Abstract
Sleep deprivation interferes with cognitive performance but the mechanisms are poorly understood. We recently reported that one night of sleep deprivation increased dopamine in striatum (measured with [11C] raclopride, a PET radiotracer that competes with endogenous dopamine for binding to D2 receptors) and that these increases were associated with impaired performance in a visual attention task. To better understand this association here we evaluate the relationship between changes in striatal dopamine (measured as changes in D2 receptor availability using PET and [11C]raclopride) and changes in brain activation to a visual attention task (measured with BOLD and fMRI) when performed during sleep deprivation versus during rested wakefulness. We find that sleep induced changes in striatal dopamine were associated with changes in cortical brain regions modulated by dopamine (attenuated deactivation of anterior cingulate gyrus and insula) but also in regions that are not recognized targets of dopaminergic modulation (attenuated activation of inferior occipital cortex and cerebellum). Moreover, the increases in striatal dopamine as well as its associated regional activation and deactivation patterns correlated negatively with performance accuracy. These findings therefore suggest that hyperstimulation of D2 receptors in striatum may contribute to the impairment in visual attention during sleep deprivation. Thus, while dopamine increases in prefrontal regions (including stimulation of D1 receptors) may facilitate attention our findings suggest that hyperstimulation of D2 receptors in striatum may impair it. Alternatively, these associations may reflect a compensatory striatal dopamine response (to maintain arousal) that is superimposed on a larger response to sleep deprivation.
Keywords: Dopamine D2 receptors, Raclopride, Visual attention, PET, fMRI, Default network, Thalamus
Sleep deprivation (SD) has significant public health implications adversely affecting health and safety (Institute of Medicine, 2006). Some of these negative consequences, which include car accidents (20% of all car accidents; equivalent to those attributed to alcohol intoxication) (Connor et al., 2002) and impaired work performance, reflect the adverse effects of SD on attention, judgment and decision-making (Durmer and Dinges, 2005). Despite its high prevalence in our modern society the neurochemical mechanisms associated with cognitive impairment with SD are poorly understood. Multiple neurotransmitters are implicated in the sleep/wake cycle including dopamine (DA) and their disruption could contribute to the cognitive impairments observed with SD (Siegel, 2004; Monti, 1983; Dzirasa et al., 2006). Indeed, we recently reported using Positron Emission Tomography (PET) and [11C]raclopride (radiotracer whose binding to D2 receptors is sensitive to endogenous DA) that D2 receptor availability decreased one night after SD, which we interpreted as an indication of striatal DA increases with SD (Volkow et al., 2008a). We also showed that the increases in striatal DA were associated with the decrease in performance (accuracy) on a visual attention (VA) task (Volkow et al., 2008a). Though this association at first sight may seem counterintuitive since stimulant medications, which increase DA, can counteract some cognitive deficits associated with SD (Boutrel and Koob, 2004) these effects most likely reflect DA increases (as well as noradrenergic increases) in prefrontal cortex (Berridge et al., 2006). Also, caffeine, which improves attention with SD (Lim and Dinges, 2008), was shown to have the opposite effects to that of SD in [11C] raclopride binding (increased striatal binding presumably from DA decreases) (Kaasinen et al., 2004). To better understand the association between striatal DA increases and disruption in VA with SD, here we assess the relationship between the striatal DA increases and the changes in brain activation responses to the VA task when performed during SD versus when performed during rested wakefulness (RW).
For this purpose we assessed the correlations between changes in [11C]raclopride binding (measured with PET and [11C]raclopride) and changes in regional brain activation to the VA task (measured with BOLD and fMRI). We contrasted this association after one night of SD and a control condition of rested wakefulness (RW) in 14 healthy male controls. We had previously shown using fMRI that task difficulty in the VA was associated with activation in parietal cortex and with deactivation in upper and middle occipital cortex, insula and anterior cingulate gyrus (CG) and that these responses were significantly lower during SD than during RW except in thalamus (activation higher for SD than RW) and in CG (no differences) (Tomasi et al., 2008). The correlation between striatal DA increases and impaired VA performance with SD (Volkow et al., 2008a) led us to hypothesize that striatal DA increases contributed to the brain activation deficits during SD. Specifically, and on the basis of prior imaging studies that showed that stimulant drugs, which increase DA, markedly decreased brain activation during a cognitive task (Mehta et al., 2000; Volkow et al., 2008b), we hypothesized that striatal DA increases with SD would be associated with decreased activation to the VA task. Also since the effects of stimulant medications on regional brain activation vary as a function of the activation pattern elicited by the specific task (Mattay et al., 1996) we expected that the striatal DA increases with SD would correlate with regional responses associated with task performance. The results of the effects of SD on striatal D2 receptor availability and on the brain activation patterns during VA have been reported elsewhere (Tomasi et al., 2008; Volkow et al., 2008a). This study focuses specifically on the correlations between these two sets of measures.
Methods
Subjects
Fourteen healthy, non-smoking, right-handed men (means±SD: age 32±8 years; education: 16±2 years; Body Mass Index=25±3) participated in the study. Participants were screened carefully with a detailed medical history, physical and neurological examination, EKG, Breath CO, routine blood tests and urinalysis, and urine toxicology for psychotropic drugs to ensure they fulfilled inclusion and exclusion criteria. Inclusion criteria were: 1) ability to understand and give informed consent; and 2) 18–50 years of age. Exclusion criteria were: 1) urine positive for psychotropic drugs; 2) present or past history of dependence on alcohol or other drugs of abuse (including current dependence on nicotine); 3) present or past history of neurological or psychiatric disorder; 4) use of psychoactive medications in the past month (i.e., opiate analgesics, stimulants, sedatives); 5) use of prescription (non-psychiatric) medication(s), i.e., antihistamines; 6) medical conditions that may alter cerebral function; 7) cardiovascular and metabolic diseases; 8) history of head trauma with loss of consciousness of more than 30 min; and 9) history of sleep disorders (if they responded affirmatively to having problems falling asleep, staying asleep, feeling tired upon wakening, and/or required medications to help them sleep and/or if they had a past or present history of sleep apnea or restless leg syndrome); and 10) work that required night shift-hours. Subjects were asked to keep a record of the number of hours slept per night for the 2 weeks duration of the study (from evaluation to completion of the study) and this corresponded to an average of 7.5±1 h per night; range 5.5–8.8 h per night; average bed time 11.45±1 PM; average wake up time 7.2±1 AM. Signed informed consents were obtained from the subjects prior to participation as approved by the Institutional Review Board at Brookhaven National Laboratory.
