Summary
Costimulation is a concept that goes back to the early 1980’s when Lafferty and others hypothesized that cell surface and soluble molecules must exist that are essential for initiating immune responses subsequent to antigen exposure. The explosion in this field of research ensued as over a dozen molecules have been identified to function as second signals following T-cell receptor engagement. By 1994, it seemed clear that the most prominent costimulatory pathway CD28, and functionally related costimulatory molecules such as CD154, were the major drivers of a positive immune response. Then the immunology world turned upside down. CD28 knockout mice, which were, in most cases, immunodeficient, led to increased autoimmunity when bred into the non-obses diabetic background. Another CD28 family member, cytotoxic T-lymphocyte-associated protein 4, which was presumed to be a costimulatorymolecule on activated T cells, turned out to be critical in down regulating immunity. These results, coupled with the vast suppressor cell literature which had been largely rebuked, suggested that the immune system was not poised for response but controlled in a way such that regulation was dominant. Over the last decade, we have learned that these costimulatory molecules play a key role in the now classical CD4+CD25+Foxp3+ regulatory T cells (Tregs) that provide critical control of unwanted autoimmune responses. In this review, we discuss the connections between costimulation and Tregs that have changed the costimulation paradigm.
Keywords: Regulatory T cells, costimulation, CD28, B7 molecules, CTLA-4
Introduction
One of the major challenges of the immune system is to preserve immune tolerance to self while maintaining the ability to fight foreign pathogens and infectious agents. As such, the immune system has evolved several mechanisms to achieve immune tolerance to self. Central tolerance refers to the process taking place in the thymus during T-cell development that leads to the elimination of most self-reactive T cells [reviewed in (1–4)]. However, this process does not eliminate all potentially harmful lymphocytes and many autoreactive T cells are found in the periphery even in healthy subjects (1–4). In the majority of the population, autoimmunity does not occur as these T cells are kept in check by peripheral tolerance mechanisms that include anergy, clonal deletion, and control by regulatory T cells (previously called suppressor T cells). Over the past 10 to 15 years, regulatory T cells (Tregs) have evolved into a major participant in the control of autoimmune diseases, cancer, transplantation and maternal-fetal tolerance (5–11). The clearest evidence for this is the fact that both humans and mice that are genetically deficient in Tregs, as a consequence of mutations in the master Treg transcription factor, Forhead box protein 3 (Foxp3), suffer from widespread autoimmune diseases (5).
The field of costimulation has evolved significantly since the original discovery by Lafferty and colleagues (12) that a productive T-cell activation requires a ‘signal 2’ provided by costimulatory molecules in addition to the T-cell receptor (TCR)-mediated ‘signal 1’. While Jenkins and Schwartz originally demonstrated that TCR-mediated activation of T cells in the absence of signal 2 resulted in antigen-specific unresponsiveness or anergy, it is now understood that costimulatory signals do not only provide a ‘on or off’ switch but rather represent a complex network of receptor-ligand interactions that qualitatively and quantitatively influence immune responses and affect all its major participants including effector T cells, B cells, and dendritic cells. Now, it is clear that costimulation is key for the development and function of Tregs as well. Like ‘classical’ effector T cells (Teff), Tregs are antigen-specific T cells expressing a TCR and thus require costimulatory signals for their function (5). In this regard, the initial demonstration that CD28 deficiency resulted in a defect in Tregs that led to aggressive autoimmunity in the non-obese diabetic (NOD) mouse opened up a new field of costimulatory biology as sets of receptors/ligands that had previously been thought to drive positive immunity [OX40 (CD134), glucocorticoid-induced tumor necrosis factor receptor (GITR), programmed cell death-1 (PD-1), CD40 ligand (CD40L), 4-1BB (CD137) and toll-like receptors as well as CD46, inducible costimulator (ICOS), and CD52 in human Tregs] [reviewed in (5, 13, 14)] are now implicated in negative regulation (15). In particular, there have been a number of other costimulatory molecules that have been implicated in Treg homeostasis and function, although the exact role of some of these pathways in Treg biology is still controversial. For example, it has been shown that tumor necrosis factor receptor (TNFR) family members OX40 and 4-1BB could promote the generation, survival or expansion of functional Tregs (16–20). Conversely, other studies report that these costimulatory molecules prevent the induction of Tregs or alter their suppressive function (21–23). Other TNFR family members CD40L and GITR have been shown to be important for Treg homeostasis and function but they are expressed by effector T cells as well, and their signals may bear a dominant effect on effector T cells (24–28). Furthermore, negative regulators such as PD-1 and cytotoxic T lymphocyte antigen-4 (CTLA-4 or CD152) now provide a basis for Treg function suggesting their disruption might enhance immunity by altering the Treg:Teff balance. In this review, we focus on the studies from many laboratories, including ours, which demonstrated that although CD28 and CTLA-4 are expressed on both regulatory and effector T cells, the CD28-CTLA-4/B7 system plays a central role in the biology of regulatory T cells.
Tregs: multi-faceted regulators of immune tolerance
Different subsets of Tregs
The notion that immune responses could be controlled by specialized suppressor T cells has lingered since the early years of modern immunology and these findings were particularly numerous in the transplantation field where the notion of ‘infectious tolerance’ was first introduced. However, issues regarding the reproducibility of data and the absence of a well-defined suppressor T-cell subset led to widespread suspicion and disbelief on the very existence of this subset. The field was revived by seminal studies from Sakaguchi and colleagues (6–8, 10, 11) who demonstrated that thymically-derived CD4+ T cells expressing the interleukin-2 (IL-2) receptor α chain (CD25) were instrumental in maintaining peripheral tolerance and protecting from autoimmunity. CD4+CD25+ Tregs were shown to inhibit effector T-cell responses in vitro and in vivo (9, 29), and similar populations of Tregs with overlapping phenotype and function were described as CD62Lhi (30), CD45RBlo (31) and later on CD127lo (32). Finally, the discovery of the lineage-specific transcription factor Foxp3 greatly contributed to establishing CD4+CD25+Foxp3+ T cells as a distinct T-cell subset in the immune system. The development of autoimmune diseases in mouse models and immunodeficiency, polyendocrinopathy, enteropathy, X-linked (IPEX) patients that was associated with Foxp3 deficiency further demonstrated that the CD4+CD25+Foxp3+ Treg subset played a unique role in keeping autoreactive T cells in check (33–38). Foxp3+ Tregs are important throughout life and not only in neonates when the immune system is still developing since acute depletion of Foxp3+ Tregs in adult mice led to rapidly lethal multi-organ autoimmunity reminiscent of the phenotype observed in Foxp3-deficient animals (39). Importantly, while the quintessential Treg is represented by the thymically-derived CD4+CD25+Foxp3+ T-cell subset (also called ‘natural Tregs’), other T-cell subsets with regulatory activity have been described such as T regulatory type 1 (Tr1) and T helper (Th) cell type 3 (Th3) cells that are believed to suppress immune responses through the production of IL-10 and transforming growth factor β (TGF-β), respectively (40). Furthermore, conventional peripheral T cells can be induced to acquire a regulatory phenotype in vivo and in vitro, notably in the presence of TGF-β, and although this conversion is not always accompanied by Foxp3 expression, these ‘adaptive Tregs’ share most features of natural Tregs in terms of phenotype and function (41). In this review, we focus on the role of costimulatory signals in the biology of natural and adaptive Tregs.
Tregs control immune responses at multiple steps and tissue sites
Defining a unifying mode of action of regulatory T cells has been prohibited by the avalanche of data revealing multiple mechanisms of suppression in in vitro and in vivo studies. These different modes of action have been addressed in detail recently in several excellent reviews (42–44) and will only be briefly summarized below, except for specific studies that relate to the effect of costimulatory molecules on Treg function. Tregs can suppress immune responses through the production of immunosuppressive cytokines (chiefly IL-10 and TGF-β), the induction of cytokine deprivation-mediated apoptosis of effector T cells, the direct killing of effector T cells or antigen-presenting cells (APCs), and the modification of functional properties of APCs [particularly dendritic cells(DCs)] (42–44). It is important to keep in mind that these different mechanisms have to be placed in the context of two major properties of immune regulation by Tregs, namely bystander suppression and infectious tolerance. Bystander suppression refers to the fact that although Tregs must be activated through their antigen-specific TCR to induce suppression, they can then do so in an antigen non-specific manner (44). Furthermore, while natural Tregs are potent regulators of T-cell responses, the long-term maintenance of immune tolerance is likely to also rely on the de novo generation of adaptive Tregs that is made possible by the immunoregulatory milieu created by the initial Treg population involved. This process, called infectious tolerance, also allows the broadening of the regulatory repertoire as additional TCR specificities are provided by the newly generated adaptive Tregs (44). Finally, just as the in vitro and in vivo modes of action of Tregs suppression appear distinct, it is likely that natural and adaptive Tregs will exploit different immunoregulatory mechanisms depending on the site (lymphoid organ versus peripheral tissue) and milieu (steady-state homeostasis versus inflammatory conditions) in which the immune response takes place (44).
CD28: the most critical costimulatory pathway for the control of Treg homeostasis
CD28 is constitutively expressed on T cells and is considered a major costimulatory pathway for T cells. CD28 interaction with its two ligands B7-1 (CD80) and B7-2 (CD86) has been implicated in many aspects of T-cell biology, including T-cell proliferation, IL-2 production, induction of the survival factor B-cell leukemia/lymphoma x (Bcl-XL), T-cell differentiation, and T-cell-B-cell interactions [reviewed in (45, 46)]. Interestingly, although CD28 is the prototypic positive costimulator of T-cell responses, the upregulation of CTLA-4 expression induced by CD28 and more recently its important role in Treg homeostasis have highlighted the unexpected role played by CD28 in the maintenance of peripheral tolerance (47).
Blockade of CD28-B7 interactions results in a reduction of the Treg population
The first evidence of the role played by CD28/B7 interactions in the homeostasis of regulatory T cells was described in the autoimmune-prone NOD mouse (15). Indeed, whereas mice deficient for CD28 or B7-1/B7-2 on non autoimmune-prone backgrounds display profoundly defective immune responses (48–50), NOD-CD28 knockout (KO) and NOD-B7-1/B7-2KO mice surprisingly developed a fulminant form of diabetes which affected all mice by eight to ten weeks of age (15, 51). Exacerbation of disease was accompanied by a dramatic decrease in the percentage of CD4+CD25+ Tregs in NOD-CD28KO and NOD-B7-1/B7-2KO mice (15). Furthermore, control of diabetes could be restored in NOD-CD28KO mice by the injection of wildtype (WT) Tregs (15, 52), indicating that CD28-B7 interactions were indeed critical for maintaining normal levels of Tregs in the periphery and consequently for prevention of autoimmune diseases. In this regard, not only did NOD-CD28KO mice develop accelerated diabetes but they were also afflicted with many other autoimmune disorders including autoimmune exocrine pancreatitis (53), peripheral neuropathy, sialadenitis and thyroiditis (Q. Tang, H. Bour-Jordan, J.A. Bluestone, unpublished observations). Thus, CD28 deficiency lowered the threshold for autoimmunity and allowed for the manifestation of autoimmune diseases usually not observed in CD28-sufficient NOD mice. This surprising observation demonstrated that the requirement for CD28 costimulatory signals was more absolute for Treg populations than for autoreactive T cells in genetically-prone individuals. The mechanisms utilized by autoreactive T cells to bypass CD28 signals are still not clearly defined, but they may result from the use of alternative costimulatory pathways and/or chronic presentation of autoantigens since continued TCR-mediated stimulation has been shown to induce T-cell activation in a viral model (54). Using a TCR-transgenic model, Lohr and colleagues (55, 56) additionally showed that constitutive expression of low levels of B7 molecules on APCs played an important role in suppressing T-cell responses to self as well as foreign antigens. Indeed, T cells stimulated with B7-sufficient mature DCs in B7-deficient hosts displayed greater effector function than in B7-sufficient recipients, and this effect was abrogated by the co-transfer of CD4+CD25+ Tregs (55). Similarly, tolerance induced by transferring ovalbumin-specific TCR-transgenic T cells into recipients expressing ovalbumin as a soluble systemic self-antigen could be broken by the administration of ovalbumin-pulsed DCs only in B7-KO recipients that were deficient in CD4+CD25+ Tregs (56). Furthermore, the critical role of CD28-B7 interactions for Tregs has been confirmed in all mouse strains tested (57, 58) and extended to many models of immune responses and pathologic conditions, including murine models for autoimmune colitis and graft-versus-host disease where CD28 costimulatory signals were shown to be uniquely important for Tregs as opposed to other costimulatory pathways such as ICOS or CD40L (59, 60).
Role of CD28 in Treg development in the thymus and survival in the periphery
When the importance of CD28-B7 interactions for Tregs homeostasis became unequivocal, the question remained whether CD28 controlled Tregs development in the thymus and/or survival in the periphery. In NOD mice, treatment with murine CTLA-4-immunoglobulin (Ig), which disrupts interaction between CD28 and B7 molecules, induced a rapid drop in Treg numbers as the percentage of CD4+CD25+ cells decreased almost 80% within only nine days of treatment (15). It should be noted that while CTLA-4-Ig treatment also precluded the binding of B7 molecules to CTLA-4, it is very likely that most of the effect observed on Treg homeostasis is due to the blockade of CD28 signals since a very similar phenotype is observed in CD28KO mice and mice treated with CTLA-4-Ig or anti-B7-1 and anti-B7-2 monoclonal antibodies (mAbs) (15, 58). However, B7-1/B7-2KO mice displayed a reduction in Tregs even greater than CD28KO mice, suggesting a possible, although minor role of CTLA-4 in Treg homeostasis (15). The role of CTLA-4 in Treg biology will be addressed in detail later in this review. The rapid kinetic of decrease in Tregs following B7 blockade suggested that CD28 controlled the peripheral homeostasis of Tregs but these experiments did not preclude a solely thymic effect associated with rapid generation and export of Tregs from the thymus to the periphery. Therefore, we blocked CD28-B7 interactions using anti-B7-1 and anti-B7-2 mAbs treatment in adult thymectomized mice (58). We showed that CD28 blockade led to a rapid reduction in Tregs that was similar in thymectomized and control animals, whereas thymectomy alone did not affect Tregs number within the same ten-day period (58). Furthermore, adoptively transferred Tregs showed similar decrease when B7 molecules were blocked. Taken together, these experiments clearly established that CD28 signals directly control the maintenance of the Treg population in the periphery. In addition, evaluation of the number of CD25+ T cells in CD4 single-positive (SP) thymocytes in CD28KO or anti-B7-treated mice demonstrated that CD28 is equally critical for the generation of this T-cell subset in the thymus (58). This finding was further extended in TCR-transgenic models where expression of a self-reactive TCR together with its cognate self-antigen led to the generation of a high percentage of CD4+CD25+ Tregs that was abrogated in mice deficient for CD28 or B7-1 and B7-2 molecules (56, 61). More recently, the importance of CD28 signals in thymic Treg development was confirmed using the lineage-specific Foxp3 marker (57), thus eliminating any doubt that may have been raised by the use of the CD25 marker given its incomplete overlap with Foxp3 expression (62). Thus, CD28 signals play a critical role for both the thymic development and peripheral maintenance of the Treg population that is non-redundant with other costimulatory pathways. We will now discuss the different mechanisms that are involved in the control of Treg homeostasis by CD28 costimulatory signals.
