Abstract
The development and functioning of the human fetoplacental vascular system are vulnerable to the maternal diabetic milieu. These vessels are in direct continuum with the fetal vascular system and are therefore also vulnerable to fetal endocrine derangements. Increased angiogenesis, altered junctional maturity and molecular occupancy, together with increased leakiness, constitute a well-described phenotype of vessels in the Type 1 diabetic human placenta and can be related to increased levels of placental vascular endothelial growth factor. The causes of these observed changes, whether maternal hyperglycaemia or fetal hyperinsulinaemia, still remain to be shown in the human placenta. Mechanistic studies using different vascular systems have shown high glucose and insulin to have profound vascular effects, with elevations in vascular endothelial growth factor, nitric oxide and protein kinase C being behind alterations in junctional adhesion molecules such as occludin and vascular endothelial-cadherin and vascular leakage of albumin. The role of advanced glycation products and oxidative stress in this vascular pathology is also discussed. The altered molecular mechanisms underlying the vascular changes in the diabetic human placenta may reflect similar consequences of high glucose and hyperinsulinaemia.
Keywords: human placental vessels, hyperglycaemia, hyperinsulinaemia, occludin, vascular endothelial-cadherin
Introduction
The human placenta is essentially a complex vascular system that allows juxtaposition of the maternal and fetal circulations without allowing actual mixing of the two. The placental fetal vessels are contained in chorionic villi composed of an outer trophoblast layer and a mesenchymal core. The villi lie bathed in maternal blood. The trophoblast and endothelium together regulate the transfer of nutrients and oxygen between maternal and fetal circuits. Therefore, the successful development, growth and maturity of the feto-placental vessels are vital to fetal growth and survival. Moreover, the feto-placental vasculature is continuous with the fetal circulation and any changes here may reflect (and affect) the vascular functioning of the fetus.
The positioning of the placental fetal vasculature renders it vulnerable to any haemodynamic modifications or metabolic endocrine derangements in both maternal and fetal environments. The development and functioning of the vessels may be directly influenced by the diabetic milieu. Altered conditions during critical periods of placental development may compromise vasculogenesis, angiogenesis and maturation of the vascular system. Vascular permeability, which relates to the degree of selectivity exhibited by the endothelium and the array of molecules able to pass through it may also be compromised (Bazzoni & Dejana, 2003). Efficient functioning throughout gestation requires the maintenance of a continuous fetal endothelium with well-defined paracellular junctions that can act as a selective barrier to hydrophilic solutes (Leach & Firth, 1992). Clearly, molecular alterations here may have ramifications for fetal growth and well-being.
Molecular regulation of endothelial permeability
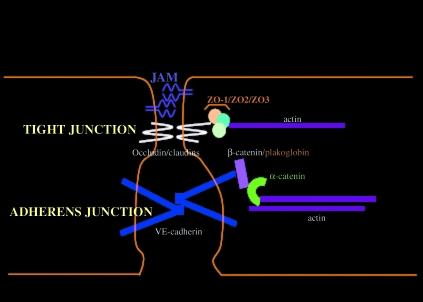
Junctional adhesion molecules play a key role in regulating endothelial monolayer integrity and paracellular permeability. They are integral constituents of cell–cell junctions as they promote homophilic cell interactions and give rise to a ‘zipper-like’ structure along the paracellular cleft (Bazzoni & Dejana, 2003). They show differential expression dependent on the type and maturity of the endothelial junctions. Tight junctions feature the transmembrane adhesion molecules, occludins and claudins (Tsukita & Furuse, 1999), whereas vascular endothelial-cadherin (VE-cadherin) is the transmembrane adhesion molecule of endothelial adherens junctions (Lampugnani et al. 1992). The extracellular portions of these molecules permit Ca2+-dependent homotypic adhesion, whereas their cytoplasmic tails allow binding to sister linking molecules and anchorage to peri-junctional cytoskeletal proteins. The key linking molecules responsible for adhesion in adherens junctions are β-catenin or plakoglobin, the latter being a later addition to fully mature junctions (Caveda et al. 1996). The catenins are of course signalling molecules in their own right, with non-junctional β-catenin being able to participate in growth and proliferation pathways (Ben-Ze’ev & Geiger 1998; Conacci-Sorrell et al. 2002). The cytoplasmic tails of adhesion molecules also contain tyrosine and serine residues, making them vulnerable to phosphorylation and internal signalling events, including tyrosine kinase pathways. This arrangement of linking molecules allows stabilization at specific membrane domains (Fig. 1) ensuring that the shape and polarity of the endothelial cell is maintained and the opening and closing of the cell junctions can be regulated (Bazzoni & Dejana 2003; Vestweber 2008).
