The central function of the aorta and all muscular arteries is to act as an efficient and durable conduit for pulsatile blood flow. As such, these vessels must preserve a nonthrombogenic lumen free of obstruction and maintain their structural integrity over a lifetime of cyclic hemodynamic stresses. The loss of structural integrity is the fundamental cause of aneurysm formation and ultimate rupture. Histologically, the complete or near-complete loss of intact medial elastic fibers has long been recognized as a distinguishing feature of aneurysms, particularly the relatively common infrarenal abdominal aortic aneurysm (AAA) (Figure 1). Mechanical studies of human tissues have confirmed the critical structural role of the elastic fiber in maintenance of arterial wall integrity.1,2 Further, animal models of aneurysms have consistently been reproduced with the initiation of inflammatory and enzymatic cascades that result in medial elastic fiber degeneration.3
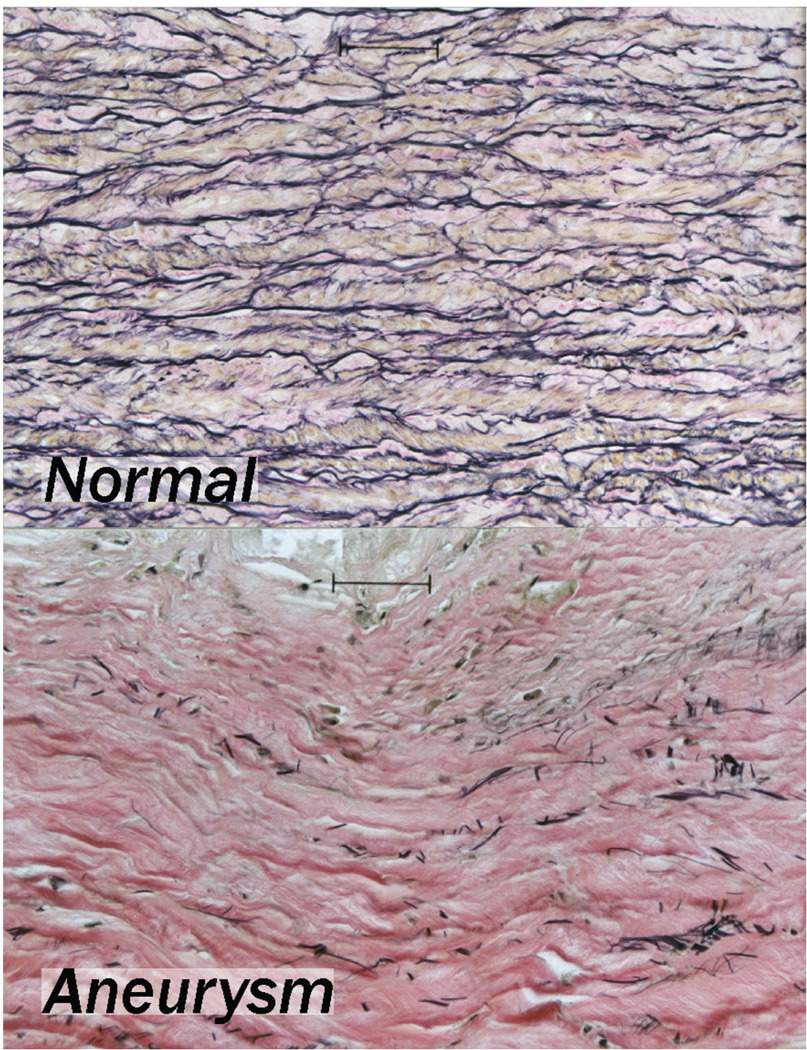
Figure 1.
Elastin-specific stain in normal and aneurysmal aorta. Note elastic fiber destruction in the aneurysm despite minimal local inflammation.
The elastolytic process in aneurysms is associated with several reflexively related features in humans: (1) an increase in expression and/or activity of elastin-degrading matrix metalloproteinases (MMPs): MMP-2,4 MMP-3,5,6 MMP-9,7–9 MMP-7,10 and MMP-1211,12; (2) an increase in tissue macrophages and other inflammatory cells12,13; and (3) a decrease in medial vascular smooth muscle cell (SMC) density (Figure 2).14–16 The prevailing conception of this disease considers that the inflammatory cell infiltrate results directly or indirectly in the elaboration of the metalloproteinases17 and that these proteases directly degrade elastin. Ultimately, pulsatile stresses are then brought to bear primarily on matrix constituents not designed to withstand this repetitive stress. Furthermore, as the aorta dilates, the distribution of force is altered, localized areas of supraphysiologic levels of wall stress develop,18,19 and rupture occurs as the result of mechanical disruption of the remaining matrix components.
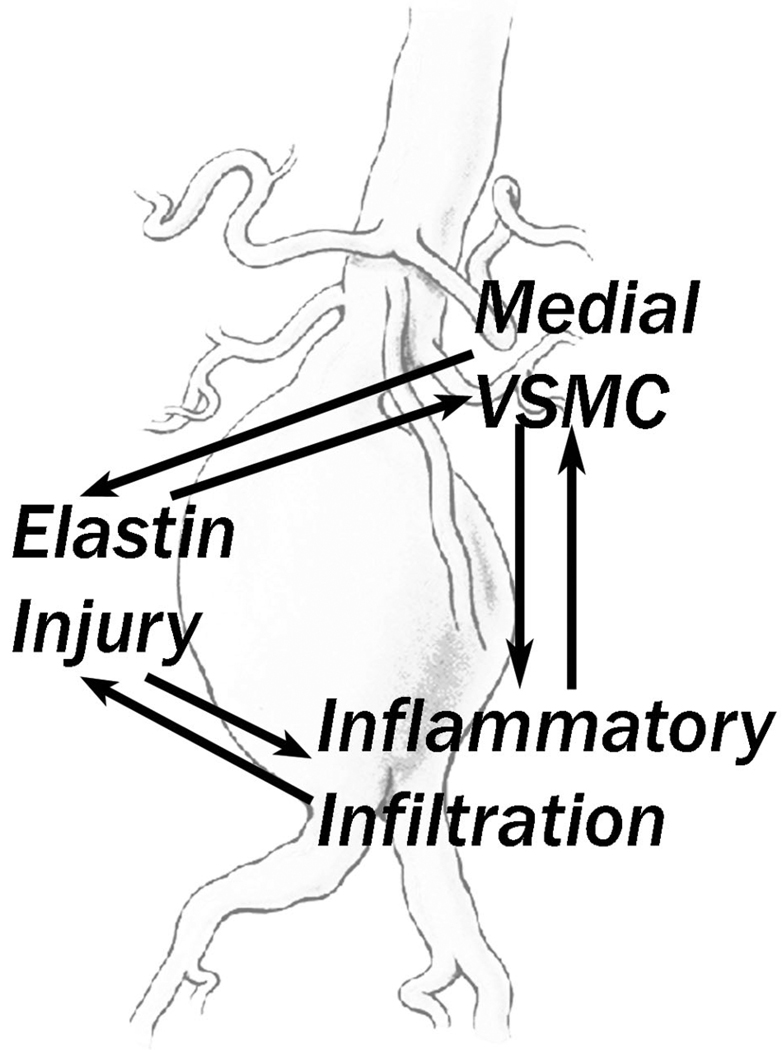
Figure 2.