CD28 control of Treg proliferation
CD28 costimulatory signals have many functional consequences on effector T-cell function and many of these could be involved in maintaining Treg homeostasis, in particular T-cell proliferation, IL-2 production and induction of the anti-apoptotic factor, Bcl-XL. Tregs were initially described as being unresponsive to anti-CD3 mAbs stimulation in vitro, and in fact, anergy was one of the hallmarks of the regulatory T-cell subset phenotype (6, 7, 29, 63). In vivo, one study demonstrated in a transfer model that CD4+CD25+ Tregs were hyporesponsive to stimulation with their cognate antigen even in the presence of complete Freund's adjuvant (64). Furthermore, observations that reversal of the Treg-unresponsive state correlated with abrogation of their regulatory potential in vitro led to the belief that anergy was a core attribute of regulatory T cells intimately linked to their suppressive function (6, 7, 29). However, additional studies of the proliferative capability of regulatory T cells in vivo rapidly established that these cells could undergo brisk expansion while fully maintaining their suppressive phenotype. Indeed, Hori et al. (65) first showed that CD4+CD25+ T cells proliferated more actively in vivo compared to CD4+CD25− T cells as evidenced by Bromodeoxyuridine (BrdU) incorporation experiments. However, these data could be subjected to an alternative interpretation of conversion between cells subsets due to the possible instability of the CD25 marker. Several studies using carboxyfluorescein succinimidyl ester (CFSE) labeling as well as congenic markers went on to unambiguously demonstrate the rapid expansion of Tregs in vivo (58, 66–68). Using non-lymphopenic hosts to avoid the complications of lymphopenia-driven proliferation, these reports clearly established that CD4+CD25+ Tregs indeed underwent expansion in vivo that was superior to their CD25− counterparts. Importantly, the Treg repertoire has been shown to be skewed towards autoreactivity (69–73), and vigorous cycling of Tregs in vivo suggested that at least a subset of these cells represent autoreactive Tregs that are continuously expanding as a result of being stimulated by tissue self-antigens (58, 66–68). In this regard, tissue self-antigen-specific Tregs selectively expand and accumulate in the corresponding draining lymph nodes (52), and Samy et al. (74) elegantly demonstrated using polyclonal populations of Tregs that regional lymph nodes were enriched in Tregs specific for self-antigens expressed in the local tissue that precluded autoimmune responses specifically directed against this tissue. Thus, it is important to keep in mind that Treg expansion is critical not only to maintain their population size as a whole but to allow for the accumulation of tissue-specific suppressor cells that can prevent the triggering of local autoimmunity. Using anti-B7 mAbs or B7-deficient recipients, we showed in an adoptive transfer model that CD28 played a critical role in Treg expansion and that blockade of CD28-B7 interactions completely abrogated Treg proliferation in vivo (58). In vitro studies further showed that proliferation of Tregs induced by DCs was dependent on the presence of B7 molecules on these APCs (75). In addition, while proliferation of Tregs was strictly dependent on CD28 costimulatory signals in vivo, survival of Tregs, but not effector T cells, was only partially affected by CD28-B7 blockade (58). Conversely, a CD28 ‘superagonistic’ mAb that display mitogenic properties for T cells in the absence of TCR-mediated stimulation preferentially expanded Tregs over effector T cells at high dose in vitro and in vivo in rats (76). In addition, administration of low doses of the CD28 superagonist to rats induced the proliferation of Tregs but not conventional T cells and resulted in increased suppressive capability of CD4+CD25+ Tregs (77), suggesting that increased CD28 signaling resulted in enhanced Treg expansion and function. The mechanism of the selective expansion of Tregs by the CD28 superagonist is unknown. The authors proposed that it could be related to the autoreactive repertoire of Tregs (77). However, Treg expansion following treatment with the CD28 superagonistic mAb was observed principally in the spleen but not in lymph nodes, which is contrary to expectations since tissue antigen-specific autoreactive Tregs have been shown to accumulate in regional lymph nodes (52, 74). It is worth noting that the CD28 superagonist used in these studies binds to the laterally-exposed C’D’ region of the molecule in contrast to B7 molecules and conventional non-mitogenic anti-CD28 mAbs which bind to distinct epitopes located distally from the T-cell surface (78–80), making these findings difficult to translate for CD28 binding to its natural ligands B7-1 and B7-2 in vivo. Furthermore, the mechanism of action of CD28 superagonistic mAbs and in particular its preferential effect on Tregs versus Teff was dramatically called into question by the catastrophic result of a phase I safety study where administration of the superagonist led to life-threatening systemic inflammatory response syndrome (SIRS) within hours of administration in healthy volunteers (81). Finally, it is important to note that the respective influence of CD28 and IL-2 signals has been investigated in regards to proliferation of CD4+CD25− Teff versus CD4+CD25+ Tregs in humans. Similarly to what was observed in murine models, CD28 costimulation was important for proliferation of Tregs and could not be substituted by addition of exogenous IL-2 (82). Indeed, Hombach et al. (82) showed that Tregs required stronger TCR and CD28 signals for induction of proliferation as compared with Teff, while IL-2 functioned principally by preventing Treg apoptosis. Furthermore, CD28 was recently shown to play a unique role in sustaining the proliferation and maintaining the phenotype and function of human peripheral-blood Tregs expanded in vitro in contrast to other costimulatory pathways such as CD27, CD40L, ICOS, 4-1BB and OX-40 (83). In agreement with the latter studies, it is noteworthy that most of the protocols currently being developed to expand Tregs in vitro for therapeutic purposes include activation of T cells with strong TCR and CD28 signals together with high doses of IL-2 (52, 83–85). Taken together, these studies underline that CD28 costimulatory signals control Treg homeostasis by several mechanisms in vivo, and while CD28 plays a unique role in Treg proliferation, other costimulatory pathways may participate in the overall survival of Tregs, particularly IL-2 and CD25 that are critically important for Treg survival and fitness.
Mechanisms of CD28 control of Treg homeostasis
Control of Treg homeostasis directly by CD28 signals versus indirectly through the upregulation of IL-2 production
One of the most important functions of CD28 costimulation is the induction of IL-2 production by T cells (45, 46). Since IL-2 seems to be required for the normal homeostasis of Treg populations and prevention of autoimmunity (86–91), it thus seemed logical to consider that the requirement for CD28 costimulation in Treg homeostasis was recapitulated by its important role for IL-2 synthesis. However, this hypothesis was challenged by the demonstration that contrary to CD28, IL-2 signals were not necessary for the generation of Tregs in the thymus, but IL-2 was critical to maintain the survival and competitive fitness of Tregs in the periphery as well as their transcriptional program including expression of critical factors such as Foxp3 and TGF-β (86, 91). This IL-2 dependency was illustrated by the catastrophic autoimmunity arising in mice deficient for IL-2 or IL-2 receptor [reviewed in (92)] but it could also play a more subtle role in preventing autoimmunity as demonstrated by defective IL-2 production by effector T cells that resulted in a local deficit in Tregs in the pancreatic tissue and ultimately contributed to the development of autoimmune diabetes in NOD mice (93). We found that steady state levels of IL-2 messenger RNA (mRNA) were greatly decreased in the spleen of Treg-deficient CD28KO mice by comparison with WT mice (58). This low level of systemic IL-2 contributed to the defective homeostasis of Tregs in CD28KO mice as demonstrated by the rapid decline in the numbers of WT Tregs adoptively transferred into CD28KO animals that could be reversed by the pre-incubation of Tregs with exogenous IL-2 prior to transfer (58). However, defective Treg homeostasis secondary to CD28 deficiency was not only due to diminished IL-2 since anti-B7 mAbs treatment induced a rapid depletion of WT Tregs even if these cells were pre-cultured in IL-2 before adoptive transfer (58). Thus, our studies demonstrate that while IL-2 signals are clearly important for Treg homeostasis, CD28 costimulation functions in part independently of IL-2 to sustain maintenance of the Treg population in the periphery. This issue was further addressed by Singer and colleagues (61) who elegantly demonstrated that CD28 signals were required for thymic development of Tregs independently of IL-2 using mixed bone marrow (BM) chimeras. Indeed, while IL-2KO Tregs developed normally in recipients of WT + IL-2KO BM, they were not generated in recipients of CD28KO + IL-2KO BM, confirming that CD28 costimulation was necessary for IL-2 production that could sustain Treg homeostasis (61). However, as mentioned above, IL-2 was necessary but not sufficient to generate a normal Treg population. Indeed, CD28KO Tregs did not develop in recipients of WT + CD28KO BM (61), demonstrating that IL-2 produced by WT cells could not compensate for the defect in CD28 and establishing that unique signals were provided by CD28 costimulation in the thymus and periphery.
Downstream pathways involved in CD28 control of Treg homeostasis
Expression of IL-2 and CD25 play both a critical role in the maintenance of Treg homeostasis and the prevention of autoimmunity (86–91). Furthermore, while CD28 signals regulated Treg development and survival independently of IL-2, as discussed previously, CD25 emerged as a key regulator of Treg homeostasis functioning downstream of the CD28 receptor. Indeed, using CFSE-labeling to track adoptively transferred Tregs independently of their CD25 expression, we showed that the level of CD25 expression diminished dramatically after anti-B7 mAbs treatment in vivo (58). Importantly, decreased CD25 expression was found to be a direct consequence of CD28 deficiency rather than limiting IL-2 levels and it occurred prior to the loss of Tregs in the periphery (58). Considering the important role of CD25 in Treg homeostasis and function, the regulation of CD25 expression by the CD28 costimulator likely represents an important mechanism of the control of Treg populations by CD28 signals. It is of note that a small residual population of Tregs is observed in the thymus and periphery of CD28KO mice (57, 58, 61). While GITR expression and suppressive function were reported to be affected in these residual CD4+CD25+ Tregs in the thymus (61), the level of expression of GITR, CD25 and Foxp3 was similar to levels observed in WT Treg cells in the periphery (57, 58), and peripheral CD28KO Tregs were found to regulate effector T-cell responses as efficiently as WT Tregs (29, 94). Taken together, these findings suggest that either CD28 signals are not required on peripheral Tregs for their suppressive function or that a subset of Tregs can develop, persist in the periphery and adopt a classical Treg phenotype and function independently of CD28 expression although the limited size of this subset prevents them from efficiently controlling autoimmunity. Finally, important insight into the pathways involved in CD28 control of Treg homeostasis was provided by the study from Tai et al. (61) who reconstituted CD28KO mice with transgenes of CD28 carrying various mutations affecting amino acids in the cytoplasmic tail of CD28 that have been associated with binding to different downstream kinases (95–98). The clear cut results from this report demonstrated that the C-terminal proline-rich region of the CD28 cytoplasmic tail that has been associated with binding to lymphocyte protein tyrosine kinase (Lck) was required to mediate the costimulatory signals necessary for the generation of Tregs in the thymus. In contrast, motifs that have been shown to mediate binding of the CD28 tail to kinases phosphatidylinositol 3-kinase (PI3K) and IL-2-inducible T-cell kinase (Itk) were not necessary for CD28 to induce Treg development in the thymus (61). Furthermore, the authors show in an in vitro system that CD28 costimulation of Tregs precursors induced the expression of the Treg lineage specific transcription factor Foxp3 and the upregulation of CTLA-4 and GITR, two typical markers of the Treg phenotype. The loop was closed by a recent report from Brumeanu and colleagues (99) who confirmed the involvement of the C-terminal Lck-binding motif but not the PI3K kinase-binding region of the CD28 cytoplasmic tail and directly showed that this motif was important for stabilization of Foxp3 mRNA in Treg precursors in thymus. Thus, CD28 signals contribute to the thymic development of Tregs by inducing the expression of Foxp3, which in turn triggers the developmental program resulting in the generation of Tregs in the thymus (100–103). It is worth noting that results from the two above-mentioned studies further suggested that the anti-apoptotic factor Bcl-XL, which has been involved in increased survival following CD28 costimulation of effector T cells, is not involved in the pathways downstream of CD28 that lead to Treg survival. Indeed, it has been shown previously that the PI3K-binding domain of the CD28 tail, but not the C-terminal proline rich motif, was required for the induction of Bcl-XL by CD28 (104). Together with the data that show it is the C-terminal proline rich motif of CD28 that is important for Treg homeostasis, these results suggest that Bcl-XL does not play a role in Treg survival. In this regard, we showed that constitutive expression of Bcl-XL in transgenic mice Tregs did not prevent depletion of Tregs secondary to blockade of CD28-B7 interactions, demonstrating that CD28 control of Treg survival was indeed independent of Bcl-XL (58).
Models of CD28 costimulation of Treg thymic development
The requirement for both high-affinity TCR interactions and CD28 costimulatory signals for the development of Tregs sheds an interesting light on the fundamental differences between the mechanisms determining the thymic selection of regulatory versus conventional T cells. Indeed, whereas high-affinity interactions of the TCR with agonist peptides result in negative selection of thymocytes, TCRs of equivalent or higher affinity for self peptides conversely induce the generation of Tregs (72, 105). Furthermore, although the role of CD28 in thymic selection is still unclear, CD28 costimulation of developing thymocytes has been shown to induce their apoptosis (106–109). Thus, it is quite intriguing that the combination of two ‘positive signals’ that individually are sufficient to induce deletion of developing thymocytes is instead required for the generation of Tregs. It is tempting to speculate that the requirement for CD28 costimulation in addition to a threshold-TCR interaction diverts thymocytes that would otherwise be rescued from negative selection and death by turning on Foxp3 leading to Treg development. This differentiation pathway would represent a pathway to the development of autoreactive regulatory T cells. Indeed, as schematically represented in Fig. 1, thymic selection based on the affinity of TCR interactions alone could result in a gray area where high-affinity peptides in principle favor negative selection except under conditions of costimulation wherein Tregs develop. Thus, CD28 costimulation during T-cell development transforms the continuum of signal strength (from positively selected conventional T cells to negatively-selected autoreactive T cells) towards a pathway that leads to positively-selected autoreactive Tregs. At this point, it is not clear whether these cells derive from high affinity or low affinity self-reactive TCRs. This model and the importance of CD28 costimulation may be particularly relevant to autoimmune-prone mouse strains such as the NOD mouse that present defects in thymic selection (110). In fact, as mentioned previously, NOD mice deficient for B7 or CD28 molecules develop an exacerbated form of diabetes as a consequence of a deficient Treg compartment (15). Treatment of NOD mice with CTLA-4-Ig or anti-B7-1 and anti-B7-2 mAbs rapidly depleted the Treg population regardless of the age of the treated mice, but treatment resulted in accelerated diabetes only if mice were treated between two-to-four weeks of age (15, 111). While NOD-B7-2KO mice are protected from diabetes but develop an autoimmune peripheral neuropathy, treatment with anti-B7-1 mAbs between two to four weeks of age resulted in Treg disappearance and as a consequence, restored diabetes and accelerated the onset of neuropathy (24, 112). However, anti-B7-1 mAbs treatment of adult NOD-B7-2KO mice induced a similar depletion of Tregs without affecting the development of either diabetes or neuropathy (B. Salomon, H. Bour-Jordan and J.A. Bluestone, unpublished observations). Thus, blockade of CD28 costimulatory signals affected Treg homeostasis uniformly during the animals’ lifespan but only resulted in exacerbated diabetes when it occurred between two-to-four weeks of age, e.g. at a time when the immune system is still maturing. Taken together with the emergence of multiple other autoimmune diseases in NOD-CD28KO mice [(53); Q. Tang, H. Bour-Jordan, J.A. Bluestone, unpublished observations] that suggested the diversification of the autoreactive Teff repertoire in addition to defective regulation of these T cells, these findings are compatible with the model depicted in Fig. 1 of the role of CD28 in thymic development. Indeed, as CD28 deficiency thwarted the development of Tregs in the thymus, it may also allow for the selection of effector T cells with high-affinity for self that would have been either negatively selected or selected into Tregs in the presence of CD28 costimulatory signals due to the expression and stabilization of Foxp3. An alternative explanation for the mechanism of CD28 costimulation of Treg development is that CD28 signals not only combine with TCR stimulation to increase the overall strength of signal but also induce a distinct transcriptional program that could play a role in the thymic selection of Tregs. This hypothesis is supported by the observation that CD28 engagement specifically induces Foxp3 expression in TCR-stimulated developing thymocytes (61, 99) and by our finding in human naive T cells that CD28 upregulates the expression of a unique set of genes after T-cell activation (J. Esensten, J.A. Bluestone, unpublished observations). If this hypothesis is correct, it remains to be determined whether CD28 costimulation functions directly by stimulating the expression of Foxp3 which in turn initiates the Treg-developmental program, or indirectly by inducing genes that favor the expression of Foxp3 and/or facilitating Treg development independently of Foxp3, such as with survival factors.
