Abstract
Background
Among Asians, including Japanese, obesity is related to dyslipidemia and insulin resistance at a lower level of body-mass index (BMI)compared with non-Hispanic whites (NHW).
Objective
We hypothesize that this ethnic difference in the relationship between BMI and metabolic risks is partly associated with the ethnic difference in fat distribution, namely, liver fat as well as visceral adipose tissue (VAT).
Design
To compare liver fat content among Japanese vs. NHW men, regional computed-tomography (CT) images were taken to measure liver CT density in population-based samples of 313 Japanese and 288NHW men aged 40–49, along with the assessment of metabolic parameters.
Results
Liver fat content was higher in Japanese than NHW men (liver to spleen attenuation ratio (lower value means higher liver fat content): 1.01 ± 0.16 vs. 1.07 ± 0.15, respectively; p<0.01), despite a lower mean BMI in Japanese men (BMI: 23.6 ± 2.9 vs. 27.8 ± 4.2 kg/m2; p<0.01). Moreover, Japanese men had a greater disposition for fatty liverwith a small increase in BMI than NHW (p<0.01), whereas both groups had a similar relationship between VAT and BMI. In both groups, liver fat content correlated with triglycerides, homeostasis model assessment-insulin resistance, and C-reactive protein.
Conclusions
Liver fat content is higher among Japanese than NHW and this ethnic difference becomes more robust with a small increase in BMI, suggesting fatty liver is a sensitive marker for the failure of the adipose tissue to expand in order to accommodate an increased energy influx, and is associated with similar metabolic risk in Japanese despite of lower BMI compared with NHW men.
Keywords: Ethnicity, Fatty liver, Fat distribution, Visceral adiposity, Dyslipidemia
INTRODUCTION
Obesity is a risk factor for dyslipidemia and insulin resistance (IR). In clinical practice and large epidemiological studies, overweight and obesity status is measured by the use of body mass index (BMI) 1. BMI correlates with fat mass (FM), though there can be a substantial inter-individual variation in the percent of weight accounted for by FM within the BMI range designated as overweight 2
Moreover, the “dose-response” relationship between BMI and metabolic risk, i.e., dyslipidemia and IR, appears to differ with ethnicity. Among many Asian groups, lower BMI is related to greater metabolic risks when compared with non-Hispanic whites (NHW) 3, 4. The reasons for the ethnic differences in the relationship between BMI and metabolic risk are uncertain. Some data, however, suggest that Asians compared to NHW have a higher percentage of body fat within the “overweight” BMI categories 5, 6 and that patterns of adipose tissue (AT) distribution differ.
Visceral adipose tissue (VAT) is associated with IR and dyslipidemia 7–12. Ethnic differences in VAT have been noted. A recent meta-analysis has shown that Japanese adults have a propensity for greater VAT, 3, 13 than subcutaneous adipose tissue (SAT) 14. We have recently reported that Japanese men have greater VAT compared with NHW after adjusting for differences in waist girth 15. Additionally, Asian-Indians also have greater VAT after adjusting for BMI, compared with NHW 16
Fatty liver has been shown to be associated with VAT, dyslipidemia, and IR 17–21. Ethnic differences in the prevalence of fatty liver have been noted between African Americans and NHW 22, 23. Very few studies, however, have reported ethnic differences in fatty liver between Asians and NHW. 23
Prior studies have shown a strong negative correlation between computed-tomography (CT) density in the liver and fatty infiltration measured by biopsy 24, 25, wherein lower values for CT density denote higher lipid content in the liver. The ratio of liver to spleen (L/S ratio) for CT attenuation value is another index, with a L/S ratio <1 considered to represent fatty liver 24–26
We hypothesize that liver fat is greater in Japanese men in Japan than in NHW men in the U.S., and this is associated with comparable metabolic risks in Japanese men despite a lower BMI 27. To test this hypothesis, we compared liver fat content evaluated using CT imaging of the liver and the spleen (L/S ratio)between population-based samples of 313 Japanese and 288 NHW men aged 40–49 (ERA JUMP Study) 27. We also explored the association of liver fat content with metabolic parameters such as triglyceride level.
METHODS
Subjects
The research design and methods of the ERA-JUMP Study have been described previously 27. Briefly, we examined population-based samples of 623 men aged 40–49 without clinical cardiovascular disease, type 1 diabetes or other severe conditions from 2002 to 2006: 313 Japanese men in Kusatsu City, Shiga, Japan and 310 NHW men living in Allegheny County, PA, U.S. Among 310 NHW men, CT images were available only for 288 subjects. The study was approved by the Institutional Review Boards of Shiga University Medical Science, Otsu, Japan and the University of Pittsburgh, Pittsburgh, PA, U.S.