SD and RW procedures
Subjects were kept overnight at Brookhaven National Laboratory prior to their scheduled SD or RW session to ensure that subjects stayed awake for the SD (one night of sleep deprivation) or had a good nights rest for the RW (mean 6.7±0.9 h slept; range 5–8.5 h) conditions. A research assistant remained with them throughout the night to ensure that they did not fall asleep for the SD condition and to observe that they slept properly for the RW condition. On the day of the RW condition the subjects were awakened at 7 AM and brought to the imaging suite. A nurse remained with the subjects to ensure that they stayed awake throughout the study. No food was given after midnight and caffeinated beverages were discontinued for 24 h prior to the study.
Behavioral measures
Subjects were asked to rate self-reports for “alertness”, on a scale of 1 to 10 where 1 being not at all and 10 being very intense. This measure was obtained twice on each day of study; prior to the PET study and 2 h later. On the day of the RW condition subjects also rated the “quality of their sleep” from 1 to 10 (1 not restful to 10 very restful).
Imaging
The PET scans were done with a Siemens HR+ tomograph (resolution 4.5×4.5×4.5 mm full width half-maximum) in 3D mode. Subjects were scanned with [11C]raclopride twice; after one night of observed RW and after one night of observed SD. The scans were performed between 12 and 1 PM, which was approximately 5 to 6 h after awakening in the RW condition and 29 to 33 h after awakening in the SD condition (12 Ss≥30 h and 2 Ss=29 h). Half the subjects had their first set of scans after RW and the other half after SD to control for order effects. Dynamic scans were started immediately after iv injection of 4–10 mCi of [11C]raclopride (specific activity>0.25 Ci/µmol at time of injection) for a total of 60min as previously described (Volkow et al., 1994).
The BOLD-fMRI scanswere done with a 4 Tesla whole-body Varian/Siemens MRI scanner using a T2*-weighted single-shot gradient-echo planar imaging sequence with ramp-sampling (TE/TR=20/1600 ms, 4 mm slice thickness, 1 mm gap, 35 coronal slices, 64×64 matrix size, 3.1×3.1 mm in-plane resolution, 90°-flip angle, 231 time points, bandwidth: 200.00 kHz) covering the whole brain. Padding was used to minimize motion. Subject motion was determined immediately after each fMRI trial, to confirm motion <1 mm-translations and <1° rotations (Caparelli et al., 2003). Anatomical images were collected using a T1-weighted 3D-MDEFT sequence (Lee et al., 1995) (TE/TR=7/15 ms, 0.94×0.94×1 mm spatial resolution, axial orientation, 256 readout and 192×96 phase-encoding steps, 16 min scan time) and a modified T2-weighted Hyperecho sequence (Hennig and Scheffler, 2001) (TE/TR=42/10,000 ms, echo train length=16, 256×256 matrix size, 30 coronal slices, 0.86×0.86 mm in-plane resolution, 5 mm thickness, 1 mm gap, 2 min scan time), which were reviewed to rule out gross morphological abnormalities in the brain.
Task activation paradigm for fMRI
The participants performed a VA with blocked design that was described previously (Tomasi et al., 2004). Briefly, the “TRACK” epochs are 1 min long and composed of five “Ball tracking” and respond periods. In these periods, subjects viewed 10 identical balls that randomly move across the central visual field, and used only their peripheral vision and attention to keep track of a subset of balls (two, three or four balls) that were briefly highlighted at the beginning of each trial. To avoid eye movements while following the balls subjects were asked to fixate on a cross at the center of the display. At the end of each trial the balls stop moving and a new set of balls is highlighted; the subject presses a button if these balls are the same as the target set. Button press events are used to record performance accuracy and reaction times. The “DO NOT TRACK” baseline epochs are 1 min long and composed of five consecutive “resting” periods. In these periods, all 10 balls move and stop in the same manner as during “TRACK” epochs; however, no balls are highlighted, and subjects are instructed to not track the balls and view them passively. The stimuli were presented on MRI-compatible goggles and the display software was synchronized with the MR acquisition using an MRI trigger pulse. Prior to testing subjects performed a brief training session (~10 min) outside of the scanner to ensure that they were able to perform the tasks. The fMRI VA task was performed two hours after completion of the PET [11C]raclopride scans in both the SD and RW conditions (performed between 1 and 3 PM; approximately 7 to 8 h after awakening for RW condition and 31 to 35 h after awakening for the SD condition).
PET image analysis
Regions of interest (ROI) were obtained directly from the [11C] raclopride images as previously described (Volkowet al., 1994). Briefly we identified and selected the ROI on summed images (dynamic images taken from 10–54 min) that were resliced along the intercommisural plane (AC–PC line). The caudate and putamen and cerebellum were measured on 4, 3, and 2 planes respectively, and right and left regions were delineated. These regions were then projected to the dynamic scans to obtain concentrations of C-11 vs. time, which were used to calculate the distribution volumes using a graphical analysis technique for reversible systems that does not require arterial blood sampling (Logan et al., 1996). We computed the ratio of the distribution volume in caudate and putamen to that in the cerebellum. The distribution volume ratio (DVR), which corresponds to Bmax/Kd +1, was used as an estimate of D2 receptor availability.
fMRI image analysis and statistics
The first four time points in each fMRI time series were discarded to avoid non-equilibrium effects in the BOLD signal. The statistical parametric mapping package SPM2 (Welcome Department of Cognitive Neurology, London UK) was used for subsequent image processing: the images were (1) realigned to the first volume in the time series (to ensure that head motion was lower than 1-mm translations and 1°-rotations for all scans); (2) spatially normalized to the Talairach frame of reference; and (3) spatially smoothed with an 8-mm FWHM Gaussian kernel.
A general linear model and a blocked design with high-pass filtering (cut-off=1/256 Hz) were used to calculate the BOLD contrast maps for each condition (2-, 3-, and 4-ball tracking) and subject in SPM2. Differential (ΔBOLD; SD minus RW) contrast volumes, reflecting the effect of SD on brain activation, were calculated for each subject and condition using the individual BOLD contrast maps and IDL (ITT Visual Information Solutions, Boulder, CO).
Multiple (linear) regression analyses in SPM2 were used to assess the effect of accuracy, and reaction time on BOLD-fMRI signals. Specifically, the estimated BOLD signal maps for each trial and subject were included in multiple regression random-effects models with three regressors: 1:(Main VA) a constant regressor modeling the average BOLD amplitude produced by the VA task; 2:(VA-load) a linear regressor (−1, 0, 1), modeling the increased BOLD amplitude produced by the increased task difficulty (2-, 3-, and 4-ball tracking). The third regressor reflected the subjects' performance (accuracy or RT) for each session (RW or SD). Separate analyses were carried out for accuracy and RT to minimize the degrees of freedom (number of fitting parameters) of the SPM multiple regression model.