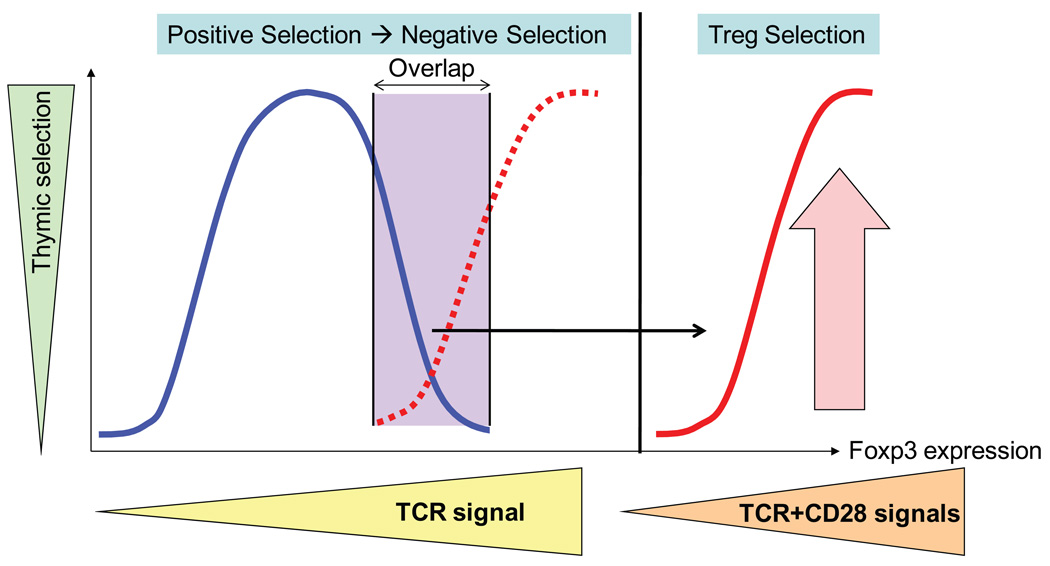
Fig. 1. Schematic model of the role of CD28 costimulation in Treg thymic development.
In the absence of CD28 costimulation, high-affinity TCR signals could have resulted in an overlap in agonist peptides inducing the selection of autoreactive effector (blue line) versus regulatory (dotted red line) T cells. CD28 costimulation raises the total strength of signal to levels not permissive for effector T-cell development but allowing the upregulation of Foxp3 expression and the effective development of Tregs (red line).
Biochemical model of the CD28-induced upregulation of Foxp3 expression
Given the critical role of Tregs in the immune system, it is surprising that very little is known about the biochemistry of signaling pathways downstream of the TCR and CD28 molecules specifically in Tregs. However, the coalescence of recent advances in the biology of effector T-cell activation together with findings identifying receptors, signaling molecules, and transcription factors important for Tregs allowed us to bring forth a model integrating CD28 costimulatory molecules and downstream signaling pathways to support Foxp3 expression and Treg homeostasis. It is important to note that this model is purely speculative and that our intent is merely to provide a conceptual framework that can be built on to formally establish the biochemistry of CD28 costimulation in Tregs. T-cell stimulation by its cognate antigen involves the formation of a stable contact between the T cell and the APC that results in the formation of an immunological synapse (IS) at their interface [reviewed in (113, 114)]. Distinct domains [supramolecular activation clusters (SMACs)] have been defined within the IS, most notably a central cluster (cSMAC) that contains TCR-CD3 complexes and a peripheral cluster (pSMAC) that contains the integrin lymphocyte function associated antigen-1 (LFA-1) (115, 116). While the cSMAC was originally believed to be the site of TCR signaling, the discovery of microclusters (MCs) containing TCR and signaling molecules and physically separated from the cSMAC led to the belief that these dynamic structures functioned as specialized signaling units in T-cell activation (117–121). In contrast, TCR molecules that accumulate in the cSMAC do not sustain signaling and appear targeted for degradation (120, 122, 123). As mentioned previously, while the mechanisms of CD28 downstream signaling are ill-defined, many kinases have been proposed to mediate CD28 costimulation including PI3K (97, 124), Lck (95, 125), TEC family kinases (126–128), thymoma viral proto-oncogene 1 (Akt) (129) as well as protein kinase C θ (PKCθ) (130). CD28 has been shown to be recruited to the immunological synapse and to colocalize with the kinase PKCθ (124, 131–135). Recently, Yokosuka et al. (136) elegantly refined the localization and role of CD28 within the immunological synapse. Indeed, although CD28 had been shown previously to accumulate in TCR microclusters before the formation of the cSMAC (131), Saito and colleagues (136) demonstrated that CD28 initially co-localized with TCR in microclusters after antigenic stimulation and segregated later on into a unique compartment of the IS located at the brim of the cSMAC. Importantly, whereas the distribution of PI3K clusters differed from CD28 MCs, PKCθ appeared to co-localize with CD28 in TCR MCs early on and at the edge of the cSMAC afterward (136). It is of note that similar results were observed using planar bilayers, artificial APCs or primary splenic DCs (136–138), demonstrating the relevance of these findings to T-cell biology in vivo. The recruitment of PKCθ into CD28-containing MCs was proposed to result in the formation of ‘costimulatory signalsomes’ that play an important role in T-cell activation; and this model is supported by the disruption of CD28/PKCθ clusters following CD28 blockade that correlated with a severe reduction in IL-2 production (136). Similarly, prevention of CD28 and PKCθ accumulation at the cSMAC secondary to a mutation in CD28 cytoplasmic tail led to a reduction in the CD28-dependent activation of nuclear factor-κB (NF-κB) (137), one of the downstream targets of PKCθ activation (139–141). Importantly, formation of the specialized MCs was influenced by the density of CD28 and B7 molecules on T cells and APCs, respectively, and by the avidity of the TCR for its cognate peptide (136). Furthermore, while the formation of CD28 MCs only hinged upon the extra-cellular interaction of CD28 with B7 molecules, the recruitment of PKCθ into CD28 MCs was completely dependent on the cytoplasmic tail of CD28 and the maintenance of these signaling units was a dynamic process requiring both new TCR-major histocompatibility complex (MHC)-peptide interactions and CD28 engagement (136–138). The residues in the CD28 tail and the associated mediators that are important for the recruitment of PKCθ into MCs are still unclear.
Although the role of lipid rafts in T-cell activation is a controversial topic in immunology (142–144), the translocation of CD28 to lipid rafts has been suggested to be important for CD28 costimulatory function (145). Recruitment of PKCθ into the IS plays a role in the ability of CD28 to localize into lipid rafts (145). Furthermore, Saito and colleagues (136) reported that the CD28-induced recruitment of PKCθ to MCs was abrogated when they used a CD28-deletion mutant lacking 16 amino acids critical for lipid raft association. Since Lck has been shown to associate with PKCθ and was proposed to be essential for PKCθ translocation into lipid rafts and the immunological synapse (125, 133, 146), it is possible that the CD28 signals that result in recruitment of PKCθ into signaling clusters and consequently T-cell activation involve CD28 association with both Lck and lipid rafts.
The link between the aforementioned studies and the role of CD28 costimulation in Tregs biology followed upon reports that described the role of lipid rafts and PKCθ, respectively, in Tregs homeostasis. Indeed, Nazarov-Stoica et al. (99) reported that the lipid components of lipid rafts were both enriched and distributed differently in Treg precursors as compared with Teff precursors in the thymus. Furthermore, the integrity of lipid rafts was necessary for the CD28-mediated stabilization of the Foxp3 message in thymic Treg precursors (99). Thus, this study suggested that lipid rafts played an integral role in the induction of Foxp3 and Treg development in the thymus by CD28 costimulatory signals. While PKCθ is critical for the activation of peripheral T cells, it is not required for their development (141). In contrast, PKCθKO mice contained greatly reduced numbers of Tregs in the thymus and the periphery and residual Tregs were characterized by a lower expression of Foxp3 by comparison with WT Tregs (147, 148), which could be significant since attenuated Foxp3 expression has been linked to defective Treg populations and development of autoimmunity (149). The Treg defect in PKCθKO mice could not be rescued by the expression of a Bcl-XL transgene (148). Furthermore, Sun and colleagues (148) found putative nuclear factor of activated T-cells (NFAT)-binding sites in the mouse Foxp3 promoter and they showed that PKCθ activated Foxp3 in a NFAT-dependent manner using a luciferase reporter assay. Although many signaling pathways are mediated through NFAT and NF-κB activity, both are believed to play a role downstream of PKCθ and they both have been proposed to play a role in Treg homeostasis (147, 150, 151). In particular, NFAT has been suggested previously to play a direct role in the induction of Foxp3 by binding to the human Foxp3 promoter and by cooperating with mothers against decapentaplegic homolog 3 (Smad3) to greatly enhance expression of Foxp3 through binding to its enhancer (151, 152).
The integration of these distinct pathways that have been described in Tregs and Teff cells allows us to propose the following hypothetical model for the role of CD28 costimulatory signals in the maintenance of Treg populations (Fig. 2): CD28 costimulation of TCR-stimulated Tregs leads to the formation of microclusters in the cSMAC where the kinase PKCθ colocalizes with CD28 and mediates its costimulatory signals. The cytoplasmic tail of CD28 is actively involved in this process, possibly for its role in docking of Lck and/or recruitment of CD28 to the lipid rafts. Downstream signals initiated by the PKCθ pathway result in the sustained expression of Foxp3 by mechanisms that are dependent on the Lck-binding domain of the CD28 cytoplasmic tail and could involve the induction of Foxp3 transcription by binding of NFAT to the Foxp3 promoter and/or enhancer as well as the stabilization of Foxp3 mRNA. Finally, the establishment of the Treg transcriptional and developmental program is ensured by the Foxp3 transcription factor and results in the development of a functional population of Tregs skewed towards the recognition of self-antigens for an efficient prevention of autoimmunity. In agreement with this model, mice deficient in CD28, PKCθ and Foxp3 are all afflicted with greatly reduced Treg populations in the thymus and periphery.
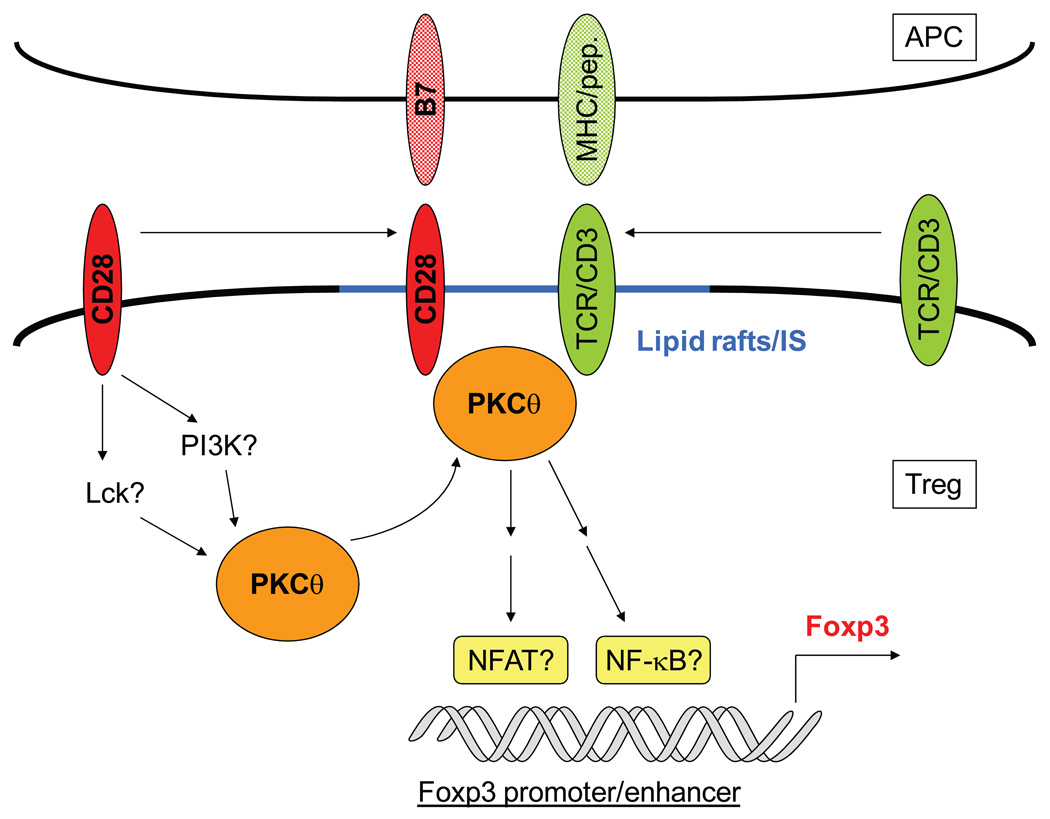
Fig. 2. Model of biochemical signals downstream of CD28 costimulation that are important for Treg homeostasis.