Metabolic Risk Factors
Height and weight were measured on calibrated scales. Adiposity was estimated using BMI, which is calculated as weight (kg)/height (m)2. Waist girth (cm) was measured at the level of the umbilicus while the participant was standing erect.
Venipuncture was performed early in the clinic visit after a 12-hour fast. Serum samples were analyzed at the Heinz Laboratory, Department of Epidemiology, University of Pittsburgh as described previously 27. Briefly, lipid levels were determined using the standardized methods of the Centers for Disease Control and Prevention, including total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Fasting serum glucose was determined by an enzymatic procedure, and fasting insulin by radioimmunoassay (Linco Research Inc., St. Charles, MO). Homeostasis model assessment (HOMA-IR) was used to estimate insulin sensitivity: HOMA-IR = [Glucose] (mg/dl) * [Insulin] (μU/ml)/405 28. Plasma samples were analyzed at the Laboratory for Clinical Biochemistry Research, University of Vermont. High-sensitivity C-reactive protein (CRP) was determined by a colorimetric competitive ELISA.
A self-administered questionnaire was used to obtain current medication use, habitual alcohol intake (Yes=1, No=0), and demographic information such as dietary habits. Alcohol intake was assessed as whether the participant drank beer, wine, liquor, sake (Japanese rice wine), or other alcoholic beverages with quantity and frequency recorded. Ethanol consumption per day was estimated, assuming that concentrations of alcohol were five percent for beer, 12 percent for wine, 40 percent for liquor, and 16 percent for sake. Habitual alcohol intake was defined as drinking 2 times per week or more.
Body Composition by CT imaging
CT images were taken to measure liver CT density. Scanning was performed using a GE-Imatron C150 Electron Beam Tomography scanner (GE Medical Systems, South San Francisco, U.S.) at both study sites. Images centered on the T12-L1 disc space and the L4–L5 disc space were used for assessing hepatic fat content and abdominal AT distribution, respectively. CT imaging provides information on spatial arrangement of tissues, with the contrast between tissues based upon differences in attenuation of energy from X-rays. Attenuation values for CT are expressed using water as a reference value of 0 Hounsfield unit (HU). Adipose tissue displays attenuation values that are negative to those of water, and in a range from −190 to −30 HU, whereas skeletal muscle in a range from 0 to 100 HU 29. The amount of fat deposition within the liver can also be characterized on the basis of its effect upon CT attenuation values of the liver. Prior studies have shown a strong negative correlation between CT density in the liver and fatty infiltration measured by biopsy 24, 25, wherein lower values for CT density denote higher lipid content in liver. In the current study, to assess hepatic fat content, CT attenuation in HU was determined in three regions of interest (ROI) for the liver and the spleen, each ROI of ~120 mm2. ROI for the liver was placed manually to avoid major vessels. By using the ratio of liver to spleen attenuation; i.e. using spleen as an internal control, possible confounding factors across images such as the effect of obesity are reduced; higher BMI is associated with lower CT attenuation 30, 31, and therefore liver to spleen ratio (L/S ratio) is used as an index of liver fat content 24–26. On the CT images of the abdomen, the area for AT and the psoas muscles were measured electronically by defining for each tissue a range of CT attenuation values: −30 to −190 HU for AT; and 0 to 100 HU for muscle, as previously described 18. To determine the respective areas of VAT and SAT, a separation line was drawn manually using a cursor along the abdominal wall musculature in continuity with fascia of the para-spinal muscles. Again, these measurements were performed by a cross-sectional image at L4–5, and were estimates for AT volume (distribution) 32, 33. All CT images were analyzed at the University of Pittsburgh using image analysis software by one trained reader (SliceOmatic, Tomovision, Montreal, Canada).
Statistical Analysis
Statistical analyses were performed using SPSS 14.0 (SPSS Inc., Chicago, IL). General linear models were used to examine ethnic differences in body composition and metabolic parameters and to assess the association of liver CT density with selected parameters. Two-way ANOVA was also used to assess the BMI-ethnicity interaction for liver fat, and BMI was used as a continuous variable, however the data were presented in categories for ease of visualization. L/S ratio was also presented as categorized despite of using as a continuous variable, and ANCOVA was used to assess the ethnic difference. A P value <0.05 was considered statistically significant. All tests were based on a two-sided level of significance.
Results
Body Composition (Table 1, unadjusted values)
Table 1.