Similarly, multiple (linear) regression analyses in SPM2 were also used to assess the effect of SD-related changes in striatal DA D2 receptor availability changes (ΔD2R) on differential ΔBOLD signals. As before the model included three regressors: 1) Main VA; 2) VA-load; and 3) ΔD2R in caudate or putamen. Separate analyses were carried out for caudate and putamen to minimize the degrees of freedom (number of fitting parameters) of the SPM multiple regression model. The continuous random field calculation implemented in SPM2 was used to perform the cluster analyses. Brain activation and deactivation clusters with at least 20 voxels (540 mm3) and p<0.05 (corrected for multiple comparisons) were considered significant.
To validate the SPM results, brain activation and deactivation clusters demonstrating significant correlations between ΔBOLD signals in the brain and ΔD2R in striatum were further evaluated with region-of-interest (ROI) analyses. Specifically, isotropic masks containing 27 imaging voxels (0.73 ml) were defined at the centers of relevant activation clusters to extract the average (and SD) % signal change from individual ΔBOLD contrast maps. These 9-mm cubic masks were created and centered at the precise coordinates listed in Table 1–Table 3, using IDL. The coordinates of the ROI masks were kept fixed across subjects and conditions. Pearson product-moment correlations were used to assess the association between ΔD2R in striatum and ΔBOLD. Since the correlations were equivalent for the 3 difficulty levels we report on the correlation with the average of the 3 measures.
Table 1.
Location of major areas of brain activation and deactivation in the Talairach coordinates (x,y,z), and average statistical significance (Z) of BOLD responses in 27 voxels (0.73 cm3; cubic) ROI centered at these cluster locations
| Brain region | BA | x | y | z | RW Main | RW Load | SD Main | SD Load | SD>RW ΔMain |
|---|---|---|---|---|---|---|---|---|---|
| z | z | z | z | z | |||||
| Activation | |||||||||
| Middle frontal gyrus | 6 | −27 | −3 | 60 | 4.9* | – | 4.9* | 1.6 | −2.1 |
| 21 | 0 | 57 | 7.2* | – | 6.5* | 2.0 | −3.4 | ||
| Middle frontal gyrus | 9 | −51 | 6 | 39 | 3.4* | – | 2.8* | 1.9 | – |
| 36 | 6 | 33 | 5.4* | 2.7 | 5.3* | 3.1 | – | ||
| Inferior parietal | 40 | −30 | −51 | 54 | 4.5* | 2.1 | 4.3* | 2.2 | −2.1§ |
| 33 | −54 | 60 | 4.1* | – | 3.8* | – | −1.7 | ||
| Superior parietal | 7 | −12 | −69 | 57 | 6.1* | 2.1 | 4.8* | – | −2.5* |
| 21 | −69 | 54 | 3.8* | – | 5.3* | 2.5‡ | −1.9 | ||
| Occipital cortex | 18 | −3 | −81 | −9 | 4.0* | – | 3.6* | – | −1.7 |
| 12 | −72 | −15 | 4.3* | – | 3.6* | – | – | ||
| Occipital cortex | 19 | −33 | −72 | −12 | 3.4* | – | 2.8* | – | −1.7 |
| 33 | −72 | −12 | 3.3* | – | 3.0* | – | −1.6 | ||
| Thalamus | −15 | −15 | 12 | 3.0* | 1.9 | 4.4* | – | 3.2* | |
| 15 | −18 | 15 | 3.7* | 2.1 | 4.7* | – | 2.8* | ||
| Cerebellum- vermis | 3 | −45 | −9 | 3.2* | 2.4 | – | – | −2.5 | |
| Cerebellum | 12 | −57 | −30 | 4.5* | 2.7* | 4.5* | 2.3 | – | |
| Deactivation | |||||||||
| Dorsal cingulate gyrus | 32 | −3 | 33 | 12 | −4.3* | −3.1 | −4.5* | – | – |
| Ventral cingulate gyrus | 24 | −6 | −9 | 39 | −4.4* | −3.3* | −4.3* | – | – |
| Insula | 13 | −36 | −21 | 18 | −5.2* | −2.3 | −5.1* | −2.0 | – |
| 30 | −12 | 15 | −4.9* | −2.8† | −4.8* | −1.8 | 1.5 | ||
| Precuneus | 31 | 3 | −63 | 21 | −6.8* | −2.0 | −6.2* | – | 1.5 |
| Middle R occipital | 18 | 12 | −81 | 12 | −1.9 | −1.6 | 1.9 | – | – |
Regions where SPM showed significant effects at p<0.05 (corrected for multiple comparisons), cluster size>20 voxels (540 mm3). Main: average BOLD response across conditions (2-, 3-, and 4-ball tracking); Load: differential BOLD response (4-ball tracking>2-ball tracking); ΔMain: differential mean signal between sessions [average (2-, 3-, and 4-ball tracking; SD)>average (2-, 3-, and 4-ball tracking; RW). Abbreviations correspond to RW = rested wakefulness, SD = sleep deprivation, BA = Brodman Area.
pcorr< 0.001
pcorr<0.005
pcorr<0.01
pcorr<0.05.
Table 3.
Brain areas and their location (Talairach coordinates, x,y,z) where changes in Bmax/Kd (ΔD2R) in caudate and in putamen were correlated with BOLD changes (ΔBOLD) and significant differences in activation responses to VA task between SD and RW (ΔBOLD Main Effect)
| Region | BA | x | y | z | Correlation Putamen DD2R vs. DBOLD |
p | Correlation Caudate DD2R vs. DBOLD |
p | ΔBOLD Main Effect (SD–RW) |
|---|---|---|---|---|---|---|---|---|---|
| Positive correlations | |||||||||
| Middle R occipital | 18 | 9 | −69 | 3 | r=0.61 | p=0.02 | r=0.65 | p=0.01 | NS |
| Middle R occipital | 18 | 12 | −81 | 12 | NS | r=0.65 | p=0.01 | Z=2.2§ | |
| Lower R occipital | 18 | 12 | −75 | −9 | NS | r=0.67 p=0.009 | p=0.009 | Z=−1.8‡ | |
| Vermis | −3 | −60 | −24 | r=0.70 | p=0.006 | r=0.64 | p=0.02 | NS | |
| L cerebellum | −27 | −69 | −14 | r=0.64 | p=0.02 | r=0.53 | p=0.05 | Z=−1.9‡ | |
| R cerebellum | 6 | −66 | −33 | r=0.77 | p=0.002 | r=0.72 | p=0.004 | NS | |
| Negative correlations | |||||||||
| Cingulate gyrus | 32 | 0 | 36 | −3 | r=−0.72 | p=0.004 | r=−0.66 | p=0.01 | NS |
| R cingulate | 24 | 9 | 33 | −3 | r=−0.84 | p=0.0002 | r=−0.79 | p=0.0008 | NS |
| R anterior insula | 13 | 33 | 12 | 6 | r=−0.67 | p=0.009 | r=−0.66 | p=0.01 | NS |
| L posterior insula | 13 | −33 | −9 | −6 | r=−0.72 | p=0.004 | r=−0.82 | p=0.0003 | NS |
For the correlations the “r” values along with their significance (p) are shown and for the BOLD “Main Effect” we show the Z values on those regions that showed significant differences between RW and SD at p<0.05 corrected for multiple comparisons and for cluster size of 27 voxels. Abbreviations correspond to BA = Brodman Area, L = left, R = right, SG = superior gyrus.
pcorr<0.005
pcorr<0.05.