Upon stimulation by MHC-peptide complexes and B7 molecules, CD28 signals result in the translocation of PKCθ into lipid rafts and its recruitment into the immunological synapse (IS). These ‘signalsomes’ structures induce downstream biochemical cascades that lead to the transcription of the Foxp3 gene and the initiation of the Treg-developmental program, with the possible involvement of transcription factors NFAT and NF-κB. See text for details.
The density of CD28/B7 molecules (that could be regulated at the level of T cells or APCs) and the avidity of the TCR (which has been shown to be different between conventional and Tregs with Tregs harboring high-affinity TCRs for self-antigens) could quantitatively influence the lipid raft-dependent formation of these microclusters in effector versus Tregs and thus control CD28-dependent upregulation and stabilization of the Foxp3 transcription factor. Namely, elevated signal strength generated by combinations of high-affinity TCRs and strong CD28 costimulation seems to favor the development of Tregs. In this regard, it is intriguing that T cells with attenuated Foxp3 expression preferentially led to the development of autoreactive Th2 cells even in Th1-skewing conditions in FILIG mice (149). T-cell differentiation in Th1 versus Th2 subsets has been shown to be greatly influenced by the overall strength of signal provided by the TCR, CD28 and CTLA-4. In particular, strong signals achieved by high-affinity TCR, high CD28 costimulation or deficient CTLA-4 inhibitory signals favor differentiation into Th2 cells (51, 153–158). In an interesting parallel with the FILIG mouse phenotype (149), we showed that increased strength of signal resulting from CTLA-4 deficiency could bypass cytokine-induced signals to generate Th2 cells (153). Thus, it is tempting to complete our model by the hypothesis that decreasing signal strengths would preferentially induce the generation of Tregs, Th2 and Th1 cells in this order, and the FILIG mice suggest that a failure to reach a certain threshold of Foxp3 expression associated with high signal strength would then redirect preferentially autoreactive T cells to a self-aggressive Th2 phenotype. Finally, we should emphasize that even if the models hypothesized above prove to be correct, they do not exclude the role of other receptors and signaling pathways in the development and maintenance of Tregs. However, the fact that CD28 signals are required for Treg development in the thymus while most other major pathways such as IL-2, CTLA-4 and TGF-β regulate mainly the function or peripheral homeostasis of Tregs suggests that CD28 costimulation and strength of signals play a unique role in the thymic generation of Tregs. In addition, signaling pathways downstream of the TCR and CD28 molecules other than PKCθ could be involved in Tregs. For example, the PI3K-regulated PIP3 pathway has been suggested to play a role in the biology of Tregs and autoimmunity, yet no report of deficient Treg development in the thymus has been made to date in mice deficient for phosphatidylinositol 3 (PIP3)-related genes [reviewed in (159)].
CD28 is important for the generation of adaptive Tregs
As mentioned previously, Tregs are composed of thymically-derived CD4+CD25+Foxp3+ Tregs as well as adaptive (also called ‘induced’) Tregs that are generated from CD4+CD25−Foxp3− naive T cells in the periphery (41, 160). While adaptive Tregs can be generated through different pathways and in the context of varied immunological milieu in vitro and in vivo, a major mechanism of Treg conversion was identified as a few years ago to be dependent on TGF-β. Indeed, stimulation of CD4+CD25− naive T cells through their TCR in the presence of TGF-β resulted in the generation of a CD4+CD25+ Treg population (161–167). These Tregs appear phenotypically similar to natural Tregs as they express CD25, CTLA-4 and Foxp3 (162–164, 166) and importantly these cells are capable of suppressing effector T-cell responses in vivo and in vitro (161, 162, 164, 166, 167). Furthermore, adaptive Tregs can themselves educate CD4+CD25− T cells to convert into CD4+CD25+Foxp3+ Tregs endowed with suppressive capabilities (165). The evidence that adaptive Tregs were truly obtained by conversion of CD4+CD25− naive T cells into Tregs rather than expansion of contaminating CD25+ cells in CD4+CD25− purified populations was provided by studies demonstrating the generation of CD4+CD25+Foxp3+ adaptive Tregs from TCR-transgenic T cells on a recombination activating gene (RAG)-deficient background, e.g. bona fide naive T cells devoid of natural Tregs (168–170). The TGF-β-dependent conversion of naive T cells into adaptive Tregs has been described in vitro and in vivo and for murine as well as human T cells (161–167), suggesting that it represents a major pathway for the generation of Tregs in the periphery. The requirement for CD28 costimulation in the generation and survival of adaptive Tregs has been studied and compared to natural Tregs. Surprisingly, the role of CD28 in the generation and survival of adaptive Tregs appeared quite controversial in the recent literature. Liang et al. (171) demonstrated in an adoptive transfer model that conversion of polyclonal CD4+CD25− T cells into Foxp3+ Tregs was abrogated in B7-deficient but not in thymectomized recipients, suggesting that CD28 signals were required for the conversion and that it was strictly peripheral and did not involve the thymus. Using CD28-deficient CD4+CD25− T cells, Liu et al. (172) showed that CD28 was dispensable for the TGF-β-dependent induction of Foxp3 and conversion to Treg phenotype but it was important for the survival of these adaptive Tregs in vitro and in vivo through undefined but IL-2-independent mechanisms. While Guo et al. (173) confirmed that CD28KO cells were defective in generating adaptive Tregs, they found that the requirement for CD28 was mediated by IL-2 and could not be substituted by any other common γ-chain cytokine. Other studies substantiated that IL-2 rather than CD28 was the major regulator of TGF-β-dependent generation of adaptive Tregs and that its effect could not be recapitulated by other cytokines (164, 174, 175). However, discrepancies were reported on the requirement for IL-2 signals for the maintenance of adaptive Tregs (174, 175). Finally, we showed that treatment with anti-CD3 mAbs induced diabetes remission in NOD mice by mechanisms that involved the TGF-β-dependent generation of adaptive Tregs (161, 167). In particular, anti-CD3 mAbs could revert disease in NOD-CD28KO mice that are deficient in natural Tregs (15) and restoration of tolerance was associated with the TGF-β-dependent generation of CD4+CD25loFoxp3+ adaptive Tregs (161, 167), suggesting that CD28 was not required in this process. It is notable that one point of agreement between most studies was that CD28 was not required for the suppressive effector functions of adaptive Tregs (161, 167, 172, 173), similar to what has been reported for natural Tregs (29, 94). It is worth noting that Noelle and colleagues (176) have suggested that adaptive Treg conversion is B7 and CD40-independent but PD-1 dependent. In summary, two major conclusions can be drawn from these studies: i) the requirement for CD28 signals is not as absolute for adaptive Tregs as compared with natural Tregs and the role of CD28 costimulation is mechanistically distinct in this subset since the pathway functions indirectly through the induction of IL-2; ii) discordant results have been reported in different studies of the CD28-dependence of CD4+CD25− T cells conversion into Tregs. While the reason for these differences is not always obvious, it is likely to reflect the fact that adaptive Tregs represent a more heterogeneous population of cells than natural Tregs. Indeed, they can be generated by a variety of experimental conditions in vivo and in vitro and although they seem phenotypically homogenous, adaptive Tregs likely belong to distinct subsets whose costimulation dependence and fine functional properties will vary depending on the site and conditions of stimulation and the immunological milieu.
CTLA-4: a once controversial but acknowledged central regulator of Treg function
Why has the role of CTLA-4 in Treg biology been so difficult to establish?
CTLA-4 (CD152) is a receptor related to CD28 that displays approximately 30% homology with CD28 at the protein levels and binds the same ligands, B7-1 and B7-2, on APCs [reviewed in (46, 177)]. In conventional T cells, CTLA-4 is not expressed in the naive and resting state but it is upregulated upon T-cell activation in a CD28-dependent manner (46, 177–179). In contrast, CTLA-4 is constitutively expressed in Tregs and represents one of the hallmarks of this cell subset (15, 94). CTLA-4 has been shown to antagonize many early outcomes of effector T-cell activation including T-cell proliferation, cell-cycle progression and IL-2 production (179–181). However, the role of CTLA-4 as a negative regulator of T-cell function was not fully acknowledged until the dramatic phenotype in CTLA-4-deficient mice was described. Indeed, CTLA-4KO mice develop a lymphoproliferative disease characterized by massive T-cell activation and expansion that leads to multi-organ infiltration and causes the animal death within two-to-four weeks after birth (182–184). It has been suggested that the uncontrolled effector T-cell proliferation leading to the CTLA-4KO disease was secondary to defective peripheral tolerance to self-antigens (185). Indeed, CTLA-4 has been proposed to be important for both the induction of anergy and the maintenance of T-cell unresponsiveness as demonstrated by the abrogation of tolerance in CTLA-4-deficient TCR-transgenic T cells or following mAbs treatments blocking CTLA-4-B7 interactions (186–189). Nevertheless, the observation that recipients that received mixed CTLA-4-deficient and CTLA-4-sufficient bone marrow or spleen cells did not develop the CTLA-4KO disease led to the hypothesis that the fatal lymphoproliferation may not be entirely due to effector T-cell intrinsic mechanisms but may also result from a defect in a CTLA-4-dependent regulatory population that would control T-cell activation (190, 191).
The discovery that CD4+CD25+ Tregs suppressed T-cell function and were critical in maintaining peripheral tolerance while constitutively expressing high levels of CTLA-4 made Tregs the perfect candidate for this CTLA-4-dependent regulatory population. However, conflicting results reported on the role of CTLA-4 in Treg function rapidly made it clear that this issue was more complex than it seemed at first. The field of the CTLA-4 dependency of suppression by CD4+CD25+ Treg has been plagued by many problems that are not always trivial to address: i) historically, it has been difficult to functionally target CTLA-4 on effector T cells in vivo and in vitro and the use of different reagents and various doses contributed to the report of apparently contradictory data on the role of CTLA-4 in Treg function; ii) since CTLA-4 has been shown to be an important inhibitor of effector T cell function, it can be difficult to distinguish whether blockade of CTLA-4 signals affects Treg or Teff in experimental conditions where both subsets are present, which by design is usually the case since Treg suppression is assessed by its ability to hamper Teff proliferation or effector function in vitro or in vivo; iii) finally, using CTLA-4-deficient Tregs or Teff to evaluate the requirement for CTLA-4 signals in each population is accompanied by its own set of intricacies due to the overlapping phenotype between Teff and Treg cells in untreated mice or the approaches employed to prevent the lymphoproliferative disease that can themselves affect the T-cell subsets subsequently examined. For example, effector T-cell activation and uncontrolled proliferation can be delayed or prevented in CTLA-4KO mice by blocking CD28-B7 interactions, but this in turn can affect the development and homeostasis of Tregs. With these caveats in mind, there appears to be a growing consensus of a role for CTLA-4 in Treg function.
CTLA-4 plays a unique role in controlling Treg function and preventing autoimmunity
The controversy surrounding CTLA-4 and Tregs began with widely diverging data reported in seemingly similar in vitro suppression assays. Indeed, many investigators tested the role for CTLA-4 in Treg suppressive function by adding anti-CTLA-4 mAbs or F(ab')2 fragments into culture of CD4+CD25− Teff stimulated with APCs plus anti-CD3 (or peptide when Teff were isolated from TCR-transgenic mice) alone or in the presence of CD4+CD25+ Tregs, usually at a 1:1 or 2:1 Teff:Treg ratio. Using this assay, several groups concluded that CTLA-4 was not necessary for human and murine Treg suppression of effector proliferation in vitro (29, 192–196). In contrast, Sakaguchi and colleagues as well as our group and others found that the suppression of Teff proliferation conferred by CD4+CD25+ Tregs could be abrogated by blocking CTLA-4 using anti-CTLA-4 mAbs or Fab fragments in vitro (94, 197–201). Comparing the blocking reagents used in equivalent experimental settings pointed up that the CTLA-4 dependence of Treg suppressive function was revealed using significantly higher concentrations of anti-CTLA-4 mAbs (100 µg/ml instead of 10 µg/ml) or Fab fragments rather the whole antibody (94, 200). The importance of the dose, form, and specific preparation of anti-CTLA-4 mAbs in their ability to block Treg suppressive function in vitro was confirmed by a direct comparison in a single experimental system (202). One concern in these experiments was that the CTLA-4 blockade affected effector T cells and decreased their threshold for activation, thus rendering them more difficult to regulate and accounting for the abrogation of suppression by Tregs. However, using CD4+CD25− responder T cells isolated from CTLA-4KO mice or CTLA-4KO CTLA-4-Ig-transgenic mice, which were protected from the lymphoproliferative disease for several weeks due to the blockade of CD28-B7 interactions (156, 200, 203, 204), we and others showed that CTLA-4 blockade abrogated suppression by WT Tregs, demonstrating that CTLA-4 was indeed necessary for Treg suppressive function (94, 200). Related studies examining Treg control of effector responses following anti-CTLA-4 mAbs treatment in vivo reported that while CD4+CD25+ Tregs could prevent T cell-induced colitis and allograft rejection, CTLA-4 blockade abrogated Teff suppression and disease protection afforded by Tregs (205–207). Similar to what was observed in vitro, anti-CTLA-4 treatment resulted in a loss of protection from disease by WT Tregs even when colitis-inducing T cells were deficient for CTLA-4 (208). Taken together, these studies strongly suggest that CTLA-4 is necessary for the suppressive function of CD4+CD25+ Tregs in vitro and in vivo, independently of its potential intrinsic effect on effector T cells. Thus, we were quite surprised when we observed that CTLA-4-KO Tregs were not affected in their suppressive function of responder T cells in vitro (200). Indeed, while Takahashi et al. (94) initially reported that CD4+CD25+ T cells isolated from CTLA-4-KO mice displayed reduced suppression in vitro, these findings were confounded by the intense T-cell activation taking place in these mice that likely resulted in the presence of significant numbers of contaminating effector T cells in the Treg population sorted solely based on CD4 and CD25 expression. To circumvent this problem, we examined CD4+CD25+ regulatory T cells in CTLA-4KO mice that were either transgenic for CTLA-4-Ig or treated with anti-B7-1/B7-2 mAbs in order to delay uncontrolled T-cell activation and proliferation. By additionally using CD62L expression to discriminate Tregs from activated T cells, we were able to show that CD4+CD25+CD62Lhi Tregs developed normally in the absence of CTLA-4 signals (200). Furthermore, CTLA-4KO Tregs displayed normal suppressive capabilities in vitro, which was at odds with the results obtained by CTLA-4 blockade of WT Tregs (200). Similarly, Kataoka et al. (209) reported normal Treg numbers and suppressive function by examining CD4+CD25+ T cells in the thymus of young CTLA-4KO mice. These apparently contradictory results were reconciled by the unexpected observation that Tregs deficient in CTLA-4 suppressed responder T-cell proliferation by alternative mechanisms dependent on TGF-β, which conversely was not necessary for WT Treg function (200). Powrie and colleagues elegantly confirmed using the Foxp3 marker that the percentage of Foxp3+ Tregs within the CD4+CD25+ T cell population was unaffected in CTLA-4-deficient cells that did not undergo uncontrolled activation and proliferation as they were isolated from bone marrow chimeras generated with mixed CTLA-4 and WT cells, which do not develop the CTLA-4KO disease (190, 208). Furthermore, in a striking parallel to our in vitro data, Read et al (208) showed that CTLA-4-deficient Tregs were as efficient as WT Tregs in controlling colitis induced by CD4+CD45RBhi T cells but that they used different mechanisms of action. Indeed, while the protection conferred by WT Tregs was independent of IL-10 signaling, suppression of colitis by CTLA-4-deficient T cells could be abrogated by treatment with anti-IL-10-receptor mAbs. Collectively, these studies suggest that CTLA-4 plays a critical role in the suppressive function of Tregs even though compensatory mechanisms exist in CTLA-4-deficient mice that allow the generation of functional Tregs that control immune responses through pathways distinct from WT Tregs. Importantly, these compensatory mechanisms are not always sufficient to generate Treg populations capable of controlling effector T-cell function. Indeed, Schmidt et al. (210) recently reported that ovalbumin-specific CTLA-4-deficient Tregs were unable to control disease in an adoptive transfer model of autoimmune diabetes into recipients expressing ovalbumin in pancreatic islets. It is unclear why CTLA-4-deficient Treg cells work in some settings and not others in vivo, but it could be due to the model used to obtain donor CTLA-4KO Tregs and/or the mechanisms of action required to control disease that could be quite different between autoimmune diabetes and colitis.