Basic Clinical Characteristics and Body Composition Assessed by CT in Japanese in Kusatsu City, Shiga, Japan and non-Hispanic white men in Allegheny County, PA, aged 40–49 in 2002–2005.
| Japanese men(n=314) | Non-Hispanic white men(n=288) | |
|---|---|---|
| Age (years) | 45.1 ± 2.8 | 45.0 ± 2.8 |
| Weight (kg) | 68.6 ± 9.6 | 90.3 ± 14.9** |
| BMI (kg/m2) | 23.6 ± 2.9 | 27.8 ± 4.2** |
| Alcohol intake (g/day) | 27 ± 29 | 10 ± 14** |
| Metabolic parameters | ||
| Triglyceride (mg/dl) | 155 ± 81 | 152 ± 100 |
| HOMA-IR | 2.77 ± 1.51 | 3.89 ± 2.76** |
| Prevalence of Diabetes (%) | 6.1% | 3.1% |
| CRP (mg/dl) | 0.74 ± 1.79 | 1.62 ± 2.36** |
| CT imaging of the Abdomen | ||
| VAT (cm2) | 80 ± 30 | 103 ± 44** |
| SAT (cm2) | 82 ± 35 | 151 ± 67** |
| V/S ratio | 1.05 ± 0.33 | 0.74 ± 0.28** |
| Abd muscle (cm2) | 25 ± 5 | 29 ± 5** |
| CT imaging of the Liver and the Spleen | ||
| Liver (HU) | 59 ± 9 | 60 ± 8 |
| Spleen (HU) | 58 ± 4 | 56 ± 5** |
| Liver/Spleen ratio | 1.01 ± 0.16 | 1.07 ± 0.15** |
p<0.05, and
p<0.01, values are unadjusted means±SD.
Clinical characteristics and body composition parameters for Japanese and NHW men are presented in Table 1. By design, the two groups were very similar for age. Japanese men were shorter (170±6 vs. 180±7 cm; p<0.01) and lighter, by about 4 BMI units, than NHW men (p<0.01). Japanese men had significantly smaller waist girth than NHW men (85 ±8 vs. 99±12 cm, respectively; p<0.01). Japanese men had significant smaller VAT by 25% and SAT by 80 % (Table 1). The ratio of VAT to SAT was higher in Japanese men, suggesting a greater disposition for storing fat calories in VAT relative to SAT among Japanese compared to NHW men. In support of this postulate, group comparisons for VAT were conducted, and—after adjusting for the effects of BMI and waist circumference—the results indicated a 7±3 cm2 greater VAT in the Japanese compared to NHW men (p<0.01).
Also shown in Table 1 are unadjusted mean values for liver attenuation, spleen attenuation and the liver-to-spleen attenuation ratio (L/S ratio). The L/S attenuation ratio was significantly lower in Japanese men, meaning greater liver fat content among Japanese compared with NHW men; mean Liver CT attenuation was similar across groups, though spleen CT attenuation was significantly higher in Japanese men. To investigate this finding, we explored the relationship between spleen CT attenuation and abdominal SAT. Higher amounts of SAT correlated with lower spleen CT attenuation (p<0.01). After adjustment for SAT, there was no significant group difference in spleen CT attenuation. This lends support to the methodological value of presenting data on liver CT attenuation as the L/S attenuation ratio, using the spleen attenuation as an internal control 25 for volume averaging effects related to abdominal SAT. The percentage of men with a low value for the L/S ratio (i.e. less than 1) that is recognized to be consistent with fatty liver as determined at biopsy 24, 25 was significantly higher in Japanese men (33.1% vs. 24.7%; p<0.05). This further supports that Japanese men had higher liver fat content than NHW men.
The L/S ratio did not correlate with habitual alcohol intake or daily ethanol intake in this study, either analyzing the two populations together or separately. Since the proportion of subjects with habitual alcohol intake and the amount of daily ethanol intake were higher among Japanese compared with NHW men (68% vs. 43%, 27±29 vs. 10±14 g/day; respectively, both p<0.01), the group difference in the L/S ratio was also analyzed among subjects without habitual alcohol intake. The L/S ratio remained significantly lower in Japanese men (1.00±0.17 vs. 1.06±0.16, unadjusted values; p<0.01). When subjects with more than 30g daily ethanol intake were excluded, the L/S ratio remained significantly lower in Japanese men. Although the use of a glucose lowering drug correlated significantly with lower L/S ratio (p<0.05), this association disappeared after adjusting for diabetes status.
