Abstract
Infectious diseases and the administration of bacterial lipopolysaccharide (LPS) result in decreased food intake and increased energy expenditure. Because the hypothalamic paraventricular nucleus (PVN) has pivotal roles in the regulation of energy homeostasis and expresses an anorexic peptide, cocaine- and amphetamine-regulated transcript (CART), we hypothesised that increased CART synthesis in this nucleus may contribute to LPS-induced changes in energy homeostasis. Therefore, we studied the effects of intraperitoneal administration of LPS on CART gene expression in the PVN by semiquantitative in situ hybridisation. LPS caused a rapid increase in CART mRNA levels in the PVN. One hour after treatment, the density of silver grains was increased by three-fold in the PVN, and remained elevated 3 h after treatment. Because the dorsal vagal complex, an important vegetative centre in the brainstem, is heavily innervated by CART-containing axons, we determined whether the retrograde tracer, cholera toxin B subunit (CTB), accumulates in CART neurons in the PVN following stereotaxic injection of the tracer into the dorsal vagal complex. One week after injection, CTB accumulated in CART neurons in the ventral, medial, and lateral parvocellular subdivisions of the PVN. In addition, LPS administration induced c-fos expression in a population of CART neurons in the PVN that project to the dorsal vagal complex. These data indicate that increased CART gene expression in neurons of PVN may contribute to LPS-induced anorexia, and suggest that this action may be mediated, at least in part, through a PVN-dorsal vagal complex pathway.
Keywords: cocaine- and amphetamine regulated transcript, bacterial lipopolysaccharide, hypothalamic paraventricular nucleus, hypothalamic arcuate nucleus, retrograde tracing, dorsal vagal complex
Cocaine- and amphetamine-regulated transcript (CART) was discovered by Douglass et al. (1) as a striatal transcript induced by psychostimulant administration. In addition to the striatum, CART is also expressed in several brainstem and hypothalamic nuclei (1, 2). The synthesis of CART peptides in specific neuronal groups that have been implicated in the central control of energy homeostasis has suggested an important role for CART in the regulation of feeding and energy metabolism. Along these lines, acute injection of CART into the ventricles induces c-fos in feeding-related regions, including the hypothalamic paraventricular nucleus (PVN), hypothalamic dorsomedial nucleus, parabrachial nucleus, nucleus tractus solitarius (3), decreases feeding and inhibits the orexigenic effects of neuropeptide Y (4, 5), whereas chronic administration induces significant weight loss (6, 7). In addition, CART has also been implicated in the central control of vegetative functions by increasing heart rate and blood pressure (8, 9).
Some of the effects of lipopolysaccharide (LPS) administration, an endotoxin that simulates bacterial infection, are similar to the effects of CART when administered centrally, including decreased food intake, increased energy expenditure and increased heart rate (10, 11). These observations raise the possibility that CART may contribute to some of the LPS-induced vegetative effects and, consistent with the findings by Sergeyev et al. (12), that CART gene expression increases in the hypothalamic arcuate nucleus 4 h after LPS treatment. CART-synthesising neurons also reside in another important hypothalamic vegetative centre, the PVN (1), where a marked increase in c-fos expression is observed following LPS administration (13). In the present study, we determined by in situ hybridisation whether CART neurons in the PVN are activated by systemic administration of LPS. Because the dorsal vagal complex, including the nucleus tractus solitarius (NTS) and the dorsal motor nucleus of vagus, are important vegetative integrator centres and densely innervated by CART-containing fibres (14), we also determined whether CART-synthesising neurons in the PVN that are activated by LPS administration innervate the dorsal vagal complex.
Materials and methods
Animals
The experiments were carried out on adult, male, Sprague–Dawley (Taconic Farms, Germantown, NY, USA) and Wistar rats (TOXI-COOP KKT, Budapest, Hungary) weighing between 250–300 g. The animals were housed in cages under a 12 : 12 h light/dark cycle (lights on 06.00 h) at 22 ± 1 °C, with rat chow and water available ad libitum. All experimental protocols were reviewed and approved by the Animal Welfare Committee at the Tufts Medical Center, Tufts University School of Medicine and the Institute of Experimental Medicine, Hungarian Academy of Sciences.
Effects of bacterial LPS on CART gene expression in the hypothalamic PVN
The Sprague–Dawley rats were divided into three groups (n = 12). One group was injected i.p. with saline (Control) and two groups with 250 µg/100 g body weight LPS in saline. The control group was perfused 2 h later, whereas the LPS-treated groups were perfused 1 and 3 h after treatment, as described below. Under sodium pentobarbital anaesthesia, the animals were perfused transcardially with 20 ml 0.01 m phosphate-buffered saline (PBS) (pH 7.4), containing 15 000 U/l heparin sulphate followed by 150 ml 4% paraformaldehyde in PBS. The brains were removed and post-fixed by immersion in the same fixative for 2 h at room temperature. Tissue blocks containing the hypothalamus were cryoprotected in 25% sucrose in PBS at 4 °C overnight, then frozen on dry ice. Serial 18 µm thick coronal sections through the rostrocaudal extent of the PVN were cut on a cryostat (Leica CM3050 S, Leica Microsystems, Nussloch GmbH, Germany) and adhered to Superfrost/Plus glass slides (Fisher Scientific Co., Pittsburgh, PA, USA) to obtain four sets of slides, each set containing every fourth section through the PVN. The tissue sections were desiccated overnight at 42 °C and stored at −80 °C until prepared for in situ hybridisation histochemistry.
In situ hybridisation histochemistry for CART
Every fourth section through the PVN was hybridised with a 866-bp, single-stranded [35S]UTP labelled cRNA probe for CART corresponding to the long splice variant of rat CART mRNA (gift from Dr Kuhar, Yerkes National Primate Research Center of Emory University, Atlanta, GA, USA) in accordance with methods previously described (15, 16). The hybridisation was performed under plastic coverslips in a buffer containing 50% formamide, a two-fold concentration of standard sodium citrate (2XSSC), 10% dextran sulphate, 0.5% sodium dodecyl sulphate, 250 µg/ml denatured salmon sperm DNA, and 5 × 105 c.p.m. of radiolabelled probe for 16 h at 54 °C. Slides were dipped into Kodak NTB autoradiography emulsion (Eastman Kodak, Rochester, NY, USA), and the autoradiograms were developed after 2 days of exposure at 4 °C.