An important step in the deciphering the role of CTLA-4 in Treg biology was achieved recently by the generation of CTLA-4 conditional KO (CKO) mice that harbored CTLA-4 deficiency specifically in Foxp3+ Tregs (211). In this important study, Sakaguchi and colleagues (211) demonstrated that the lack of CTLA-4 expression in Tregs leads to a fatal lymphoproliferative disease and development of multi-organ autoimmunity. Although the phenotype of CTLA-4 CKO was reminiscent of the disorder observed in CTLA-4KO mice, the later onset of disease and prolonged survival of conditional KO mice suggested that CTLA-4 is important for both effector and regulatory T cell subsets. Importantly, Wing et al (211) demonstrated that deregulated immunity in CTLA-4 CKO mice was associated with a profound defect in Treg function in vivo and in vitro. Consistent with the typical Yin-Yang outcome of the immune system on tumor surveillance versus autoimmunity, the Treg functional defect that led to autoimmune diseases in CTLA-4 CKO mice conversely resulted in enhanced tumor rejection by CTLA-4 CKO splenocytes. Of course, like all CTLA-4 studies, there remains the caveat that Foxp3 expression in these mice might be leaky and thus expressed in a small percentage of conventional T cells as has been shown in humans.
In conclusion, while the role of CTLA-4 in Treg biology remained controversial for several years, the exploitation of increasingly refined experimental systems has now established unambiguously that CTLA-4 plays a critical role in the suppressive function Tregs. Furthermore, CTLA-4 does not appear to be necessary for the development and survival of Tregs. On the contrary, it has recently been suggested that CTLA-4 could dampen the homeostasis of Tregs by regulating their expansion since defective CTLA-4 signals following mAbs blockade or in CTLA-4-deficient cells led to amplified proliferation of Tregs (210, 212). In this regard, it is intriguing that a 30–40% increase in the frequency of CD4+CD25+ Tregs was observed in the blood of healthy individuals homozygous for a single nucleotide CTLA-4 polymorphism associated with decreased susceptibility to autoimmune disease (213). In mice, CTLA-4 was selectively upregulated on recently dividing Tregs and this increase in CTLA-4 expression appeared to be directly secondary to Treg expansion (212), suggesting that CTLA-4 may be involved in a negative feedback loop to maintain the size of the Tregs compartment. Furthermore, it was recently shown that, similarly to WT mice, CD4+CD25+ cells unexpectedly contained a majority of Foxp3+ cells in untreated CTLA-4KO mice (210, 214). The overall percentage of CD4+CD25+Foxp3+ T cells was thus greatly increased in untreated CTLA-4KO mice, suggesting that CTLA-4 was important to limit the expansion and homeostasis of Tregs (210). It is notable that CTLA-4 deficiency limited to Tregs in CTLA-4 conditional KO mice did not reveal any Treg-intrinsic role of CTLA-4 with regard to Treg development, homeostatic proliferation and survival in non-inflammatory conditions, although the frequency of CD4+Foxp3+ T cells was increased in unmanipulated CTLA-4 CKO mice that develop the lymphoproliferative disease (211). To reconcile these different findings, it is tempting to speculate that the increase in the frequency of CD4+CD25+Foxp3+ T cells observed in CTLA-4KO mice (210) is not due to the preferential expansion of bona fide Tregs. Indeed, we and others have recently described using reporter mice the presence of a small but significant subset of CD4+ T cells that expressed Foxp3 at one point during their lifespan but subsequently lost Foxp3 expression (215, 216). One possible explanation for the generation of this subset is that these cells represent conventional CD4+ T cells that transiently expressed Foxp3. In fact, human CD4+CD25− T cells have been shown to transiently upregulate Foxp3 expression upon activation usually without acquisition of a suppressive phenotype (217–221). Furthermore, we performed complementation experiments in bone marrow chimera recipients of CTLA-4KO and/or Scurfy cells and demonstrated that expression of CTLA-4 and Foxp3 in cis (i.e. on the same cell) was required to completely protect recipients from developing a lymphoproliferative disease (222). Thus, it is conceivable that CTLA-4 plays a specific role in controlling conventional T cells that transiently upregulated Foxp3 following T-cell activation, either by regulating their expansion or by playing a role in the down-regulation of Foxp3. If this hypothesis proves correct, it could explain the poor suppressive ability of CD4+CD25+ isolated from untreated CTLA-4KO mice (94) despite their normal frequency of Foxp3+ cells (210). Furthermore, it is unclear if this phenomenon could play a part in the development of the lymphoproliferative disease and autoimmunity observed in CTLA-4 conditional KO mice (211).
The mechanisms of action of CTLA-4 on Tregs
Treg-intrinsic function of CTLA-4
It is quite puzzling that a consensus has never been reached on the mechanisms of action of CTLA-4 in conventional T cells more than 20 years after its initial discovery. Many distinct modes of action have been proposed to explain the inhibition of T-cell responses by CTLA-4, including cell intrinsic and cell extrinsic pathways. They have been reviewed recently and thus will only be briefly summarized below (14, 185, 223–225). The major possibilities that have been put forward are the following: i) due to its higher affinity for ligands B7-1 and B7-2, CTLA-4 effectively competes with CD28 for binding of B7 molecules and thus interferes with CD28 costimulatory signals; ii) the biochemistry of CTLA-4 signaling has been extensively studied and it has been suggested that CTLA-4 could directly inhibit TCR signaling either in a proximal or distal manner and in association with various proteins such as phosphatases; iii) CTLA-4 interaction with B7 molecules (in particular B7-1) could result in the generation of a lattice-like structure that could interfere with the formation of the immunological synapse; iv) CTLA-4 could interact with B7 molecules on dendritic cells and phenotypically and functionally modify the APCs in part through reverse-signaling; v) finally, adding to the confusion is the notion that CTLA-4 could operate in a ligand-independent manner to a certain extent although interactions with B7 seem necessary to fully recapitulate a number of CTLA-4 functional outcomes. Needless to say, it is likely that all these different mechanisms can play a part in CTLA-4 biology but the parameters that direct the use of a given mode of action in a given immunological context are unknown. The biochemical basis and functional mechanisms underlying the role of CTLA-4 in Treg function is not clearly defined either. Since CD28 and CTLA-4 seem to have assumed a certain division of labor in Treg biology, with CD28 controlling Treg development and homeostasis and CTLA-4 governing Treg function in the periphery, it seems unlikely that the role of CTLA-4 in Treg function is achieved only through competition with CD28 for B7 molecules or direct inhibition of CD28 signaling. However, the findings that strong CD28 signals provided by activated DCs can induce Treg proliferation and impair their function suggest that CTLA-4 could contribute to Treg suppression by competing with CD28 for B7 interactions and maintaining the anergic state of Tregs that has been associated with their suppressive function (226, 227). A key research area remains in deciphering the signaling pathways involved in the transduction of TCR-mediated signals in regulatory T cells. Defects in TCR-induced tyrosine phosphorylation of many proteins downstream of the TCR have been reported in CD4+CD25+ Tregs as compared with CD4+CD25− T cells, including CD3 ζ chain, Src homology 2 domain containing leukocyte protein of 76 kDa (SLP76) and extracellular signal-related kinase 1/2 (Erk1/2) while the jury is still out regarding impaired Akt phosphorylation (228–231). Flores-Borja et al. (231) recently reported that CTLA-4 ligation further dampened signaling pathways downstream of the TCR in Tregs. It is difficult to predict the consequences of CTLA-4 signals on TCR-induced biochemical pathways specifically in Tregs given the number of unknowns both in the very existence of Treg-specific TCR signaling pathways and the possible influence of CTLA-4 on these pathways (Fig. 3). However, it is plausible that the biochemical mechanisms of CTLA-4 function in Tregs could be quite different from conventional T cells. Indeed, the different pattern of CTLA-4 expression characterized by constitutive expression in Tregs versus upregulation following T-cell activation in naive T cells conceivably could provide a distinct set of biochemical targets for CTLA-4 in Tregs versus conventional T cells. While the biochemistry of CTLA-4 signaling in Tregs is ill-defined, three major functional outcomes of CTLA-4 signals have been proposed to be important for its control of Treg function. While many mechanisms have been proposed to be responsible for Treg suppressive function, a number of studies have emphasized the importance of the immunosuppressive cytokine TGF-β. Indeed, TGF-β has been shown to be produced by Tregs and to be critical for their control of effector function in a number of in vivo settings, including inflammatory bowel disease (IBD), autoimmune diabetes, leishmania skin infection and tumor immunity [reviewed in (44)]. Furthermore, CTLA-4 ligation has been proposed to induce the production of TGF-β by murine T cells that could contribute to the suppression of immune responses (232, 233), although CTLA-4 was found to function independently of TGF-β in a distinct TCR-transgenic model (234). Taken together, these data led to the hypothesis that CTLA-4 could at least in part function by favoring the production of TGF-β by Tregs. In this regard, CTLA-4 signals were shown to selectively increase the production of TGF-β but not other cytokines by CD4+CD25+ Tregs in a setting where TGF-β was required for Treg-mediated suppression (235). Conversely, we found that suppression by CTLA-4KO Tregs but not WT Tregs was dependent on TGF-β in vitro (200), suggesting that while both pathways could be important for Tregs suppression, they may do so through independent pathways (Fig. 3). The direct comparison of the role of CTLA-4 and TGF-β in Treg-mediated prevention of autoimmune diabetes further supported this hypothesis (236). Indeed, while CTLA-4 and TGF-β synergized to control autoimmunity in NOD mice, the blockade of TGF-β but not CTLA-4 resulted in acceleration of diabetes in natural Treg-deficient NOD-CD28KO mice (236). Taken together with the report that CTLA-4 is required for the TGF-β-mediated induction of adaptive Tregs (237) and with our previous observation that TGF-β was important for the generation of adaptive Tregs in NOD-CD28KO mice (161, 167), these results suggest that CTLA-4 and TGF-β could function differently in natural and adaptive Tregs. Thus, CTLA-4 could control the suppressive function of natural Tregs independently of TGF-β, while CTLA-4 and TGF-β could synergize for the generation and/or function of adaptive Tregs (Fig. 3).
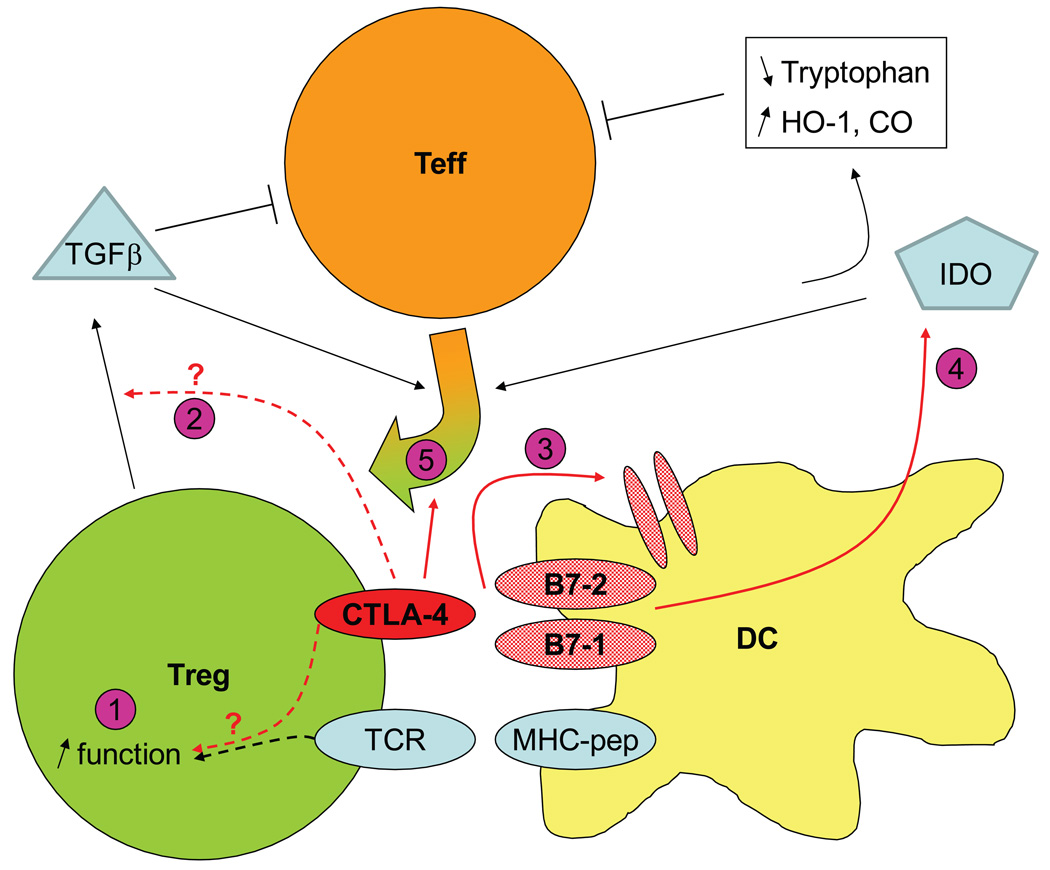
Fig. 3. Model of pathways involved in CTLA-4 control of Treg function.
CTLA-4 could influence Treg suppressive through different pathways. In Treg-intrinsic pathways, CTLA-4 could alter TCR-mediated signaling and lead to increased suppression (1) and induce the production of immunosuppressive TGF-β, although TGF-β may function independently from CTLA-4 (2). In Treg-extrinsic pathways, CTLA-4 alters DCs by triggering the down-modulation of B7-1 and B7-2 (3) and the production of IDO (4) that interferes with activation of Teff by activating tryptophan catabolism and/or through downstream mediators HO-1 and CO. Finally, CTLA-4 is necessary for the generation of adaptive Tregs induced by TGF-β or IDO (5). See text for details.