Ethnicity-by-BMI interaction for Liver fat content (Figure 1, Table 2)
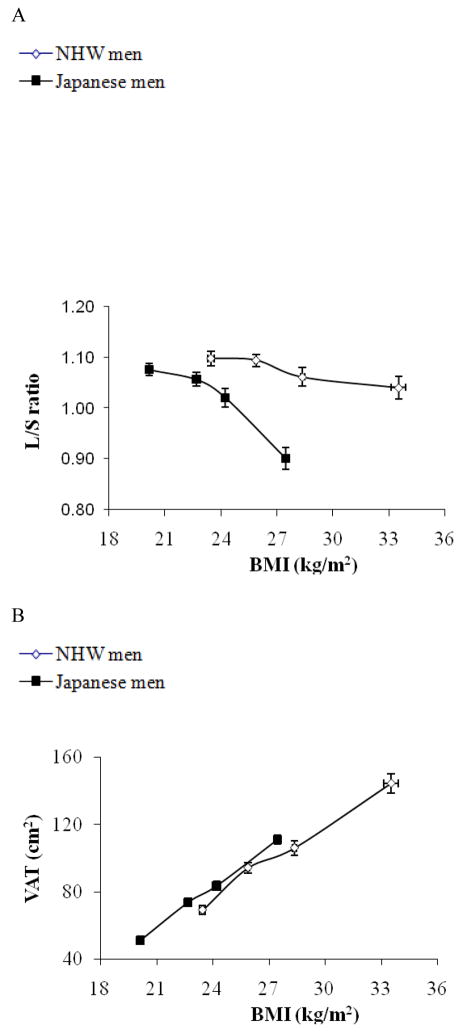
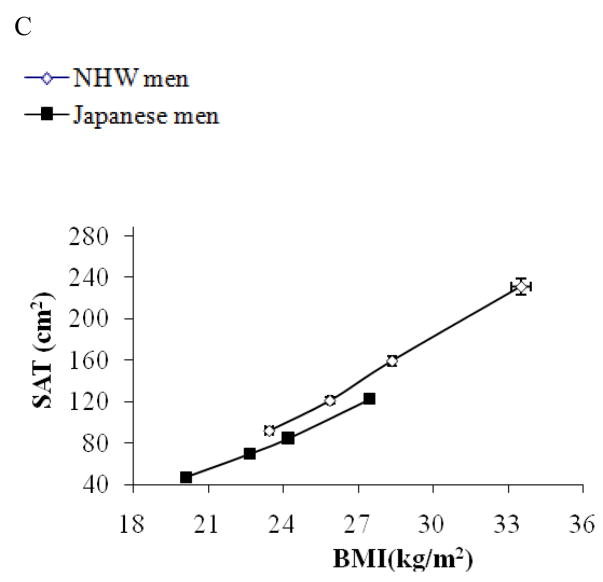
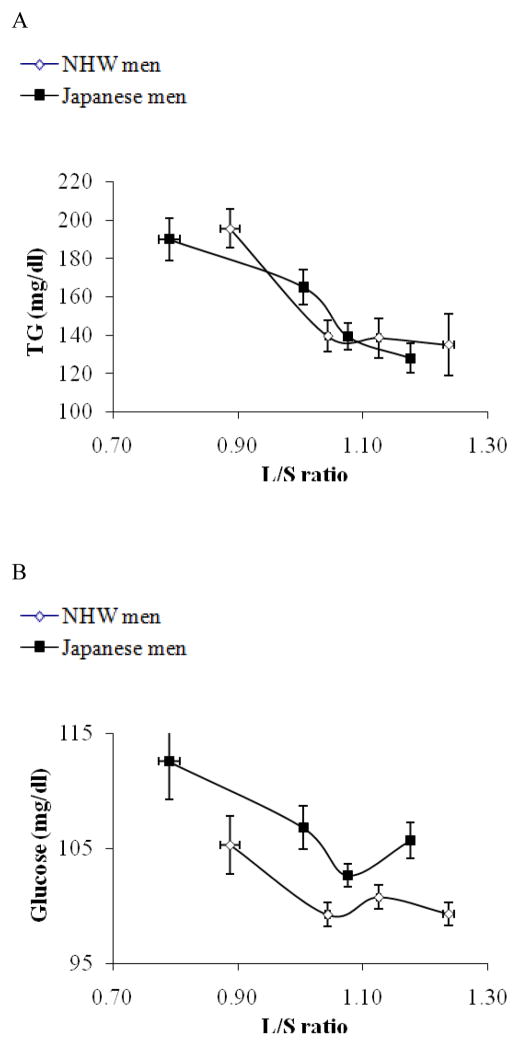
Figure 1.
Subjects were stratified by BMI in Japanese men (solid rectangle) and non-Hispanic white men (open rectangle) separately and mean values (±SEM) of BMI for each quartile are plotted against mean values (±SEM) of liver to spleen ratio (L/S ratio) for respective quartiles (1A), those of VAT (1B), and SAT (1C). As shown in Figure 1A, there was a significant ethnicity by BMI interaction for L/S ratio (p<0.01); the decrease in L/S ratio was more robust in response to the increase in BMI, though in both population, L/S ratio was negatively correlated with BMI (p<0.01). There was no ethnicity by BMI interaction for VAT or SAT (Figure 1B, and 1C).
Table 2.
