Abstract
The development of adult-onset diseases such as type II diabetes, obesity and cardiovascular disease is traditionally attributed to adult lifestyle characteristics such as a lack of physical exercise, poor diet and smoking. However, evidence from both human and animal model studies has demonstrated that environmental factors such as an imbalance or reduction in maternal nutrition during gestation can have adverse effects on offspring metabolism and cardiovascular health. The severity and nature of the phenotypic changes induced in offspring is influenced by the period of gestation manipulated. In particular, the mammalian preimplantation embryo in different animal models displays particular sensitivity to environmental factors, either in vivo (maternal diet) or in vitro (embryo culture) that is associated with the onset of cardiovascular dysfunction in adult life. The detailed mechanisms by which environmental conditions can alter postnatal cardiovascular physiology are poorly understood. However, various factors including endothelial function, vascular responsiveness, the renin-angiotensin system, kidney structure and early postnatal growth dynamics have all been recognize as potential contributors. Here, we review the relationship between preimplantation embryo environment and postnatal cardiovascular disease risk, and consider biochemical, molecular, genetic and physiological pathways implicated in this association.
Keywords: blastocyst, in vitro culture, kidney, maternal diet, preimplantation embryo, renin-angiotensin system, vascular responsiveness
Introduction
Data from human and animal studies have demonstrated that a strong association exists between the onset of specific adult diseases and poor maternal nutrition during pregnancy. Initial observations made by Professor David Barker and colleagues in the late 1980s identified the relationship between low birth weight in humans and the risk of developing type II diabetes (Hales et al. 1991; Ravelli et al. 1998) and cardiovascular disease (Barker & Osmond, 1986; Barker et al. 1989). Since then, the use of animal models has further increased our understanding of the relationships between a deficient maternal environment and the long-term health of offspring. Typically, the nature of the animal studies employed have focused upon altering specific aspects of the maternal diet (e.g. reduction in protein content, global restriction of food and/or caloric intake) during critical windows of gestational (e.g. early development, periods of organogenesis, fetal growth in late gestation), and then examining the resultant health and physiology of the offspring.
In response to these findings from both human and animal studies, the Developmental Origins of Health and Disease (DOHaD) hypothesis has emerged, proposing that the fetus can adapt its own development in direct response to the uterine environment, mediated by factors such as maternal nutrition and physiology (Gluckman & Hanson, 2004; McMillen & Robinson, 2005; Hanson & Gluckman, 2008). These responses to maternal diet allow the fetus to make adjustments to its growth and metabolism so that fetal properties and size are compatible with the predicted availability of postnatal nutrients. However, under conditions of maternal undernutrition, the predictive responses may become maladaptive if the postnatal nutritional environment then provides a relative abundance of nutrients. In such circumstances, a discordance exists between nutrient intake and homeostatic processes regulating energy consumption, leading to an increased risk of adult cardiovascular and metabolic disease (Gluckman & Hanson, 2004; McMillen & Robinson, 2005; Hanson & Gluckman, 2008).
In recent years, a new focus has emerged concerning the sensitivity to environmental factors during preimplantation development and their effect on the long-term health of the offspring both in vivo (maternal diet) and in vitro (embryo culture) (Fleming et al. 2004; Sinclair & Singh, 2007; Thompson et al. 2007; Watkins et al. 2008a). During preimplantation development (between fertilization of the egg and implantation of the embryo into the uterus), the embryo forms a blastocyst in which are established the first two cell lineages; the outer polarize epithelial trophectoderm (TE) and the pluripotent inner cell mass (ICM), which give rise predominantly to placental and fetal tissues, respectively. Thus, during zygotic cleavage (approximately 4 days in the mouse and 6 days in the human), the role of the embryo has been classically viewed as initiation of the developmental programme comprising embryonic gene activation, cell cycling, lineage diversification and blastocyst morphogenesis including TE differentiation (Eckert & Fleming, 2008). However, with recent work, this concept has been revised such that the intrinsic programme appears responsive to extrinsic cues from the maternal environment to fine-tune the course of development, using a range of cellular, physiological and epigenetic mechanisms. These responses to the environment may facilitate changes in the development and behaviour of early lineages to compensate for nutrient deficits and/or to optimize phenotype for later development and the postnatal environment. For example, poor periconceptual nutrition can stimulate the extra-embryonic visceral yolk sac lineage to develop with enhanced endocytic capacity, thereby facilitating increased nutrient retrieval in later pregnancy (Watkins et al. 2008b). In a stable environment, the responses may be beneficial, acting in a compensatory way to protect fetal growth and maintain reproductive fitness and competitiveness. However, in line with the DOHaD concept, responses may become inappropriate and lead to adult disease if a mismatch exists between periconceptual and postnatal environments.
