Abstract
At the time of blastocyst implantation the uterine spiral arteries have already undergone morphological changes in the absence of any extravillous trophoblast invasion. Only 2 weeks after implantation, extravillous trophoblast cells develop and come into first contact with decidual tissues. Invading through the decidual interstitium, extravillous trophoblasts potentially reach and transform spiral arteries into uteroplacental arteries. Spiral arterial erosion starts at about mid-first trimester, whereas flow of maternal blood into the intervillous space is continuously established only at the beginning of the second trimester. One key regulator of the number of extravillous trophoblasts is oxygen. The steep gradient in oxygen concentration within the first trimester placenta is diminished with the onset of maternal blood flow. This gradient is used by the trophoblast to generate a large number of invasive cells to adapt the arterial vasculature in the placental bed to the growing needs of the fetus. Changes in oxygen concentrations or other factors leading to alterations in the rates of proliferation and/or apoptosis of extravillous trophoblast clearly impact on the remodelling of the vessels. The respective consequences of a failure in trophoblast invasion are growth restrictions of the baby and perhaps other pregnancy complications.
Keywords: extravillous trophoblast, fetal growth restriction, impaired invasion, oxygen, spiral artery
Development of the extravillous trophoblast
In humans the first invasion of trophoblast into maternal tissues occurs during implantation. It seems as if this first invasion is performed by the early multinucleated syncytiotrophoblast rather than by mononucleated trophoblast cells. The early syncytiotrophoblast clearly displays invasive properties. It not only penetrates the uterine epithelium but further invades into the upper part of the endometrium, the stratum functionalis.
The next 2 weeks of placental development are characterized by an internal reorganization process: mononucleated trophoblast cells, now referred to as cytotrophoblasts, push forward through the syncytiotrophoblastic mass and reach the maternal side of the placenta at about day 15 post conception (Benirschke et al. 2006). As soon as they come into contact with maternal tissues they reorganize themselves and develop multilayered structures, the trophoblastic cell columns. The cells in contact with the basement membrane of the developing villi keep their stem cell character and continue to proliferate. Those cells losing contact with the basement membrane also lose their generative potential, differentiate towards an invasive phenotype and migrate into maternal tissues. Thus, with the appearance of the first cytotrophoblasts outside the placenta proper, a new cell type is generated: the extravillous trophoblast (Kaufmann et al. 2003).
Now that the uterine epithelium has been successfully penetrated, the early syncytiotrophoblast loses its invasive phenotype and develops into an epithelial-like surface of the chorionic villi. In contrast to the early syncytiotrophoblast, the extravillous trophoblasts only need to invade connective tissues and endothelial linings. Thus there is no need for an extravillous trophoblast to have the potential to penetrate an epithelium.
Is there any in vivo or in vitro proof for such a statement?
Alan Enders (2007) has very clearly shown that in the macaque it is indeed the syncytiotrophoblast that penetrated the uterine epithelium and only later mononucleated extravillous trophoblasts derived from trophoblastic cell columns further invade into the decidua and myometrium. In the human these stages of development have not been investigated so far, thus it can only be deduced from other species such as the macaque that the human trophoblast may behave similarly.
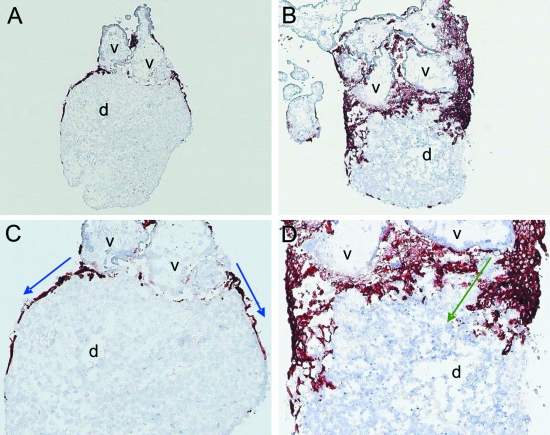
Moser et al. (2008) have established a co-culture system of human tissues where first trimester villous explants are placed on top of a piece of decidua parietalis from the same pregnancy. If the decidual pieces are precultured for 72 h they gain a new epithelium by outgrowth of epithelial cells from the glands embedded in the tissue. Villous explants confronted to such re-epithelialized decidual pieces start to develop trophoblastic cell columns at the attachment sites with the decidual tissues. Attachment of villi and development of cell columns is also seen if the villous explants are confronted to decidual pieces directly after harvesting the tissues, i.e. decidual pieces without an epithelial coverage. But after 72 h of co-culture, invasion of extravillous trophoblasts differs between the two models. If extravillous trophoblasts come into direct contact with decidual stroma, they deeply invade these tissues. However, if the trophoblast cell columns are attached to the newly formed uterine epithelium, migration of extravillous trophoblasts can mostly be found on top of the epithelium (Fig. 1).