Prevalence of fatty liver (L/S ratio<1) stratified by obesity criteria for Asians
| BMI | Japanese men (n=314) | Non-Hispanic white men(n=288) |
|---|---|---|
| < 23.5 | 19.1 % ( 30/157 ) | 25.9 % ( 7/27 ) |
| 23.5 – 25 | 33.3 % ( 22/66 ) | 4.0 % ( 2/50 ) |
| 25 – 30 | 55.0 % ( 44/80 ) | 22.1 % ( 30/136 ) |
| 30 + | 73.0 % ( 8/11 ) | 42.7 % ( 32/75 ) |
Since there was a difference in BMI by 4 kg/m2, an estimate of total fat mass, we also analyzed the data stratifying the subjects by BMI. As shown in Figure 1A, the change in liver fat content was more pronounced in response to increased BMI in Japanese compared to NHW men (P for interaction <0.01) although both Japanese and NHW men had an inverse association of BMI with L/S ratio. Also, as shown in Table 2, among Japanese, the prevalence of fatty liver (L/S ratio <1) was increased as BMI exceeded to the overweight range as Asian criteria of obesity 4 (BMI >23.5), while among NHW men, the prevalence was rather decreased in the range of BMI 23.5~25.0. The increase in VAT or SAT in response to the increase in BMI was similar between the two populations (Figure 1B, and 1C, respectively). As a result, the association of VAT as well as BMI with liver fat content was significantly different by ethnicity (p<0.01).
Association of L/S ratio with metabolic parameters (Figure 2)
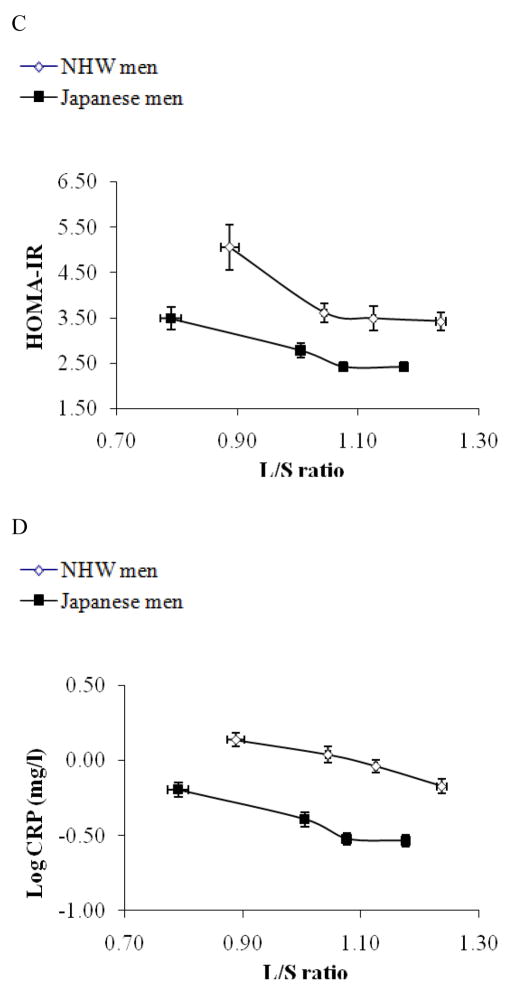
Figure 2.
Subjects were stratified by liver fat content (L/S ratio) in Japanese men (solid rectangle) and non-Hispanic white men (open rectangle) and mean values (±SEM) of L/S ratio for each quartile are plotted against mean values (±SEM) of TG (mg/dl, 2A), Fasting glucose (2B), HOMA-IR (2C), and logCRP (mg/dl, 2D), respectively for respective quartiles. As shown in figure 2A, serum fasting TG level was strongly correlated with L/S ratio and the two plotted lines were super-imposable each other. As shown in figure 2B, there was not a linear correlation between L/S ratio and fasting glucose. As shown in figure 2C and 2D, HOMA-IR and log CRP were strongly associated with L/S ratio, however, at any given L/S ratio, Japanese had lower HOMA-IR and lower log CRP (p<0.01).
The associations of L/S ratio with several metabolic parameters were next explored, focusing upon fasting triglycerides (TG), fasting glucose, HOMA-IR and CRP. These associations are shown in Figure 2, stratified by L/S ratio. Other metabolic profiles are described in detail previously 27.
Among both Japanese and NHW men, liver fat content had a significant positive correlation with fasting TG (Figure 2A). Across L/S ratio, TG was similar between Japanese and NHW men, further indicating that liver fat is a good marker for reduced capacity to store excess energy (TG) inside the adipose tissue. Fasting glucose was much greater among subjects with fatty liver (L/S ratio <1) than those without fatty liver (L/S ratio >1) in each population (both p <0.01), though fasting glucose was not linearly correlated with liver fat content (Figure 2B). Fasting glucose tended to be higher among Japanese than NHW men regardless of the degree of liver fat deposition. HOMA-IR, an index of insulin resistance (IR), and CRP, an index of systemic inflammation, showed strong correlations with L/S ratio (Figure 2C and 2D, respectively), when analyzed with the two ethnic groups together or separately (all p<0.01). At any given L/S ratio, however, both HOMA-IR and CRP were significantly lower among Japanese men (both p<0.01).
