JW. Evaluation of diagnostic procedures. BMJ. 2002;324:477–480. doi: 10.1136/bmj.324.7335.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Dinant GJ, Maag I, Gantner L, Meyer FJ, Szecsenyi J. The added value of C-reactive protein to clinical signs and symptoms in patients with obstructive airway disease: results of a diagnostic study in primary care. BMC Fam Pract. 2006;7:28. doi: 10.1186/1471-2296-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–770. doi: 10.1016/0895-4356(91)90128-V. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, Grinten CP van der, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, Grinten CP van der, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, Grinten CP van der, Gustafsson P, Hankinson J, Jensen R, Johnson D, MacIntyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- American Association for Respiratory Care AARC clinical practice guideline. Body plethysmography: 2001 revision and update. Resp Care. 2001;46:506–513. [Google Scholar]
- Siafakas NM, Vermeire P, Pride NB, Paoletti P, Gibson J, Howard P, Yernault JC, Decramer M, Higenbottam T, Postma DS. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J. 1995;8:1398–1420. doi: 10.1183/09031936.95.08081398. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Buhl R, Criee CP, Gillissen A, Kardos P, Kohler D, Magnussen H, Morr H, Nowak D, Pfeiffer-Kascha D, Petro W, Rabe K, Schultz K, Sitter H, Teschler H, Welte T, Wettengel R, Worth H. Guidelines for the diagnosis and therapy of COPD issued by Deutsche Atemwegsliga and Deutsche Gesellschaft fur Pneumologie und Beatmungsmedizin. Pneumologie. 2007;61:e1–40. doi: 10.1055/s-2007-959200. [DOI] [PubMed] [Google Scholar]
- Quanjer PH. EGKS: standardized lung function testing. Bull Eur Physiopathol Respir. 1983;5:1–92. [PubMed] [Google Scholar]
- Hunter CJ, Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID. A comparison of the validity of different diagnostic tests in adults with asthma. Chest. 2002;121:1051–1057. doi: 10.1378/chest.121.4.1051. [DOI] [PubMed] [Google Scholar]
- James A, Ryan G. Testing airway responsiveness using inhaled methacholine or histamine. Respirology. 1997;2:97–105. doi: 10.1111/j.1440-1843.1997.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Perpina M, Pellicer C, de Diego A, Compte L, Macian V. Diagnostic value of the bronchial provocation test with methacholine in asthma. A Bayesian analysis approach. Chest. 1993;104:149–154. doi: 10.1378/chest.104.1.149. [DOI] [PubMed] [Google Scholar]
- Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. doi: 10.2307/2276774. [DOI] [Google Scholar]
- Altman DG, Machin D, Bryant TN, Gardner MJ. Statistics with confidence. 2. London: BMJ Books; 2000. [Google Scholar]
- Dales RE, Vandemheen KL, Clinch J, Aaron SD. Spirometry in the primary care setting: influence on clinical diagnosis and management of airflow obstruction. Chest. 2005;128:2443–2447. doi: 10.1378/chest.128.4.2443. [DOI] [PubMed] [Google Scholar]
- Dales RE, Aaron SD, Vandemheen KL, Mehdizadeh A, Clinch J. The prevalence of airflow obstruction in rural primary care. Respir Med. 2006;100:754–759. doi: 10.1016/j.rmed.2005.06.015. [DOI] [PubMed] [Google Scholar]
- den Otter JJ, Reijnen GM, Bosch WJ van den, van Schayck CP, Molema J, van Weel C. Testing bronchial hyper-responsiveness: provocation or peak expiratory flow variability? Br J Gen Pract. 1997;47:487–492. [PMC free article] [PubMed] [Google Scholar]
- Kunzli N, Stutz EZ, Perruchoud AP, Brandli O, Tschopp JM, Bolognini G, Karrer W, Schindler C, Ackermann-Liebrich U, Leuenberger P. Peak flow variability in the SAPALDIA study and its validity in screening for asthma-related conditions. The SPALDIA Team. Am J Respir Crit Care Med. 1999;160:427–434. doi: 10.1164/ajrccm.160.2.9807008. [DOI] [PubMed] [Google Scholar]
- Kharitonov SA, Yates D, Robbins RA, Logan-Sinclair R, Shinebourne EA, Barnes PJ. Increased nitric oxide in exhaled air of asthmatic patients. Lancet. 1994;343:133–135. doi: 10.1016/S0140-6736(94)90931-8. [DOI] [PubMed] [Google Scholar]
- Schneider A, Tilemann L, Schermer T, Gindner L, Laux G, Szecsenyi J, Meyer FJ. Diagnosing asthma in general practice with portable exhaled nitric oxide measurement – results of a prospective diagnostic study. Respir Res. 2009;10:15. doi: 10.1186/1465-9921-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P, Rutjes AW, Reitsma JB, Glas AS, Bossuyt PM, Kleijnen J. Sources of variation and bias in studies of diagnostic accuracy: a systematic review. Ann Intern Med. 2004;140:189–202. doi: 10.7326/0003-4819-140-3-200402030-00010. [DOI] [PubMed] [Google Scholar]
- Schermer TR, Smeele IJ, Thoonen BP, Lucas AE, Grootens JG, van Boxem TJ, Heijdra YF, van Weel C. Current clinical guideline definitions of airflow obstruction and COPD overdiagnosis in primary care. Eur Respir J. 2008;32:945–952. doi: 10.1183/09031936.00170307. [DOI] [PubMed] [Google Scholar]
- Cerveri I, Corsico AG, Accordini S, Niniano R, Ansaldo E, Anto JM, Kunzli N, Janson C, Sunyer J, Jarvis D, Svanes C, Gislason T, Heinrich J, Schouten JP, Wjst M, Burney P, de Marco R. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008;63:1040–1045. doi: 10.1136/thx.2008.095554. [DOI] [PubMed] [Google Scholar]
- Miravitlles M, de la RC, Naberan K, Lamban M, Gobartt E, Martin A. Use of spirometry and patterns of prescribing in COPD in primary care. Respir Med. 2007;101:1753–1760. doi: 10.1016/j.rmed.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Schermer TR, Jacobs JE, Chavannes NH, Hartman J, Folgering HT, Bottema BJ, van Weel C. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease (COPD) Thorax. 2003;58:861–866. doi: 10.1136/thorax.58.10.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavannes N, Schermer T, Akkermans R, Jacobs JE, van de GG, Bollen R, van Schayck O, Bottema B. Impact of spirometry on GPs' diagnostic differentiation and decision-making. Respir Med. 2004;98:1124–1130. doi: 10.1016/j.rmed.2004.04.004. [DOI] [PubMed] [Google Scholar]