Fig. 1.
Double tissue confrontation assay using placental tissues at a gestational age of 6 weeks. Cryosections were stained with the monoclonal antibody MEM-G9 to visualize HLA-G, a specific marker for extravillous trophoblast. (A,B) Placental villous explants (v) firmly attach to the re-epithelialized decidual tissue (d). Outgrowing extravillous trophoblasts do not invade decidual tissues but rather only migrate on top of the decidual epithelium (blue arrows in B). (C,D) Placental villous explants (v) firmly attach to the non-epithelialized decidual tissue (d). Outgrowing extravillous trophoblasts deeply invade into the decidual tissues (green arrows in D). Magnification (A,C) 50×, (B,D) 100×.
Both the in vivo findings in the macaque and the in vitro findings using human tissues point to the fact that extravillous trophoblasts of human origin may not have the potential to penetrate a simple epithelium.
Transformation of the uterine spiral arteries
The maternal uterine spiral arteries need to be transformed into large inelastic conduits to ensure adequate blood supply to the placenta. Only then can normal growth and development of the fetus be guaranteed. The physiological changes of uterine spiral arteries into uteroplacental arteries occur in consecutive stages.
Pijnenborg et al. (2006) very elegantly described the historical pathway of how we viewed the placenta. Theories started with a joint blood system of the mother and fetus and only in the 18th century did the separation of both blood systems become apparent (Hunter, 1980). In the mid 20th century, further evidence was provided suggesting that the uterine spiral arteries undergo morphological alterations even prior to the presence of any extravillous trophoblast cell near such vessels (Boyd & Hamilton 1956, 1967; Brettner 1964; Harris & Ramsey, 1966). These trophoblast-independent alterations comprised endothelial vacuolation and swelling of smooth muscle cells. Later Craven et al. (1989) added more information on the subject by showing that there is a generalized disorganization of these arteries including endothelial basophilia and expression of VCAM-1 combined with a perturbation of the vascular smooth muscle layer Craven et al. (1989). All these changes lead to a vasodilatation of a certain degree, which is considered to be further increased by the activation of the local decidual artery renin-angiotensin systems of the mother (Morgan et al. 1998) linked to early endometrial decidualization (Brettner 1964).
Only as a second step of the arterial transformation changes due to trophoblast invasion come into play. Extravillous trophoblasts reach the media of spiral arteries by invading the interstitium of the decidua basalis. The first contact of these mononucleated cells with maternal tissues occurs at about week 5 post menstruation (Kaufmann et al. 2003).
It seems as if extravillous trophoblasts in close vicinity to uteroplacental arteries are responsible for further vascular alterations. Such changes have been described in the guinea pig (Hees et al. 1987; Moll et al. 1988; Nanaev et al. 1995), where trophoblasts close to spiral arteries induce the reduction of media smooth muscle cells and the deposition of fibrinoid material prior to infiltration. Respective observations in the human are still missing.
Finally, as a third stage of uteroplacental vascular remodelling infiltration of extravillous trophoblasts into the arterial wall takes place. The further degeneration of the vascular smooth muscle layer together with the loss of elastic fibres (Robertson & Manning 1974; Robertson 1976) enables a further dilation of the vessel lumen up to several times wider compared to the original diameter (Brosens et al. 1967; Hirano et al. 2002; Benirschke et al. 2006). There is still an ongoing debate whether smooth muscle cells die and are replaced by intramural trophoblast or whether they undergo a temporary molecular and structural de-differentiation as long as trophoblasts are present in this layer (Nanaev et al. 1995). A similar question was raised for the replacement of the arterial endothelium. Tuttle et al. (1985) presented evidence for the presence of large pleomorphic cells that line the lumen of the proximal decidual segments of uteroplacental arteries. These cells express the factor VIII-related antigen but could not be shown to express hCG. Thus it may be tempting to speculate that not all of the morphologically altered cells lining transformed uteroplacental arteries are, in fact, trophoblast cells, even in the absence of an obvious endothelial phenotype (Tuttle et al. 1985).