Image analysis of autoradiograms
Autoradiograms were visualised under dark field illumination using a COHU 4910 video camera (COHU, Inc., San Diego, CA, USA). The images were captured with a colour PCI frame grabber board (Scion Corporation, Frederick, MD, USA) and analysed with a Macintosh G4 computer using Scion Image. Background density points were removed by thresholding the image and integrated density values (density × area) of hybridised neurons in the same region on each side of the parvocellular part of the PVN were measured in three consecutive sections and the sum of these values was calculated for each animal. Nonlinearity of radioactivity in the emulsion was evaluated by comparing density values with a calibration curve created from autoradiograms of known dilutions of the radiolabelled probes immobilised on glass slides in 2% gelatin fixed with 4% paraformaldehyde, and exposed and developed simultaneously with the in situ hybridisation autoradiograms.
Statistical analysis
Comparison of groups was performed with one-way ANOVA followed by multiple comparisons (Newman–Keuls post-hoc test). P < 0.05 was considered statistically significant. Results are presented as the mean ± SEM.
Innervation of the dorsal vagal complex by CART-containing neurons residing in the PVN
To determine whether CART neurons residing in the PVN innervate the dorsal vagal complex, the retrograde transport marker, cholera toxin subunit B (CTB) (List Biological Laboratories, Inc.; Campbell, CA, USA) was injected into nine Wistar rats by iontophoresis (Stoelting apparatus, Wood Dale, IL, USA; 6.0 MA, pulsed at 7s intervals) through a stereotaxically positioned glass micropipette (tip diameter 20 µm) into the dorsal vagal complex (anteroposterior, −5.0 mm; lateral, −0.5 mm; dorsoventral, +1.4 mm from the interauricular line), based on the coordinates of the atlas of Paxinos and Watson (17). Following 10–14 days transport times, the animals received 60 µg of colchicine by stereotaxic injection into the lateral ventricle and, 24 h later, they were deeply anaesthetised with sodium pentobarbital (50 mg/kg of body weight, i.p.) and perfused through the ascending aorta with 20 ml of 0.01 m PBS (pH 7.4), followed sequentially by 100 ml of 3% paraformaldehyde and 1% acrolein in 0.1 m phosphate buffer (pH 7.4), and 30 ml of 3% paraformaldehyde in the same buffer. Freezing microtome (Leica Microsystems AG, Wetzlar, Germany) sections (25 µm) through the PVN were collected in freezing solution (30% ethylene glycol; 25% glycerol; 0.05 m phosphate buffer) in four identical sets of sections from each of the brains and stored at −20 °C until used. The sections were treated with iso sodium borohydride followed by a mixture of 0.5% H2O2 /0.5% Triton–X 100 in PBS for 15 min at room temperature. To block nonspecific antibody binding, the sections were treated with 2.0% normal horse serum in PBS for 15 min, and then further processed as described below.
Detection of CTB injection sites
Every fourth section through the medulla was incubated in goat anti-CTB serum (List Biological Laboratories, Inc.) at 1 : 20 000 dilution for 1 day at room temperature. After rinses in PBS, the sections were immersed in biotinylated donkey-anti sheep IgG (1 : 500; Jackson Immunoresearch, West Grove, PA, USA) for 2 h and then in avidin-biotin complex (ABC, 1 : 1000; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. The immunoreaction product was developed in 0.05% diaminobenzidine/0.15% Ni-ammonium-sulphate/0.005% H2O2 in 0.05 m Tris buffer (pH 7.6). After mounting, the sections were counterstained using Nissl’s method. Brain sections from three animals with an injection site in the dorsal vagal complex were used for further studies.
The specificity of the detection of CTB was demonstrated by the absence of any immunoreactivity signal on sections of rats without CTB deposition.
Double-labelling immunofluorescence of CART and CTB in the PVN
Every fourth section through the PVN was incubated in a mixture of goat anti-CTB serum (List Biological Laboratories Inc.) at 1 : 10 000 dilution and mouse monoclonal antibody to CART at 3.34 µg/ml in PBS (a gift from Jes Thorn Clausen, Novo Nordisk, Bagsvaerd, Denmark) containing 2.0% normal horse serum/0.2% sodium azide for 2 days at 4 °C. After rinses in PBS, the sections were incubated in biotinylated donkey anti-sheep IgG for 2 h (1 : 500; Jackson ImmunoResearch) followed by ABC (1 : 1000; Vector Laboratories) in PBS for 2 h at room temperature. After intensification of the peroxidase signal using the TSA kit according to the manufacturer’s instruction (NEN Life Sciences Products, Boston, MA, USA) for 10 min, the sections were incubated in the mixture of avidin-fluorescein-isothiocyanate (FITC) DCS (1 : 300; Vector Laboratories) and Cy3-conjugated donkey anti-mouse IgG (1 : 200; Jackson Immunoresearch).
Image analysis of immunofluorescent preparations
Brightfield illumination images of the CTB injection sites were taken using a Zeiss Axiophot microscope equipped with a RT SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). The double-labelling fluorescent preparations were examined using a Radiance 2100 confocal microscope (Bio-Rad Laboratories, Hemel Hempstead, UK) using a 20 × lens and 40 × oil immersion lens with the following laser lines and filters: laser excitation lines 488 nm for FITC, 543 nm for Cy3; dichroic/emission filters, 560 nm/500–530 nm for FITC and 570 nm long pass filter for Cy3. Pinhole sizes were set to obtain 2-µm thick optical slices with a 20 × lens and 1-µm thick optical slices with a 40 × lens, and the series of optical slices were recorded with a 2 µm (20 ×) or 1 µm (40 ×) Z step.