Results
Effects of SD on the behavioral measures and on the VA task
During RW scores for “sleep quality” corresponded to 8.3±2 and the self-reports of alertness (average of the two measures) were significantly higher than during SD (7.9±2 vs. 5.9±2; p<0.005). The comparisons within a given day between the measures obtained prior to the PET scan to those obtained 2 h later were significantly lower for the SD measures (6.3±2; and 5.4±2 p<0.05), but did not differ for RW (8.2±2 and 7.6±3).
Performance accuracy, averaged across the three task difficulty levels, decreased significantly during SD (92±8% for RW, 77±20% for SD; p=0.001, repeated measures ANOVA). In contrast, there were no differences in reaction time between RW and SD (849±333% for RW, 918±370ms for SD; p=0.422). The relationship between accuracy and reaction time also differed between conditions; whereas these two measures were not correlated during RW (r=0.29, p=0.31) during SD they showed a negative correlation (r=−0.70, p<0.005) for the two less demanding task levels (2-balls and 3-balls), such that the faster the responses during SD the greater the accuracy.
Effects of SD on brain activation to the VA task
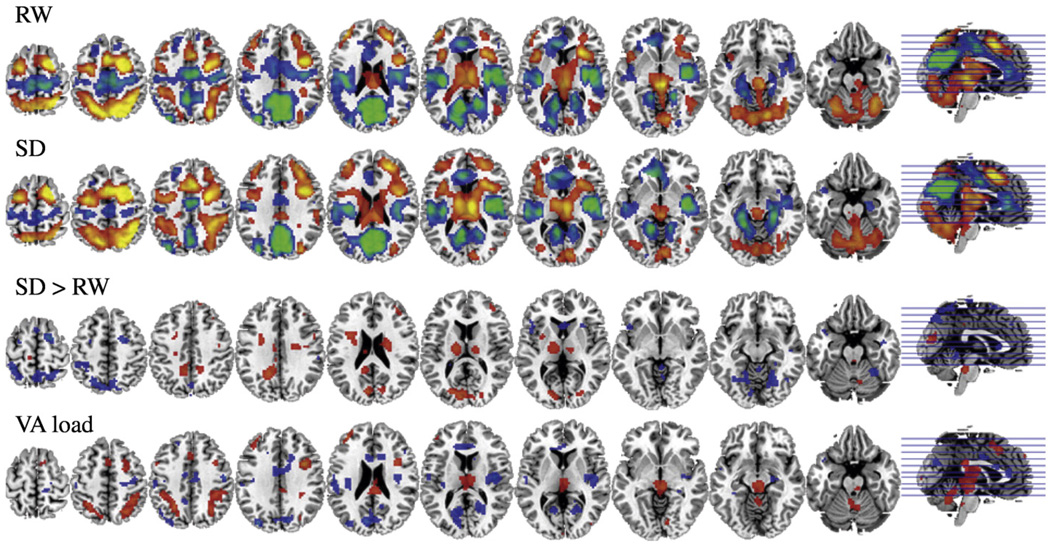
As previously reported the VA task activated thalamus, superior and inferior parietal gyri, cerebellar vermis and lower occipital cortex and deactivated precuneus, upper and middle occipital cortex, anterior cingulate gyrus (CG), and insula (Tomasi et al., 2008). Analysis on the “VA load effect” revealed that with increased difficulty there was enhanced activation in thalamus and parietal cortex and enhanced deactivation in upper and middle occipital cortex and in the insula (Fig. 1, Table 2).
Fig. 1.
SPM activation maps with VA during RW(first row) and during SD conditions (second row); SPM activation maps showing areas where SD>RW(third row); and SPM activation map showing areas that changed with increased VA attentional load (fourth row). Significance for all comparisons corresponds to p<0.005 not corrected. VA = visual attention, RW=rested wakefulness, SD = sleep deprivation.
Table 2.
Brain regions and their location in the Talairach coordinate space (x,y,z) where BOLD signals were positively and negatively correlated with performance accuracy in the VA task for the RW and SD conditions and effects of VA task on bold signal (main effect)
| Region | BA | x | y | z | Correlation accuracy vs. BOLD (RW) | Correlation accuracy vs. BOLD (SD) | p | Main effect RW | Main effect SD |
|---|---|---|---|---|---|---|---|---|---|
| Positive correlations | |||||||||
| Lingual gyrus | 17, 18 | 3 | −81 | −6 | NS | r=0.67 | p=0.009 | Z=3.6* | Z=3.9* |
| R cerebellum | 27 | −72 | −18 | NS | r=0.62 | p=0.02 | Z=3.2* | Z=2.6* | |
| L cerebellum | −12 | −72 | −18 | NS | r=0.65 | p=0.01 | Z=3.3* | Z=3.6* | |
| Negative correlations | |||||||||
| L cingulate | 32 | −12 | 12 | 36 | NS | r=−0.84 | p=0.0002 | NS | Z=−1.9 |
| R insula | 13 | 42 | −21 | 21 | NS | r=−0.76 | p=0.002 | Z=−2.6* | Z=−2.9* |
| Precuneus | 7 | −3 | −66 | 42 | NS | r=−0.65 | p=0.01 | Z=−2.3* | Z=−3.9* |
For the correlations the “r” values along with their significance (p) are shown and for the BOLD “Main Effect” we show the Z values for the ROI (27 voxels) centered at these cluster locations. Abbreviations correspond to BA = Brodman Area, L = left, R = right.
pcorr< 0.005.
Comparison between SD and RW for the “main effect” revealed that during SD there was enhanced activation of thalamus, attenuated activation of parietal gyri, cerebellar vermis and lower occipital and attenuated deactivation of upper and middle occipital cortex and precuneus (Fig. 1, Table 1). In contrast the “load effect” did not differ between RW and SD (data not shown).