Fig. 1.
Schematic diagram of key tight and adherens junctional adhesion molecules in quiescent mature endothelial paracellular clefts. Tight junctions here also include the endothelial junctional molecule (JAM)-A. This arrangement of linking molecules allows stabilization at specific membrane domains ensuring that the shape and polarity of the endothelial cell is maintained and the opening and closing of the cell junctions can be regulated.
Although claudins constitute the backbone of tight junctional strands, occludins play a permeability regulatory role by incorporation into the claudin-based strands. This plays a key fence and permeability barrier role in epithelial cells and endothelial cells of the blood–brain and blood–retinal barriers (see review Forster 2008). Since the identification of zonula occludens (ZO)-1 as the first tight junction-associated plaque protein (Stevenson et al. 1986), almost 30 additional proteins have been described to be associated with the cytoplasmic aspect of tight junctions. They can be grouped into two major categories: peripherally associated scaffolding proteins (like ZO-1, ZO-2, ZO-3, afadin and cingulin), which stabilize the transmembrane proteins and couple them to other cytoplasmic proteins and peri-junctional actin, and numerous signalling proteins (ZO-1 associated nucleic acid binding protein (ZONAB), Ras homolog gene family, member A (RhoA) and Ras effector 1 (Raf-1)), which are proposed to be involved in junction assembly, barrier regulation and gene transcription (Schneeberger & Lynch 2004).
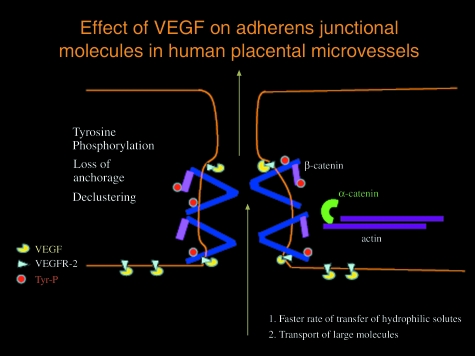
The proximity of the transmembrane junctional molecules to surface receptors of pro-angiogenic and pro-permeability growth factors, such as vascular endothelial growth factor (VEGF), allows the junctional molecules to act as receptors for external signalling. Indeed, VEGF has been shown to induce the phosphorylation of VE-cadherin, β-catenin, plakoglobin and p120, resulting in increased paracellular permeability (Fig. 2) and endothelial cell migration, which is suggested to be due to a loosening of cell–cell contacts (Esser et al. 1998; Cohen et al. 1999; Vestweber, 2008). Similarly, VEGF can phosphorylate the tight junctional proteins occludin and ZO-1 directly or via protein kinase C (PKC) (Harhaj et al. 2006).
Fig. 2.
Schematic diagram showing how VEGF may disrupt adherens junctions, the major regulator of paracellular permeability in human placental exchange vessels. Occupation of VEGFR-2 by VEGF may lead to autophosphorylation of VE-cadherin with loss of anchorage to actin, declustering and translocation of VE-cadherin and β-catenin. This would alter cleft dimension and paracellular filter properties, affecting rate of transfer and allowing transport of larger hydrophilic proteins.
In the human placenta, the frequency, position or dimensions of tight junctions are similar in all vessels; however, molecular occupancy studies revealed the differential expression of occludin with preferential localization in tight junctions in large conduit vessels only (Leach et al. 2000). The adherens junctions of the placental exchange vessels (the terminal villous capillaries) have an immature phenotype with β-catenin, rather than plakoglobin, being the linking partner of the transmembrane adhesion molecule VE-cadherin. These terminal vessels and the adjacent trophoblast are also rich in angiogenic growth factors and their receptors, including VEGF and its receptors (Leach et al. 2002;Demir et al. 2006); physiological angiogenesis during the latter part of gestation occurs at this terminal end of the villous tree. In-vitro studies and ex-vivo perfusion studies have shown that placental endothelial adhesion molecules are susceptible to external angiogenic and inflammatory stimuli and even maternal nutritional status (Leach et al. 1995; Dye et al. 2001, 2004; Rutland et al. 2007).