CTLA-4-dependent modification of APCs by Tregs
As mentioned previously, the mechanisms of action of Treg suppressive function are still a matter of intense debate. While early data obtained in suppression assays in vitro led to the hypothesis that Treg suppressive function may involve direct contact with effector T cells [reviewed in (44)], imaging studies revealed that Tregs did not interact directly with effector T cells but instead aggregated around APCs in vitro (238, 239). Furthermore, we demonstrated that direct contact between Tregs and APCs rather than effector T cells was not a peculiarity of in vitro systems but could be observed in lymph nodes in situ using two-photon microscopy (240). Importantly, interactions of Tregs and APCs were associated with a dramatic decrease in the ability of APCs to stimulate effector T cells, suggesting that an important mean of Treg suppression of immune responses is achieved through their alteration of APC function (240). Considering the critical role of CTLA-4 in Treg-suppressive function and the expression of its B7 ligands on APCs, it became apparent that CTLA-4 could play a role in the cross-talk between Tregs and APCs that leads to their diminished stimulatory function. However, CTLA-4 did not appear to control the aggregation of Tregs around DCs whereas LFA-1 was critical for this process (238). Alternatively, two major mechanisms have been proposed to support a role for CTLA-4-mediated Treg alteration of APC function, namely the downregulation of costimulatory molecules on APCs and the production of the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO). Indeed, it has been shown in vitro that culture of CD4+CD25+ Tregs, but not CD4+CD25− effector T cells, with dendritic cells led to a significant reduction in the expression levels of costimulatory molecules B7-1 and B7-2 on DCs (241). The down-modulation of B7 molecules on DCs induced by Tregs was observed using murine or human Tregs as well as natural or adaptive Tregs and it was dependent on direct contacts between Tregs and APCs (241–243). Importantly, downregulation of B7 expression on DCs was dependent on expression of CTLA-4 on Tregs (210, 238, 244) (Fig. 3). Indeed, both the decrease in B7 expression on DCs and the subsequent alteration in APC function induced by Tregs could be prevented by the use of anti-CTLA-4 mAbs or CTLA-4-deficient Tregs (210, 238, 244). Similar results were observed using CTLA-4 conditional KO Tregs (211). Downmodulation of B7 molecules can be induced by Tregs on DCs as well as B cells, although the extent of downmodulation was more pronounced in DCs (210, 238). Finally, it is notable that the modification of APCs phenotype and function by Tregs resulted in decreased expression of B7-1 and B7-2 molecules but not other proteins important for APC function such as MHC class II molecules or CD40 (210, 211, 238, 242). While the mechanisms underlying the diminution in expression of B7 molecules on DCs after direct interaction with Tregs are unknown, they were shown to result from down-modulation of B7 molecules rather than masking by soluble CTLA-4 (211). It has been shown previously that T cells can uptake various molecules from the APC surface membrane, a phenomenon more generally referred to as trogocytosis, including MHC molecules and B7 molecules through CD28 [reviewed in (245)]. Thus, given the unique role of CTLA-4 in Treg function and the selective Treg-mediated down-modulation of B7 molecules on APCs, it is tempting to speculate that CTLA-4-mediated absorption of B7 molecules on APCs by Tregs could contribute to altering APC stimulatory function and thus to Treg suppression of effector T-cell responses. The second mechanism that has been invoked in the CTLA-4-mediated alteration of APCs by Tregs entails the production of IDO by APCs. Indeed, Fallarino et al. (246) demonstrated that mouse CD4+CD25+ Tregs initiated tryptophan catabolism in DCs through the production of IDO and that this process was dependent on interactions between CTLA-4 on Tregs and B7 molecules on DCs (246) (Fig. 3). These results were reminiscent of previous studies showing that treatment with CTLA-4-Ig could induce IDO production in DCs (247) and that IDO-producing DCs seem to be important in downregulating T-cell responses and maintaining T-cell tolerance in a number of settings such as fetal tolerance, transplantation, autoimmunity, and tumor immunity [reviewed in (248)]. The exact mechanism(s) by which IDO would regulate effector T-cell responses is still unknown and while it is clear that Tregs can stimulate IDO production by DCs in a CTLA-4-dependent manner (249, 250), the formal demonstration that IDO is necessary for Treg function has yet to come. IDO was originally proposed to inhibit effector T cell by triggering the degradation of tryptophan and thus affect T-cell function and/or survival (Fig. 3). An interesting alternative hypothesis has been recently put forward by several investigators (251, 252). A metabolite of IDO has been shown to increase the expression of hemeoxygenase-1 (HO-1) (253), an enzyme that catalyzes the formation of carbon monoxide and that is upregulated on Tregs and has been proposed to play a role in their suppressive function (254–257). Interestingly, HO-1-deficient mice exhibited defective immune regulation that was not due to an intrinsic Treg defect but involved HO-1 deficiency in APCs (258). Taken together with the report that carbon monoxide (CO) could inhibit T-cell proliferation and IL-2 production (259), these findings suggest that Treg-induced and CTLA-4-mediated production of IDO by DCs could suppress immune responses partially through the influence of downstream metabolites HO-1 and CO (Fig. 3). Furthermore, since the inhibitory action of CO on T cells was effective only if administered early in T-cell activation (259), it is possible that the role of CTLA-4 in Treg function involves concerted actions on DCs that lead to down-modulation of B7 molecules, thus dampening and delaying efficient effector T-cell stimulation, and inhibition of effector T-cell activation or survival through IDO, HO-1 and CO. Finally, the role of IDO in the cross-talk between Tregs and DCs could also entail a positive feedback loop, since IDO has been proposed to be essential for the generation of adaptive Tregs and to augment the suppressive capability of natural Tregs in a pathway that required CTLA-4 (260–262) (Fig. 3). Hence, CTLA-4 could control Tregs function at various levels in Treg intrinsic and extrinsic fashion, and it is likely that his unique role for Treg suppression ability stems from its influence in these multiple pathways.
The overlapping but not identical role of CD28/CTLA-4 ligands B7-1 and B7-2
The biology of B7-1 and B7-2 interactions with CD28 and CTLA-4
The complexity of the CD28/CTLA-4/B7 system is greatly widened by the biochemical and biological properties of the B7-1 and B7-2 ligands [reviewed in (45, 46)]. Indeed, both B7-1 and B7-2 can bind the conserved amino acid motif MYPPPY in the extracellular domain of CD28 and CTLA-4, resulting in partially overlapping function (203, 263). However, the relative avidity of the different receptor-ligand pairs as well as distinct kinetics of expression provide a structural basis for the profound differences that have been reported regarding the role of B7-1 and B7-2 in T-cell function (45, 46). Major clues to the characteristics of B7-1 and B7-2 binding to CD28 and CTLA-4 were provided by the crystal structure of B7-1 and B7-2 complexes with CTLA-4 and biophysical studies that quantitatively addressed the attribute of each receptor-ligand interaction (264–268). These reports revealed striking dissimilarities in the affinity and avidity of the different combinations. Indeed, the B7-1 binding affinity to both CD28 and CTLA-4 is five-to-ten fold greater than the affinity of B7-2, and CTLA-4 binds both ligands with higher affinity than CD28 (267). When considering the relative affinity of B7 molecules for CD28 and CTLA-4, it turns out that B7-2 binds CD28 more efficiently than B7-1. Furthermore, although both B7-1 and B7-2 were originally proposed to dimerize (264, 265, 268), the stability of B7-2 dimers was subsequently questioned and it is now clear that B7-1 forms homodimers while B7-2 functions in a monomeric form (266, 267). On the T cell side, both CD28 and CTLA-4 can form homodimers but CD28 homodimers appear monovalent while CTLA-4 homodimers are bivalent (267). Taken together, these results suggest gradually increasing levels of complexity from the interaction of a single CD28 homodimer with a single B7-2 monomer up to an organized lattice resulting from the binding of bivalent CTLA-4 dimers to B7-1 homodimers. It is likely that this very distinct organization between different ligand-receptor pairs greatly modifies the avidity of these various interactions, which already display a vast array of affinities (267). Thus, this model predicts that B7-1 will interact with CTLA-4 with an avidity that could be several orders of magnitude greater than the binding of B7-2 to CD28. However, the relative binding ratio of CD28 compared to CTLA-4 will be higher when B7-2 is expressed on APCs than when B7-1 is expressed. In addition to this biochemical component, the biology of B7-1 and B7-2 expression is bound to influence the attributes of their interaction with CD28 and CTLA-4. Indeed, B7-2 is constitutively expressed on APCs while B7-1 in up-regulated following their activation and maturation. Considering the parallel pattern of expression of CD28 and CTLA-4 on conventional T cells, this led to the model that CD28 interactions with B7-2 will govern early T-cell activation events in lymph nodes whereas CTLA-4 binding to B7-1 will play a more prominent role in the termination of immune responses, particularly in peripheral tissues. This model has been validated in the NOD mouse where blocking B7-2 leads to protection from diabetes while blocking B7-1 accelerates disease in NOD mice and in other in vivo models of autoimmunity or transplantation (46, 111, 269–272). However, other studies have observed a worsening of disease following anti-B7-2 but not anti-B7-1 mAbs treatment, emphasizing the complexity of these interactions and the difficulty to predict the outcome depending on the nature of the T-cell stimulus and the immunological milieu. Furthermore, many of these studies ought to be re-examined in the context of the knowledge of the importance of CD28 and CTLA-4 signals in Treg biology. The general model of preferential interaction between CD28/B7-2 and CTLA-4/B7-1 that has been established based on data obtained in conventional T cells seems to hold true for regulatory since similar outcome on Treg function were found after blockade of CD28 and B7-2 versus CTLA-4 and B7-1 (227, 273). However, a clear difference between naive and regulatory T cells is the constitutive expression of CD28 and CTLA-4 in Tregs. Therefore, it is the differential expression of B7-1 and B7-2 ligands on APCs that will likely govern the hierarchy of interactions between B7-1/B7-2 and CD28/CTLA-4. However, there is not doubt that the biology is complicated on Tregs. In the broadest sense a major question remains. If CTLA-4 is constitutively expressed in Tregs and has a higher affinity for both the B7-1 and B7-2 ligands, then why does CTLA-4 effectively competes with CD28 for binding of B7. In other words, how do Tregs receive the CD28 costimulatory signal? There will need to be more work in this area where context of the two molecules on the cell surface, intracellular signaling and altered avidity are all likely to influence the integration of these signals within the Treg cell.
Another crucial distinction between Tregs and conventional T cells is that whereas CD28 and CTLA-4 respectively induce positive and negative signals in conventional T cells, the two receptors influence Treg biology at different levels since CD28 is necessary for Treg homeostasis and CTLA-4 for Treg function. Furthermore, CD28 interaction with B7 molecules and especially B7-2 has been shown to induce proliferation of Tregs, breaking their anergic state and suppressive function, when Tregs are stimulated in the presence of mature activated dendritic cells (226, 227). This process allows the full activation of effector T cells in order to mount an effective immune response until IL-2 produced by Teff increases the competitive fitness of Tregs (86) and initiates the negative feedback loops necessary to terminate immune responses and reset the system to normal homeostasis. The influence of the CD28/CTLA-4/B7 system of costimulation on immune responses has thus reached a new level of complexity, since the prediction of the outcome of costimulation on a given response will now have to take into account not only the differential interaction of B7-1 and B7-2 with CD28 and CTLA-4 on effector T cells but also which cell subset will be dominant as a result of B7 interactions with CD28/CTLA-4 on effector versus Tregs. This paradigm is well illustrated by our findings in the NOD mouse model that we describe below.
Common versus specific features of B7-1 and B7-2 costimulation in the balance of effector and Tregs
Although B7-1 and B7-2 bind the same receptors, it has become clear that they have distinct effects. NOD mice deficient for B7-2 are completely protected from diabetes, which is in striking contrast with the acceleration of disease observed in NOD-B7-1KO and NOD-CD28KO mice (15, 24). We found that whereas a profound Treg deficiency allowed autoreactive T cells to aggressively induce diabetes in NOD-CD28KO mice, the balance was shifted towards regulation in NOD-B7-2KO mice. B7-2 deficiency led to a 20 to 30% decrease in the frequency of CD4+CD62LhiCD25+ Tregs cells but the effect was more pronounced on effector T cells with a striking defect in autoreactive T-cell activation that allowed even a reduced Treg population to keep them in check (24). Interestingly, Tregs were depleted and diabetes was restored after treatment of NOD-B7-2KO mice with anti-B7-1 mAbs, suggesting that B7-1 alone was sufficient to maintain a peripheral Treg population capable of controlling autoimmunity. A similar decrease in the percentage of Tregs has been reported in NOD-B7-1KO mice (274) and this does not appear to be a peculiarity of the autoimmune-prone NOD background since a 20–30% reduction in the percentage of Tregs was observed in C57BL/6 mice deficient for B7-1 or B7-2 (275). Thus, the expression of B7-1 only or B7-2 only is sufficient to support the generation of a significant population of Tregs. It is unclear whether the small defect in Treg numbers in single B7-deficient mice is due to a unique non-overlapping function of each molecule or to the overall level of expression of B7 molecules. Indeed, we showed that heterozygous NOD-B7-1/2+/− mice displayed a 37% reduction in the percentage of CD4+CD25+ Tregs, suggesting that Tregs homeostasis is affected when B7 expression is maintained below a certain threshold (15). It is intriguing that although they display a similar reduction in the frequency of Tregs (24, 274), NOD mice deficient in B7-1 or B7-2 display completely opposite phenotypes (24, 46) Similar results were observed in mice treated with anti-B7-1 or anti-B7-2 mAbs (111). Considering what is now known regarding the preferential interaction of CTLA-4 with B7-1 and the important role of CTLA-4 in Treg function but not homeostasis, it is tempting to revisit, and perhaps reinterpret these studies to suggest that blockade of B7-1 mainly affected its interaction with CTLA-4 and resulted in defective intrinsic inhibition of autoreactive T cells and insufficient suppression by Tregs that both contributed to acceleration of disease. In agreement with this hypothesis, we observed that B7-1 blockade was associated with an earlier and more severe insulitis and with increased activation of infiltrating T cells (111). In contrast, B7-2 deficiency primarily altered the activation of autoreactive T cells while preserving immune regulation, thus leading to prevention of diabetes (24). We examined whether this model would hold true in NOD mice heterozygous for each or both B7 molecules, as we reasoned that this might more accurately reflect a therapeutic situation where only partial blockade of B7 molecules can be achieved in vivo. After having verified that the expression of B7 molecules was decreased in the respective NOD-B7-heterozygous mice (data not shown), we compared the percentage of CD4+Fopx3+ Tregs in the spleen as well as the frequency of IGRP-specific CD8+ T cells, which have been shown to be important for the development of diabetes in NOD mice (276). Similar to what we had shown previously using CD25 as a Treg marker, the frequency of CD4+Foxp3+ Tregs was decreased by approximately 40% in NOD mice heterozygous for both B7-1 and B7-2 (Fig. 4A) (H. Bour-Jordan, J.A. Bluestone, unpublished observations). In contrast, the influence of single B7-1 or B7-2 deficiency on Tregs was minor with reductions of only 5 to 18% in NOD mice heterozygous for B7-1 or B7-2 as compared to WT mice. The pattern observed for effector T cells was quite different. Indeed, while decreased expression of B7-1 hardly affected the frequency of IGRP-specific T cells, suboptimal expression of B7-2 in NOD-B7-2+/− mice strikingly reduced this population of autoreactive T cells, suggesting that expression of B7-2 had fallen below a threshold required to efficiently costimulate the activation of autoreactive T cells (Figure 4B). Thus, these results suggest that B7-1 interactions with CD28 were sufficient to maintain almost normal Treg numbers but not to generate effector T cells. It is unclear why the threshold of B7-2/CD28 signaling was different in Treg and Teff cells but it is possible that it is related to the skewing of Tregs for high-affinity autoreactive TCRS (69–73). In NOD-B7-1/2+/− mice, we found an ‘all or none’ pattern where most mice had very reduced islet specific glucose-6-phosphatase (IGRP)-specific T cells, similar to NOD-B7-2+/− mice, but one mouse had a frequency of NRP-V7 tetramer positive cells falling within the range observed in normal mice. We interpret these data by examining the individual influence of B7-1 and B7-2 on the balance of Treg versus Teff cells. Indeed, as illustrated in Fig. 4C, reduced expression of B7-1 had a limited and equivalent influence on the frequency of Tregs and Teff as compared to WT mice whereas decreased B7-2 levels greatly affected Teff cells but not the Treg population. In NOD-B7-1/2+/− mice, examining the average values showed that both Tregs and Teff were altered as a consequence of reduced expression of both B7-1 and B7-2 (Fig. 4C) but analyzing individual mice revealed that while suboptimal B7-2 expression had a dominant effect on Teff cells in most cases, the reduction in Treg cells could allow the generation CD28-independent autoreactive cells in some cases (Fig. 4B). This result is reminiscent of the exacerbated form of disease developing in NOD-CD28KO mice and the restoration of insulitis and diabetes in NOD-CD28KO-CD40LKO by comparison with NOD-CD40LKO mice which are completely protected from disease and free of insulitis (15, 24). From a clinical standpoint, these results suggest that targeting B7-2 alone rather than both B7-1 and B7-2 may be a better option to achieve immunological tolerance, especially in settings where initial antigen presentation can take place under the cover of immunotherapy such as transplantation. Taken together, these different studies emphasize the critical role of costimulation in the biology of Tregs and their balance with effector T cells, and they underline the unexpected finding that altered costimulation resulting in deficient Treg populations can bypass the need for major costimulatory signals such as CD28 and CD40L for the generation of autoreactive T cells leading to autoimmune attacks in genetically-prone individuals.