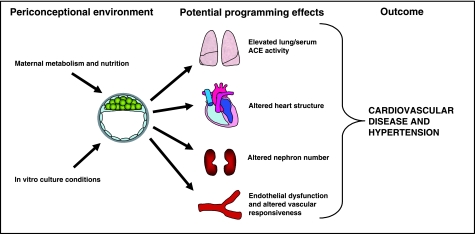
From a range of animal models and from different laboratories it has been demonstrated that manipulation of preimplantation environment through dietary or in vitro culture treatments can adversely affect postnatal phenotype (Fleming et al. 2004; Sinclair et al. 2007; Thompson et al. 2007; Watkins et al. 2008a). In particular, poor fetal or adult cardiovascular and metabolic health, and notably hypertension, constitute a common outcome phenotype from diverse preimplantation treatments (Kwong et al. 2000, 2007; Edwards & McMillen, 2002 a,b; Fernandez-Gonzalez et al. 2004, 2007; Gardner et al. 2004, 2006; Cleal et al. 2007; Sinclair et al. 2007; Watkins et al. 2007, 2008b). This is summarized in Fig. 1 and Table 1. This review focuses on the long-term changes in offspring cardiovascular phenotype following altered periconceptual and preimplantation embryo development, and the various pathways and physiological mechanisms underlying the hypertensive state induced.
Fig. 1.
Diagram representing the importance of environmental factors, either in vivo or in vitro, during the perioconceptional period on embryo development and adult cardiovascular phenotype, mediated potentially through diverse organ systems leading to hypertension and cardiovascular ill health. See Table 1 for details and references.
Table 1.
Effect of periconceptional environment on fetal and postnatal cardiovascular physiology
| Model | Observed effects | Hypertension | Reference(s) |
|---|---|---|---|
| Rodent | |||
| Embryo culture | Increased serum ACE activity. | Yes | Watkins et al. (2007) |
| Maternal LPD | Altered nephron number. | Yes | Langley-Evans et al. (1996) |
| Attenuated mesenteric responsiveness to isoprenaline and sodium nitroprusside. | Kwong et al. (2000, 2007) | ||
| Elevated ACE activity. | Watkins et al. (2008b, unpublished observations) | ||
| Absencel of Ped gene expression | Elevated serum ACE activity. | Yes | Watkins et al. (2006) |
| Sheep/cattle | |||
| Maternal periconceptual undernutrition | Elevated fetal and postnatal arterial blood pressure. | Yes | Edwards et al. (2005) |
| Increased activation of fetal hypothalamic-pituitary-adrenal (HPA) axis. | Edwards & McMillen (2002a) | ||
| Increased postnatal plasma cortisol and altered cardiovascular function. | Gardner et al. (2004, 2006) | ||
| Increased thoracic sensitivity to phenylephrine and acetylcholine. | Cleal et al. (2007) | ||
| Increased heart wall thickness. | Sinclair et al. (2007) | ||
| Human | |||
| Assisted reproductive techniques (ART) | Elevated systolic and diastolic blood pressure and altered glucose homeostasis. | Yes | Ceelen et al. (2008) |
Manipulation of maternal diet
Analysis of historical human data from women who became pregnant during the Dutch Hunger winter famine, a period of 5 months of malnutrition in the winter of 1944–45 in Amsterdam, the Netherlands, found that grown children of mothers who experienced the famine during embryonic and/or early fetal development had increased levels of coronary heart disease, an increased body mass index, and were glucose intolerant (Ravelli et al. 1999; Painter et al. 2006; De Rooij et al. 2006). Interestingly, children of mothers who became pregnant prior to the famine and experienced malnutrition during the later stages of pregnancy had reduced birth weights and became obese as adults (Ravelli et al. 1999; McMillen & Robinson, 2005). These data suggest that different periods of gestation appear differentially sensitive to the effects of maternal undernutrition with regard to the nature of the changes in offspring health and phenotype induced.