Endothelial adhesion molecules in diabetes
The tight junctional molecule occludin has been shown to be decreased in the intercellular junctions of retinal endothelial cells of streptozotocin (STZ)-induced diabetic rats coinciding with periods of increased permeability (Antonetti et al. 1998). In primary retinal endothelial cells VEGF stimulated the phosphorylation of occludin, whereas chemical inhibitors of PKC activity blocked the VEGF-induced increase in occludin phosphorylation (Harhaj et al. 2006). Matrix metalloproteinases (MMPs) are enzymes of the endopeptidase family and are known to be involved in the cleavage of cell surface receptors. The expression and activity of MMP-2 and MMP-9 have been shown to increase in diabetes and their involvement in the degradation of occludin in cultured bovine retinal microvessel endothelial cells has been demonstrated (Giebel et al. 2005). Occludin has been shown to be downregulated from large vessels of the placenta in pregnancies complicated by gestational diabetes (Babawale et al. 2000).
The retinal endothelial cells of STZ-induced diabetic rats exhibit a decrease in VE-cadherin content that inversely correlates with vascular permeability (Navaratna et al. 2007). The authors proposed that this is also due to the upregulation of MMPs that cleave VE-cadherin from the cell surface. Endothelial cells in Type 1 diabetic placental vessels have been shown to exhibit altered junctional occupancy of key adhesion molecules, including VE-cadherin and β-catenin in more than 50% of vessels (Leach et al. 2004). The endothelium here and in perivascular cells shows increased levels of VEGF-A. Concomitantly, these fetoplacental vessels also exhibit leakiness to large macromolecules (76 kDa), slightly greater than albumin, which are normally retained in the vascular compartment of perfused vessels. Immunoblot studies revealed that the loss of VE-cadherin and β-catenin was not due to the downregulation of junctional proteins but occurred as a consequence of phosphorylation and loss of these molecules from junctional regions (Leach et al. 2004).
In a study by Aiello et al. (1994) 69 of 136 samples of ocular fluid from patients with diabetic retinopathy contained detectable levels of VEGF and concentrations were higher in those patients with signs of retinal disease. In addition to the retina, VEGF and its receptors have been found to be increased in the renal glomeruli of STZ-induced diabetic rats (Cooper et al. 1999). The microvasculature of the cochlea in STZ-induced diabetic rats also shows a significant increase in VEGF (Liu et al. 2008). The reported increases in placental VEGF in the diabetic placenta may well be behind the junctional changes and increased vascular leakage (Babawale et al. 2000; Leach et al. 2004).
Increased angiogenesis of fetoplacental vessels is a feature of human placenta complicated by Type 1 diabetes (Teasdale, 1983; Mayhew, 2002; Leach et al. 2004; Jauniaux & Burton, 2006). Stereological data from Mayhew (2002) showed that there was increased angiogenesis even in well-controlled Type 1 diabetic placenta and that this occurred predominantly by means of longitudinal growth (Mayhew, 2002; Leach et al. 2004). VEGF-A levels are also increased in the Type 1 diabetic placenta, where VEGF is found in both the endothelium and trophoblast layer of terminal, intermediate and stem villi, as opposed to terminal villi only in normal pregnancies (Leach et al. 2004). Mechanistically, in-vitro angiogenesis of human placental and umbilical endothelial cells has been linked to both exogenous addition of VEGF and altered dynamics of VE-cadherin and β-catenin (Wright et al. 2002; Dye et al. 2004). Breakage of cell–cell junctions is a first step in angiogenesis.
Causes of vascular dysfunction in the diabetic placenta: hyperglycaemia
What in the diabetic milieu causes vascular dysfunction? Increased angiogenesis and the preponderance of leaky vessels remain unresolved. Maternal (and fetal) hyperglycaemia may well impact on placental vascular permeability. In diabetes per se the rise in blood glucose has several effects on the surrounding vasculature. Hyperglycaemia has been shown to have a direct effect, acting as a pro-constrictor (Boden et al. 2007; Singh et al. 2008), pro-coagulatory (Kwaan, 1992; Kario et al. 1995;Boden et al. 2007), pro-inflammatory (Sweet et al. 2009; Yang et al. 2009), pro-angiogenic (Ettelaie et al. 2008; Ejaz et al. 2008) and pro-permeability (Chiarelli et al. 2000; Sung et al. 2006) agent.
One of the molecular mechanisms affected by high glucose is the PKC pathway. High glucose causes de-novo synthesis of diacylglycerol in various tissues in the rat, which in turn causes an increase in the production of PKC (Craven et al. 1990; Inoguchi et al. 1992; Shiba et al. 1993; Derubertis & Craven, 1994). This leads to an increase in the release of several growth factors including VEGF (Kelly et al. 2007; Xia et al. 2007). The diabetic changes to nitric oxide (NO) production (Bohlen & Nase, 2001) and VEGF can be reversed by inhibiting the PKC pathway (Kelly et al. 2007; Xia et al. 2007).