Diagrammatic representation of the reflexive relationships that exist between the elastin matrix destruction (mediated by specific enzymes), loss of normal smooth muscle cell function and number, and inflammatory infiltration found in aortic aneurysmal disease. Each of those invariate findings of aneurysmal tissue can induce or increase the other findings, resulting in a destructive cycle leading to aortic wall failure. VSMC = vascular smooth muscle cell.
The vascular SMC must play a critical role in aneurysm pathobiology since it is capable of (1) matrix synthesis, (2) proteinase (and inhibitor) elaboration, and (3) inflammatory cell recruitment. As seen in the simplified schematic of Figure 3, the SMC plays a central role in its interactions (both positive and negative) with matrix-degrading enzymes and medial inflammation. Furthermore, SMCs are the principal cell type involved in the production of the extracellular matrix (ECM) components of the media, particularly elastin and collagen. Given that the most likely therapeutic window for medical therapy of aneurysm disease is after dilatation has been detected, stabilization of the aortic wall is likely to require both enzymatic inhibition and the synthetic, reparative activities of the SMCs producing ECM.
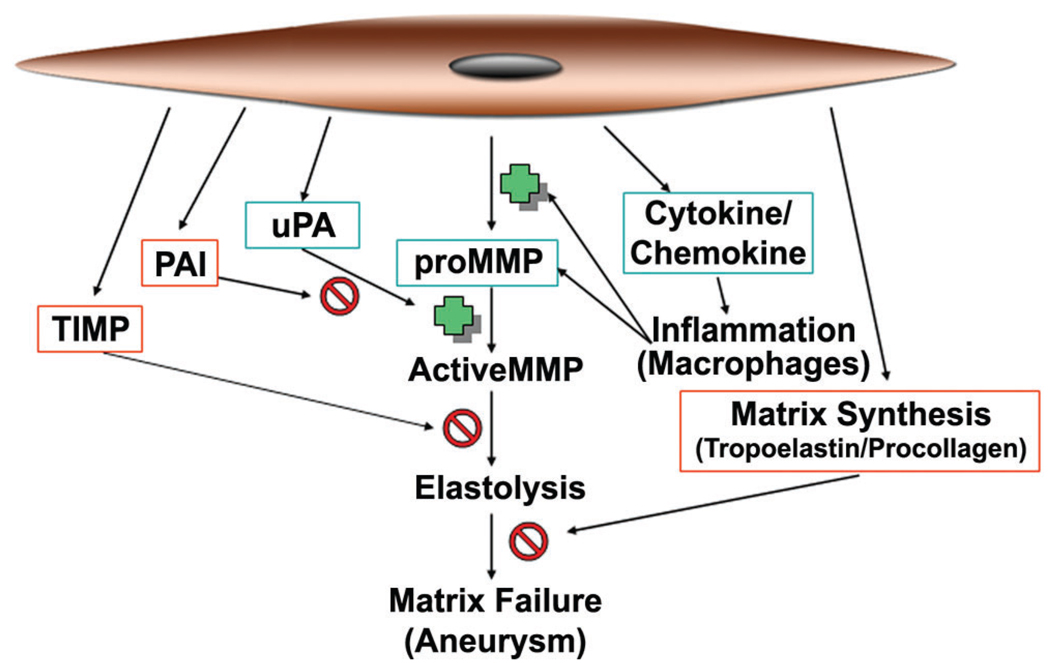
Figure 3.
Vascular smooth muscle cell products involved in matrix metalloproteinase (MMP)-mediated matrix injury in aneurysms. Products that may promote matrix injury are in green, and products that may reduce limit or repair matrix injury are in red. PAI = plasminogen activator inhibitor; TIMP = tissue inhibitor of metalloproteinase; uPA = urokinase.
Differentiation of SMCs in AAA
Diverse Phenotypes of the Vascular SMC
The term smooth muscle cell encompasses a rather diverse population of cells that perform a variety of functions within each tissue, including mechanical responses (eg, contraction, migration, and proliferation) and maintenance of the ECM (eg, synthesis of matrix proteins, growth factors, and cytokines). These cells tend to have unique tissue-specific functional phenotypes, such as found in the bladder, the gastrointestinal tract, the small airways of the lung, and vascular tissue. It is also now recognized that even among the vascular SMC population, there is no homogeneous phenotype.20–30 These cells appear to undergo differentiation relative to their location in the vascular tree and their embryologic origin.
The relative differentiation of vascular SMCs may be critical to the health and stability of the matrix of a blood vessel for several reasons. First, the cells are, by far, the majority cell type present within the normal vessel wall, and they perform important mechanical functions integrated with the matrix. Second, these cells are responsible for the production of the structural proteins of the ECM. Third, these cells are capable of releasing cytokines and chemokines, which can result in the recruitment of the other cell types to the vascular wall. Fourth, these cells are capable of migration and the expression of matrix-degrading enzymes. Each of these functions will require the regulation of expression of a distinct set of genes unique to each process.
It is well recognized that SMCs retain significant plasticity with regard to their expression profiles, which may allow them to modulate gene expression to participate in this myriad of functions. In particular, the phenotype of these cells in vivo and in vitro appears to be significantly affected by local environmental conditions. In tissue culture, vascular SMCs have been found to alter their phenotype significantly based on culture conditions. Generically, there have been two in vitro phenotypes referred to as the “contractile” and “synthetic” phenotypes with different expression and activity profiles, and in certain circumstances, particular SMC clones can undergo reversible conversion between these two phenotypes.31
There are limits to phenotypic modulation for specific SMCs, however, which are dictated by the differentiation state of the cell. Thus, under identical culture conditions, SMCs from different vascular mural compartments are found to have unique phenotypes and can be distinguished in culture. Frid and colleagues established that there is heterogeneity of cultured SMC phenotype even within a single segment of bovine pulmonary arterial media.30 This evidence for local heterogeneity of cultured SMCs was recently extended to the internal thoracic artery in humans.32 This suggests an important level of specialization of the medial SMC for particular tasks of vascular maintenance.