Detection of CTB, c-fos and CART mRNA in the PVN of LPS-treated rats
CTB was administered into the dorsal vagal complex of Wistar rats as described above, followed by placement of an indwelling intraperitoneal cannula under the same anaesthesia. Animals (n = 24) were handled daily over the duration of the transport time. Ten days later, twelve animals were administered saline through the i.p. cannula (Control) and the remaining twelve animals treated with 250 µg/100 g body weight LPS in saline. Injections were given through the i.p. cannula to prevent the stress induced by the intraperitonial injection. Two hours after treatment, the animals were perfused with 20 ml diethylpyrocarbonate-treated PBS, followed by 150 ml 4% paraformaldehyde in PBS. The brains were removed and post-fixed by immersion in the same fixative for 2 h at room temperature. Tissue blocks containing the hypothalamus were cryoprotected in 20% sucrose in PBS at 4 °C overnight, then frozen on powered dry ice. Serial 20-µm thick coronal sections through the rostrocaudal extent of the PVN were cut with a freezing microtome and collected in anti-freeze solution and stored at −20 °C until used.
CTB injection sites were detected as described above. Hypothalamic sections from control rats and four LPS-treated rats where the injection site was centred in the dorsal vagal complex were used for combined in situ hybridisation histochemistry and immunocytochemistry.
Serial sections through the PVN of LPS treated rats were washed in 2 × SSC, acetylated with 0.25% acetic anhydride in 0.1 m triethanolamine for 10 min and then treated in 50%, 70% and 50% acetone, for 5, 10 and 5 min, respectively. After further washing in 2 × SSC for 2 × 5 min, the hybridisation was performed in polymerase chain reaction tubes in a buffer containing 50% formamide, 2 × SSC, 0.25 m Tris (pH 8.0), Denhardt’s solution, 10% dextran sulphate, 0.5% sodium dodecyl sulphate, 265 µg/ml denatured salmon sperm DNA, 40 mm dithiothreitol and 3 × 104 c.p.m/µl of the [35S]UTP labelled cRNA probe for CART. Hybridised sections were placed in humid chambers in ovens at 52 °C for 16 h. Following hybridisation, sections were washed in 1 × SSC for 15 min and then incubated for 1 h at 37 °C in RNase buffer (10 mm Tris, 0.5 m NaCl, 1 mm EDTA, pH 8.0) containing 50 µg/ml RNase A.
After additional washes in 1 × SSC (15 min), 0.5 × SSC (15 min) and 0.1 × SSC (2 × 30 min) at 65 °C, sections were washed in PBS, treated with the mixture of 0.5% Triton X-100 and 0.5% H2O2 for 15 min and then with 1% blocking reagent (Roche Applied Sciences, Basel, Switzerland) for 10 min. The sections were then incubated in a mixture of goat anti-CTB serum (1 : 10.000; List Biological Laboratories, Inc.) and rabbit anti-c-fos (1 : 10.000; Calbiochem, San Diego, CA, USA) diluted in 1% blocking reagent, overnight at 4 °C. Then, the sections were transferred to biotinylated donkey anti-sheep IgG (1 : 500, Jackson) and CY3-conjugated donkey anti-rabbit IgG (1 : 200; Jackson Immunoresearch). After rinses in PBS, the sections were immersed in the avidin-biotin peroxidase complex (ABC Elite, at 1 : 1000; Vector) in PBS for 2 h at room temperature. After intensification of the peroxidase signal using the TSA kit for 30 min, according to the manufacturers instructions (NEN Life Sciences Products), the sections were incubated in FITC-conjugated Streptavidin DCS (1 : 300; Jackson Immunoresearch).
Sections were mounted onto gelatin coated glass slides and dipped into Kodak NTB autoradiography emulsion (Eastman Kodak), placed in light-tight boxes containing desiccant and stored at 4 °C. After 7 days of exposure, the autoradiograms were developed using Kodak D19 developer, and slides were coverslipped with DPX mounting medium (Sigma-Aldrich Ltd, St Louis, MO, UK).
The fluorescent signals were studied under a Zeiss Axio Imager M1 epifluorescent microscope (Carl Zeiss Ltd, Göttingen, Germany) using the following filter sets: for Cy3, excitation of 538–562 nm, beamsplitter of 570 nm and emission of 570–640 nm; for FITC, excitation of 450–490 nm, beamsplitter of 495 nm and emission of 500–550 nm. Autoradiograms of the same field were studied under darkfield illumination. The images were captured with an AxioCam MRc 5 digital camera (Carl Zeiss Ltd). Adobe Photoshop 7.0 software (Adobe Systems Incorporated, San Jose, CA, USA) was used to create composite images for analysis.
To demonstrate the lack of neuronal activation in the PVN of control animals, sections from the PVN of the control group were immunostained for c-fos and imaged as described above.