The polyol pathway is also increased under hyperglycaemia leading to an overproduction of fructose and sorbitol (Gabbay, 1973). The latter two sugars create a pseudohypoxic environment, where there is an increase in the NADH : NAD+ ratio (Ido & Williamson, 1997; Ido et al. 2004). In this state, the endothelium increases its production of prostaglandin and decreases the availability of NO (Pugliese et al. 1991). NO is an inhibitor of VEGF production, so this leads to more VEGF being produced. Moreover, alteration in the NADH : NAD+ ratio also directly stimulates the PKC pathway, thereby producing a double-edged sword for the production of VEGF.
Nitric oxide and the PKC pathway also play a part in glucose-induced oxidative stress. Glucose auto-oxidates to form free radical hydroxilic anions (Thornalley et al. 1984; Dominguez et al. 1998), which overwhelm the antioxidant cellular responses in diabetes. This leads to the endothelium being more sensitive to free radicals and the latter react with NO to form peroxinitrates, one of the most damaging free radicals (Nishikawa et al. 2000; Vareniuk et al. 2008). Intense nitrotyrosine staining is a feature of the vascular endothelium and villous stroma of diabetic placentas (Lyall et al. 1998).
All of these changes can be seen under relatively short-duration hyperglycaemia. If, however, high glucose is sustained, the formation of advanced glycation products (AGEs) occurs (Brownlee et al. 1988). Formation of AGEs is greatly accelerated in diabetes mellitus and is directly proportional to the glycaemic status (Stitt et al. 1998). Binding of AGEs to their receptors can stimulate the production of nuclear factor kappa – light-chain-enhancer of activated beta cells (NFκB) and various cytokines (Yan et al. 1994), leading to inflammation (Ettelaie et al. 2008) and inhibition of the production of NO (Bucala et al. 1991; Chakravarthy et al. 1998). The human placenta is capable of responding to AGEs and the trophoblast of the first trimester placenta does contain receptors to AGEs (Konishi et al. 2004). AGE–glycated bovine serum albumin (BSA) has been shown to induce release of the pro-inflammatory cytokines and prostaglandins in human placental tissue explants (Lappas et al. 2007). Moreover, the serum levels of AGEs are high in pre-eclamptic women and two known AGEs, pentosidine and N-carboxymethyllysine protein, have been found in the pre-eclamptic placenta (Chekir et al. 2006). Their presence and importance in the diabetic placental vasculature require investigation.
Vascular endothelial growth factor and NO appear to be the main players in the pro-angiogenic and pro-permeability aspects of hyperglycaemia. In a normal endothelial cell, if VEGF is produced at a basal rate it increases the production of NO by endothelial constitutive NO synthase (NOS), and NO then feeds back to inhibit further VEGF production (Ziche et al. 1997; Parenti et al. 1998; Ghiso et al. 1999; Ferrara, 2002, 2004). However, in diabetes, this balance is lost. VEGF leads to an increase in permeability, whereas inducible NOS produces large quantities of NO, which also leads to increased permeability (Kroll & Waltenberger, 1998). Endothelial constitutive NOS and inducible NOS have been located in the placental microvascular endothelium, syncytiotrophoblast and trophoblast cells although there is controversy about whether the levels of these are elevated in pregnancies complicated by pre-eclampsia (see review by Escudero & Sobrevia, 2008). The authors suggest that the reduced availability of NO in pre-eclampsia may neutralize the pro-angiogenic effects of VEGF here. The converse may well be true for the diabetic placenta.
The alteration of these mechanisms in hyperglycaemia is a major contributor to the diabetic phenotype and, in tissues that are insulin-independent for glucose uptake (such as the eye, kidney and nervous tissues), high glucose causes a range of vascular pathologies. In the eye, hyperglycaemia can lead to retinopathy (Ido & Williamson, 1997; Chakravarthy et al. 1998; Ido et al. 2004), where excessive angiogenesis and permeability of newly formed vessels cause visual problems. In the kidney, diabetic patients might experience nephropathy (Derubertis & Craven, 1994; Chiarelli et al. 2000; Kelly et al. 2007; Xia et al. 2007), where the kidneys become leaky to blood proteins. In a skin chamber preparation (in Sprague-Dawley rats) glucose has been shown to increase albumin permeation and blood flow, which could be blocked by anti-VEGF antibodies (Tilton et al. 1997). The study of the direct effects of high glucose on the perfused placental vessels is underway in our laboratory, preliminary studies show an increase in VEGF and increased albumin permeation after a 4 h hyperglycaemic insult.