The understanding of SMC differentiation and its role in vascular pathology has been advanced primarily through the study of atherosclerosis. Atherosclerotic plaque has long been recognized to be a pathologic process that involves SMCs that populate the lesion and produce ECM and metalloproteinases. In atherosclerosis research, it has become clear that the SMCs that populate an intimal plaque consist primarily of a monoclonal or oligoclonal population of cells.33–36 Moreover, these cells derived from atherosclerotic plaque are now known to have a unique phenotype compared with SMCs obtained from arterial media.37,38 It is believed that the unique phenotype of these cells is critical for the development of atherosclerotic disease in humans.35
Unresolved Pathobiology: Role of the SMC in AAA
The predominant paradigm of aneurysm pathobiology describes the medial degeneration as developing secondary to an imbalance between proteolytic and matrix synthetic activities. Obviously, the imbalance is weighted in favor of the proteolytic activity, with metalloproteinases appearing to be the most significant participating enzyme family. Within this context, SMCs, as a class, could participate on either side of the balance, either accentuating the matrix-degrading or the matrix-synthesizing/antiprotease roles. Therefore, the SMCs in AAA must be participating in the process of matrix degradation either through enhancement of the matrix proteolysis or through insufficient matrix repair or protection.
Despite their central role, study of the activity of the SMC in human aortic aneurysm tissue has been relatively superficial. Most of the human studies of SMC in aneurysm disease describe a decrease in the concentration of the cells within the media, evidence of SMC apoptosis, and poor in vitro propagation of SMC lines derived from aneurysmal tissue.16,39–41 It has therefore been hypothesized that the matrix destruction seen in AAA in part results from a reduction in the quantity or activity of SMCs.14–16,39,40 The implication here is that given adequate numbers or activity levels, the medial SMCs of the aorta are capable of mitigating the proteolytic injury either through the matrix-synthesizing capabilities of the SMC or enzymatic inhibition. Supporting this concept is evidence that SMC seeding can prevent AAA in a decellularized xenograft transplantation model of aneurysms.42–44
Alternatively, there is a body of evidence that directly implicates the SMC in the matrix destructive process of aneurysms. The ability of SMCs to degrade medial matrix has been well described in relation to migration of SMCs.45,46 Furthermore, there is important evidence that protease expression by SMCs is critical to aneurysm development in humans and in certain models of aneurysmal degeneration.47–51
Thus, there is contradictory information on the function of these cells in the complex environment of the aneurysmal aortic wall. Ultimately, the true role of the medial SMC in human aneurysm development remains unknown. Deciphering the activities of these cells with respect to the medial ECM changes seen in aneurysms has great potential for assisting in devising therapies and improving our animal models of aneurysm disease. Fortunately, extensive studies of SMCs in the pathobiology of atherosclerotic disease can offer some important insights and techniques to understand the role of these cells in AAA.
SMC Differentiation in AAA
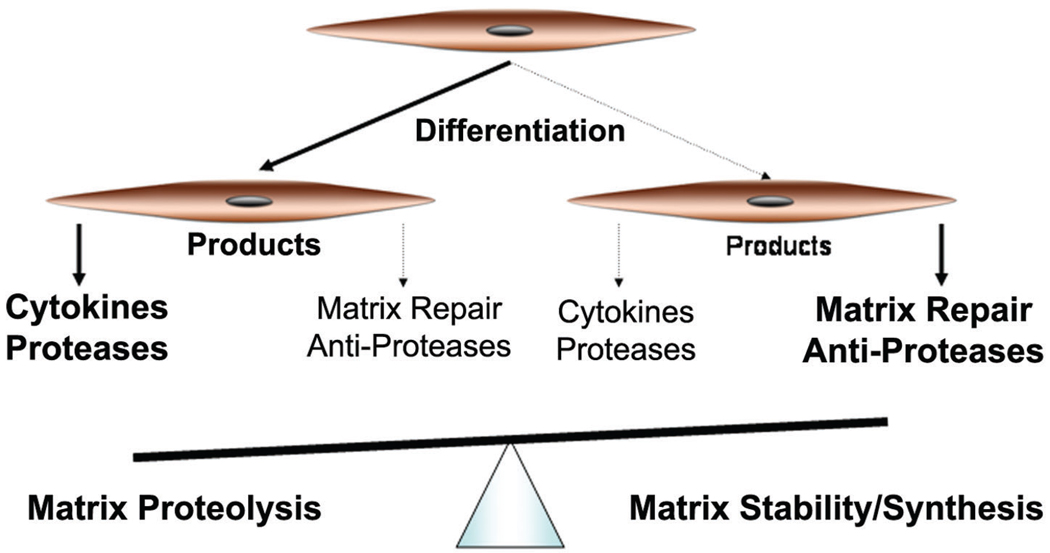
As shown in Figure 4, the prevalence of a differentiated phenotype of SMCs that predominantly express proteins associated with matrix degradation could result in a medial environment that results in elastin fiber degradation typical of aneurysms. Given the central role of the SMC in maintenance of the medial ECM of the aorta, understanding the phenotype of the resident SMC may be a critical link in our understanding of aneurysm pathobiology. There is evidence that SMCs derived from the wall of an AAA may be unique compared with cells from a nonaneurysmal artery, as suggested by the in vitro growth characteristics16,39–41 and tissue studies of SMCs in aneurysms.48 In studies performed over 10 years ago, SMCs explanted from aneurysms and normal aortas were compared under interleukin-1β (IL-1β) stimulation.52 These studies examined a limited set of metalloproteinases and inhibitors with Western blotting but did identify unique responses of the aneurysm cells to cytokine stimulation.52 However, there has been no ongoing systematic attempt to define this population of SMCs.
Figure 4.
In this simplified schematic, unbalanced differentiation of SMC could result in a predominance of cells that have a tendency to express proinflammatory and matrix-degrading products. These cells would feed into the cycle of aneurysmal degeneration once it is initiated by a secondary injury. A more balanced phenotype of SMC could express products that predominantly act to put a brake on the cycle and prevent inappropriate matrix damage.
Postulating a unique phenotype for SMCs populating aneurysmal segments of the arterial tree begs the question of the origins of the diversity of these cells. Unlike the endothelial cells that appear to derive from primary endothelial or endodermal tubes, in the embryo, SMCs seem to arise locally from the surrounding mesenchyme53,54 or from the neural crest.55 It has also been reported that certain situations can result in endothelial cells delaminating, migrating, and transdifferentiating into SMCs.56 Finally, SMCs populating intimal plaque can derive from circulating bone marrow–derived stem cells,57–59 although this has been specifically disputed.60 Any or all of these may have an impact on the development of unique populations of SMCs in aneurysms.