Results
Effect of LPS on CART mRNA in the PVN
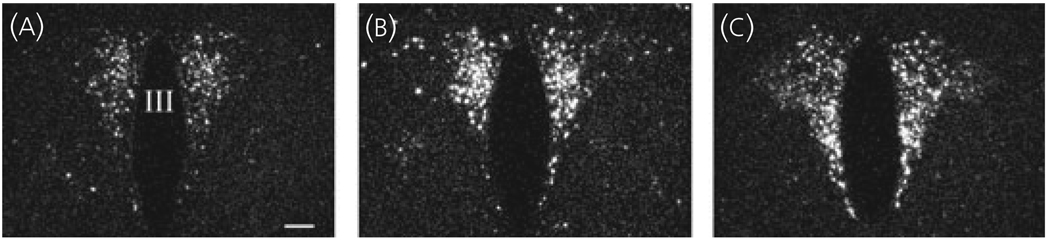
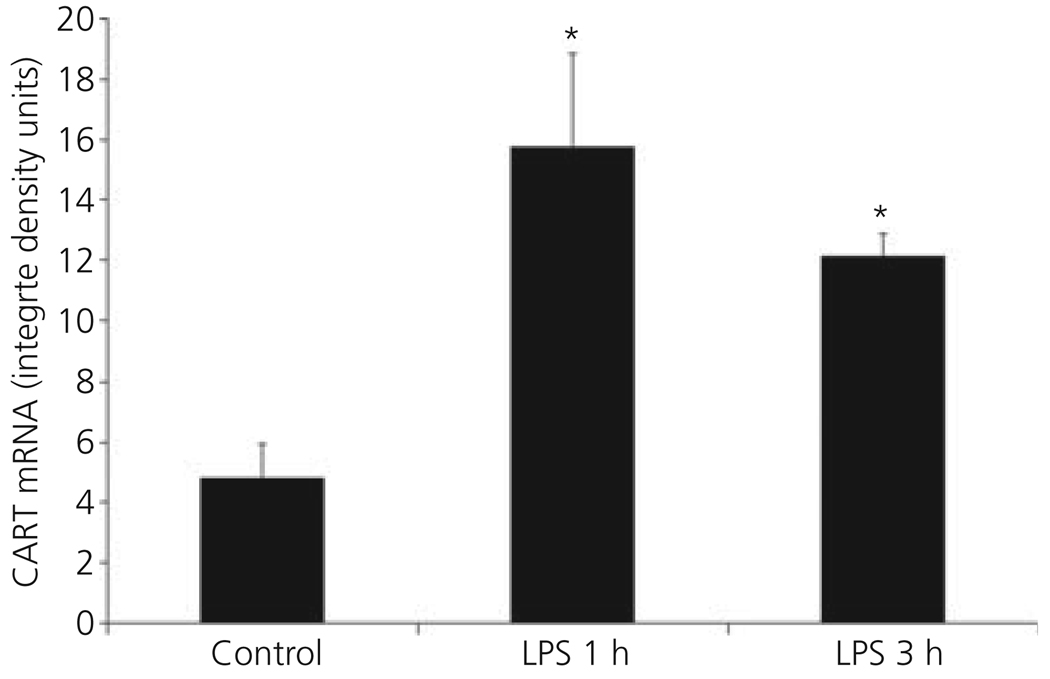
In control animals, a large number of CART mRNA-containing neurons were readily visualised in the parvocellular part of the PVN (Fig. 1a). Following LPS, a rapid rise in CART mRNA was apparent at 1 h (Fig. 1b) and was still increased after 3 h (Fig. 1c). By image analysis (Fig. 2), a significant three-fold increase in CART mRNA was observed 1 h after LPS treatment [Control versus LPS 1 h (integrated density units): 4.79 ± 1.16 versus 15.76 ± 3.09, P < 0.01], which was sustained after 3 h (12.13 ± 0.73; LPS 1 h versus LPS 3 h, P = 0.22).
Fig. 1.
Dark-field photomicrographs showing the presence of cocaine- and amphetamine-regulated transcript (CART) mRNA in the hypothalamic paraventricular nucleus (a–c) by in situ hybridisation histochemistry of control animals (a) and 1 (b) and 3 h (c) after lipopolysaccharide (LPS). Note increased CART mRNA 1 and 3 h after LPS treatment. III, Third ventricle. Scale bar = 200 µm.
Fig. 2.
Computerised image analysis of density values of cocaine- and amphetamine-regulated transcript (CART) mRNA in Control and lipopolysaccharide (LPS)-treated animals in the hypothalamic paraventricular nucleus. *Significantly different from control.
Innervation of the dorsal vagal complex by CART neurons residing in the PVN
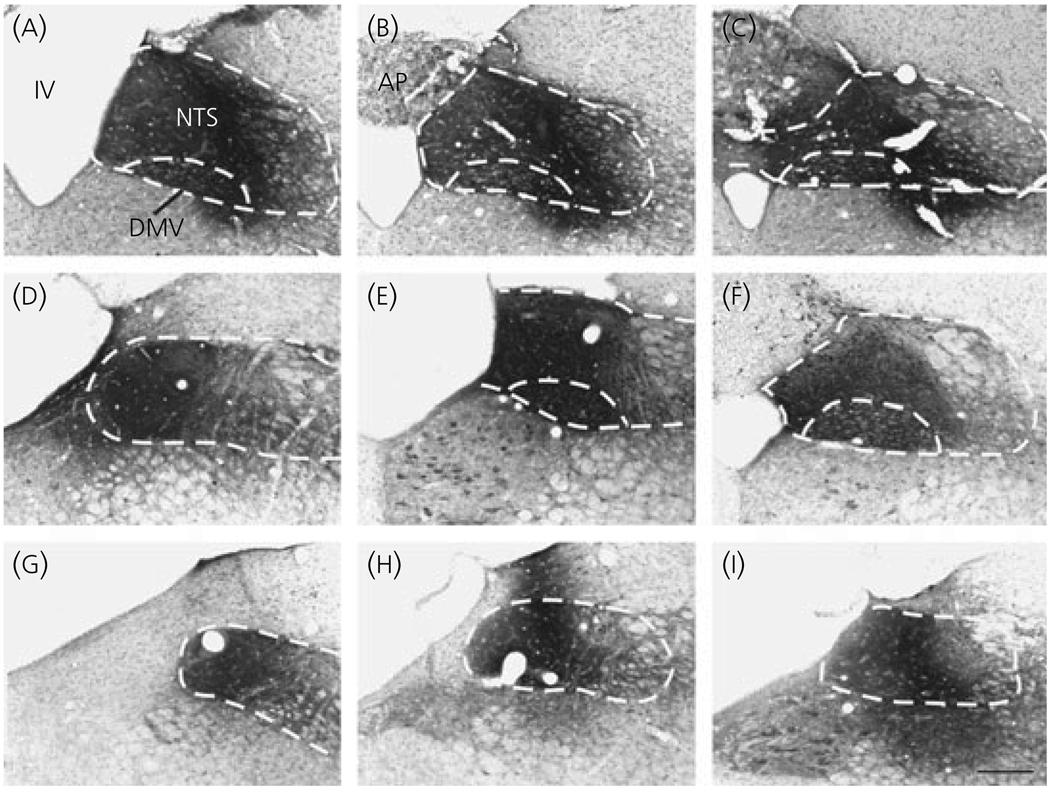
In three animals, the CTB injection site was centred in the nucleus tractus solitarius and confined within the boundaries of the dorsal vagal complex. The injection site filled the NTS and the dorsal motor nucleus of vagus in two animals (Fig. 3a–f) and filled the NTS without spreading into the dorsal motor nucleus of vagus in one animal (Fig. 3g–i). The brains of these three animals were used for further studies.