All of these glucose-mediated changes may have an effect on the fetus. A recent study by Ericsson et al. (2007) showed that, in rat placenta, the most vulnerable time for the fetus is in the first trimester, when just three injections of high glucose in early pregnancy caused an increase in fetal weight at term. However, despite the important role of hyperglycaemia in the diabetic placenta, high glucose is only one of a range of complications in diabetes. In our experience (Babawale et al. 2000; Leach et al. 2004) and as stated by Jansson et al. (2006), fetal macrosomia, which is the main consequence of the pathological changes seen in the diabetic placenta, cannot be explained fully by maternal hyperglycaemia, as diabetic patients with optimal glucose control still present with macrosomic neonates. Hyperglycaemia in the diabetic placenta must be seen as a player in a range of diabetic complications.
Causes of vascular dysfunction in the diabetic placenta: hyperinsulinaemia
Increasingly, fetal hyperinsulinaemia is being entertained as a factor behind the observed placental vascular dysfunction. In the second and third trimesters of pregnancy, when the fetal pancreas starts insulin secretion and when expansion of the villous trees is mostly based on longitudinal growth and coiling of fetal capillaries, insulin receptor expression switches to the luminal surfaces of fetal capillaries (Desoye et al. 1994; Hiden et al. 2009, see review in this issue). This suggests that, from this period onwards, fetal insulin can be involved in the control of placental angiogenesis and vascular permeability. Poor glycaemic control in the mother results in periods of hyperglycaemia, to which the fetus is subsequently exposed due to the transplacental flux of glucose down the concentration gradient, into the fetal circulation. This results in fetal hyperglycaemia. According to Pedersen's hypothesis, this hyperglycaemia results in an increased insulin secretion from the fetal pancreas, in order to achieve normoglycaemia (see review by Nold & Georgieff, 2004). Pancreatic β-cell hypertrophy occurs in the fetus in order to meet this demand for increased insulin secretion. Although the subsequent high levels of insulin may succeed in correcting the fetal hyperglycaemia, the increased uptake of glucose into the fetal cells results in an increase in adipose tissue synthesis and macrosomia (Jansson et al. 2006). The concept of fetal hyperinsulinaemia in infants of diabetic mothers is supported by research showing that such infants have higher cord C-peptide and therefore insulin levels, despite normoglycaemia, than infants of non-diabetic mothers (Sosenko et al. 1979). This high circulating level of insulin has direct access to both the fetal and placental vascular endothelium.
There is evidence demonstrating that insulin enhances VEGF protein expression and secretion. Clinical trials have demonstrated that acute intensive insulin therapy exacerbates diabetic blood–retinal barrier breakdown via activation of the hypoxia inducible factor-1alpha (HIF-1α)/VEGF pathway and is accountable for blindness (Poulaki et al. 2002). Intensive insulin therapy increases retinal HIF-1α levels and promotes HIF-1α translocation into the nucleus, which in turn increases VEGF mRNA expression and protein synthesis via the PI3-kinase and mitogen activated protein kinase (MAPK) pathways. HIF-1α is normally induced by hypoxia; however, activation of PI3-kinase/Akt can upregulate the expression of HIF-1α, which in turn increases VEGF expression through a direct interaction with the VEGF promoter (Poulaki et al. 2002). Insulin may also act to vasodilate vessels by a receptor-dependent mechanism that stimulates endothelial constitutive NOS and thus produces NO (Kuboki et al. 2000). Although a relationship between a local increase in intraocular VEGF and retinopathy progression in diabetes has been proposed, systemic levels provide a different perspective. A study by Lee et al. (2006) showed that, although there was an increase in systemic VEGF plasma levels in Type 2 diabetes, this did not correlate with the severity of retinopathy. This dissociation had also been reported in Type 1 diabetics (Chaturvedi et al. 2001). Both studies suggest that circulating VEGF levels are not an adequate marker of the progression of diabetic microvascular disease and local increases in VEGF may have a more profound effect.
Whether insulin can upregulate VEGF in the human placenta requires direct investigation and is the focus of study in our laboratory. Preliminary findings show that perfusion of the fetal circulation of term placentae with high insulin results in increased endothelial VEGF, junctional disruption and increased vascular leaks (Lucas et al. 2008). Given that maternal hyperglycaemia induces fetal hyperglycaemia and hyperinsulinaemia, the latter may play a key role in inducing vascular dysfunction in the fetal vessels of the human placenta in utero. Hyperinsulinaemia in the developing fetus may also directly affect the vascular system therein. Studies into fetal regulation of the placental vasculature may also shed light on the puzzle as to why vascular dysfunction is a feature of placenta from well-controlled diabetic pregnancies.