The embryologic origins of the SMC during development may be particularly useful in explaining localized differences in arterial pathology. For example, mesenchyme of the head and neck derives from ectoderm, whereas mesenchyme of the lower body derives from mesoderm.61 Differences in aortic SMCs persist and can be identified topographically even in young adults without significant aortic disease.62
In the context of aneurysm disease, it is remarkable that the vast majority of arterial aneurysms occur in an anatomically defined segment, including the distal aorta, common iliac, and internal iliac arteries. The predisposition of aneurysms for this portion of the aorta has been explained in many different ways, including blood flow and wall stress dynamics, as well as differences in medial structure.63–66 However, these explanations do not generally allow for an understanding of the development of aneurysms in the adjacent iliac system. On the other hand, differential SMC ontogeny related to the local properties of the mesenchyme could result in a regional predominance of cells with a differentiation bias toward matrix degradation. Given the anatomic continuity of the most affected segments of the vascular tree, this might reasonably account for the localized anatomic distribution of aneurysm disease. The localized differences in matrix of the infrarenal aorta could be accounted for by uniquely differentiated SMCs as well.64 Furthermore, clonal expansion of pathologic SMCs has not been demonstrated in animals,36,53,67 which may relate to the absence of spontaneous aneurysmal disease in animals.
Elastolysis and SMCs in AAA
As noted above, an SMC population within the media of the wall of an aneurysm may have a phenotype that promotes matrix degeneration. One means by which a unique population of SMCs in AAA may impact the course of the disease is through production of active metalloelastases. Alternatively, SMCs may participate in matrix degeneration through an inability to produce appropriate antiproteases. Several lines of evidence suggest that SMCs may play an integral role in the MMP-mediated characteristic degradation of elastin in AAA.
Metalloelastases in AAA
Although a complete review of MMP activities in AAA is beyond the scope of this monograph, briefly, both constitutive and inducible production of elastolytic enzymes MMP-2, MMP-9, MMP-7, and MMP-12 can be identified within human AAA tissues. Davis and colleagues showed that large amounts of MMP-2 are bound to the ECM.68 A large portion of this matrix-bound MMP-2 is found in the activated form, suggesting that MMP-2 is activated in AAAs and tightly sequestered within the extracellular space. MMP-9 has attracted particular interest because it is abundantly produced by human AAA tissues in vitro.69 Elevated amounts of enzymatically active MMP-12 are also produced in human AAA tissue.12 Importantly, MMP-12 is prominently localized to residual elastin fiber fragments within aneurysm tissue by immunohistochemistry, a pattern distinct from other elastolytic MMPs. Stromelysin has been detected in human AAAs by immunoblotting and messenger ribonucleic acid analysis,9,70–72 but its role in aneurysm development remains unclear. Stromelysin may play a significant role, however, by activating the proenzyme form of other MMPs, particularly MMP-9.73
The regulation of MMP activities is critical to prevent widespread tissue destruction, both in normal tissues undergoing remodeling and in disease.74–76 MMPs are thereby controlled at several levels, including the induction and suppression of MMP gene transcription, extracellular activation, and interaction with natural inhibitors. MMPs are secreted as zymogens (pro-MMPs) and are maintained in an inactive state by the presence of the amino-terminal propeptide domain. Enzymatic cleavage of the propeptide is the most likely mechanism of MMP activation in vivo, indicating that extracellular processing of pro-MMPs is required, with few exceptions, to achieve full catalytic activity.77 Proteases known to be effective activators of one or more elastolytic MMPs include plasmin,78,79 trypsin,80,81 chymase,82 active MMPs,48 and others. Several lines of evidence suggest that these MMP activators are present in significantly elevated quantities in aneurysm tissue, particularly the plasminogen activators urokinase (uPA) and tissue plasminogen activator (tPA).83,84 Evidence exists to suggest that increases in expression of the fibrinolytic proteins are important for the progression of small aortic aneurysms.85,86 Furthermore, in multiple models, plasminogen activators appear to play a crucial role in the development of experimental aneurysms.87–89
SMC and MMP Expression
Proteolytic enzyme secretion in SMCs is a well-recognized capability. Unstimulated SMCs produce MMP-2 both in vivo and in vitro,90,91 whereas MMP-3, MMP-7, MMP-9, and MMP-12 are expressed in vivo in vessel walls associated with inflammation, shear stress, or injury.92–96 In cell culture, SMCs can be induced to express the elastolytic MMPs 3, 9, and 12, under specific cytokine or other receptor stimulation.90,91,97–100 Expression of MMP-7, however, has not been demonstrated from human vascular SMCs in culture. As noted, MMP-2 is expressed constitutively by SMCs, although augmented expression can be stimulated by platelet-derived growth factor (PDGF).101 Although MMP-12 is not expressed constitutively by SMCs, its expression can also be stimulated by PDGF through an AP-1-dependent mechanism.98 Expression and synthesis of both MMP-9 and MMP-3 in SMCs are induced by IL-1β and tumor necrosis factor α90 through nuclear factor (NF)-κB.102,103 All of these cytokines have been found to be upregulated in human aneurysm tissue and thus may have a role in stimulating SMCs in vivo.104,105
In addition to producing these potentially elastolytic metalloproteases, SMCs are also capable of producing the enzymes that can activate them as well. Vascular SMCs may effect activation of MMPs produced through the expression and activity of plasminogen activators, uPA and tPA, or a membrane-bound metalloproteinase, MT1-MMP.11,47,106–108
Protease Expression in Aneurysm-Derived SMCs
As noted above, the role of the SMC as it relates to the degradation of medial elastin in aneurysms is vaguely understood, and contrary hypotheses exist in regard to their participation in aneurysm genesis. Yet there are data to suggest that SMCs in patients with aneurysms are unique and, moreover, are associated with alterations in protease or inhibitor expression. In an early study by Keen and colleagues, SMCs grown in explant culture from the aortas of patients with aneurysms and from the aortas of elderly individuals donating organs demonstrated differential expression of tissue inhibitor of metalloproteinase 1 (TIMP-1) in response to IL-1β stimulation.52 In fact, it was found that SMCs from AAA had a significant increase in TIMP-1 production, whereas no effect was seen in cells from normal aortas, which has been confirmed by others.90 Although this may suggest an antiproteolytic bias of these cells, expression of TIMP-1 and certain MMPs are known to be coordinately regulated through NF-κB and AP-1 transcription factors, thus suggesting that there may have been significant increases in elastolytic MMPs that were not recognized.109,110
In a study by the same group the following year, evidence supporting this later interpretation was presented.111 It was found that unstimulated SMCs derived from AAA were found to produce detectable amounts of uPA, whereas control SMCs did not express uPA. There was also significantly more tPA produced by the aneurysm-derived SMCs than the SMCs from normal aortic tissue.111 Although not evaluated in this study, this may have increased the activity of the MMPs secreted from these same cells. This suggests that SMCs from aneurysms may actively participate in the production of a proproteolytic environment in the aortic media.