Fig. 3.
Photomicrographs of rostro-caudal sections of the dorsal vagal complex (illustrated by dotted lines), showing the location of the injection sites in three animals. The core of the cholera toxin B subunit injection sites covers the nucleus tractus solitarius (NTS) and the dorsal motor nucleus of vagus (dmv) in two animals (a–c and d–f), and confined to the boundaries of the NTS in a third animal (g–i). AP, Area postrema; IV, fourth ventricle; Scale bar = 200 µm.
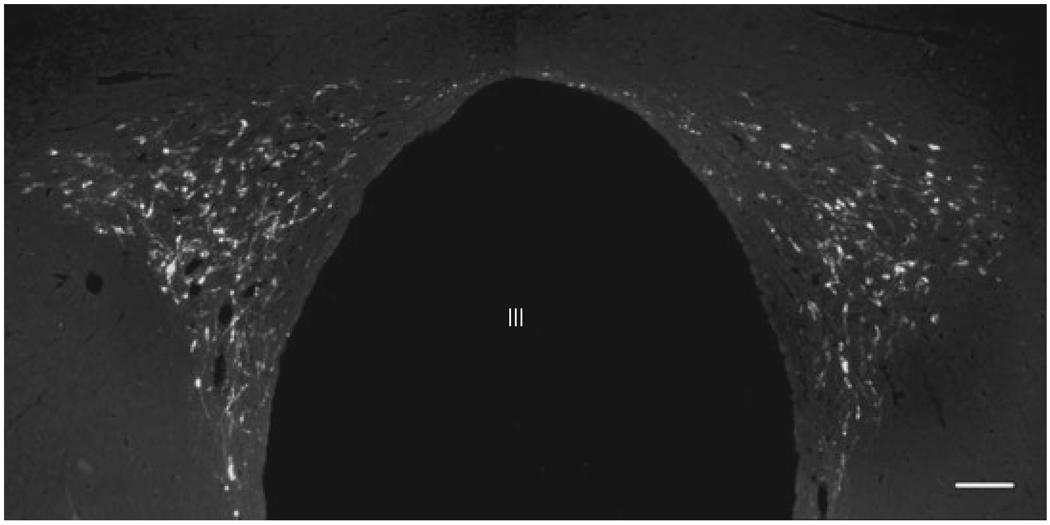
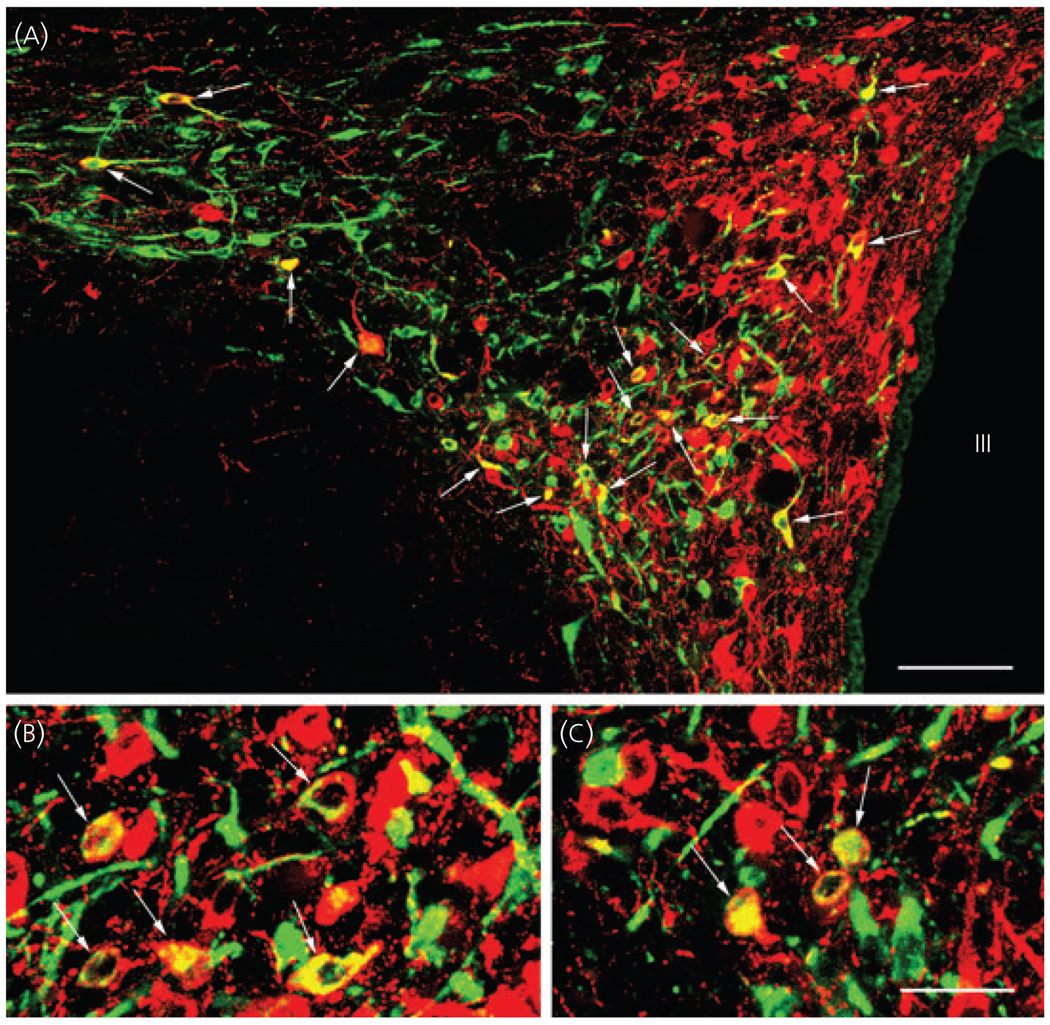
The distribution of retrogradely labelled neurons in the PVN was similar in all three brains. However, CTB-containing cells were more numerous in the two brains with injection sites extending into the dorsal motor nucleus of vagus. CTB-containing cells were observed in both the contra- and ipsilateral sides of the PVN in all parvocellular subdivisions at the mid level of the PVN, but the density of the retrogradely labelled neurons was the lowest in the periventricular parvocellular subdivision (Fig. 4). The majority of double-labelled CART/CTB neurons were found in the ventral part of the medial parvocellular subdivision and in the ventral and lateral parvocellular subdivisions. The lowest density of retrogradely labelled CART neurons was present in the periventricular parvocellular subdivision (Fig. 5a–c).