Oxygen as modulator of trophoblast invasion
Prior to onset of maternal blood flow
The development of the early embryo during the first trimester of pregnancy takes place in a low oxygen environment (Rodesch et al. 1992; Jauniaux et al. 2000). It is only at the beginning of the second trimester when maternal blood flow into the intervillous space of the placenta is fully established and fetal development starts. It is believed that oxygen is one of the key regulators of trophoblast differentiation and that during the first trimester a low oxygen environment is mandatory for successful development of embryo and placenta. A premature supply of maternal blood to the placenta and thus an increase in oxygen too early during pregnancy accounts for a loss in placental mass or even spontaneous abortion (Watson et al. 1998; Jauniaux et al. 2000, 2003).
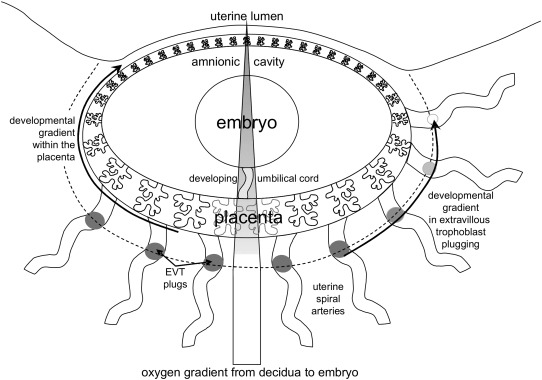
In a first trimester placenta, prior to the onset of maternal blood flow into the intervillous space, an oxygen gradient exists from the decidua towards the developing embryo (Fig. 2). The maternal decidua is vascularized and hence supplied with oxygen like any other maternal organ. Using a multiparameter sensor, Jauniaux et al. (1999) determined a pO2 in the placental bed during the first trimester of about 70 mm Hg. Due to the absence of any supply of maternal blood cells to the placenta there is a steep gradient in the oxygen concentration from the decidua to the placenta, resulting in a mean pO2 in the placenta of less than 20 mm Hg (Jauniaux et al. 2000).
Fig. 2.
Schematic representation of placenta and embryo at about weeks 8–10 of gestation. The embryo rests in its amnionic cavity and is connected to the placenta via the developing umbilical cord. The placenta shows a clear developmental gradient (arrow on the left) with the highest level of development at the site of implantation, i.e. at the embryonic pole. This developmental gradient can be found as well in the extent of extravillous trophoblast plugging of spiral arteries (arrow on the right). The bar in the centre represents the oxygen gradient from the placental bed (pO2 70 mm Hg) to placenta (pO2 < 20 mm Hg) and embryo (pO2 << 20 mm Hg). The dotted line indicates where plugging of spiral arteries generates the oxygen gradient towards the embryo. For further details on the changes at the end of the first trimester see also Figure 2 in Jauniaux et al. (2006) and Figure 4 in Burton (in press).
However, it needs to be stressed that these oxygen concentrations of the placenta during the first trimester of pregnancy are low but normal for this tissue at that stage of development. Thus, the term hypoxia, defined as a pathological deficiency of oxygen, should not be used for the low oxygen environment of the placenta during early stages of development.
The oxygen gradient from decidua to embryo during the first trimester of pregnancy puts the placenta into an intermediate level of oxygen. Low levels of oxygen may be important for placental development as the following features are associated with low oxygen levels:
higher proliferative activity of trophoblast,
lower invasive activity of trophoblast, and
reduced levels of reactive oxygen species, thus less DNA damage.
Furthermore, there is evidence for a histiotroph nourishing of the embryo by secretion products of the uterine glands at that stage of gestation (Burton et al. 2001).
A high rate of proliferation is mandatory for the rapid growth of the placenta at that stage of development. Another indicator for the rapid growth of the placenta during the first trimester is the almost complete absence of apoptosis in the syncytiotrophoblast (Smith et al. 1997, 2000). All the material introduced into this layer by fusion of cytotrophoblasts is used for growth rather than for maintenance of a steady-state.
At that stage not only the villous trophoblast grows rapidly, also the developing extravillous trophoblast uses this low oxygen environment. The trophoblastic cell columns develop at the border between placenta proper and decidua and thus develop within the oxygen gradient. Proliferation of trophoblast is restricted to those cells resting on the basement membrane separating the trophoblast from the villous stroma. The proximal parts of the cell columns are those parts of the columns experiencing the lowest oxygen levels of the whole columnar structures. Thus, they are well placed to generate as many extravillous trophoblasts as possible.