Fig. 4. Influence of B7-1 and B7-2 molecules on Treg homeostasis versus generation of autoreactive T cells.
Spleen and pancreatic lymph nodes were isolated from WT NOD mice or NOD mice heterozygous for B7-1 or B7-2 alone or for both B7-1 and B7-2. (A) The percentage of CD4+Foxp3+ Tregs was examined by flow cytometry in the spleen. (B) The percentage of autoreactive IGRP-specific CD8+ T cells was evaluated by flow cytometry using NRP-V7-H-2Kd tetramers (provided by the NIH MHC Tetramer Core Facility, Atlanta, GA) and anti-CD8 and anti-CD44 mAbs. Results shown were obtained in pancreatic lymph nodes and similar results were obtained in the spleen. (C) Ratio of the mean frequency of Treg (closed bar) and Teff (IGRP-specific T cells, hatched bar) in indicated NOD-B7-het mice divided by the frequency in NOD WT mice.
Conclusions
A clearer picture of the role of CD28/CTLA-4/B7 costimulation in Treg biology is starting to emerge after an initial period of confusion. Both CD28 and CTLA-4 are now established as critical regulators of Treg homeostasis and function. The critical role of CD28 for the generation and maintenance of Tregs in the periphery shed a new light on the overall function of CD28 in the immune system. Originally described as the quintessential positive costimulator of immune responses, CD28 now appears to exert dual actions in T-cell biology, allowing immune responses to efficiently mount T-cell responses against pathogens while at the same time setting the regulatory mechanisms needed to terminate responses and prevent autoimmunity. CTLA-4, on the other hand, combines immunoregulatory mechanisms by controlling Treg suppressive function as well as intrinsically inhibiting effector T-cell function and thus emerges as one of the central inhibitory molecules of the immune system. Manipulating these pathways for therapeutic purposes thus still holds tremendous promises but designing these immunotherapies will have to be carefully tailored to account for the remarkable impact of costimulation on both effector and regulatory arms of immune responses.
Acknowledgements
The authors thank the members of the Bluestone laboratory for the many experiments that represent the basis for this review. We also wish to thank Drs. Abul Abbas, Qizhi Tang, Xuyu Zhou, Cristina Penaranda and Jonathon Esensten for critical reading of the manuscript and helpful suggestions. This research was supported by grants from the JDRF, NIAID and NIDDK.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 3.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng MH, Shum AK, Anderson MS. What's new in the Aire? Trends Immunol. 2007;28:321–327. doi: 10.1016/j.it.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 6.Itoh M, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 7.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 9.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 10.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 12.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Newell KA. The role of positive costimulatory molecules in transplantation and tolerance. Curr Opin Organ Transplant. 2008;13:366–372. doi: 10.1097/MOT.0b013e328306115b. [DOI] [PubMed] [Google Scholar]
- 14.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 15.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 16.Hippen KL, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroemer A, et al. OX40 controls functionally different T cell subsets and their resistance to depletion therapy. J Immunol. 2007;179:5584–5591. doi: 10.4049/jimmunol.179.8.5584. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol Lett. 2005;101:210–216. doi: 10.1016/j.imlet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Nitric oxide induces CD4+CD25+ Foxp3 regulatory T cells from CD4+CD25 T cells via p53, IL-2, and OX40. Proc Natl Acad Sci U S A. 2007;104:15478–15483. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, et al. Agonistic anti-4-1BB antibody promotes the expansion of natural regulatory T cells while maintaining Foxp3 expression. Scand J Immunol. 2007;66:435–440. doi: 10.1111/j.1365-3083.2007.01994.x. [DOI] [PubMed] [Google Scholar]
- 21.Choi BK, et al. 4-1BB-dependent inhibition of immunosuppression by activated CD4+CD25+ T cells. J Leukoc Biol. 2004;75:785–791. doi: 10.1189/jlb.1003491. [DOI] [PubMed] [Google Scholar]
- 22.Chen M, Xiao X, Demirci G, Li XC. OX40 controls islet allograft tolerance in CD154 deficient mice by regulating FOXP3+ Tregs. Transplantation. 2008;85:1659–1662. doi: 10.1097/TP.0b013e3181726987. [DOI] [PubMed] [Google Scholar]
- 23.Vu MD, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–2510. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumanogoh A, et al. Increased T cell autoreactivity in the absence of CD40-CD40 ligand interactions: a role of CD40 in regulatory T cell development. J Immunol. 2001;166:353–360. doi: 10.4049/jimmunol.166.1.353. [DOI] [PubMed] [Google Scholar]
- 26.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 27.Shevach EM, Stephens GL. The GITR-GITRL interaction: costimulationor contrasuppression of regulatory activity? Nat Rev Immunol. 2006;6:613–618. doi: 10.1038/nri1867. [DOI] [PubMed] [Google Scholar]
- 28.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci U S A. 2008;105:973–978. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L. Mature mainstream TCR alpha beta+CD4+ thymocytes expressing L-selectin mediate “active tolerance” in the nonobese diabetic mouse. J Immunol. 1998;161:2620–2628. [PubMed] [Google Scholar]
- 31.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 34.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 36.Bacchetta R, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 38.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 39.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 40.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 46.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 47.Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7. doi: 10.1023/a:1014256417651. [DOI] [PubMed] [Google Scholar]
- 48.Tada Y, et al. CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–208. [PubMed] [Google Scholar]
- 49.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahinian A, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 51.Lenschow DJ, et al. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 52.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meagher C, et al. Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J Immunol. 2008;180:7793–7803. doi: 10.4049/jimmunol.180.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kundig TM, et al. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 55.Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664–669. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- 56.Lohr J, Knoechel B, Kahn EC, Abbas AK. Role of B7 in T cell tolerance. J Immunol. 2004;173:5028–5035. doi: 10.4049/jimmunol.173.8.5028. [DOI] [PubMed] [Google Scholar]
- 57.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 58.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 59.de Jong YP, et al. Blocking inducible co-stimulator in the absence of CD28 impairs Th1 and CD25+ regulatory T cells in murine colitis. Int Immunol. 2004;16:205–213. doi: 10.1093/intimm/dxh019. [DOI] [PubMed] [Google Scholar]
- 60.Johnson BD, Konkol MC, Truitt RL. CD25+ immunoregulatory T-cells of donor origin suppress alloreactivity after BMT. Biol Blood Marrow Transplant. 2002;8:525–535. doi: 10.1053/bbmt.2002.v8.pm12434947. [DOI] [PubMed] [Google Scholar]
- 61.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 62.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 63.Kuniyasu Y, Takahashi T, Itoh M, Shimizu J, Toda G, Sakaguchi S. Naturally anergic and suppressive CD25(+)CD4(+) T cells as a functionally and phenotypically distinct immunoregulatory T cell subpopulation. Int Immunol. 2000;12:1145–1155. doi: 10.1093/intimm/12.8.1145. [DOI] [PubMed] [Google Scholar]
- 64.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 65.Hori S, Haury M, Lafaille JJ, Demengeot J, Coutinho A. Peripheral expansion of thymus-derived regulatory cells in anti-myelin basic protein T cell receptor transgenic mice. Eur J Immunol. 2002;32:3729–3735. doi: 10.1002/1521-4141(200212)32:12<3729::AID-IMMU3729>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 66.Fisson S, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci U S A. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 70.Hsieh CS, Rudensky AY. The role of TCR specificity in naturally arising CD25+ CD4+ regulatory T cell biology. Curr Top Microbiol Immunol. 2005;293:25–42. doi: 10.1007/3-540-27702-1_2. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 72.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 73.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 74.Samy ET, Parker LA, Sharp CP, Tung KS. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamazaki S, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin CH, Hunig T. Efficient expansion of regulatory T cells in vitro and in vivo with a CD28 superagonist. Eur J Immunol. 2003;33:626–638. doi: 10.1002/eji.200323570. [DOI] [PubMed] [Google Scholar]
- 77.Beyersdorf N, et al. Selective targeting of regulatory T cells with CD28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202:445–455. doi: 10.1084/jem.20051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Evans EJ, et al. Crystal structure of a soluble CD28-Fab complex. Nat Immunol. 2005;6:271–279. doi: 10.1038/ni1170. [DOI] [PubMed] [Google Scholar]
- 79.Linsley PS. New look at an old costimulator. Nat Immunol. 2005;6:231–232. doi: 10.1038/ni0305-231. [DOI] [PubMed] [Google Scholar]
- 80.Luhder F, et al. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med. 2003;197:955–966. doi: 10.1084/jem.20021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 82.Hombach AA, Kofler D, Hombach A, Rappl G, Abken H. Effective proliferation of human regulatory T cells requires a strong costimulatory CD28 signal that cannot be substituted by IL-2. J Immunol. 2007;179:7924–7931. doi: 10.4049/jimmunol.179.11.7924. [DOI] [PubMed] [Google Scholar]
- 83.Golovina TN, et al. CD28 costimulation is essential for human T regulatory expansion and function. J Immunol. 2008;181:2855–2868. doi: 10.4049/jimmunol.181.4.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Earle KE, et al. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin Immunol. 2005;115:3–9. doi: 10.1016/j.clim.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Putnam AL, et al. Expansion of Human Regulatory T Cells from Patients with Type 1 Diabetes. Diabetes. 2008 doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 87.Furtado GC, Curottode Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 89.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3+ CD25+ CD4+ regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolf M, Schimpl A, Hunig T. Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(−) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol. 2001;31:1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 91.D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 92.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 93.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holdorf AD, et al. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J Exp Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marengere LE, et al. The SH3 domain of Itk/Emt binds to proline-rich sequences in the cytoplasmic domain of the T cell costimulatory receptor CD28. J Immunol. 1997;159:3220–3229. [PubMed] [Google Scholar]
- 97.Pages F, et al. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 98.Prasad KV, et al. T-cell antigen CD28 interacts with the lipid kinase phosphatidylinositol 3-kinase by a cytoplasmic Tyr(P)-Met-Xaa-Met motif. Proc Natl Acad Sci U S A. 1994;91:2834–2838. doi: 10.1073/pnas.91.7.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nazarov-Stoica C, Surls J, Bona C, Casares S, Brumeanu TD. CD28 signaling in T regulatory precursors requires p56lck and rafts integrity to stabilize the Foxp3 message. J Immunol. 2009;182:102–110. doi: 10.4049/jimmunol.182.1.102. [DOI] [PubMed] [Google Scholar]
- 100.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 101.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8:277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 102.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 103.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 104.Burr JS, et al. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 105.Apostolou I, Sarukhan A, Klein L, Von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 106.Graham DB, et al. CD28 ligation costimulates cell death but not maturation of double-positive thymocytes due to defective ERK MAPK signaling. J Immunol. 2006;177:6098–6107. doi: 10.4049/jimmunol.177.9.6098. [DOI] [PubMed] [Google Scholar]
- 107.Kishimoto H, Sprent J. Several different cell surface molecules control negative selection of medullary thymocytes. J Exp Med. 1999;190:65–73. doi: 10.1084/jem.190.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Punt JA, Havran W, Abe R, Sarin A, Singer A. T cell receptor (TCR)-induced death of immature CD4+CD8+ thymocytes by two distinct mechanisms differing in their requirement for CD28 costimulation: implications for negative selection in the thymus. J Exp Med. 1997;186:1911–1922. doi: 10.1084/jem.186.11.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anderson MS, Bluestone JA. The NOD Mouse: A Model of Immune Dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 111.Lenschow DJ, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- 113.Bromley SK, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 114.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 115.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 116.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 117.Bunnell SC, et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 120.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 122.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 123.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 124.Sanchez-Lockhart M, et al. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 125.Liu Y, Witte S, Liu YC, Doyle M, Elly C, Altman A. Regulation of protein kinase Ctheta function during T cell activation by Lck-mediated tyrosine phosphorylation. J Biol Chem. 2000;275:3603–3609. doi: 10.1074/jbc.275.5.3603. [DOI] [PubMed] [Google Scholar]
- 126.August A, Gibson S, Kawakami Y, Kawakami T, Mills GB, Dupont B. CD28 is associated with and induces the immediate tyrosine phosphorylation and activation of the Tec family kinase ITK/EMT in the human Jurkat leukemic T-cell line. Proc Natl Acad Sci U S A. 1994;91:9347–9351. doi: 10.1073/pnas.91.20.9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gibson S, August A, Branch D, Dupont B, Mills GM. Functional LCK Is required for optimal CD28-mediated activation of the TEC family tyrosine kinase EMT/ITK. J Biol Chem. 1996;271:7079–7083. doi: 10.1074/jbc.271.12.7079. [DOI] [PubMed] [Google Scholar]
- 128.Yang WC, Ghiotto M, Barbarat B, Olive D. The role of Tec protein-tyrosine kinase in T cell signaling. J Biol Chem. 1999;274:607–617. doi: 10.1074/jbc.274.2.607. [DOI] [PubMed] [Google Scholar]
- 129.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 130.Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P, Altman A. A novel functional interaction between Vav and PKCtheta is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 131.Andres PG, Howland KC, Dresnek D, Edmondson S, Abbas AK, Krummel MF. CD28 signals in the immature immunological synapse. J Immunol. 2004;172:5880–5886. doi: 10.4049/jimmunol.172.10.5880. [DOI] [PubMed] [Google Scholar]