The employment of animal models has enabled the study of fetal and postnatal development in response to precise and controlled manipulation of the maternal diet to be conducted in greater detail. Along with dietary constituents, the use of rodent and sheep models permits the targeting of maternal undernutrition to discrete periods of embryonic and/or fetal development. Langley-Evans et al. (1996) demonstrate that different periods of gestation in the rat showed differential sensitivity with regard to the severity of the hypertension induced in weanling rats exposed to maternal low-protein diet (LPD). They observed that maternal LPD given at discrete 7-day intervals, or throughout gestation, resulted in hypertensive offspring. However, the magnitude of hypertension induced was greatest when LPD was given either during the last 7 days of gestation, or for the entirety of gestation. In a range of studies, LPD targeted exclusively to the preimplantation stage of rodent development has been shown to alter blastocyst cell number, increase the expression of 11β-hydroxysteroid dehydrogenase type 1 (Hsd11b1) and phosphoenolpyruvate carboxykinase (Pepck, Pck1), genes involved in activating glucocorticoid in the fetal liver and gluconeogenesis, respectively, which alter fetal and postnatal growth, induce relative hypertension in the adult offspring, increase patterns of anxiety-related behaviour in an open field test, and alter the relative sizes of organs (Kwong et al. 2000, 2006, 2007; Watkins et al. 2008b). In the sheep, maternal undernutrition from days 0 to 30/31 of pregnancy increases circulating plasma cortisol levels in female lambs and altered cardiovascular function of lambs at 1 year of age (Gardner et al. 2004, 2006), whilst in the adult, an increased interventricular septal wall thickness and increased mean left ventricular wall thickness were observed (Cleal et al. 2007). Maternal dietary deficiency in B vitamins and methionine during the periconceptional period in sheep also induced heavier and fatter offspring with associated hypertension with evidence of epigenetic changes associated with the DNA methylation profile of many genes (Sinclair et al. 2007).
Data from the human and animal studies have also revealed the significance of early postnatal growth dynamics upon adult health and physiology. In humans, children born small for gestational age and who remained small at 1 year of age but then underwent rapid gains in weight and body mass index (BMI), but not height, had a higher prevalence of coronary heart disease as adults when compared to adults of average birth weight (Eriksson et al. 1999, 2001). In mice, offspring from mothers fed LPD exclusively during preimplantation development (Emb-LPD) differ significantly in their associations between perinatal body weight and adult systolic blood pressure (SBP) from those of controls (Watkins et al. 2008b). Here, control offspring who were small at 3 weeks of age developed with a higher SBP as adults. However, Emb-LPD offspring showed positive associations, such that increased perinatal growth was associated with a higher adult SBP, highlighting the cardiovascular risk derived from excessive peri- and postnatal growth and body weight.
Not only do the very earliest stages of development appear sensitive to the programming effects of maternal undernutrition, but also dietary challenges during the period of oocyte maturation may result in changes in offspring cardiovascular health. In the mouse, maternal LPD given exclusively during oocyte maturation (3.5 days prior to mating) has been shown to result in the development of hypertensive adult offspring (Watkins et al. 2008c). In the sheep, maternal undernutrition during the periconceptual period (45 days prior to conception until 7 days after) alters maternal and placental/fetal growth dynamics, increases fetal arterial blood pressure, and increases the activation of the fetal hypothalamo-pituitary-adrenal axis (Edwards & McMillen, 2002a,b; Edwards et al. 2005, McMillen et al. 2008).
In vitro culture
In vitro culture and manipulation of mammalian preimplantation embryos is a necessary requirement for diverse procedures ranging from human assisted reproductive techniques (ART) such as IVF, the derivation of embryonic stem cell lines, biotechnological treatments to improve production of important domestic animal strains, and ultimately increasing our understanding of essential developmental pathways and mechanisms. However, whilst the development of morphologically ‘normal’ blastocysts may be achieved in vitro with relatively high success rates, the short- and long-term developmental capacity of those tissues, and ultimately the offspring derived from them, may be compromised. Perhaps the most dramatic display of how fetal and postnatal development can be altered in response to manipulating the preimplantation embryo is the phenomenon of ‘large offspring syndrome’ (LOS). LOS has been observed in cattle and sheep, and occurs as a consequence of in vitro culture in the presence of serum, the introduction of the preimplantation embryo to an altered uterine environment, or the production of cloned embryos following nuclear transfer (Holm et al. 1996; Sinclair et al. 1999, 2000; Wells et al. 1999). LOS is associated with significantly increased fetal and postnatal growth, altered organ sizing, changes in patterns of gene expression, and increased rates of perinatal death (Holm et al. 1996; Sinclair et al. 1999; Niemann & Wrenzycki, 2000; Farin et al. 2006, Smith et al. 2008). It has also been reported that mice produced from cloned embryos also display an increased rate of fetal and postnatal growth (Eggan et al. 2001).