The post proliferative daughter cells are simply pushed forward by the enormous proliferative capacity of those cells resting on the basement membrane. There is no need for these cells to start invasion as early as at the second cell layer as they are passively pushed forward towards maternal tissues. Only at the distal portions of the cell columns do the extravillous trophoblasts really become invasive and start their route through the maternal decidual interstitium (Kaufmann et al. 2003).
The localization of the cell columns at the border between the low oxygen placenta and the high oxygen decidua is exactly what is needed for the shift from proliferation in the proximal part of the cell columns to invasion in the distal part of the columns. There is evidence that low oxygen maintains the trophoblast in a proliferative, non-invasive phenotype (Genbacev et al. 1997), up-regulating factors such as hypoxia-inducible factor-1 (HIF-1) (Caniggia et al. 2000). There are at least two mechanisms by which the daughter cells are forced to stop their proliferation: loss of contact with the basement membrane and experiencing higher oxygen levels while being pushed forward towards the decidua.
Finally, at the distal part of the cell columns the extravillous trophoblasts have completely lost their generative capacity, but have gained a high invasive potential. Consequently, they invade towards the myometrium and potentially reach spiral arteries.
After the onset of maternal blood flow
Only at the beginning of the second trimester (11–14 weeks, Burton & Jauniaux 2004) have the plugs of extravillous trophoblasts blocking the spiral arteries (Fig. 2) dissolved, and the placental pO2 rises to values above 50 mmHg. The placental pO2 stays at about this level until delivery. The fast increase of oxygen concentrations within the placenta is associated with increased oxidative stress of the placental villi. This has been shown by an increased expression of genes associated with oxidative stress (Jauniaux et al. 2003). Higher oxygen levels drive trophoblast differentiation as well as placental maturation into an exchange organ.
With the onset of maternal blood flow the steep oxygen gradient between decidua and placenta mostly disappears. As can be seen from the values mentioned above, a small gradient remains. Under these conditions the proximal parts of the cell columns reduce their proliferative activity. The daughter cells start to invade earlier because they are experiencing a higher oxygen level earlier during their way towards the decidua.
This can be followed by the appearance of the trophoblast cell columns throughout pregnancy. They start as real columns that attach anchoring villi to decidual tissues. Throughout the second and third trimesters the columns reduce their height and at the end of gestation most of them are gone completely. Even though some may be left, their shape is different compared to those of the first trimester. Now the anchoring villi are embedded in the tissues of the basal plate with only a few extravillous trophoblasts directly attached to the villus. Most of them have invaded the decidua and thus have left the anchoring villus.
The shift from low to high oxygen at the onset of the second trimester thus helps to keep the number of invading cells high, although the proliferative activity of the proximal parts of the cell columns diminishes. However, at around mid gestation the cell columns in the central part of the placenta are mostly exhausted. An exhausted pool of cells abolishes the chance to increase the number of invading cells at that stage of pregnancy in case something went wrong during early invasion.
Failure of trophoblast invasion
Reduced invasive potential of the extravillous trophoblast is due to a lower number of invading extravillous trophoblasts. The two main mechanisms to reduce cell numbers are increased apoptosis and/or decreased proliferation.
Apoptosis of extravillous trophoblast may be upregulated in early-onset pre-eclampsia as well as pure fetal growth restriction, although at present no consensus exists in the literature. Cases of spontaneous abortion are mostly associated with a complete absence of trophoblast invasion (Hustin et al. 1990; Jauniaux et al. 2003), and less severe reductions in cell numbers have been found in early-onset fetal growth restriction, with or without co-existent pre-eclampsia (Brosens 1988; Sheppard & Bonnar 1988; Kadyrov et al. 2003). The failure in trophoblast invasion can non-invasively be monitored by uterine artery Doppler. More than 85% of such cases show an abnormal Doppler, which in turn correlates with the extent of decidual vasculopathy in the uterine wall below the placenta (Reister et al. 2001).
Reduced invasion of extravillous trophoblasts into the uteroplacental arteries may result from intrinsic trophoblastic factors in combination with extrinsic factors of the maternal uterus. Such extrinsic factors include impaired remodelling of the decidua (Aplin 1991; Brosens et al. 2002), macrophage-dependent defence mechanisms against trophoblast invasion (Aplin 1991; Reister et al. 1999; Reister et al. 2001; Brosens et al. 2002), as well as a variety of other mechanisms.
There is a huge variability in the rates of extravillous trophoblast apoptosis, ranging between 4% (Kadyrov et al. 2003) and more than 50% (DiFederico et al. 1999). Such contrasting reports give room for new and more appropriate hypotheses to explain reduced numbers of extravillous trophoblast leading to impaired adaptation of uteroplacental arteries during pregnancy.