Correlations between BOLD signals and VA performance
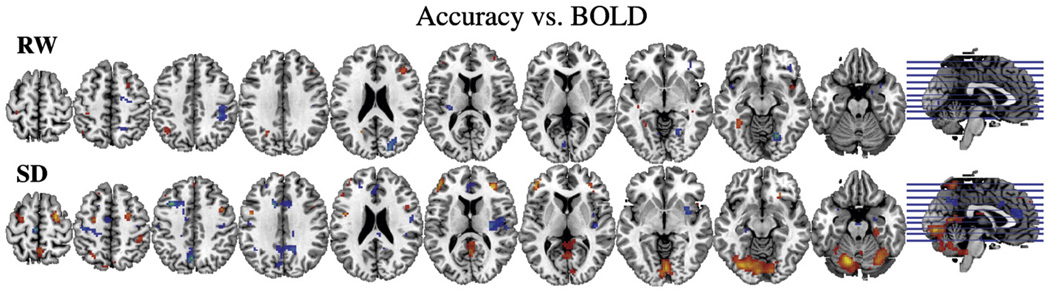
Brain activation and performance accuracy were significantly correlated for the SD condition but not for the RW condition (Fig. 2). During SD, accuracy was positively correlated with BOLD responses in cerebellum and lower occipital cortex (the higher the activation the better the accuracy) (pcorr <0.001, corrected for multiple comparisons) and negatively correlated in right insula, left CG (BA 32) and precuneus (the higher the deactivation the better accuracy) (pcorr <0.05) (Fig. 2, Table 2). None of the correlations with reaction time reached significance after correction for multiple comparisons and cluster size (data not shown).
Fig. 2.
SPM correlation maps between VA performance (accuracy) and BOLD signals for the RW (first row) and the SD (second row) conditions. Significance for all comparisons corresponds to p<0.005 not corrected.
Effects of SD on striatal D2 receptor availability
As previously reported D2 receptor availability (Bmax/Kd) was significantly lower for SD than for RW in caudate (2.30±0.20 vs. 2.43±0.19; t=3.4 df 13, p<0.005) and putamen (2.93±0.16 vs. 3.05±0.18; t=2.2 df 13, p<0.05) (Volkow et al., 2008a). The changes in D2 receptor availability (ΔD2R) in putamen were negatively correlated with changes in accuracy (r=−0.79, df 14, p<0.001) and changes in caudate showed a trend (r=−0.51, df 14, p<0.06). The negative correlation means that larger ΔD2R were associated with worse performance. The correlations with reaction time were not significant.
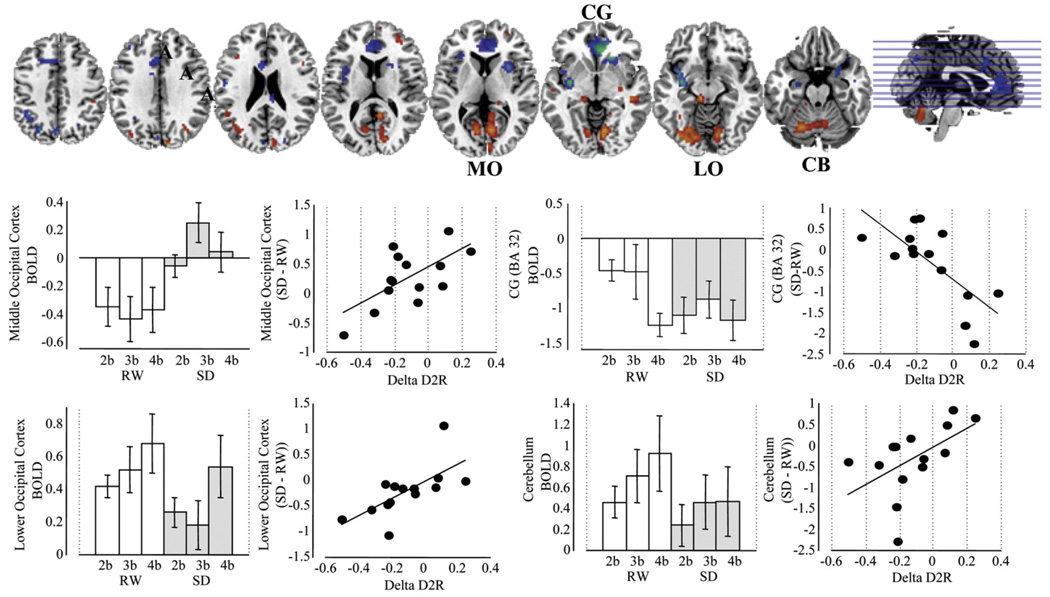
Correlations between ΔBOLD (SD–RW) and ΔD2R
Since the correlations between SD and RW were similar for caudate and putamen, we describe them together (Fig. 3, Table 3). Striatal ΔD2R was positively correlated with ΔBOLD in occipital cortex (including upper, middle and lower occipital cortex) and cerebellum (pcorr <0.001) and negatively correlated in anterior CG and insula (Table 3, Fig. 3). The positive correlation in the lower occipital cortex and cerebellum, which were regions activated more in RW than SD, indicated that decreases in D2 receptor availability were associated with decreased activation in these regions during SD. The positive correlation in the upper and middle occipital cortex, which were deactivated more in RW than SD indicated that subjects with larger decreases in D2 receptor availability tended to show more deactivation in this region during SD than subjects with less or no changes in D2 receptor availability. The negative correlations in the CG and insula, which were regions deactivated during VA but that did not differ between SD and RW indicated that decreases in D2 receptor availability during SD were associated with less deactivations.
Fig. 3.
SPM correlation maps between the changes in D2R availability in putamen and the changes in activation/deactivation BOLD (SD vs. RW) and histograms showing the activation/deactivation responses to the 3 difficulty levels (2 balls, 3 ball, 4 balls) during RW and SD and the corresponding regression plots between putamen ΔD2R and ΔBOLD (averaged across the 3 difficulty levels). Significance for SPM maps correspond to p<0.005 not corrected. Abbreviations correspond to CG cingulate gyrus, LO = lower occipital, MO = middle occipital, CB = cerebellum, 2b = 2 balls, 3b = 3 balls, and 4b = 4 balls.
Discussion
Here we show that decreases in striatal D2 receptor availability (interpreted as DA increases and hyperstimulation of D2 receptors) were associated with decreased activation in lower occipital cortex and cerebellum (regions activated by the VA task to a greater extent in RW than SD) and with decreased deactivation in CG and insula (regions that did not differ between RW and SD).
Since activation of lower occipital cortex and cerebellum and deactivation in CG and insula were associated with accuracy during SD one interpretation could be that striatal hyperstimulation of D2 receptors by attenuating these regional brain responses contributed to the VA deficit during SD. However, another interpretation is that striatal DA increases reflect counteracting effects to maintain arousal as the drive to sleep increases (greater fatigue greater DA increases), masking DA true effects. In the former interpretation the association between striatal ΔD2R and blunted regional brain responses may reflect the mechanisms by which DA interferes with VA attention during SD, while in the latter interpretation this may reflect DA's counteracting effects on the drive to sleep in order to maintain arousal.