In vitro culture and transfer of mouse preimplantation embryos has also been associated with significant changes to offspring phenotype. We reported elevation of offspring SBP following either prolonged (two-cell to blastocyst stage) or brief (2 h prior to uterine transfer) in vitro culture and embryo transfer (Watkins et al. 2007). Changes in embryo gene expression and regulation (Stojanov & O’Neill, 2001; Rinaudo & Schultz, 2004), reduced rates of development and survival post transfer (Bowman & McLaren, 1970; Li et al. 2007), altered fetal growth (Caro & Trounson, 1984; Arny et al. 1987; Khosla et al. 2001) and disturbed postnatal memory characteristics (Ecker et al. 2004; Fernandez-Gonzalez et al. 2004) have also been documented following in vitro culture of preimplantation embryos. Recently, culture and transfer of mouse preimplantation embryos was shown to result in altered postnatal growth and organ sizing in the offspring which persisted into a second generation, indicating epigenetic modifications (Mahsoudi et al., 2007).
The well-documented sensitivity of rodent and large animal embryos to differing in vitro manipulations raises several questions about the possible long-term development and cardiovascular health of children conceived using ART. Follow-up studies have demonstrated that ART children have a higher risk of being born smaller, even after accounting for the incidences of multiple pregnancies (Schieve et al. 2002). There are also several reports indicating increased rates of congenital abnormalities for infants conceived by intracytoplasmic sperm injection (ICSI) (Hansen et al. 2002; Winston & Hardy, 2002) and increased incidence of syndromes caused by errors in gene imprinting (Cox et al. 2002; De Rycke et al. 2002; DeBaun et al. 2003; Lucifero et al. 2004). Conversely, others report no differences in the psychological status of school-age children conceived by IVF as compared with children conceived naturally (Montgomery et al. 1999). At present it is difficult to gauge the detailed long-term impact of ART treatments primarily due to the relatively young age of the children born. Whilst there have been several systematic follow-up studies examining physical and cognitive development and rates of diseases such as cancer occurring in ART children (Schieve et al. 2002; Koivurova et al. 2003; Ludwig et al. 2006), there has only been one study examining postnatal blood pressure and metabolic profile. This study reported that pre-pubertal IVF children displayed significantly elevated systolic and diastolic blood pressure, and elevated fasting glucose levels independent of early life factors or parental characteristics (Ceelen et al. 2008). It will be important to extend such cardiovascular studies on ART children as they reach adulthood, particularly as the tracking of childhood blood pressure into adulthood is known to occur (Law et al. 1993).
Mechanisms central to the hypertensive state
The mechanisms and causal pathways that underlie the hypertensive state and associated cardiovascular diseases in response to developmental programming are likely to be complex and numerous and have been described in detail elsewhere (Langley-Evans, 2001; Brawley et al. 2003a, McMillen & Robinson 2005). Therefore, we highlight here the central components demonstrated to be affected in response to periconceptual environment.
Endothelial dysfunction and vascular responsiveness
As peripheral vascular dysfunction is associated with, and often precedes, the development of hypertension (Brawley et al. 2003a), an understanding of the role of vascular responsiveness and endothelial and smooth muscle function in the homeostatic regulation of blood pressure is essential. Offspring of diet-restricted mothers have consistently demonstrated elevations in their SBP (Langley-Evans et al. 1996, Kwong et al. 2000, Torrens et al. 2003, 2006; Watkins et al. 2008c) and, as a result, numerous studies have examined the production of and responsiveness to a range of vasoactive compounds. Isolated mesenteric arteries from male mice exposed to maternal LPD either exclusively during preimplantation development or throughout gestation, display attenuated responsiveness to the β-adrenoceptor agonist, isoprenaline, and the nitric oxide (NO) donor, sodium nitroprusside (Watkins et al. 2008, unpublished observations), suggestive of a vascular defect in smooth muscle signalling via cAMP. Maternal LPD given exclusively during mouse oocyte maturation results in impaired responsiveness to the endothelial-dependent vasodilator acetylcholine, and isoprenaline, in male isolated mesenteric arteries (Watkins et al. 2008c). It is of interest to note that no difference in vasoconstriction mediated through the α1-adrenergic vasoagonist, phenylephrine, was observed in these two studies. These findings are in line with previous studies of offspring from dams fed a globally restricted diet (50% reduction) during the second half of pregnancy (Holemans et al. 1999), or LPD throughout pregnancy (Brawley et al. 2003b), where no difference in α1-adrenoceptor-mediated constriction was observed.