One hypothesis could be forwarded keeping the important observations of Jauniaux and Burton in mind (Burton et al. 2003; Jauniaux et al. 2003). These authors described the adverse effects of a premature onset of maternal blood flow towards the placenta before the beginning of the second trimester of pregnancy. A premature rise of placental oxygen may damage the stem cell pool of extravillous trophoblast in the proximal parts of the cell columns. These highly proliferative cells rest on the basement membranes of anchoring villi and show high rates of proliferation during the first trimester, i.e. under low oxygen. An early onset of maternal blood flow leading to a premature rise in oxygen may reduce the generative potency of these cells and hence impair the number of cells available to invade maternal tissues. Thus impaired trophoblast invasion – perhaps even in the absence of any increase in extravillous trophoblast apoptosis – could be due to a premature rise in placental oxygen (Huppertz & Kingdom 2004).
Conclusions
There is evidence that the first changes of the uterine spiral arteries occur already during the secretory phase of the menstrual cycle, clearly in advance of any trophoblast invasion. At the same time the transformation of spiral arteries into inelastic and wide tubes can only be achieved by adequate invasion of extravillous trophoblast. Oxygen is one of the main regulators to define the number of these cells. This in turn controls their capacity to remodel the walls of spiral arteries, which is decisive for a regular remodelling to result in the development of uteroplacental arteries. Only then can adequate flow of maternal blood into the intervillous space guarantee normal growth of the fetus. Any changes in the amount of invading trophoblasts may impair maternal blood supply to the placenta and in turn have major negative effects on the growth of the fetus.
References
- Aplin JD. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P, Baergen R. Pathology of the Human Placenta. 5th edn. New York: Springer; 2006. [Google Scholar]
- Boyd JD, Hamilton WJ. Cells in the spiral arteries of the pregnant uterus. J Anat. 1956;90:595. [Google Scholar]
- Boyd JD, Hamilton WJ. Development and structure of the human placenta from the end of the 3rd month of gestation. J Obstet Gynaecol Br Commonw. 1967;74:161–226. doi: 10.1111/j.1471-0528.1967.tb14864.x. [DOI] [PubMed] [Google Scholar]
- Brettner A. Zum Verhalten der sekundaeren Wand der Uteroplacentargefaesse bei der decidualen Reaktion. Acta Anat. 1964;57:367–376. [PubMed] [Google Scholar]
- Brosens IA. The utero-placental vessels at term – the distribution and extent of physiological changes. Trophoblast Res. 1988;3:61–67. [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–1423. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- Burton GJ. Oxygen, the Janus gas; its effects on human placental development and function. J Anat. doi: 10.1111/j.1469-7580.2008.00978.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Hempstock J, Jauniaux E. Nutrition of the human fetus during the first trimester – a review. Placenta. 2001;22(Suppl. A):S70–S77. doi: 10.1053/plac.2001.0639. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Skepper JN, Hempstock J, Cindrova T, Jones CJ, Jauniaux EA. Reappraisal of the contrasting morphological appearances of villous cytotrophoblast cells during early human pregnancy; evidence for both apoptosis and primary necrosis. Placenta. 2003;24:297–305. doi: 10.1053/plac.2002.0882. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(Suppl. A):S25–S30. doi: 10.1053/plac.1999.0522. [DOI] [PubMed] [Google Scholar]
- Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1998;19:241–252. doi: 10.1016/s0143-4004(98)90055-8. [DOI] [PubMed] [Google Scholar]
- DiFederico E, Genbacev O, Fisher SJ. Preeclampsia is associated with widespread apoptosis of placental cytotrophoblasts within the uterine wall. Am J Pathol. 1999;155:293–301. doi: 10.1016/S0002-9440(10)65123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders AC. Implantation in the macaque: expansion of the implantation site during the first week of implantation. Placenta. 2007;28:794–802. doi: 10.1016/j.placenta.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277:1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- Harris JWS, Ramsey EM. The morphology of human uteroplacental vasculature. Contrib Embryol Carnegie Inst Wash. 1966;38:43–58. [Google Scholar]