Further support of the unique properties of SMCs from AAA comes from a more recent study by Goodall and colleagues.49 In these experiments, migration through a Matrigel layer was compared between SMCs derived from patients with AAA and normal controls. The SMCs from aneurysm patients showed significantly increased invasiveness under PDGF stimulation. This effect was associated with increased MMP-2 production compared with the normal controls. These enhanced responses to inflammatory stimuli may be an important feature of the SMCs in AAA as even crude extracts of aneurysm tissue demonstrate high levels of chemotactic and proinflammatory molecules.104,105,112
Summary
As described by Schwartz and colleagues over 10 years ago, the unique properties of the “soil” (the cellular responses) of the intima provide for the development of intimal arterial diseases.53 Now a significant amount of evidence points to the likelihood that the SMCs that populate the media of aneurysms are unique and that they directly or indirectly participate in the medial elastin degeneration characteristic of AAA. Because maintenance of a functional aortic matrix depends on the SMC, restoration of the normal activities of these cells may be essential to stabilize and potentially repair the damage that is associated with aneurysmal degeneration. Evidence for this effect can be seen in recent studies in murine models with both repopulation of the aorta with normal SMCs113 and alteration of SMC activities by modifying intracellular inflammatory response pathways.114 Finally, identification of SMC differentiation profiles associated with aneurysms may provide the unique ability to identify those individuals with an increased propensity for aneurysm development prior to the phenotypic expression of the disease.
Acknowledgments
Supported by grants from the National Institutes for Health (5K08HL084004–02), Department of Veterans’ Affairs, Flight Attendants Medical Research Institute, and the American Heart Association (0765432Z).
References
- 1.Dobrin PB, Mrkvicka R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 1994;2:484–488. [PubMed] [Google Scholar]
- 2.Dobrin PB, Baker WH, Gley WC. Elastolytic and collagenolytic studies of arteries: implications for the mechanical properties of aneurysms. Arch Surg. 1984;119:405–409. doi: 10.1001/archsurg.1984.01390160041009. [DOI] [PubMed] [Google Scholar]
- 3.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 4.McMillan WD, Patterson BK, Keen RR, Pearce WH. In situ localization and quantification of seventy-two-kilodalton type IV collagenase in aneurysmal, occlusive, and normal aorta. J Vasc Surg. 1995;22:295–305. doi: 10.1016/s0741-5214(95)70144-3. [DOI] [PubMed] [Google Scholar]
- 5.Higashikata T, Yamagishi M, Sasaki H, et al. Application of real-time RT-PCR to quantifying gene expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human abdominal aortic aneurysm. Atherosclerosis. 2004;177:353–360. doi: 10.1016/j.atherosclerosis.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Carrell TW, Burnand KG, Wells GM, et al. Stromelysin-1 (matrix metalloproteinase-3) and tissue inhibitor of metalloproteinase-3 are overexpressed in the wall of abdominal aortic aneurysms. Circulation. 2002;105:477–482. doi: 10.1161/hc0402.102621. [DOI] [PubMed] [Google Scholar]
- 7.Hovsepian DM, Ziporin SJ, Sakurai MK, et al. Elevated plasma levels of matrix metalloproteinase-9 in patients with abdominal aortic aneurysms: a circulating marker of degenerative aneurysm disease. J Vasc Interv Radiol. 2000;11:1345–1352. doi: 10.1016/s1051-0443(07)61315-3. [DOI] [PubMed] [Google Scholar]
- 8.McMillan WD, Patterson BK, Keen RR, et al. In situ localization and quantification of mRNA for 92-kD type IV collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 1995;15:1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- 9.Elmore JR, Keister BF, Franklin DP, et al. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann Vasc Surg. 1998;12:221–228. doi: 10.1007/s100169900144. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine V, Jacob M-P, Houard X, et al. Involvement of the mural thrombus as a site of protease release and activation in human aortic aneurysms. Am J Pathol. 2002;161:1701–1710. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annabi B, Shedid D, Ghosn P, et al. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J Vasc Surg. 2002;35:539–546. doi: 10.1067/mva.2002.121124. [DOI] [PubMed] [Google Scholar]
- 12.Curci JA, Liao S, Huffman MD, et al. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998;102:1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch AE, Haines GK, Rizzo RJ, et al. Human abdominal aortic aneurysms. Immunophenotypic analysis suggesting an immunemediated response. Am J Pathol. 1990;137:1199–1213. [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RW, Liao S, Curci JA. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8:623–631. doi: 10.1097/00019501-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Liao S, Curci JA, Kelley BJ, et al. Accelerated replicative senescence of medial smooth muscle cells derived from abdominal aortic aneurysms compared to the adjacent inferior mesenteric artery. J Surg Res. 2000;92:85–95. doi: 10.1006/jsre.2000.5878. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Candales A, Holmes DR, Liao S, et al. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol. 1997;150:993–1007. [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong W, Zhao Y, Prall A, et al. Key roles of CD4+ T cells and IFN-gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2004;172:2607–2612. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 18.Vorp DA, Raghavan ML, Webster MW. Mechanical wall stress in abdominal aortic aneurysm: influence of diameter and asymmetry. J Vasc Surg. 1998;27:632–639. doi: 10.1016/s0741-5214(98)70227-7. [DOI] [PubMed] [Google Scholar]
- 19.Vorp DA, Trachtenberg JD, Webster MW. Arterial hemodynamics and wall mechanics. Semin Vasc Surg. 1998;11:169–180. [PubMed] [Google Scholar]