The renin-angiotensin system (RAS)
Along with vascular responsiveness, the renin-angiotensin system is also sensitive to the programming effects of an altered embryo environment. Angiotensin-converting-enzyme (ACE), a metallopeptidase, has been identified as a central component of the RAS through its predominant actions of cleaving bradykinin, and the generation of angiotensin II from angiotensin I (Corvol et al. 1995; Coates, 2003). Upon binding to its receptor (AT1), located in vascular smooth muscle cells, kidney, heart, adrenal glands and the brain, angiotensin II induces vasoconstriction, renal tubular sodium re-absorption, and aldosterone and vasopressin secretion (Unger, 2002). Angiotensin II has also been implicated in endothelial dysfunction through its ability to increase oxidative stress (Mehta & Griendling, 2007), whilst the cleavage of the vasodilator bradykinin also contributes to the establishment of a hypertensive state (Corvol et al. 1995; Coates, 2003). In mice, ACE−/− animals are hypotensive with an SBP ~30 mmHg lower than control animals (Esther et al. 1997; Cole et al. 2000), whilst in humans, serum ACE levels are significantly higher in hypertensive subjects (Forrester et al. 1997). The RAS and ACE function have been demonstrated to be involved in the programming of hypertension following maternal dietary manipulation in rodents and sheep. Elevated activity of serum and lung ACE has been observed in mice from mothers fed LPD either exclusively during preimplantation development or throughout gestation (Watkins et al. 2008, unpbublished observations). Elevated ACE activity also occurs in mice in response to in vitro culture and embryo transfer procedures (Watkins et al. 2007) and in vivo in response to expression of the ‘Ped’ genes that contribute to embryo survival (Watkins et al. 2006). The blockade of the AT1 receptor with the agonist losartan (Sherman & Langley-Evans, 2000), or the use of the ACE inhibitor captopril (Langley-Evans & Jackson, 1995), significantly reduces the SBP in offspring rats from LPD-fed mothers. In the sheep, significantly elevated blood pressure responses to RAS stimulation by ferusamide is observed in offspring of mothers with early gestational nutrient restriction (Cleal et al. 2007). This elevation in SBP was blocked by prior administration of captopril, further indicating the central role of the RAS in the hypertensive state.
Kidney development and function
The notion that a reduced birth weight and associated reduction in nephron number could increase the risk of adult hypertension and chronic renal disease was first proposed by Brenner & Chertow (1994). Autopsy studies from intrauterine growth-retarded (IUGR) infants have demonstrated associations with a reduction in nephron number (Hinchcliffe et al. 1991, 1992), whilst in a range of animal species, experimental maternal undernutrition or placental insufficiency have now been reproducibly associated with reduced nephron number (Jones et al. 2001; Woods et al. 2001; Bauer et al. 2002; Gilbert et al. 2005), which is then associated with elevated SBP (Woods et al. 2001; Gilbert et al. 2005). However, several studies have demonstrated programmed elevated blood pressure effects in the absence of significant changes in glomerular number (Ortiz et al. 2003, Pladys et al. 2005; Woods et al. 2005). Whilst we have found no change in glomerular number in response to maternal LPD given exclusively during the mouse preimplantation period (Watkins et al. 2008, unpublished observations), targeting LPD to the period of oocyte maturation induced both reduced kidney size and an increase in glomerula number coincident with relative hypertension (Watkins et al. 2008c). As kidney development occurs late in gestation and this periconceptional dietary challenge actually precedes the onset of development, the response of increased glomerula numbers may be compensatory to guard against adverse programming, although the mechanism of this response is currently unknown.
Conclusions
Evidence is growing from many laboratories that the periconceptual period is a window of enhanced sensitivity to environmental conditions both in vivo and in vitro. This sensitivity reflects the emerging concept that the early embryo, before implantation, responds to maternal cues important in setting homeostatic regulation of metabolic, physiologic and growth criteria for gestation. We have found that the cardiovascular system is particularly vulnerable to adverse programming during the periconceptual window (Fig. 1). Such compensatory responses by embryos identified in animal models may also occur in the human and underlie epidemiological and related studies concerning the developmental origin of adult chronic disease. Further research is clearly needed to identify and understand the range of mechanisms contributing to the link between embryo environment and adult health.