The regions where striatal ΔD2R were positively correlated with ΔBOLD (occipital cortex and cerebellum) are regions that are not typically considered main targets for DA innervation or regulation, which could be taken to suggest that the association reflects parallel processes rather than processes that are causally linked. However, the association could reflect either downstream effects from regions modulated by DA or direct DA modulation since, though low, there are D2 receptors in occipital cortex (Gil-Martin et al., 1994; Lidow and Goldman-Rakic, 1994; Meador-Woodruff et al., 1997; Parkinson, 1989; Mukherjee et al., 2002), and in cerebellum (Pinborg et al., 2007).
In the occipital cortex DA modulates visual processing in part via D2 receptors (Müller and Huston, 2007; Reyes et al., 2002). DA also regulates the transfer of retinal information in the dorsal lateral geniculate nucleus (major relay nucleus of the visual system (Zhao et al., 2002)) (Govindaiah and Cox, 2006), where it exerts a predominant inhibitory action (Albrecht et al., 1996). In turn the dorsal geniculate nucleus modulates the transfer of information into the visual system as a function of attentional demands and behavioral state (Sherman and Guillery, 2002).
Regions in the occipital cortex where ΔBOLD correlated positively with striatal ΔD2R included both areas deactivated (upper and middle occipital) and activated (lower occipital) by the VA task and these responses were attenuated during SD (Fig.1). The association between ΔD2R and ΔBOLD showed an opposite pattern in upper and middle occipital (larger ΔD2R greater deactivation and hence less differences between SD and RW) than in the inferior occipital (larger ΔD2R less activation and hence greater differences between SD and RW) (Fig. 3). This most likely reflects the functional heterogeneity of the visual cortex (Grill-Spector and Malach, 2004) and its impairment by SD (Corsi-Cabrera et al., 1999; Chuah and Chee, 2008; Chee et al., 2008).
Higher activity in the middle occipital cortex has been associated with lower arousal (Hofle et al., 1997). Thus it is possible that higher activity in the upper and middle occipital cortex associated with decreased arousal during SD could have interfered with the deactivation required for maintaining performance with increasing task difficulty (Fig. 1). Interestingly transcranial magnetic stimulation (rTMS) of the upper middle occipital cortex, but not the lower occipital, during SD was shown to remediate the deficits in a visual working memory task (Luber et al., 2008).
Decreased activation of the inferior occipital cortex while performing a VA task during SD, has been reported by others (Chee et al., 2008). Moreover, it has been suggested that it is contingent on the engagement of selective attention since decreased activation during SD was not observed with passive visual stimulation (Chee et al., 2008). Cholinergic neurotransmission, which plays a key role in regulating the visual cortex (Sato et al., 1987) and is reduced with prolonged wakefulness (Jones, 2005) was shown to contribute to the reduced activation of the visual system during SD (Chuah and Chee, 2008). Our finding suggests that DA neurotransmission (directly or indirectly) may also contribute to decreased activation of the visual system during SD.
The association between striatal ΔD2R and cerebellar activation most likely reflects striatal modulation of cerebellar activity (Perciavalle et al., 1987). The correlation with ΔD2R was centered in the inferior posterior hemisphere (Fig. 3) where its activation by the VA task during SD was associated with performance accuracy (Fig. 2). This is consistent with prior imaging studies linking the activity of the cerebellar hemispheres with VA processes (Dieterich et al., 2000). Similarly, in patients with attention deficit hyperactivity disorders (ADHD) and dyslexia the cerebellar abnormalities (including posterior cerebellar hemispheres) have been linked with symptoms of inattention (Mackie et al., 2007; Ashtari et al., 2005; Kibby et al., 2008). Thus striatal hyperstimulation of D2 receptors may have contributed to impairments in VA by disrupting cerebellar activation.
The anterior CG where striatal ΔD2R was negatively correlated with ΔBOLD is a target of the DA mesocortical system (Hurd et al., 2001) and plays a key role in attention (Rueda et al., 2005; Mason et al., 2007). The association could reflect direct or indirect modulation via striato-thalamo-cortical pathways (Alexander et al., 1990). The area in the CG that correlated with ΔD2R corresponded to BA 24 and BA 32. BA 24 has been shown to deactivate during cognitive tasks in proportion to emotional interference (Bush et al., 2003; Simpson et al., 2001a,b). Though deactivation of the CG did not differ for the RW and SD conditions, individual analysis revealed that subjects with the largest ΔD2R showed less BOLD differences in CG between SD and RW whereas subjects with less or no ΔD2R deactivated more during SD than RW (Fig. 3). Since CG deactivation was associated with performance accuracy during SD this suggests that striatal hyperstimulation of D2 receptors by interfering with further CG deactivation was detrimental for performance.
In the insula, striatal ΔD2R was also negatively correlated with ΔBOLD (Fig. 3). The insula is implicated in the awareness of the physiological condition of the physical body (interoception) (Craig, 2002). Thus awareness of fatigue and sleepiness may have contributed to the blunted deactivation of the insula during SD. The insula has very low levels of D2 receptors (Hurd et al., 2001) and thus the association with striatal ΔD2R most likely reflects the striatal connectivity with the insula (Postuma and Dagher, 2006). The role of DA and/or of striato-insular pathways in interoceptive function to our knowledge has not been investigated.
Studies on the role of DA on SD-induced cognitive impairment
The deterioration in performance in the VA task with SD, which is consistent with prior studies (Durmer and Dinges, 2005) was associated with decreases in striatal ΔD2R (interpreted as DA increases). This finding may seem paradoxical since it is opposite to the beneficial effects that stimulant medications can have on attention during SD (Bonnet et al., 2005). However, stimulant medication and DA agonists are not always beneficial and in some subjects they impair performance (Oranje et al., 2006). Also, the beneficial effects of stimulant drugs in attention are believed to reflect its dopaminergic as well as its noradrenergic effects on the prefrontal cortex rather than the striatum (Berridge et al., 2006; Corbetta et al., 2008) and to require the stimulation of D1 receptors (or D1 and D2) (Levy, 2008) whereas here we show DA stimulation of D2 receptors in striatum with SD. Indeed, microdialysis studies in rodents showed that low doses of methylphenidate that improved cognitive function but were devoid of locomotor effects increased DA and norepinephrine in prefrontal cortex with minimal effects in striatum (Berridge et al., 2006). In contrast, high doses of stimulant medications, which induce robust DA increases in striatum, impair attention (Martinez and Sarter, 2008).