- 20.Mahoney WM, Schwartz SM. Defining smooth muscle cells and smooth muscle injury. J Clin Invest. 2005;115:221–224. doi: 10.1172/JCI24272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bochaton-Piallat ML, Clowes AW, Clowes MM, et al. Cultured arterial smooth muscle cells maintain distinct phenotypes when implanted into carotid artery. Arterioscler Thromb Vasc Biol. 2001;21:949–954. doi: 10.1161/01.atv.21.6.949. [DOI] [PubMed] [Google Scholar]
- 22.Bochaton-Piallat ML, Gabbiani F, Ropraz P, Gabbiani G. Age influences the replicative activity and the differentiation features of cultured rat aortic smooth muscle cell populations and clones. Arterioscler Thromb. 1993;13:1449–1455. doi: 10.1161/01.atv.13.10.1449. [DOI] [PubMed] [Google Scholar]
- 23.Lemire JM, Covin CW, White S, et al. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994;144:1068–1081. [PMC free article] [PubMed] [Google Scholar]
- 24.Orlandi A, Ehrlich HP, Ropraz P, et al. Rat aortic smooth muscle cells isolated from different layers and at different times after endothelial denudation show distinct biological features in vitro. Arterioscler Thromb. 1994;14:982–989. doi: 10.1161/01.atv.14.6.982. [DOI] [PubMed] [Google Scholar]
- 25.Villaschi S, Nicosia RF, Smith MR. Isolation of a morphologically and functionally distinct smooth muscle cell type from the intimal aspect of the normal rat aorta. Evidence for smooth muscle cell heterogeneity. In Vitro Cell Dev Biol Anim. 1994;30A:589–595. doi: 10.1007/BF02631257. [DOI] [PubMed] [Google Scholar]
- 26.Ehler E, Jat PS, Noble MD, et al. Vascular smooth muscle cells of H-2Kb-tsA58 transgenic mice. Characterization of cell lines with distinct properties. Circulation. 1995;92:3289–3296. doi: 10.1161/01.cir.92.11.3289. [DOI] [PubMed] [Google Scholar]
- 27.Holifield B, Helgason T, Jemelka S, et al. Differentiated vascular myocytes: are they involved in neointimal formation? J Clin Invest. 1996;97:814–825. doi: 10.1172/JCI118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-beta. Dev Biol. 1996;178:430–445. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 29.Majesky MW, Dong XR, Topouzis S. Smooth muscle cell diversity and the extracellular matrix in a rat model of restenosis. P R Health Sci J. 1996;15:187–191. [PubMed] [Google Scholar]
- 30.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res. 1997;81:940–952. doi: 10.1161/01.res.81.6.940. [DOI] [PubMed] [Google Scholar]
- 31.Li S, Sims S, Jiao Y, et al. Evidence from a novel human cell clone that adult vascular smooth muscle cells can convert reversibly between noncontractile and contractile phenotypes. Circ Res. 1999;85:338–348. doi: 10.1161/01.res.85.4.338. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Fan Y-S, Chow LH, et al. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res. 2001;89:517–525. doi: 10.1161/hh1801.097165. [DOI] [PubMed] [Google Scholar]
- 33.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murry CE, Gipaya CT, Bartosek T, et al. Monoclonality of smooth muscle cells in human atherosclerosis. Am J Pathol. 1997;151:697–705. [PMC free article] [PubMed] [Google Scholar]
- 35.Chung IM, Schwartz SM, Murry CE. Clonal architecture of normal and atherosclerotic aorta: implications for atherogenesis and vascular development. Am J Pathol. 1998;152:913–923. [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz SM, Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu Rev Med. 1998;49:437–460. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- 37.Mulvihill ER, Jaeger J, Sengupta R, et al. Atherosclerotic plaque smooth muscle cells have a distinct phenotype. Arterioscler Thromb Vasc Biol. 2004;24:1283–1289. doi: 10.1161/01.ATV.0000132401.12275.0c. [DOI] [PubMed] [Google Scholar]
- 38.Bonin LR, Madden K, Shera K, et al. Generation and characterization of human smooth muscle cell lines derived from atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 1999;19:575–587. doi: 10.1161/01.atv.19.3.575. [DOI] [PubMed] [Google Scholar]
- 39.Henderson EL, Gang YJ, Sukhova GK, et al. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 40.Holmes DR, Lopez-Candales A, Liao S, Thompson RW. Smooth muscle cell apoptosis and p53 expression in human abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:286–287. doi: 10.1111/j.1749-6632.1996.tb33334.x. [DOI] [PubMed] [Google Scholar]
- 41.Jacob T, Hingorani A, Ascher E. Examination of the apoptotic pathway and proteolysis in the pathogenesis of popliteal artery aneurysms. Eur J Vasc Endovasc Surg. 2001;22:77–85. doi: 10.1053/ejvs.2001.1344. [DOI] [PubMed] [Google Scholar]
- 42.Allaire E, Forough R, Clowes M, et al. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–1420. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allaire E, Muscatelli-Groux B, Guinault AM, et al. Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Ann Surg. 2004;239:417–427. doi: 10.1097/01.sla.0000114131.79899.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Losy F, Dai J, Pages C, et al. Paracrine secretion of transforming growth factor-beta1 in aneurysm healing and stabilization with endovascular smooth muscle cell therapy. J Vasc Surg. 2003;37:1301–1309. doi: 10.1016/s0741-5214(02)75336-6. [DOI] [PubMed] [Google Scholar]
- 45.Lijnen HR. Metalloproteinases in development and progression of vascular disease. Pathophysiol Haemostas Thromb. 2003;33:275–281. doi: 10.1159/000083814. [DOI] [PubMed] [Google Scholar]
- 46.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 47.Sinha I, Hannawa KK, Eliason JL, et al. Early MT-1 MMP expression following elastase exposure is associated with increased cleaved MMP-2 activity in experimental rodent aortic aneurysms. Surgery. 2004;136:176–182. doi: 10.1016/j.surg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 48.Nollendorfs A, Greiner T, Nagase H, Baxter B. The expression and localization of membrane type-1 matrix metalloproteinase in human abdominal aortic aneurysms. J Vasc Surg. 2001;34:316–322. doi: 10.1067/mva.2001.115962. [DOI] [PubMed] [Google Scholar]
- 49.Goodall S, Porter KE, Bell PR, Thompson MM. Enhanced invasive properties exhibited by smooth muscle cells are associated with elevated production of MMP-2 in patients with aortic aneurysms. Eur J Vasc Endovasc Surg. 2002;24:72–80. doi: 10.1053/ejvs.2002.1675. [DOI] [PubMed] [Google Scholar]