Acknowledgments
This work was supported by grants to T.P.F. from (a) the National Institutes of Health, USA as part of the NICHD National Cooperative Program on Female Health and Egg Quality under cooperative agreement U01 HD044635, (b) the Gerald Kerkut Charitable Trust and (c) the BBSRC, UK (BB/F007450).
References
- Arny M, Nachtigall L, Quagliarello J. The effect of preimplantation culture conditions on murine embryo implantation and fetal development. Fertil Steril. 1987;48:861–865. doi: 10.1016/s0015-0282(16)59545-4. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;i:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Walter B, Bauer K, Klupsch R, Patt S, Zwiener U. Intrauterine growth restriction reduces nephron number and renal excretory function in newborn piglets. Acta Physiol Scand. 2002;176:83–90. doi: 10.1046/j.1365-201X.2002.01027.x. [DOI] [PubMed] [Google Scholar]
- Bowman P, McLaren A. Viability and growth of mouse embryos after in vitro culture and fusion. J Embryol Exp Morphol. 1970;23:693–704. [PubMed] [Google Scholar]
- Brawley L, Poston L, Hanson MA. Mechanisms underlying the programming of small artery dysfunction: review of the model using low protein diet in pregnancy in the rat. Arch Physiol Biochem. 2003a;111:23–35. doi: 10.1076/apab.111.1.23.15138. [DOI] [PubMed] [Google Scholar]
- Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, Hanson M. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003b;54:83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Chertow GM. Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis. 1994;23:171–175. [PubMed] [Google Scholar]
- Caro CM, Trounson A. The effect of protein on preimplantation mouse embryo development in vitro. J In Vitro Fert Embryo Transf. 1984;1:183–187. doi: 10.1007/BF01139212. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008;93:1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, et al. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA. 2007;104:9529–9533. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates D. The angiotensin converting enzyme (ACE) Int J Biochem Cell Biol. 2003;35:769–773. doi: 10.1016/s1357-2725(02)00309-6. [DOI] [PubMed] [Google Scholar]
- Cole J, Ertoy D, Bernstein KE. Insights derived from ACE knockout mice. J Renin Angiotensin Aldosterone Syst. 2000;1:137–141. doi: 10.3317/jraas.2000.016. [DOI] [PubMed] [Google Scholar]
- Corvol P, Williams TA, Soubrier F. Peptidyl dipeptidase A: angiotensin I-converting enzyme. Methods Enzymol. 1995;248:283–305. doi: 10.1016/0076-6879(95)48020-x. [DOI] [PubMed] [Google Scholar]
- Cox GF, Burger J, Lip V, et al. Intracytoplasmic sperm injection may increase the risk of imprinting defects. Am J Hum Genet. 2002;71:162–164. doi: 10.1086/341096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rooij SR, Painter RC, Roseboom TJ, et al. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia. 2006;49:637–643. doi: 10.1007/s00125-005-0136-9. [DOI] [PubMed] [Google Scholar]
- De Rycke M, Liebaers I, Van SA. Epigenetic risks related to assisted reproductive technologies: risk analysis and epigenetic inheritance. Hum Reprod. 2002;17:2487–2494. doi: 10.1093/humrep/17.10.2487. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci U S A. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert JJ, Fleming TP. Tight junction biogenesis during early development. Biochim Biophys Acta. 2008;1778:717–728. doi: 10.1016/j.bbamem.2007.09.031. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Impact of maternal undernutrition during the periconceptional period, fetal number, and fetal sex on the development of the hypothalamo-pituitary adrenal axis in sheep during late gestation. Biol Reprod. 2002a;66:1562–1569. doi: 10.1095/biolreprod66.5.1562. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Periconceptional nutrition programs development of the cardiovascular system in the fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2002b;283:R669–R679. doi: 10.1152/ajpregu.00736.2001. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McFarlane JR, Kauter KG, McMillen IC. Impact of periconceptional nutrition on maternal and fetal leptin and fetal adiposity in singleton and twin pregnancies. Am J Physiol Regul Integr Comp Physiol. 2005;288:R39–R45. doi: 10.1152/ajpregu.00127.2004. [DOI] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, III, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci USA. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318:427–431. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esther CR, Marino EM, Howard TE, Machaud A, Corvol P, Capecchi MR, Bernstein KE. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J Clin Invest. 1997;99:2375–2385. doi: 10.1172/JCI119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farin PW, Piedrahita JA, Farin CE. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology. 2006;65:178–191. doi: 10.1016/j.theriogenology.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira P, Bilbao A, et al. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci U S A. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Gonzalez R, Ramirez MA, Bilbao A, De Fonseca FR, Gutiérrez-Adán A. Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects. Mol Reprod Dev. 2007;74:1149–1156. doi: 10.1002/mrd.20746. [DOI] [PubMed] [Google Scholar]