Moreover, since SD has been shown to disrupt prefrontal activity (Horne, 1993) and this disruption has also been associated with cognitive impairment (Thomas et al., 2000, 2003) it is possible that the association between VA performance and striatal ΔD2R reflects reduced prefrontal activity occurring concomitantly to striatal hyperstimulation of D2 receptors with SD. Indeed, D2 receptor blockade (using sulpiride) was shown to alleviate the impairment in attention in prefrontal lesioned animals, but not in intact animals, suggesting that striatal hyperstimulation of D2 receptors may contribute to attention deficits in the prefrontal damaged animal (Passetti et al., 2003). Inasmuch as the prefrontal cortex regulates DA cell firing and its damage increases striatal DA (Bertolino et al., 2000) further studies are required to assess if decreases in prefrontal activity during SD contribute to the striatal DA increases.
However, it is also possible that striatal DA increases reflect a counteracting effect to maintain arousal as the drive to sleep increases. Thus greater fatigue would produce greater striatal DA increases. This might mask the effect of DA increases, or even make them appear to be opposite to the true effects. Thus even though the effect of DA may be to improve performance, it may be associated with decreased performance due to the correlated and greater effect of SD onto which the DA effects are superimposed.
Study limitations
(1) The limited sensitivity of the [11C]raclopride did not allow us to measure DA changes in the prefrontal cortex nor did it allow us to assess regions predominantly modulated by D1 receptors, which are crucial for attention. (2) The [11C]raclopride method does not allow us to ascertain if changes in binding reflect changes in DA, in D2 receptor levels or in affinity (Gjedde et al., 2005). (3) In our subjects we did not obtain electroencephalographic measures nor did we obtain information on chronotype, the Epworth score, nor on sleep latencies. These would have provided additional information on sleeping patterns of our subjects that may have been relevant to understanding the intersubject variability in the responses to SD. (4) The PET and the fMRI were done sequentially (2 h apart from each other) and we cannot necessarily assume that the state of the brain was the same at both measurements. This is relevant since alertness and cognitive performance fluctuate with SD and may deteriorate abruptly after 30 h of wakening (Doran et al., 2001). Indeed our data showed fluctuation in alertness during the SD condition. However, since prior imaging studies did not show regional brain activation differences during a working memory task performed after 24 h versus 35 h of SD (Chee et al., 2006) it is unlikely that the 2 hour time difference invalidates our findings. In the future with the development of dual PET-MRI scanners it will be possible to simultaneously assess these two measures, which will eliminate this confound.
Conclusion
Striatal DA increases during SD were associated with decreased activation in cerebellum and lower occipital cortex and with decreased deactivation of CG while performing the VA task (Fig. 3). Since performance accuracy during the VA task correlated with activation of the cerebellum and lower occipital cortex and with deactivation of CG (Fig. 2) this suggests that striatal hyperstimulation of D2 receptors may contribute to VA impairment by attenuating the regional brain activation responses necessary for optimal task performance. The mechanism(s) by which striatal hyperstimulation of D2 receptors during SD result in attenuated activation responses to the VA task require further investigation.
References
- Albrecht D, Quaschling U, Zippel U, Davidowa H. Effects of dopamine on neurons of the lateral geniculate nucleus: an iontophoretic study. Synapse. 1996;23:70–78. doi: 10.1002/(SICI)1098-2396(199606)23:2<70::AID-SYN2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, Rhinewine J, Kane JM, Adesman A, Milanaik R, Maytal J, Diamond A, Szeszko P, Ardekani BA. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol. Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Breier A, Callicott JH, Adler C, Mattay VS, Shapiro M, Frank JA, Pickar D, Weinberger DR. The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000;22:125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Balkin TJ, Dinges DF, Roehrs T, Rogers NL, Wesensten NJ. Sleep Deprivation and Stimulant Task Force of the American Academy of Sleep Medicine. The use of stimulants to modify performance during sleep loss: a review by the Sleep Deprivation and Stimulant Task Force of the American Academy of Sleep Medicine. Sleep. 2005;28:1163–1187. doi: 10.1093/sleep/28.9.1163. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27:1181–1194. doi: 10.1093/sleep/27.6.1181. [DOI] [PubMed] [Google Scholar]
- Bush G, Shin L, Holmes J, Rosen B, Vogt B. The Multi-Source Interference Task: validation study with fMRI in individual subjects. Mol. Psychiatry. 2003;8:60–70. doi: 10.1038/sj.mp.4001217. [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. NeuroImage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chuah LY, Venkatraman V, Chan WY, Philip P, Dinges DF. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: correlations of fronto-parietal activation with performance. NeuroImage. 2006;31:419–428. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. J. Neurosci. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah LY, Chee MW. Cholinergic augmentation modulates visual task performance in sleep-deprived young adults. J. Neurosci. 2008;28:11369–11377. doi: 10.1523/JNEUROSCI.4045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J, Norton R, Ameratunga S, Robinson E, Civil I, Dunn R, Bailey J, Jackson R. Driver sleepiness and risk of serious injury to car occupants: population based case control study. BMJ. 2002;324:1125–1130. doi: 10.1136/bmj.324.7346.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Arce C, Del Rio-Portilla IY, Perez-Garci E, Guevara MA. Amplitude reduction in visual event-related potentials as a function of sleep deprivation. Sleep. 1999;22:181–189. [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev., Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dieterich M, Bucher SF, Seelos KC, Brandt T. Cerebellar activation during optokinetic stimulation and saccades. Neurology. 2000;54:148–155. doi: 10.1212/wnl.54.1.148. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch. Ital. Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005;25:117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ribeiro S, Costa R, Santos LM, Lin SC, Grosmark A, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA. Dopaminergic control of sleep-wake states. J. Neurosci. 2006;26:10577–10589. doi: 10.1523/JNEUROSCI.1767-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Martin E, Fernandez-Briera A, Fernandez-Lopez A, Calvo P. Effect of chronic treatment with ethanol and withdrawal of ethanol on binding of [3H] SCH23390 to D1 dopamine receptor in rat visual cortex and hippocampus. An autoradiographic study. Neuropharmacology. 1994;33:1203–1209. doi: 10.1016/s0028-3908(05)80011-5. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Wong DF, Rosa-Neto P, Cumming P. Mapping neuroreceptors at work: on the definition and interpretation of binding potentials after 20 years of progress. Int. Rev. Neurobiol. 2005;63:1–20. doi: 10.1016/S0074-7742(05)63001-2. [DOI] [PubMed] [Google Scholar]