- 50.Patel MI, Melrose J, Ghosh P, Appleberg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1996;24:82–92. doi: 10.1016/s0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- 51.Crowther M, Goodall S, Jones JL, et al. Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J Vasc Surg. 2000;32:575–583. doi: 10.1067/mva.2000.108010. [DOI] [PubMed] [Google Scholar]
- 52.Keen R, Nolan K, Cipollone M, et al. Interleukin-1 beta induces differential gene expression in aortic smooth muscle cells. J Vasc Surg. 1994;20:774–786. doi: 10.1016/s0741-5214(94)70165-2. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 54.Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989;140:1097–1103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- 55.Mann KM, Ray JL, Moon ES, et al. Calcineurin initiates smooth muscle differentiation in neural crest stem cells. J Cell Biol. 2004;165:483–491. doi: 10.1083/jcb.200402105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeRuiter MC, Poelmann RE, VanMunsteren JC, et al. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res. 1997;80:444–451. doi: 10.1161/01.res.80.4.444. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu K, Sugiyama S, Aikawa M, et al. Host bone-marrow cells are a source of donor intimal smooth-muscle-like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 58.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 59.Hu Y, Davison F, Ludewig B, et al. Smooth muscle cells in transplant atherosclerotic lesions are originated from recipients, but not bone marrow progenitor cells. Circulation. 2002;106:1834–1839. doi: 10.1161/01.cir.0000031333.86845.dd. [DOI] [PubMed] [Google Scholar]
- 60.Bentzon JF, Weile C, Sondergaard CS, et al. Smooth muscle cells in atherosclerosis originate from the local vessel wall and not circulating progenitor cells in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2006;26:2696–2702. doi: 10.1161/01.ATV.0000247243.48542.9d. [DOI] [PubMed] [Google Scholar]
- 61.Majesky MW, Schwartz SM. An origin for smooth muscle cells from endothelium? Circ Res. 1997;80:601–603. [PubMed] [Google Scholar]
- 62.Rao RN, Falls DG, Gerrity RG, et al. Intimal thickness and layering, and smooth muscle cell phenotypes in aorta of youth. Pathobiology. 2000;68:18–28. doi: 10.1159/000028111. [DOI] [PubMed] [Google Scholar]
- 63.Halloran BG, Davis VA, McManus BM, et al. Localization of aortic disease is associated with intrinsic differences in aortic structure. J Surg Res. 1995;59:17–22. doi: 10.1006/jsre.1995.1126. [DOI] [PubMed] [Google Scholar]
- 64.Godfrey M, Nejezchleb PA, Schaefer GB, et al. Elastin and fibrillin mRNA and protein levels in the ontogeny of normal human aorta. Connect Tissue Res. 1993;29:61–69. doi: 10.3109/03008209309061967. [DOI] [PubMed] [Google Scholar]
- 65.Hoshina K, Sho E, Sho M, et al. Wall shear stress and strain modulate experimental aneurysm cellularity. J Vasc Surg. 2003;37:1067–1074. doi: 10.1016/s0741-5214(03)70052-4. [DOI] [PubMed] [Google Scholar]
- 66.Nakahashi TK, Hoshina K, Tsao PS, et al. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22:2017–2022. doi: 10.1161/01.atv.0000042082.38014.ea. [DOI] [PubMed] [Google Scholar]
- 67.Thomas WA, Lee KT, Kim DN. Cell population kinetics in atherogenesis. Cell births and losses in intimal cell mass-derived lesions in the abdominal aorta of swine. Ann N Y Acad Sci. 1985;454:305–315. doi: 10.1111/j.1749-6632.1985.tb11870.x. [DOI] [PubMed] [Google Scholar]
- 68.Davis V, Persidskaia R, Baca-Regen L, et al. Matrix metalloproteinase-2 production and its binding to the matrix are increased in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1998;18:1625–1633. doi: 10.1161/01.atv.18.10.1625. [DOI] [PubMed] [Google Scholar]
- 69.Thompson RW, Holmes DR, Mertens RA, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms: an elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997;122:264–271. doi: 10.1016/s0039-6060(97)90017-9. discussion 71–2. [DOI] [PubMed] [Google Scholar]
- 71.Newman KM, Ogata Y, Malon AM, et al. Identification of matrix metalloproteinases 3 (stromelysin-1) and 9 (gelatinase B) in abdominal aortic aneurysm. Arterioscler Thromb. 1994;14:1315–1320. doi: 10.1161/01.atv.14.8.1315. [DOI] [PubMed] [Google Scholar]
- 72.Newman KM, Malon AM, Shin RD, et al. Matrix metalloproteinases in abdominal aortic aneurysm: characterization, purification, and their possible sources. Connect Tissue Res. 1994;30:265–276. doi: 10.3109/03008209409015042. [DOI] [PubMed] [Google Scholar]
- 73.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 74.Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 75.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- 76.Birkedal-Hansen H, Moore WGI, Bodden HK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 77.Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151–160. [PubMed] [Google Scholar]
- 78.Davis G, Pintar Allen K, Salazar R, Maxwell S. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–930. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- 79.Collen D. Ham-Wasserman Lecture: role of the plasminogen system in fibrin-homeostasis and tissue remodeling. Hematology. 2001;2001:1–9. doi: 10.1182/asheducation-2001.1.1. [DOI] [PubMed] [Google Scholar]
- 80.Moilanen M, Sorsa T, Stenman M, et al. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry. 2003;42:5414–5420. doi: 10.1021/bi020582s. [DOI] [PubMed] [Google Scholar]
- 81.Nyberg P, Moilanen M, Paju A, et al. MMP-9 activation by tumor trypsin-2 enhances in vivo invasion of human tongue carcinoma cells. J Dent Res. 2002;81:831–835. doi: 10.1177/154405910208101207. [DOI] [PubMed] [Google Scholar]
- 82.Tchougounova E, Lundequist A, Fajardo I, et al. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- 83.Falkenberg M, Holmdahl L, Tjarnstrom J, Risberg B. Abnormal levels of urokinase plasminogen activator protein and tissue plasminogen activator activity in human aortic aneurysms. Eur J Surg. 2001;167:10–14. doi: 10.1080/110241501750069747. [DOI] [PubMed] [Google Scholar]
- 84.Tromholt N, Jorgensen SJ, Hesse B, Hansen MS. In vivo demonstration of focal fibrinolytic activity in abdominal aortic aneurysms. Eur J Vasc Surg. 1993;7:675–679. doi: 10.1016/s0950-821x(05)80715-7. [DOI] [PubMed] [Google Scholar]
- 85.Lindholt JS, Jorgensen B, Shi GP, Henneberg EW. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2003;25:546–551. doi: 10.1053/ejvs.2002.1872. [DOI] [PubMed] [Google Scholar]