Fig. 4.
Distribution of the cholera toxin B subunit (CTB)- containing cells in the hypothalamic paraventricular nucleus retrogradely labelled after tracer injection into the dorsal vagal complex. III, Third ventricle; Scale bar = 100 µm.
Fig. 5.
Double-labelling immunofluorescence of cholera toxin B subunit-containing cells (green) retrogradely labelled after (CTB) injection into the dorsal vagal complex, and cocaine- and amphetamine-regulated transcript-immunofluorescent neurons (red) in the hypothalamic paraventricular nucleus (PVN). Due to the colour mixing, the double-labelled neurons (arrows) appear yellow. Note the large number of double-labelled neurons in the PVN. III, Third ventricle. Scale bars: (a) 90 µm; 30 µm in (c) corresponds to (b) and (c).
Effect of LPS treatment on CART neurons innervating the dorsal vagal complex
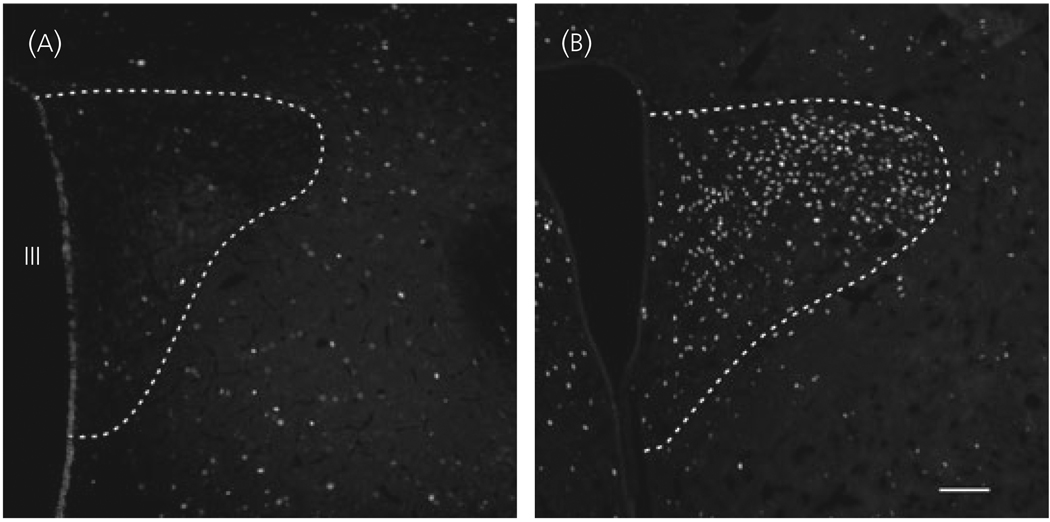
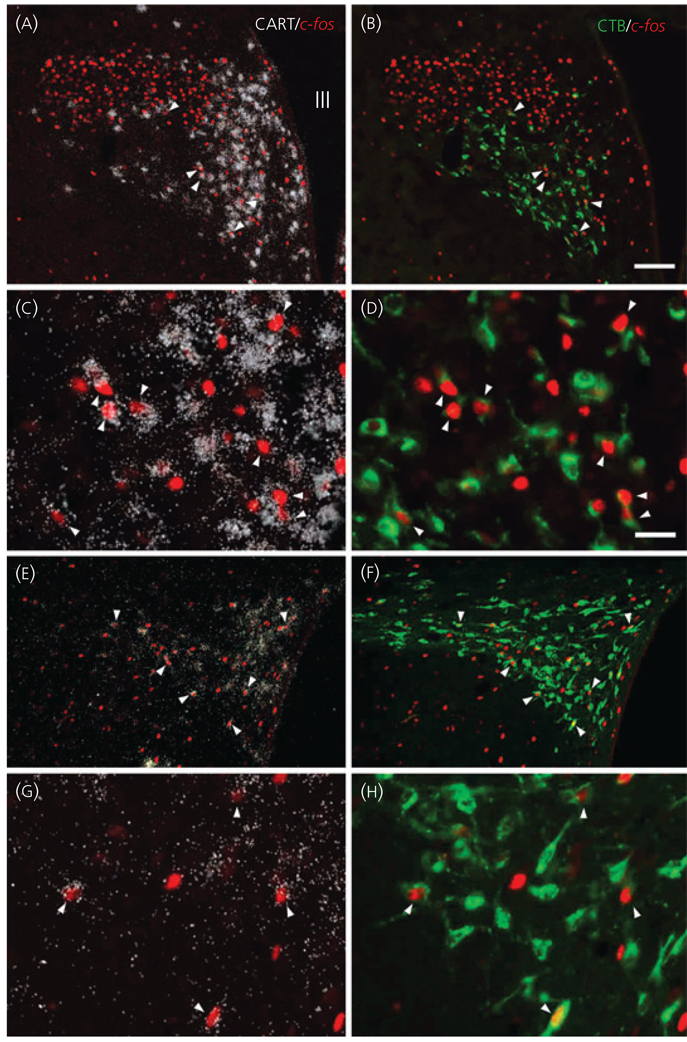
The brains of four LPS-treated rats where CTB administration was accurately placed into the dorsal vagal complex were used for further studies. The distribution of retrogadely labelled CTB-IR, and double-labelled CTB/CART neurons in the PVN was similar to that described above. In the PVN of the control group, only very few c-fos-IR neurons were observed, whereas, in LPS treated animals, numerous c-fos-IR neurons were present in the parvocellular division (Fig. 6). Triple labelled (CART mRNA/CTB/c-fos) neurons were primarily found in the ventral parvocellular subdivision of the PVN, and in the ventral part of the medial parvocellular subdivision, but some triple labelled neurons were also observed in the lateral parvocellular subdivision (Fig. 7).