- Govindaiah G, Cox CL. Depression of retinogeniculate synaptic transmission by presynaptic D(2)-like dopamine receptors in rat lateral geniculate nucleus. Eur. J. Neurosci. 2006;23:423–434. doi: 10.1111/j.1460-9568.2005.04575.x. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. The human visual cortex. Ann. Rev. Neurosci. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. [DOI] [PubMed] [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magn. Reson. Med. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Covariation of regional cerebral blood flow with delta and spindle activity during slow wave sleep in humans. J. Neurosci. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorders. Br. J. Psychiatry. 1993;162:413–419. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J. Chem. Neuroanat. 2001;22(1–2):127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine Committee on Sleep Medicine and Research Board on Health Sciences Policy. Sleep Disorders and Sleep Deprivation: an Unmet Public Health Problem. Washington, DC: National Academies Press; 2006. Extent and health consequences of chronic sleep loss and sleep disorders; pp. 67–166. [Google Scholar]
- Jones BE. Basic mechanisms of sleep–wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Elsevier Saunders; 2005. pp. 136–153. [Google Scholar]
- Kaasinen V, Aalto S, Någren K, Rinne JO. Dopaminergic effects of caffeine in the human striatum and thalamus. NeuroReport. 2004;15:281–285. doi: 10.1097/00001756-200402090-00014. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Fancher JB, Markanen R, Hynd GW. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. J. Child Neurol. 2008;23:368–380. doi: 10.1177/0883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Garwood M, Menon R, Adriany G, Andersen P, Truwit CL, Ugurbil K. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn. Reson. Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- Levy F. Pharmacological and therapeutic directions in ADHD: specificity in the PFC. Behav. Brain Funct. 2008;28:4–12. doi: 10.1186/1744-9081-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. A common action of clozapine, haloperidol, and remoxipride on D1- and D2-dopaminergic receptors in the primate cerebral cortex. Proc. Natl. Acad. Sci. U. S. A. 1994;91:4353–4356. doi: 10.1073/pnas.91.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann. N.Y. Acad. Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding Y-S, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Luber B, Stanford AD, Bulow P, Nguyen T, Rakitin BC, Habeck C, Basner R, Stern Y, Lisanby SH. Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb. Cortex. 2008;18:2077–2085. doi: 10.1093/cercor/bhm231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, III, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Detection of the moderately beneficial cognitive effects of low-dose treatment with haloperidol or clozapine in an animal model of the attentional impairments of schizophrenia. Neuropsychopharmacology. 2008;33:2635–2647. doi: 10.1038/sj.npp.1301661. [DOI] [PubMed] [Google Scholar]
- Mason M, Norton M, Van Horn J, Wegner D, Grafton S, Macrae C. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR. Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J. Neurosci. 1996;16:4816–4822. doi: 10.1523/JNEUROSCI.16-15-04816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch. Gen. Psychiatry. 1997;54:1089–1095. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, et al. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J. Neurosci. 2000;20:RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM. Catecholamines and the sleep–wake cycle. II. REM sleep. Life Sci. 1983;32:1401–1415. doi: 10.1016/0024-3205(83)90905-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test–retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- Müller CP, Huston JP. Dopamine activity in the occipital and temporal cortices of rats: dissociating effects of sensory but not pharmacological stimulation. Synapse. 2007;61:254–258. doi: 10.1002/syn.20366. [DOI] [PubMed] [Google Scholar]
- Oranje B, Gispen-de Wied CC, Westenberg HG, Kemner C, Verbaten MN, Kahn RS. No effects of l-dopa and bromocriptine on psychophysiological parameters of human selective attention. J. Psychopharmacol. 2006;20:789–798. doi: 10.1177/0269881106061712. [DOI] [PubMed] [Google Scholar]
- Parkinson D. Evidence for a dopaminergic innervation of cat primary visual cortex. Neuroscience. 1989;30:171–179. doi: 10.1016/0306-4522(89)90363-1. [DOI] [PubMed] [Google Scholar]
- Passetti F, Levita L, Robbins TW. Sulpiride alleviates the attentional impairments of rats with medial prefrontal cortex lesions. Behav. Brain Res. 2003;138:59–69. doi: 10.1016/s0166-4328(02)00229-2. [DOI] [PubMed] [Google Scholar]
- Perciavalle V, Berretta S, Li VG, Polizzi MC. Basal ganglia influences on the cerebellum of the cat. Arch. Ital. Biol. 1987;125:29–35. [PubMed] [Google Scholar]
- Pinborg LH, Videbaek C, Ziebell M, Mackeprang T, Friberg L, Rasmussen H, Knudsen GM, Glenthoj BY. [123I]epidepride binding to cerebellar dopamine D2/D3 receptors is displaceable: implications for the use of cerebellum as a reference region. NeuroImage. 2007;34:1450–1453. doi: 10.1016/j.neuroimage.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Reyes E, Rossell S, Paredes D, Rada P, Tucci S, Gonzalez LE, Hernandez L. Haloperidol abolished glutamate release evoked by photic stimulation of the visual cortex in rats. Neurosci. Lett. 2002;327:149–152. doi: 10.1016/s0304-3940(02)00316-6. [DOI] [PubMed] [Google Scholar]
- Rueda M, Rothbart M, McCandliss B, Saccomanno L, Posner M. Training, maturation, and genetic influences on the development of executive attention. Proc. Natl. Acad. Sci. U. S. A. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J. Neurophysiol. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. The neurotransmitters of sleep. J. Clin. Psychiatry. 2004;65 Suppl 16:4–7. [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc. Natl. Acad. Sci. U. S. A. 2001a;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc. Natl. Acad. Sci. U. S. A. 2001b;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, Wagner H, Thorne D, Popp K, Rowland L, Welsh A, Balwinski S, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation onwaking human regional brain activity. J. Sleep Res. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Wagner HN, Thorne DR, Popp KA, Rowland LM, Welsh AB, Balwinski SM, Redmond DP. Neural basis of alertness and cognitive performance impairments during sleepiness. II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus Relat. Sys. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 Tesla. NeuroImage. 2004;23:1414–1421. doi: 10.1016/j.neuroimage.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Tomasis D, Wang R, Telang F, Boronikolas V, Jayne M, Wang GJ, Fowler JS, Volkow ND. Impairment of attentional networks after 1 night of sleep deprivation. Cereb. Cortex. 2008;19:233–240. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, Ma J, Pradhan K, Tomasi D, Thanos PK, Ferré S, Jayne M. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J. Neurosci. 2008a;28:8454–8461. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM. Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS ONE. 2008b;3(4):e2017. doi: 10.1371/journal.pone.0002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Kerscher N, Eysel U, Funke K. D1 and D2 receptor-mediated dopaminergic modulation of visual responses in cat dorsal lateral geniculate nucleus. J. Physiol. 2002;539:223–238. doi: 10.1113/jphysiol.2001.012721. [DOI] [PMC free article] [PubMed] [Google Scholar]