DISCUSSION
Our main findings are that liver fat content was higher in Japanese compared with NHW men and that liver fat increases with increasing BMI at a higher rate in Japanese men.
For Asians, including Japanese adults, obesity has been defined with a different BMI cut off point (BMI >25) from WHO criteria (BMI >30, 3, 4). Only 3% of the Japanese population has a BMI >30. This percentage has changed little during the last 40 years despite a recent rapid increase in incidence of obesity-associated metabolic risks such as dyslipidemia and type 2 DM in Japan3, 13. On the other hand, about 20% of the population has a BMI >25 and the percentage has increased more than three-fold in the last 40 years 4. These data imply that Asians have reduced capacity to store fat in response to positive net energy balance as a consequence of overeating and physical inactivity. Subsequently, BMI >30 is rarely exceeded among Asians. In turn, ectopic fat such as liver fat 34 is rapidly accumulated among people with BMI >25, which is consistent with our current findings (Figure 1 and Table 2).
Few previous studies have reported ethnic differences in liver fat content between Asians and NHW although some studies reported that African Americans have less liver fat as well as less VAT than NHW men 22, 23. Weston et al. reported that the incidence of fatty liver in Asians tended to be higher compared with NHW men, but the result was not conclusive due to the lack of direct statistical comparison for this ethnic difference 22, 23. In the current study, we have observed higher liver fat content among Japanese compared with NHW men in both adjusted and unadjusted values for the effect of age, and BMI. Other possible confounding factors such as medication and alcohol drinking did not explain the observed ethnic difference.
It is unclear why the propensity for liver fat accumulation with increasing BMI differs between Japanese and NHW men, especially since visceral fat accumulation with increasing adiposity is similar between the two populations. It has been shown that liver fat content dynamically changes in response to moderate weight reduction. Additionally, the absolute amount of fat accumulation is much less for the liver (<200g) compared with visceral adiposity (~2kg) or subcutaneous adiposity (~20kg), provided there is plenty of blood circulation in the liver. 35 Therefore, due tothe current homogenous population and indirect body composition assessment by BMI and CT imaging, only the ethnic difference in liver fat can be seen, and we may miss the potential difference in visceral/subcutaneous adiposity.
Emerging data convincingly demonstrate that hepatic fat content is strongly associated with metabolic risks 17, 18, 20, 21, even among subjects with lipodystrophy 36, who do not have any adipose tissue. Indeed, we have found that liver fat content is strongly associated with higher TG, higher fasting insulin, and higher systemic inflammation in both populations. Moreover, TG levels in the liver and the blood are closely correlated in a similar manner regardless of the ethnicity, suggesting that liver fat is indeed a good marker to detect inability to store fat (TG) inside the adipose tissue. There seems to be, however, some ethnic differences in these associations as well.
HOMA-IR, an index of insulin resistance, was much lower among Japanese compared with NHW men. This holds true at any given liver fat content. No previous epidemiological study has compared insulin resistance between Japanese and NHW. Only four epidemiological studies have examined the difference in insulin resistance between NHW and Japanese Americans and the results were inconsistent37–40. Conversely, Japanese have higher liver fatthan NHW men despite having a lower BMI and less insulin resistance, which is considered to be most important for hepatic fat accumulation. This may further emphasize that Japanese adults have had a phenotype for lower capacity to store fat in the body in response to positive net energy balance, a similar phenotype to acquired lipodystrophy34. Another possibility is that HOMA-IR varies between populations. In fact, when looking at the 75th percentile of HOMA-IR in non-diabetic Japanese (298/314) and NHW (277/288) men, it was significantly lower among Japanese compared with NHW (2.61±1.19 vs. 3.75±2.22, p<0.01).
CRP, an index of systemic inflammation, was also much lower among Japanese compared with NHW men at a given liver fat content, despite a very strong correlation with liver fat content in both populations. This is consistent with a prior report41 and probably due to genetic42 and environmental factors. For the latter possibility, the quality of liver fat rather than the quantity might be important, mainly due to a difference in dietary intake. Although we do not have daily caloric intake data, or macronutrient balance data, the INTERMAP Study showed that Japanese and NHW men have similar total daily energy intake per body weight (2300kcal/day, 34kcal/kg/BW vs. 2800kcal/day, 31kcal/kg/BW, respectively), yet Japanese compared with NHW men consume more carbohydrate as a percentage of total calories (52% vs. 48%, respectively), less total and saturated fat, and less sugar 43. The INTERMAP Study provides one of the most comprehensive international dietary data in middle-aged men and women in the US, Japan and other countries 43. The INTERMAP Study also shows that omega-3 fatty acid intake is greater in Japanese men compared to NHW men (1.3g vs. 0.7g, respectively), whereas saturated fat intake is much less in the Japanese men (16g vs. 35g, respectively). The results are consistent with our food questionnaire that shows Japanese men had a much higher fish and soy consumption compared to NHW. One study showed that saturated fatty acid intake leads to steatohepatitis rather than simple steatosis44. In a mice model, saturated fatty acids in the liver may be associated with liver injury and ER stress 45, leading to inflammation and atherosclerosis 46. This may be one reason why there is much higher prevalence of fatty liver in Japanese men but less systemic inflammation and no reported higher incidence of steatohepatitis. Further study, including liver biopsy, will elucidate whether it is indeed less inflammation in the liver in Japanese due to a difference in quality of fat, not quantity of fat in the liver.