Fig. 6.
Effect of lipopolysaccharide (LPS) treatment on the activity of neurons residing in the hypothalamic paraventricular nucleus (PVN). Only a few scattered c-fos labelled neurons are present in the PVN of control animals (a), but their number markedly increases following LPS (b). III, Third ventricle. Scale bar = 100 µm.
Fig. 7.
Location of cocaine- and amphetamine-regulated transcript (CART)-synthesising neurons in the hypothalamic paraventricular nucleus (PVN) that project to the dorsal vagal complex [CART/cholera toxin B subunit (CTB)/c-fos-immunoreactive (IR)] and express c-fos following lipopolysaccharide administration. Pairs of images of the same field under low (a,b) and high (c,d) magnification at the mid level of the PVN demonstrate triple-labelled neurons (arrowheads) containing CART mRNA (white silver grains), c-fos-IR (red immunofluorescence) nuclei, and retrogladely transported tracer CTB (green immunofluorescence) in the ventral parvocellular subdivision and in the ventral part of the medial parvocellular subdivision. Low (e,f) and high (g,h) magnification images of the caudal level of the PVN demonstrate c-fos containing CTB/CART neurons in the medial and lateral parvocellular subdivisions. Scale bars: 100 µm in (b) corresponds to (a), (e) and (f); 25 µm in (d) corresponds to (c), (g) and (h).
Discussion
LPS causes widespread expression of the immediate early gene, c-fos, in brain areas including centres involved in the central regulation of energy balance and vegetative functions (3) and results in effects on thermogenesis, sympathetic activity and heart rate, inhibiting the hypothalamic-pituitary-thyroid axis, reducing food intake and activating the hypothalamic-pituitary-adrenal axis (8, 11, 18, 19). It is generally accepted that the primary central targets of LPS and LPS-induced circulating cytokines are the circumventricular organs and the wall of brain blood vessels (20), and then the signal spreads to other brain regions. However, the mechanisms by which LPS induces effects on energy balance and vegetative functions are largely unknown.
In the present study, we demonstrate that LPS administration rapidly increases CART gene expression in the parvocellular sub divisions of the PVN. Very little is currently known about the role of CART synthesised in PVN neurons. CART released from hypophysiotrophic TRH and somatostatin neurons may be involved in the regulation of prolactin release from lactotrophs in the anterior pituitary gland (21–23). CART has a short-term, inhibitory effect on prolactin release (24) and is able to block the potent prolactin stimulatory effects of TRH (21). As LPS administration is known to have inhibitory effects on prolactin secretion (25, 26), it is conceivable that the LPS-induced increase in CART synthesis in the PVN contributes to the inhibitory actions of LPS on lactotrophs in the pituitary.
CART neurons of the PVN may also give collaterals to innervate neurons within the PVN. Indeed, administration of CART into the PVN has been shown to have potent anorexigenic effects (9), suggesting that LPS-induced release of CART from terminals in the PVN may be at least partly responsible for the LPS-induced anorexia. In addition, increased CART release in the PVN may contribute to LPS-induced activation of the hypothalamic-pituitary-adrenal axis. Central CART administration has potent stimulatory effects on the HPA axis, increasing CRH gene expression and ACTH and corticosterone levels in the circulating blood (27–29).
To gain further insight into the potential importance of the observed LPS-induced increase in CART gene expression in the PVN, we determined whether PVN CART neurons innervate the dorsal vagal complex, a region known to be involved in the central regulation of the effects of LPS (30–32). Indeed, a select population of CART neurons in the parvocellular PVN residing largely within the ventral and medial parvocellular subdivisions were shown to contribute to the dense CART-IR innervation of the dorsal vagal complex and a portion of these neurons were activated by LPS administration. As the only other adjacent area to the dorsal vagal complex that contains a dense CART-IR innervation is the area postrema (33) and none of the stereotaxic injections spread into this brain region, it is unlikely that tracer spread outside the dorsal vagal complex significantly contributed to the retrograde accumulation of CTB in the CART-containing neurons.
Because fourth ventricular administration of CART induces c-fos in the dorsal vagal complex and decreases the sucrose intake of rats (34, 35), we suggest that the activation of CART neurons in the PVN that project to the dorsal vagal complex may play an important role in the mediation of LPS induced anorexia. In addition, the administration of CART into the dorsal vagal complex (8) and peripheral administration of subseptic doses of LPS both result in tachycardia and increased blood pressure (8, 10), and it is conceivable that CART-containing neurons in the PVN that contribute to the PVN-dorsal vagal complex pathway mediate the effects of LPS on the cardiovascular system. Unfortunately, the lack of specific CART antagonists makes it impossible to determine with certainty whether inhibition of CART signalling in the NTS attenuates LPS-induced inhibition of food intake and/or affects cardiovascular function.
In conclusion, CART-synthesising neuronal populations in the PVN are rapidly stimulated by peripheral LPS administration. As a subpopulation of CART-synthesising neurons in the PVN innervate key vegetative centres in the brainstem, we propose that these neurons may contribute to some of the central effects of LPS on energy homeostasis and vegetative functions.
Acknowledgment
This work was supported by Grants NIH DK-37021, Hungarian Science Foundation (OTKA T046492) and the Sixth EU Research Framework Programme (LSHM-CT-2003-503041).