- Fleming TP, Kwong WY, Porter R, et al. The embryo and its future. Biol Reprod. 2004;71:1046–1054. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- Forrester T, McFarlane-Anderson N, Bennett FI, et al. The angiotensin converting enzyme and blood pressure in Jamaicans. Am J Hypertens. 1997;10:519–524. doi: 10.1016/s0895-7061(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Pearce S, Dandrea J, et al. Peri-implantation undernutrition programs blunted angiotensin II evoked baroreflex responses in young adult sheep. Hypertension. 2004;43:1290–1296. doi: 10.1161/01.HYP.0000126991.67203.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DS, Van Bon BW, Dandrea J, et al. Effect of periconceptional undernutrition and gender on hypothalamic-pituitary-adrenal axis function in young adult sheep. J Endocrinol. 2006;190:203–212. doi: 10.1677/joe.1.06751. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Lang AL, Grant AR, Nijland MJ. Maternal nutrient restriction in sheep: hypertension and decreased nephron number in offspring at 9 months of age. J Physiol. 2005;565:137–147. doi: 10.1113/jphysiol.2005.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777–784. [PubMed] [Google Scholar]
- Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- Holemans K, Gerber R, Meurrens K, De CF, Poston L, Van Assche FA. Maternal food restriction in the second half of pregnancy affects vascular function but not blood pressure of rat female offspring. Br J Nutr. 1999;81:73–79. [PubMed] [Google Scholar]
- Holm P, Walker SK, Seamark RF. Embryo viability, duration of gestation and birth weight in sheep after transfer of in vitro matured and in vitro fertilized zygotes cultured in vitro or in vivo. J Reprod Fertil. 1996;107:175–181. doi: 10.1530/jrf.0.1070175. [DOI] [PubMed] [Google Scholar]
- Jones SE, Bilous RW, Flyvbjerg A, Marshall SM. Intra-uterine environment influences glomerular number and the acute renal adaptation to experimental diabetes. Diabetologia. 2001;44:721–728. doi: 10.1007/s001250051681. [DOI] [PubMed] [Google Scholar]
- Khosla S, Dean W, Brown D, Reik W, Feil R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes 21. Biol Reprod. 2001;64:918–926. doi: 10.1095/biolreprod64.3.918. [DOI] [PubMed] [Google Scholar]
- Koivurova S, Hartikainen AL, Sovio U, Gissler M, Hemminki E, Jarvelin MR. Growth, psychomotor development and morbidity up to 3 years of age in children born after IVF. Hum Reprod. 2003;18:2328–2336. doi: 10.1093/humrep/deg445. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Ursell E, et al. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction. 2006;132:265–277. doi: 10.1530/rep.1.01038. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Miller DJ, Wilkins AP, et al. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev. 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc Nutr Soc. 2001;60:505–513. doi: 10.1079/pns2001111. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci (Lond) 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- Law CM, de SM, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH. Initiation of hypertension in utero and its amplification throughout life. BMJ. 1993;306:24–27. doi: 10.1136/bmj.306.6869.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Chandrakanthan V, Chami O, O’Neill C. Culture of zygotes increases TRP53 [corrected] expression in B6 mouse embryos, which reduces embryo viability. Biol Reprod. 2007;76:362–367. doi: 10.1095/biolreprod.106.056838. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Chaillet JR, Trasler JM. Potential significance of genomic imprinting defects for reproduction and assisted reproductive technology. Hum Reprod Update. 2004;10:3–18. doi: 10.1093/humupd/dmh002. [DOI] [PubMed] [Google Scholar]