- 132.Bromley SK, et al. The immunological synapse and CD28–CD80 interactions. Nat Immunol. 2001;2:1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 133.Huang J, et al. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc Natl Acad Sci U S A. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 135.Tseng SY, Liu M, Dustin ML. CD80 cytoplasmic domain controls localization of CD28, CTLA-4, and protein kinase Ctheta in the immunological synapse. J Immunol. 2005;175:7829–7836. doi: 10.4049/jimmunol.175.12.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanchez-Lockhart M, Graf B, Miller J. Signals and sequences that control CD28 localization to the central region of the immunological synapse. J Immunol. 2008;181:7639–7648. doi: 10.4049/jimmunol.181.11.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28–CD80 clusters that recruit protein kinase C theta. J Immunol. 2008;181:4852–4863. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci U S A. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin X, O'Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3–CD28 costimulation through selective activation of IkappaB kinase beta. Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun Z, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 142.He HT, Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:525–530. doi: 10.1038/embor.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kenworthy AK. Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:531–535. doi: 10.1038/embor.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shaw AS. Lipid rafts: now you see them, now you don't. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 145.Sadra A, Cinek T, Imboden JB. Translocation of CD28 to lipid rafts and costimulation of IL-2. Proc Natl Acad Sci U S A. 2004;101:11422–11427. doi: 10.1073/pnas.0403792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bi K, et al. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 147.Schmidt-Supprian M, et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gupta S, Manicassamy S, Vasu C, Kumar A, Shang W, Sun Z. Differential requirement of PKC-theta in the development and function of natural regulatory T cells. Mol Immunol. 2008;46:213–224. doi: 10.1016/j.molimm.2008.08.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 150.Schmidt-Supprian M, et al. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 151.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 152.Mantel PY, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 153.Bour-Jordan H, Grogan JL, Tang Q, Auger JA, Locksley RM, Bluestone JA. CTLA-4 regulates the requirement for cytokine-induced signals in T(H)2 lineage commitment. Nat Immunol. 2003;4:182–188. doi: 10.1038/ni884. [DOI] [PubMed] [Google Scholar]
- 154.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kato T, Nariuchi H. Polarization of naive CD4+ T cells toward the Th1 subset by CTLA-4 costimulation. J Immunol. 2000;164:3554–3562. doi: 10.4049/jimmunol.164.7.3554. [DOI] [PubMed] [Google Scholar]
- 156.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162:5784–5791. [PubMed] [Google Scholar]
- 157.Oosterwegel MA, et al. The role of CTLA-4 in regulating Th2 differentiation. J Immunol. 1999;163:2634–2639. [PubMed] [Google Scholar]
- 158.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 159.Kashiwada M, Lu P, Rothman PB. PIP3 pathway in regulatory T cells and autoimmunity. Immunol Res. 2007;39:194–224. doi: 10.1007/s12026-007-0075-2. [DOI] [PubMed] [Google Scholar]
- 160.Walker MR, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25− T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 162.Chen W, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 164.Fu S, et al. TGF-beta induces Foxp3 + T-regulatory cells from CD4 + CD25 − precursors. Am J Transplant. 2004;4:1614–1627. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 165.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 166.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci U S A. 2007;104:6335–6340. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 169.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 171.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Y, Amarnath S, Chen W. Requirement of CD28 signaling in homeostasis/survival of TGF-beta converted CD4+CD25+ Tregs from thymic CD4+CD25− single positive T cells. Transplantation. 2006;82:953–964. doi: 10.1097/01.tp.0000232330.46688.37. [DOI] [PubMed] [Google Scholar]
- 173.Guo F, Iclozan C, Suh WK, Anasetti C, Yu XZ. CD28 controls differentiation of regulatory T cells from naive CD4 T cells. J Immunol. 2008;181:2285–2291. doi: 10.4049/jimmunol.181.4.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 175.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 176.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 178.Alegre ML, et al. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 179.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 182.Chambers CA, Sullivan TJ, Allison JP. Lymphoproliferation in CTLA-4-deficient mice is mediated by costimulation-dependent activation of CD4+ T cells. Immunity. 1997;7:885–895. doi: 10.1016/s1074-7613(00)80406-9. [DOI] [PubMed] [Google Scholar]
- 183.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 184.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 185.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 186.Eagar TN, Karandikar NJ, Bluestone JA, Miller SD. The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immunol. 2002;32:972–981. doi: 10.1002/1521-4141(200204)32:4<972::AID-IMMU972>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 187.Fife BT, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 189.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 190.Bachmann MF, Kohler G, Ecabert B, Mak TW, Kopf M. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. J Immunol. 1999;163:1128–1131. [PubMed] [Google Scholar]
- 191.Tivol EA, Gorski J. Re-establishing peripheral tolerance in the absence of CTLA-4: complementation by wild-type T cells points to an indirect role for CTLA-4. J Immunol. 2002;169:1852–1858. doi: 10.4049/jimmunol.169.4.1852. [DOI] [PubMed] [Google Scholar]
- 192.Chai JG, Tsang JY, Lechler R, Simpson E, Dyson J, Scott D. CD4+CD25+ T cells as immunoregulatory T cells in vitro. Eur J Immunol. 2002;32:2365–2375. doi: 10.1002/1521-4141(200208)32:8<2365::AID-IMMU2365>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 193.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Birebent B, et al. Suppressive properties of human CD4+CD25+ regulatory T cells are dependent on CTLA-4 expression. Eur J Immunol. 2004;34:3485–3496. doi: 10.1002/eji.200324632. [DOI] [PubMed] [Google Scholar]
- 198.Loser K, et al. An important role of CD80/CD86-CTLA-4 signaling during photocarcinogenesis in mice. J Immunol. 2005;174:5298–5305. doi: 10.4049/jimmunol.174.9.5298. [DOI] [PubMed] [Google Scholar]
- 199.Manzotti CN, et al. Inhibition of human T cell proliferation by CTLA-4 utilizes CD80 and requires CD25+ regulatory T cells. Eur J Immunol. 2002;32:2888–2896. doi: 10.1002/1521-4141(2002010)32:10<2888::AID-IMMU2888>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 200.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 201.Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- 202.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 203.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tivol EA, et al. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J Immunol. 1997;158:5091–5094. [PubMed] [Google Scholar]
- 205.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4-and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 206.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu Z, et al. B7 interactions with CD28 and CTLA-4 control tolerance or induction of mucosal inflammation in chronic experimental colitis. J Immunol. 2001;167:1830–1838. doi: 10.4049/jimmunol.167.3.1830. [DOI] [PubMed] [Google Scholar]
- 208.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kataoka H, et al. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17:421–427. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 210.Schmidt EM, et al. Ctla-4 controls regulatory T cell peripheral homeostasis and is required for suppression of pancreatic islet autoimmunity. J Immunol. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 211.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 212.Tang AL, et al. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806–1813. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Atabani SF, et al. Association of CTLA4 polymorphism with regulatory T cell frequency. Eur J Immunol. 2005;35:2157–2162. doi: 10.1002/eji.200526168. [DOI] [PubMed] [Google Scholar]
- 214.Kolar P, et al. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009;60:123–132. doi: 10.1002/art.24181. [DOI] [PubMed] [Google Scholar]
- 215.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 218.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 219.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gavin MA, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chikuma S, Bluestone JA. Expression of CTLA-4 and FOXP3 in cis protects from lethal lymphoproliferative disease. Eur J Immunol. 2007;37:1285–1289. doi: 10.1002/eji.200737159. [DOI] [PubMed] [Google Scholar]
- 223.Chikuma S, Bluestone JA. CTLA-4 and tolerance: the biochemical point of view. Immunol Res. 2003;28:241–253. doi: 10.1385/IR:28:3:241. [DOI] [PubMed] [Google Scholar]
- 224.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 225.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 226.Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int Immunol. 2004;16:1769–1780. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- 227.Zheng Y, Manzotti CN, Liu M, Burke F, Mead KI, Sansom DM. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172:2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 228.Li L, et al. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tsang JY, et al. Altered proximal T cell receptor (TCR) signaling in human CD4+CD25+ regulatory T cells. J Leukoc Biol. 2006;80:145–151. doi: 10.1189/jlb.0605344. [DOI] [PubMed] [Google Scholar]
- 230.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 231.Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4(+) T cells. J Exp Med. 1998;188:1849–1857. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gomes NA, Gattass CR, Barreto-De-Souza V, Wilson ME, DosReis GA. TGF-beta mediates CTLA-4 suppression of cellular immunity in murine kalaazar. J Immunol. 2000;164:2001–2008. doi: 10.4049/jimmunol.164.4.2001. [DOI] [PubMed] [Google Scholar]
- 234.Sullivan TJ, et al. Lack of a role for transforming growth factor-beta in cytotoxic T lymphocyte antigen-4-mediated inhibition of T cell activation. Proc Natl Acad Sci U S A. 2001;98:2587–2592. doi: 10.1073/pnas.051632398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.You S, et al. Immunoregulatory pathways controlling progression of autoimmunity in NOD mice. Ann N Y Acad Sci. 2008;1150:300–310. doi: 10.1196/annals.1447.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 238.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr Opin Immunol. 2006;18:496–502. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 240.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate costimulatorymolecules on antigen-presenting cells. Eur J Immunol. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 242.DiPaolo RJ, Brinster C, Davidson TS, Andersson J, Glass D, Shevach EM. Autoantigen-specific TGFbeta-induced Foxp3+ regulatory T cells prevent autoimmunity by inhibiting dendritic cells from activating autoreactive T cells. J Immunol. 2007;179:4685–4693. doi: 10.4049/jimmunol.179.7.4685. [DOI] [PubMed] [Google Scholar]
- 243.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–4680. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 244.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sprent J. Swapping molecules during cell-cell interactions. Sci STKE. 2005;2005:pe8. doi: 10.1126/stke.2732005pe8. [DOI] [PubMed] [Google Scholar]
- 246.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 247.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 248.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 249.Feunou P, Vanwetswinkel S, Gaudray F, Goldman M, Matthys P, Braun MY. Foxp3+CD25+ T regulatory cells stimulate IFN-gamma-independent CD152-mediated activation of tryptophan catabolism that provides dendritic cells with immune regulatory activity in mice unresponsive to staphylococcal enterotoxin B. J Immunol. 2007;179:910–917. doi: 10.4049/jimmunol.179.2.910. [DOI] [PubMed] [Google Scholar]
- 250.Mellor AL, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 251.Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 252.Wood KJ, Sawitzki B. Interferon gamma: a crucial role in the function of induced regulatory T cells in vivo. Trends Immunol. 2006;27:183–187. doi: 10.1016/j.it.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 253.Oh GS, et al. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem Biophys Res Commun. 2004;320:1156–1162. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 254.Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem Biophys Res Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 255.Lee SS, et al. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007;21:3450–3457. doi: 10.1096/fj.07-8472com. [DOI] [PubMed] [Google Scholar]
- 256.Pae HO, Oh GS, Choi BM, Chae SC, Chung HT. Differential expressions of heme oxygenase-1 gene in CD25-and CD25+ subsets of human CD4+ T cells. Biochem Biophys Res Commun. 2003;306:701–705. doi: 10.1016/s0006-291x(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 257.Xia ZW, et al. Heme oxygenase-1-mediated CD4+CD25high regulatory T cells suppress allergic airway inflammation. J Immunol. 2006;177:5936–5945. doi: 10.4049/jimmunol.177.9.5936. [DOI] [PubMed] [Google Scholar]
- 258.George JF, et al. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pae HO, et al. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 260.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Curti A, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 262.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Borriello F, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 264.Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for costimulationby the human CTLA-4/B7-2 complex. Nature. 2001;410:604–608. doi: 10.1038/35069112. [DOI] [PubMed] [Google Scholar]
- 265.Stamper CC, et al. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 266.Zhang X, Schwartz JC, Almo SC, Nathenson SG. Crystal structure of the receptor-binding domain of human B7-2: insights into organization and signaling. Proc Natl Acad Sci U S A. 2003;100:2586–2591. doi: 10.1073/pnas.252771499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Collins AV, et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17:201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 268.Ikemizu S, et al. Structure and dimerization of a soluble form of B7-1. Immunity. 2000;12:51–60. doi: 10.1016/s1074-7613(00)80158-2. [DOI] [PubMed] [Google Scholar]
- 269.Judge TA, Wu Z, Zheng XG, Sharpe AH, Sayegh MH, Turka LA. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol. 1999;162:1947–1951. [PubMed] [Google Scholar]
- 270.Karandikar NJ, Vanderlugt CL, Eagar T, Tan L, Bluestone JA, Miller SD. Tissue-specific up-regulation of B7-1 expression and function during the course of murine relapsing experimental autoimmune encephalomyelitis. J Immunol. 1998;161:192–199. [PubMed] [Google Scholar]
- 271.Peterson KE, Sharp GC, Tang H, Braley-Mullen H. B7.2 has opposing roles during the activation versus effector stages of experimental autoimmune thyroiditis. J Immunol. 1999;162:1859–1867. [PubMed] [Google Scholar]
- 272.Saegusa K, et al. Treatment with anti-CD86 costimulatory molecule prevents the autoimmune lesions in murine Sjogren's syndrome (SS) through up-regulated Th2 response. Clin Exp Immunol. 2000;119:354–360. doi: 10.1046/j.1365-2249.2000.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Montagnoli C, et al. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol. 2002;169:6298–6308. doi: 10.4049/jimmunol.169.11.6298. [DOI] [PubMed] [Google Scholar]
- 274.Yadav D, Fine C, Azuma M, Sarvetnick N. B7-1 mediated costimulation regulates pancreatic autoimmunity. Mol Immunol. 2007;44:2616–2624. doi: 10.1016/j.molimm.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Zeng M, Guinet E, Nouri-Shirazi M. B7-1 and B7-2 differentially control peripheral homeostasis of CD4(+)CD25(+)Foxp3(+) regulatory T cells. Transpl Immunol. 2009;20:171–179. doi: 10.1016/j.trim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 276.Santamaria P. Kinetic evolution of a diabetogenic CD8+ T cell response. Ann N Y Acad Sci. 2003;1005:88–97. doi: 10.1196/annals.1288.010. [DOI] [PubMed] [Google Scholar]