- Hees H, Moll W, Wrobel KH, Hees I. Pregnancy-induced structural changes and trophoblastic invasion in the segmental mesometrial arteries of the guinea pig (Cavia porcellus L. Placenta. 1987;8:609–626. doi: 10.1016/0143-4004(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Hirano H, Imai Y, Ito H. Spiral artery of placenta: development and pathology – immunohistochemical, microscopical, and electron-microscopic study. Kobe J Med Sci. 2002;48:13–23. [PubMed] [Google Scholar]
- Hunter W. Birmingham, AL: Gryphon Editions; Anatomia uteri humani gravidi tabulis illustrata e the anatomy of the human gravid uterus exhibited in figures. Facsimile edition of the original edition (Birmingham: John Baskerville 1774) [Google Scholar]
- Huppertz B, Kingdom JC. Apoptosis in the trophoblast – role of apoptosis in placental morphogenesis. J Soc Gynecol Investig. 2004;11:353–362. doi: 10.1016/j.jsgi.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hustin J, Jauniaux E, Schaaps JP. Histological study of the materno-embryonic interface in spontaneous abortion. Placenta. 1990;11:477–86. doi: 10.1016/s0143-4004(05)80193-6. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson A, Ozturk O, Quick D, Burton G. In-vivo measurement of intrauterine gases and acid-base values early in human pregnancy. Hum Reprod. 1999;14:2901–2904. doi: 10.1093/humrep/14.11.2901. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress; a possible factor in human early pregnancy failure. Am J Pathol. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Hempstock J, Greenwold N, Burton GJ. Trophoblastic oxidative stress in relation to temporal and regional differences in maternal placental blood flow in normal and abnormal early pregnancies. Am J Pathol. 2003;162:115–125. doi: 10.1016/S0002-9440(10)63803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrov M, Schmitz C, Black S, Kaufmann P, Huppertz B. Pre-eclampsia and maternal anaemia display reduced apoptosis and opposite invasive phenotypes of extravillous trophoblast. Placenta. 2003;24:540–548. doi: 10.1053/plac.2002.0946. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1e7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- Moll W, Nienartowicz A, Hees H, Wrobel K-H, Lenz A. Blood flow regulation in the uteroplacental arteries. Trophoblast Res. 1988;3:83–96. [Google Scholar]
- Morgan T, Craven C, Ward K. Human spiral artery renin-angiotensin system. Hypertension. 1998;32:683–687. doi: 10.1161/01.hyp.32.4.683. [DOI] [PubMed] [Google Scholar]
- Moser G, Theuerkauf RS, Flieser N, Huppertz B. Mononuclear extravillous trophoblast cells do not penetrate the decidual epithelium in a double tissue confrontation assay. Placenta. 2008;29:A88. [Google Scholar]
- Nanaev AK, Chwalisz K, Frank HG, Kohnen G, Hegele-Hartung C, Kaufmann P. Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 1995;282:407–421. doi: 10.1007/BF00318873. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Heyl W, et al. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta. 1999;20:229–233. doi: 10.1053/plac.1998.0373. [DOI] [PubMed] [Google Scholar]
- Reister F, Frank HG, Kingdom JCP, et al. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest. 2001;81:1143–1152. doi: 10.1038/labinvest.3780326. [DOI] [PubMed] [Google Scholar]
- Robertson WB. Uteroplacental vasculature. J Clin Pathol. 1976;29(Suppl):9–17. doi: 10.1136/jcp.s3-10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson WB, Manning PJ. Elastic tissue in uterine blood vessels. J Pathol. 1974;112:237–243. doi: 10.1002/path.1711120408. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol. 1992;80:283–285. [PubMed] [Google Scholar]
- Sheppard BL, Bonnar J. The maternal blood supply to the placenta in pregnancy complicated by intrauterine fetal growth retardation. Trophoblast Res. 1988;3:69–81. [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–65. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- Smith SC, Leung TN, To KF, Baker PN. Apoptosis is a rare event in first-trimester placental tissue. Am J Obstet Gynecol. 2000;183:697–699. doi: 10.1067/mob.2000.106555. [DOI] [PubMed] [Google Scholar]
- Tuttle SE, O'Toole RV, O'Shaughnessy RW, Zuspan FP. Immunohistochemical evaluation of human placental implantation: an initial study. Am J Obstet Gynecol. 1985;153:239–244. doi: 10.1016/s0002-9378(85)80104-6. [DOI] [PubMed] [Google Scholar]
- Watson AL, Skepper JN, Jauniaux E, Burton GJ. Susceptibility of human placental syncytiotrophoblastic mitochondria to oxygen-mediated damage in relation to gestational age. J Clin Endocrinol Metab. 1998;83:1697–705. doi: 10.1210/jcem.83.5.4830. [DOI] [PubMed] [Google Scholar]