- Ludwig AK, Sutcliffe AG, Diedrich K, Ludwig M. Post-neonatal health and development of children born after assisted reproduction: a systematic review of controlled studies. Eur J Obstet Gynecol Reprod Biol. 2006;127:3–25. doi: 10.1016/j.ejogrb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Mahsoudi B, Li A, O’Neill C. Assessment of the long-term and transgenerational consequences of perturbing preimplantation embryo development in mice. Biol Reprod. 2007;77:889–896. doi: 10.1095/biolreprod.106.057885. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- McMillen IC, MacLaughlin SM, Muhlhausler BS, Gentili S, Duffield JL, Morrison JL. Developmental origins of adult health and disease: the role of periconceptional and foetal nutrition. Basic Clin Pharmacol Toxicol. 2008;102:82–89. doi: 10.1111/j.1742-7843.2007.00188.x. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Montgomery TR, Aiello F, Adelman RD, et al. The psychological status at school age of children conceived by in-vitro fertilization. Hum Reprod. 1999;14:2162–2165. doi: 10.1093/humrep/14.8.2162. [DOI] [PubMed] [Google Scholar]
- Niemann H, Wrenzycki C. Alterations of expression of developmentally important genes in preimplantation bovine embryos by in vitro culture conditions: implications for subsequent development. Theriogenology. 2000;53:21–34. doi: 10.1016/s0093-691x(99)00237-x. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter RC, de Rooij SR, Bossuyt PM, et al. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2006;84:322–327. doi: 10.1093/ajcn/84.1.322. [DOI] [PubMed] [Google Scholar]
- Pladys P, Sennlaub F, Brault S, Checchin D, et al. Microvascular rarefaction and decreased angiogenesis in rats with fetal programming of hypertension associated with exposure to a low-protein diet in utero. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1580–R1588. doi: 10.1152/ajpregu.00031.2005. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction. 2004;128:301–311. doi: 10.1530/rep.1.00297. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98:269–275. [PubMed] [Google Scholar]
- Sinclair KD, Singh R. Modelling the developmental origins of health and disease in the early embryo. Theriogenology. 2007;67:43–53. doi: 10.1016/j.theriogenology.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, McEvoy TG, Maxfield EK, et al. Aberrant fetal growth and development after in vitro culture of sheep zygotes. J Reprod Fertil. 1999;116:177–186. doi: 10.1530/jrf.0.1160177. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Young LE, Wilmut I, McEvoy TG. In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod. 2000;15(Suppl. 5):68–86. doi: 10.1093/humrep/15.suppl_5.68. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Allegrucci C, Singh R, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SL, Everts RE, Sung LY, et al. Gene expression profiling of single bovine embryos uncovers significant effects of in vitro maturation, fertilization and culture. Mol Reprod Dev. (E published ahead of print) [DOI] [PubMed]
- Stojanov T, O’Neill C. In vitro fertilization causes epigenetic modifications to the onset of gene expression from the zygotic genome in mice. Biol Reprod. 2001;64:696–705. doi: 10.1095/biolreprod64.2.696. [DOI] [PubMed] [Google Scholar]
- Thompson JG, Mitchell M, Kind KL. Embryo culture and long-term consequences. Reprod Fertil Dev. 2007;19:43–52. doi: 10.1071/rd06129. [DOI] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Barker AC, Itoh S, Poston L, Hanson MA. Maternal protein restriction in the rat impairs resistance artery but not conduit artery function in pregnant offspring. J Physiol. 2003;547:77–84. doi: 10.1113/jphysiol.2002.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Anthony FW, et al. Folate supplementation during pregnancy improves offspring cardiovascular dysfunction induced by protein restriction. Hypertension. 2006;47:982–987. doi: 10.1161/01.HYP.0000215580.43711.d1. [DOI] [PubMed] [Google Scholar]
- Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89:3A–9A. doi: 10.1016/s0002-9149(01)02321-9. [DOI] [PubMed] [Google Scholar]
- Watkins A, Wilkins A, Osmond C, et al. The influence of mouse Ped gene expression on postnatal development. J Physiol. 2006;571:211–220. doi: 10.1113/jphysiol.2005.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Platt D, Papenbrock T, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci U S A. 2007;104:5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Papenbrock T, Fleming TP. The preimplantation embryo: handle with care. Semin Reprod Med. 2008a;26:175–185. doi: 10.1055/s-2008-1042956. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod. 2008b;78:299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- Watkins AJ, Wilkins A, Cunningham C, et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J Physiol. 2008c;586:2231–2244. doi: 10.1113/jphysiol.2007.149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999;60:996–1005. doi: 10.1095/biolreprod60.4.996. [DOI] [PubMed] [Google Scholar]
- Winston RM, Hardy K. Are we ignoring potential dangers of in vitro fertilization and related treatments? Nat Cell Biol. 2002;4(Suppl):s14–s18. doi: 10.1038/ncb-nm-fertilityS14. [DOI] [PubMed] [Google Scholar]
- Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]