Concluding remarks
This review highlights the possible cellular/molecular mechanisms by which hyperglycaemia and hyperinsulinaemia may alter vascular permeability and angiogenesis in the diabetic placenta. It also reveals the paucity of mechanistic information regarding the effects of diabetes on the growth, development and functioning of the human placenta. Given the acceptance of the consequences of maternal diabetes on the growing fetus and also on later vascular diseases in adulthood, this area of placental research needs to be prioritized.
Acknowledgments
We wish to thank the Wellcome Trust and the Anatomical Society of Great Britain and Ireland for funding the research from the laboratory of L.L. reported in this review.
References
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Khin SE, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content. Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- Babawale MO, Lovat S, Mayhew TM, Lammiman MJ, James DK, Leach L. Effects of gestational diabetes on junctional adhesion molecules in human term placenta vasculature. Diabetologia. 2000;43:1185–1196. doi: 10.1007/s001250051511. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2003;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Ben-Ze’ev A, Geiger B. Differential molecular interactions of β-catenin and plakoglobin in adhesion, signalling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- Boden G, Vaidyula VR, Homko C, Cheung P, Rao AK. Circulating tissue factor procoagulant activity and thrombin generation in patients with type 2 diabetes: effects of insulin and glucose. J Clin Endocrinol Metab. 2007;92:4352–4358. doi: 10.1210/jc.2007-0933. [DOI] [PubMed] [Google Scholar]
- Bohlen HG, Nase GP. Arteriolar nitric oxide concentration is decreased during hyperglycemia-induced betaII PKC activation. Am J Physiol Heart Circ Physiol. 2001;280:H621–627. doi: 10.1152/ajpheart.2001.280.2.H621. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Bucala R, Tracey KJ, Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caveda L, Martin-Padura I, Navarro P, et al. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin) J Clin Invest. 1996;98:886–893. doi: 10.1172/JCI118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Hayes RG, Stitt AW, McAuley E, Archer DB. Constitutive nitric oxide synthase expression in retinal vascular endothelial cells is suppressed by high glucose and advanced glycation end products. Diabetes. 1998;47:945–952. doi: 10.2337/diabetes.47.6.945. [DOI] [PubMed] [Google Scholar]
- Chaturvedi N, Fuller JH, Pokras F, et al. Circulating plasma vascular endothelial growth factor and microvascular complications of Type 1 diabetes mellitus: the influence of ACE inhibition. Diabet Med. 2001;18:288–294. doi: 10.1046/j.1464-5491.2001.00441.x. [DOI] [PubMed] [Google Scholar]
- Chekir C, Nakatsuka M, Noguchi S, et al. Accumulation of advanced glycation end products in women with preeclampsia: possible involvement of placental oxidative and nitrative stress. Placenta. 2006;27:225–233. doi: 10.1016/j.placenta.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Chiarelli F, Cipollone F, Romano F, et al. Increased circulating nitric oxide in young patients with type 1 diabetes and persistent microalbuminuria: relation to glomerular hyperfiltration. Diabetes. 2000;49:1258–1263. doi: 10.2337/diabetes.49.7.1258. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Carbajal JM, Schaeffer RC. VEGF stimulates tyrosine phosphorylation of β-catenin and small-pore endothelial barrier dysfunction. Am J Physiol. 1999;277:H2038–2049. doi: 10.1152/ajpheart.1999.277.5.H2038. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben-Ze’en A. The cadherin-catenin adhesion system in signalling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ME, Vranes D, Youssef S, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- Craven PA, Davidson CM, DeRubertis FR. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990;39:667–674. doi: 10.2337/diab.39.6.667. [DOI] [PubMed] [Google Scholar]
- Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27:535–539. doi: 10.1016/j.placenta.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Derubertis FR, Craven PA. Activation of protein kinase C in glomerular cells in diabetes. Mechanisms and potential links to the pathogenesis of diabetic glomerulopathy. Diabetes. 1994;43:1–8. doi: 10.2337/diab.43.1.1. [DOI] [PubMed] [Google Scholar]