The strength of this study includes that this is the first international comparison of liver fat content using population-based sample with rigid standardization of the assessment for body composition and metabolic parameters. The limitation of this study includes that the samples are limited to men in their 40s, and it is unclear whether this finding is applicable to women or different age groups. We used CT density as an index for liver fat, but this is not a quantitative measure. Liver attenuation can be caused by other conditions than the presence of fat. For example, liver glycogen content raises CT density 47. Given the fact that Japanese consume more carbohydrate than NHW, however, this is unlikely to cause a lower CT density among Japanese and may rather minimize the difference between the two populations. We used a cross sectional CT image for estimations of VAT and SAT volume and due to homogeneity of sample population, we may miss the potential importance of VAT and SAT. We did not check HCV infection, which is one of the major causes for liver steatosis. However, the prevalence of HCV infection is similarly very low (< 2%) in both population 48 and this does not seem to affect the result.
In summary, we have observed that liver fat content was higher among Japanese compared with NHW men. Additionally, Japanese have a greater propensity of liver fat disposition with a smaller increase in positive net energy balance, which is partly associated with similar metabolic risk such as triglyceride levels between the two populations.
Acknowledgments
This research was supported by grants R01 HL68200 from the US National Institutes of Health, and Grant-in-aid for Scientific Research (A) 17209023 and Grant-in aid for Young Scientists (B) 18790396 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seidell JC. Obesity, insulin resistance and diabetes--a worldwide epidemic. Br J Nutr. 2000;83 (Suppl 1):S5–8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 2.Jackson AS, Stanforth PR, Gagnon J, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord. 2002;26(6):789–96. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- 3.Gill TP. Cardiovascular risk in the Asia-Pacific region from a nutrition and metabolic point of view: abdominal obesity. Asia Pac J Clin Nutr. 2001;10(2):85–9. doi: 10.1111/j.1440-6047.2001.00231.x. [DOI] [PubMed] [Google Scholar]
- 4.Kanazawa M, Yoshiike N, Osaka T, et al. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pac J Clin Nutr. 2002;11 (Suppl 8):S732–S737. doi: 10.1159/000088200. [DOI] [PubMed] [Google Scholar]
- 5.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22(12):1164–71. doi: 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Thornton JC, Russell M, et al. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr. 1994;60(1):23–8. doi: 10.1093/ajcn/60.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Albu JB, Kovera AJ, Johnson JA. Fat distribution and health in obesity. Ann N Y Acad Sci. 2000;904:491–501. doi: 10.1111/j.1749-6632.2000.tb06505.x. [DOI] [PubMed] [Google Scholar]
- 8.Cnop M, Landchild MJ, Vidal J, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations : distinct metabolic effects of two fat compartments. Diabetes. 2002;51(4):1005–15. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 9.Despres JP, Lemieux S, Lamarche B, et al. The insulin resistance-dyslipidemic syndrome: contribution of visceral obesity and therapeutic implications. Int J Obes Relat Metab Disord. 1995;19 (Suppl 1):S76–86. [PubMed] [Google Scholar]
- 10.Despres JP, Moorjani S, Lupien PJ, et al. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10(4):497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 11.Misra A, Vikram NK. Clinical and pathophysiological consequences of abdominal adiposity and abdominal adipose tissue depots. Nutrition. 2003;19(5):457–66. doi: 10.1016/s0899-9007(02)01003-1. [DOI] [PubMed] [Google Scholar]
- 12.Ross R, Aru J, Freeman J, et al. Abdominal adiposity and insulin resistance in obese men. Am J Physiol Endocrinol Metab. 2002;282(3):E657–63. doi: 10.1152/ajpendo.00469.2001. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto WY. Overview of non-insulin-dependent diabetes mellitus (NIDDM) in different population groups. Diabet Med. 1996;13(9 Suppl 6):S7–10. [PubMed] [Google Scholar]
- 14.Tanaka S, Horimai C, Katsukawa F. Ethnic differences in abdominal visceral fat accumulation between Japanese, African-Americans, and Caucasians: a meta-analysis. Acta Diabetol. 2003;40 (Suppl 1):S302–4. doi: 10.1007/s00592-003-0093-z. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes (Lond) 2006;30(7):1163–5. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj M, Banerji MA. Type 2 diabetes in South Asians: a pathophysiologic focus on the Asian-Indian epidemic. Curr Diab Rep. 2004;4(3):213–8. doi: 10.1007/s11892-004-0026-4. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 18.Kelley DE, McKolanis TM, Hegazi RA, et al. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285(4):E906–16. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 19.Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue--emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2(6):273–80. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- 20.Toledo FG, Sniderman AD, Kelley DE. Influence of hepatic steatosis (fatty liver) on severity and composition of dyslipidemia in type 2 diabetes. Diabetes Care. 2006;29(8):1845–50. doi: 10.2337/dc06-0455. [DOI] [PubMed] [Google Scholar]