References
- 1.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- 3.Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res. 1999;818:499–509. doi: 10.1016/s0006-8993(98)01349-3. [DOI] [PubMed] [Google Scholar]
- 4.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 6.Larsen PJ, Vrang N, Petersen PC, Kristensen P. Chronic intracerebroventricular administration of recombinant CART(42–89) peptide inhibits and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes Res. 2000;8:590–596. doi: 10.1038/oby.2000.76. [DOI] [PubMed] [Google Scholar]
- 7.Rohner-Jeanrenaud F, Craft LS, Bridwell J, Suter TM, Tinsley FC, Smiley DL, Burkhart DR, Statnick MA, Heiman ML, Ravussin E, Caro JF. Chronic central infusion of cocaine- and amphetamine-regulated transcript (CART 55–102): effects on body weight homeostasis in lean and high-fat-fed obese rats. Int J Obes Relat Metab Disord. 2002;26:143–149. doi: 10.1038/sj.ijo.0801863. [DOI] [PubMed] [Google Scholar]
- 8.Scruggs P, Dun SL, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide attenuates phenylephrine-induced bradycardia in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1496–R1503. doi: 10.1152/ajpregu.00183.2003. [DOI] [PubMed] [Google Scholar]
- 9.Wang C, Billington CJ, Levine AS, Kotz CM. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. Neuroreport. 2000;11:3251–3255. doi: 10.1097/00001756-200009280-00040. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, Krukoff TL. Cardiovascular responses to subseptic doses of endotoxin contribute to differential neuronal activation in rat brain. Brain Res Mol Brain Res. 2001;89:71–85. doi: 10.1016/s0169-328x(01)00065-1. [DOI] [PubMed] [Google Scholar]
- 11.Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am J Physiol. 1999;276:R864–R871. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- 12.Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res Mol Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 13.Rivest S, Laflamme N. Neuronal activity and neuropeptide gene transcription in the brains of immune-challenged rats. J Neuroendocrinol. 1995;7:501–525. doi: 10.1111/j.1365-2826.1995.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 14.Koylu EO, Couceyro PR, Lambert PD, Ling NC, DeSouza EB, Kuhar MJ. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. [DOI] [PubMed] [Google Scholar]
- 15.Dyess EM, Segerson TP, Liposits Z, Paull WK, Kaplan MM, Wu P, Jackson IM, Lechan RM. Triiodothyronine exerts direct cell-specific regulation of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology. 1988;123:2291–2297. doi: 10.1210/endo-123-5-2291. [DOI] [PubMed] [Google Scholar]
- 16.Kakucska I, Rand W, Lechan RM. Thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is dependent upon feedback regulation by both triiodothyronine and thyroxine. Endocrinology. 1992;130:2845–2850. doi: 10.1210/endo.130.5.1572297. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 18.Sarkar S, Wittmann G, Fekete C, Lechan RM. Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotropin-releasing hormone-producing neurons but not in prothyrotropin-releasing hormone-producing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;999:181–192. doi: 10.1016/j.brainres.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 19.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- 21.Raptis S, Fekete C, Sarkar S, Rand WM, Emerson CH, Nagy GM, Lechan RM. Cocaine- and amphetamine-regulated transcript co-contained in thyrotropin-releasing hormone (TRH) neurons of the hypothalamic paraventricular nucleus modulates TRH-induced prolactin secretion. Endocrinology. 2004;145:1695–1699. doi: 10.1210/en.2003-1576. [DOI] [PubMed] [Google Scholar]
- 22.Vrang N, Larsen PJ, Clausen JT, Kristensen P. Neurochemical characterization of hypothalamic cocaine- amphetamine-regulated transcript neurons. J Neurosci. 1999;19:RC5. doi: 10.1523/JNEUROSCI.19-10-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez F, Fekete C, Lechan RM, Joseph-Bravo P. Cocaim and amphetamine-regulated transcript (CART) expression is differentially regulated in the hypothalamic paraventricular nucleus of lactating rats exposed to suckling or cold stimulation. Brain Res. 2001;1132:120–128. doi: 10.1016/j.brainres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuriyama G, Takekoshi S, Tojo K, Nakai Y, Kuhar MJ, Osamura RY. Cocaine- and amphetamine-regulated transcript peptide in the rat anterior pituitary gland is localized in gonadotrophs and suppresses prolactin secretion. Endocrinology. 2004;145:2542–2550. doi: 10.1210/en.2003-0845. [DOI] [PubMed] [Google Scholar]
- 25.Hollis JH, Lightman SL, Lowry CA. Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic-pituitary-adrenal axis and inhibition of prolactin secretion. J Endocrinol. 2005;184:393–406. doi: 10.1677/joe.1.05839. [DOI] [PubMed] [Google Scholar]
- 26.Thim L, Kristensen P, Nielsen PF, Wulff BS, Clausen JT. Tissue-specific processing of cocaine- and amphetamine-regulated transcript peptides in the rat. Proc Natl Acad Sci USA. 1999;96:2722–2727. doi: 10.1073/pnas.96.6.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- 28.Stanley SA, Small CJ, Murphy KG, Rayes E, Abbott CR, Seal LJ, Morgan DG, Sunter D, Dakin CL, Kim MS, Hunter R, Kuhar M, Ghatei MA, Bloom SR. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001;893:186–194. doi: 10.1016/s0006-8993(00)03312-6. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, Vaughan JM, Donaldson CJ, Rivier J, Li C, Chen A, Vale WW. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology. 2004;145:5202–5209. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- 30.Molina-Holgado F, Guaza C. Endotoxin administration induced differential neurochemical activation of the rat brain stem nuclei. Brain Res Bull. 1996;40:151–156. doi: 10.1016/0361-9230(96)00043-3. [DOI] [PubMed] [Google Scholar]
- 31.Wiertelak EP, Roemer B, Maier SF, Watkins LR. Comparison of the effects of nucleus tractus solitarius and ventral medial medulla lesions on illness-induced and subcutaneous formalin-induced hyperalgesias. Brain Res. 1997;748:143–150. doi: 10.1016/s0006-8993(96)01289-9. [DOI] [PubMed] [Google Scholar]
- 32.Lacroix S, Rivest S. Functional circuitry in the brain of immune-challenged rats: partial involvement of prostaglandins. J Comp Neurol. 1997;387:307–324. doi: 10.1002/(sici)1096-9861(19971020)387:2<307::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 33.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- 34.Zheng H, Patterson C, Berthoud HR. Fourth ventricular injection of CART peptide inhibits short-term sucrose intake in rats. Brain Res. 2001;896:153–156. doi: 10.1016/s0006-8993(00)03256-x. [DOI] [PubMed] [Google Scholar]
- 35.Zheng H, Patterson LM, Berthoud HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res. 2002;957:298–310. doi: 10.1016/s0006-8993(02)03640-5. [DOI] [PubMed] [Google Scholar]