- Desoye G, Hartmann M, Blaschitz A, et al. Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry. 1994;101:277–285. doi: 10.1007/BF00315915. [DOI] [PubMed] [Google Scholar]
- Dominguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care. 1998;21:1736–1742. doi: 10.2337/diacare.21.10.1736. [DOI] [PubMed] [Google Scholar]
- Dye JF, Leach L, Clark P, Firth JA. Cyclic AMP and acidic fibroblast growth factor have opposing effects on tight and adherens junctions in microvascular endothelial cells in vitro. Microvasc Res. 2001;62:94–113. doi: 10.1006/mvre.2001.2333. [DOI] [PubMed] [Google Scholar]
- Dye JF, Laurence L, Leach L, Linge LC, Firth JA, Clark P. Distinct patterns of in vitro angiogenic behaviour of human microvascular endothelial cells are determined by extracellular matrices. Endothelium. 2004;11:151–167. doi: 10.1080/10623320490512093. [DOI] [PubMed] [Google Scholar]
- Ejaz S, Chekarova I, Ejaz A, Sohail A, Lim CW. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10:53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Säljö K, Sjöstrand E, et al. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007;581:1323–1332. doi: 10.1113/jphysiol.2007.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero C, Sobrevia L. A hypothesis for preeclampsia: Adenosine and inducible nitric oxide synthase in human placental microvascular endothelium. Placenta. 2008;29:469–483. doi: 10.1016/j.placenta.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Ettelaie C, Su S, Li C, Collier ME. Tissue factor-containing microparticles released from mesangial cells in response to high glucose and AGE induce tube formation in microvascular cells. Microvasc Res. 2008;76:152–160. doi: 10.1016/j.mvr.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay KH. The sorbitol pathway and the complications of diabetes. N Engl J Med. 1973;288:831–836. doi: 10.1056/NEJM197304192881609. [DOI] [PubMed] [Google Scholar]
- Ghiso N, Rohan RM, Amano S, Garland R, Adamis AP. Suppression of hypoxia-associated vascular endothelial growth factor gene expression by nitric oxide via cGMP. Invest Ophthalmol Vis Sci. 1999;40:1033–1039. [PubMed] [Google Scholar]
- Giebel SJ, Menicucci G, McGuire PG, Das A. Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood–retinal barrier. Lab Invest. 2005;85:597–607. doi: 10.1038/labinvest.3700251. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- Hiden U, Glitzner E, Hartmann M, Desoye G. Insulin and the IGF system in the human placenta of normal and diabetic pregnancies. J Anat. 2009 doi: 10.1111/j.1469-7580.2008.01035.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido Y, Williamson JR. Hyperglycemic cytosolic reductive stress ‘pseudohypoxia’: implications for diabetic retinopathy. Invest Ophthalmol Vis Sci. 1997;38:1467–1470. [PubMed] [Google Scholar]
- Ido Y, Chang K, Williamson JR. NADH augments blood flow in physiologically activated retina and visual cortex. Proc Natl Acad Sci USA. 2004;101:653–658. doi: 10.1073/pnas.0307458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson T, Cetin I, Powell TL, et al. Placental transport and metabolism in fetal overgrowth – a workshop report. Placenta. 2006;27:S109–113. doi: 10.1016/j.placenta.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Burton GJ. Villous histomorphometry and placental bed biopsy investigation in Type I diabetic pregnancies. Placenta. 2006;27:468–474. doi: 10.1016/j.placenta.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kario K, Matsuo T, Kobayashi H, Matsuo M, Sakata T, Miyata T. Activation of tissue factor-induced coagulation and endothelial cell dysfunction in non-insulin-dependent diabetic patients with microalbuminuria. Arterioscler Thromb Vasc Biol. 1995;15:1114–1120. doi: 10.1161/01.atv.15.8.1114. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Buck D, Cox AJ, Zhang Y, Gilbert RE. Effects on protein kinase C-beta inhibition on glomerular vascular endothelial growth factor expression and endothelial cells in advanced experimental diabetic nephropathy. Am J Physiol Renal Physiol. 2007;293:F565–574. doi: 10.1152/ajprenal.00397.2006. [DOI] [PubMed] [Google Scholar]
- Konishi H, Nakatsuka M, Chekir C, et al. Advanced glycation end products induce secretion of chemokines and apoptosis in human first trimester trophoblasts. Hum Reprod. 2004;19:2156–2162. doi: 10.1093/humrep/deh389. [DOI] [PubMed] [Google Scholar]