- 21.Yki-Jarvinen H. Fat in the liver and insulin resistance. Ann Med. 2005;37(5):347–56. doi: 10.1080/07853890510037383. [DOI] [PubMed] [Google Scholar]
- 22.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 23.Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41(2):372–9. doi: 10.1002/hep.20554. [DOI] [PubMed] [Google Scholar]
- 24.Longo R, Ricci C, Masutti F, et al. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol. 1993;28(4):297–302. [PubMed] [Google Scholar]
- 25.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27(1):108–13. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 26.Piekarski J, Goldberg HI, Royal SA, et al. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137(3):727–9. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 27.Sekikawa A, Ueshima H, Kadowaki T, et al. Less Subclinical Atherosclerosis in Japanese Men in Japan than in White Men in the United States in the Post-World War II Birth Cohort. Am J Epidemiol. 2007 doi: 10.1093/aje/kwk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250(6 Pt 1):E736–45. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 30.Nelson JC, Kronmal RA, Carr JJ, et al. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235(2):403–14. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- 31.Stanford W, Burns TL, Thompson BH, et al. Influence of body size and section level on calcium phantom measurements at coronary artery calcium CT scanning. Radiology. 2004;230(1):198–205. doi: 10.1148/radiol.2301020807. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol. 2004;97(3):948–54. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- 33.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80(2):271–8. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–78. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 35.Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–8. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg A. Lipodystrophies. Am J Med. 2000;108(2):143–52. doi: 10.1016/s0002-9343(99)00414-3. [DOI] [PubMed] [Google Scholar]
- 37.Chiu KC, Chuang LM, Yoon C. Comparison of measured and estimated indices of insulin sensitivity and beta cell function: impact of ethnicity on insulin sensitivity and beta cell function in glucose-tolerant and normotensive subjects. J Clin Endocrinol Metab. 2001;86(4):1620–5. doi: 10.1210/jcem.86.4.7432. [DOI] [PubMed] [Google Scholar]
- 38.Chiu KC, Cohan P, Lee NP, Chuang LM. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care. 2000;23(9):1353–8. doi: 10.2337/diacare.23.9.1353. [DOI] [PubMed] [Google Scholar]
- 39.Jensen CC, Cnop M, Hull RL, et al. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U. S Diabetes. 2002;51(7):2170–8. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 40.Torrens JI, Skurnick J, Davidow AL, et al. Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women’s Health Across the Nation (SWAN) Diabetes Care. 2004;27(2):354–61. doi: 10.2337/diacare.27.2.354. [DOI] [PubMed] [Google Scholar]
- 41.Yamada S, Gotoh T, Nakashima Y, et al. Distribution of serum C-reactive protein and its association with atherosclerotic risk factors in a Japanese population : Jichi Medical School Cohort Study. Am J Epidemiol. 2001;153(12):1183–90. doi: 10.1093/aje/153.12.1183. [DOI] [PubMed] [Google Scholar]
- 42.Austin MA, Zhang C, Humphries SE, et al. Heritability of C-reactive protein and association with apolipoprotein E genotypes in Japanese Americans. Ann Hum Genet. 2004;68(Pt 3):179–88. doi: 10.1046/j.1529-8817.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 43.Stamler J, Elliott P, Dennis B, et al. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary) J Hum Hypertens. 2003;17(9):591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vilar L, Oliveira CP, Faintuch J, et al. High-fat diet: a trigger of non-alcoholic steatohepatitis? Preliminary findings in obese subjects. Nutrition. 2008;24(11–12):1097–102. doi: 10.1016/j.nut.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–51. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 46.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26(5):995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 47.Leander P, Sjoberg S, Hoglund P. CT and MR imaging of the liver. Clinical importance of nutritional status. Acta Radiol. 2000;41(2):151–5. doi: 10.1080/028418500127345172. [DOI] [PubMed] [Google Scholar]
- 48.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]