- 86.Schneiderman J, Bordin GM, Engelberg I, et al. Expression of fibrinolytic genes in atherosclerotic abdominal aortic aneurysm wall: a possible mechanism for aneurysm expansion. J Clin Invest. 1995;96:639–645. doi: 10.1172/JCI118079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Allaire E, Hasenstab D, Kenagy RD, et al. Prevention of aneurysm development and rupture by local overexpression of plasminogen activator inhibitor-1. Circulation. 1998;98:249–255. doi: 10.1161/01.cir.98.3.249. [DOI] [PubMed] [Google Scholar]
- 88.Deng GG, Martin-McNulty B, Sukovich DA, et al. Urokinase-type plasminogen activator plays a critical role in angiotensin II-induced abdominal aortic aneurysm. Circ Res. 2003;92:510–517. doi: 10.1161/01.RES.0000061571.49375.E1. [DOI] [PubMed] [Google Scholar]
- 89.Wang YX, Martin-McNulty B, Freay AD, et al. Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am J Pathol. 2001;159:1455–1464. doi: 10.1016/S0002-9440(10)62532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galis ZS, Muszynski M, Sukhova GK, et al. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann N Y Acad Sci. 1995;748:501–507. doi: 10.1111/j.1749-6632.1994.tb17348.x. [DOI] [PubMed] [Google Scholar]
- 91.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23:769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 92.Zempo N, Kenagy R, Au Y, et al. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20:209–217. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 93.Zempo N, Koyama N, Kenagy RD, et al. Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol. 1996;16:28–33. doi: 10.1161/01.atv.16.1.28. [DOI] [PubMed] [Google Scholar]
- 94.Johnson JL, van Eys GJJM, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001;21:1146–1451. doi: 10.1161/hq0701.092106. [DOI] [PubMed] [Google Scholar]
- 95.Henney A, Wakeley P, Davies M, et al. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. PNAS. 1991;88:8154–8188. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bendeck MP, Zempo N, Clowes AW, et al. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 97.Hussain S, Assender JW, Bond M, et al. Activation of protein kinase Czeta is essential for cytokine-induced metalloproteinase-1, -3, and -9 secretion from rabbit smooth muscle cells and inhibits proliferation. J Biol Chem. 2002;277:27345–27352. doi: 10.1074/jbc.M111890200. [DOI] [PubMed] [Google Scholar]
- 98.Wu L, Tanimoto A, Murata Y, et al. Matrix metalloproteinase-12 gene expression in human vascular smooth muscle cells. Genes Cells. 2003;8:225–234. doi: 10.1046/j.1365-2443.2003.00628.x. [DOI] [PubMed] [Google Scholar]
- 99.Bendeck MP, Irvin C, Reidy M, et al. Smooth muscle cell matrix metalloproteinase production is stimulated via alpha(v)beta(3) integrin. Arterioscler Thromb Vasc Biol. 2000;20:1467–1472. doi: 10.1161/01.atv.20.6.1467. [DOI] [PubMed] [Google Scholar]
- 100.Schonbeck U, Mach F, Sukhova GK, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res. 1997;81:448–454. doi: 10.1161/01.res.81.3.448. [DOI] [PubMed] [Google Scholar]
- 101.Uzui H, Lee J-D, Shimizu H, et al. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis. 2000;149:51–59. doi: 10.1016/s0021-9150(99)00295-6. [DOI] [PubMed] [Google Scholar]
- 102.Wu C-Y, Hsieh H-L, Jou M-J, Yang C-M. Involvement of p42/ p44 MAPK, p38 MAPK, JNK and nuclear factor-kappa B in interleukin-1beta-induced matrix metalloproteinase-9 expression in rat brain astrocytes. J Neurochem. 2004;90:1477–1488. doi: 10.1111/j.1471-4159.2004.02682.x. [DOI] [PubMed] [Google Scholar]
- 103.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1{beta} J Biol Chem. 2004;279:39513–39519. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 104.Tung WS, Lee JK, Thompson RW. Simultaneous analysis of 1176 gene products in normal human aorta and abdominal aortic aneurysms using a membrane-based complementary DNA expression array. J Vasc Surg. 2001;34:143–150. doi: 10.1067/mva.2001.113310. [DOI] [PubMed] [Google Scholar]
- 105.Pearce WH, Sweis I, Yao JS, et al. Interleukin-1 beta and tumor necrosis factor-alpha release in normal and diseased human infrarenal aortas. J Vasc Surg. 1992;16:784–789. [PubMed] [Google Scholar]
- 106.Clowes A, Clowes M, Au Y, et al. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990;67:61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- 107.Kenagy RD, Vergel S, Mattsson E, et al. The role of plasminogen, plasminogen activators, and matrix metalloproteinases in primate arterial smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 1996;16:1373–1382. doi: 10.1161/01.atv.16.11.1373. [DOI] [PubMed] [Google Scholar]
- 108.Shofuda T, Shofuda K-I, Ferri N, et al. Cleavage of focal adhesion kinase in vascular smooth muscle cells overexpressing membranetype matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2004;24:839–844. doi: 10.1161/01.ATV.0000126680.78500.4c. [DOI] [PubMed] [Google Scholar]
- 109.Onodera S, Nishihira J, Iwabuchi K, et al. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and -13 in rat osteoblasts. Relevance to intracellular signaling pathways. J Biol Chem. 2002;277:7865–7874. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 110.Papathoma AS, Zoumpourlis V, Balmain A, Pintzas A. Role of matrix metalloproteinase-9 in progression of mouse skin carcinogenesis. Mol Carcinog. 2001;31:74–82. doi: 10.1002/mc.1042. [DOI] [PubMed] [Google Scholar]
- 111.Louwrens HD, Kwaan HC, Pearce WH, et al. Plasminogen activator and plasminogen activator inhibitor expression by normal and aneurysmal human aortic smooth muscle cells in culture. Eur J Vasc Endovasc Surg. 1995;10:289–293. doi: 10.1016/s1078-5884(05)80044-9. [DOI] [PubMed] [Google Scholar]
- 112.Hance KA, Tataria M, Ziporin SJ, et al. Monocyte chemotactic activity in human abdominal aortic aneurysms: role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J Vasc Surg. 2002;35:254–261. doi: 10.1067/mva.2002.120382. [DOI] [PubMed] [Google Scholar]
- 113.Allaire E, Muscatelli-Groux B, Mandet C, et al. Paracrine effect of vascular smooth muscle cells in the prevention of aortic aneurysm formation. J Vasc Surg. 2002;36:1018–1026. doi: 10.1067/mva.2002.127347. [DOI] [PubMed] [Google Scholar]
- 114.Yoshimura K, Aoki H, Ikeda Y, et al. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]