- Kuboki K, Jiang ZY, Takahara N, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo. A specific vascular action of insulin. Circulation. 2000;101:676–681. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- Kwaan HC. Changes in blood coagulation, platelet function, and plasminogen-plasmin system in diabetes. Diabetes. 1992;41:32–35. doi: 10.2337/diab.41.2.s32. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, et al. A novel-endothelial specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Rice GE. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-κB and extracellular signal-regulated kinase 1/2. J Endocrinol. 2007;193:269–277. doi: 10.1677/JOE-06-0081. [DOI] [PubMed] [Google Scholar]
- Leach L, Firth JA. Fine structure of the paracellular junctions of terminal villous capillaries in the perfused human placenta. Cell Tissue Res. 1992;268:447–452. doi: 10.1007/BF00319151. [DOI] [PubMed] [Google Scholar]
- Leach L, Eaton BM, Westcott EDA, Firth JA. The effects of histamine on endothelial permeability, structure and adhesion molecules of the paracellular junctional complexes of perfused term human placental microvessels. Microvasc Res. 1995;50:323–337. doi: 10.1006/mvre.1995.1062. [DOI] [PubMed] [Google Scholar]
- Leach L, Lammiman MJ, Babawale MO, et al. Molecular organisation of tight and adherens junctions in the human placental vascular tree. Placenta. 2000;21:547–557. doi: 10.1053/plac.2000.0541. [DOI] [PubMed] [Google Scholar]
- Leach L, Babawale MO, Anderson M, Lammiman M. Vasculogenesis, angiogenesis and the molecular organisation of endothelial junctions in the early human placenta. J Vasc Res. 2002;39:246–259. doi: 10.1159/000063690. [DOI] [PubMed] [Google Scholar]
- Leach L, Gray C, Staton S, et al. Vascular endothelial cadherin and β-catenin in human fetoplacental vessels of pregnancies complicated by Type 1 diabetes: associations with angiogenesis and perturbed barrier function. Diabetologia. 2004;47:695–709. doi: 10.1007/s00125-004-1341-7. [DOI] [PubMed] [Google Scholar]
- Lee IG, Chae SL, Kim JC. Involvement of circulating endothelial progenitor cells and vasculogenic factors in the pathogenesis of diabetic retinopathy. Eye. 2006;20:546–552. doi: 10.1038/sj.eye.6701920. [DOI] [PubMed] [Google Scholar]
- Liu F, Xia M, Xu A. Expression of VEGF, iNOS, and eNOS is increased in cochlea of diabetic rat. Acta Otolaryngol. 2008;128:1178–1186. doi: 10.1080/00016480801901774. [DOI] [PubMed] [Google Scholar]
- Lucas J, Thomas R, Ikram A, Leach L. The effect of fetal hyperinsulemia on human placental vascular function: Perfusion of fetal microvascular bed results in increased vascular leakage and loss of junctional beta-catenin. Microcirculation. 2008;15:672–673. [Google Scholar]
- Lyall F, Gibson JL, Greer IA, Brockman DE, Eis AL, Myatt L. Increased nitrotyrosine in the diabetic placenta: evidence for oxidative stress. Diabetes Care. 1998;21:1753–1758. doi: 10.2337/diacare.21.10.1753. [DOI] [PubMed] [Google Scholar]
- Mayhew TM. Enhanced fetoplacental angiogenesis in pre-gestational diabetes mellitus: the extra growth is exclusively longitudinal and not accompanied by microvascular remodelling. Diabetologia. 2002;45:1434–1439. doi: 10.1007/s00125-002-0927-1. [DOI] [PubMed] [Google Scholar]
- Navaratna D, McGuire PG, Menicucci G, Das A. Proteolytic degradation of VE-cadherin alters the blood-retinal barrier in diabetes. Diabetes. 2007;56:2380–2387. doi: 10.2337/db06-1694. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Nold JL, Georgieff MK. Infants of diabetic mothers. Pediatr Clin North Am. 2004;51:619–637. doi: 10.1016/j.pcl.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Cui XL, et al. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- Poulaki V, Qin WY, Joussen AM, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-l alpha and VEGF. J Clin Invest. 2002;109:805–815. doi: 10.1172/JCI13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese G, Tilton RG, Williamson JR. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabetes Metab Rev. 1991;7:35–59. doi: 10.1002/dmr.5610070106. [DOI] [PubMed] [Google Scholar]
- Rutland CS, Latunde-Dada AO, Thorpe A, Plant R, Langley-Evans S, Leach L. Effect of gestational nutrition on vascular integrity in the murine placenta. Placenta. 2007;28:734–742. doi: 10.1016/j.placenta.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, King GL. Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol. 1993;265:E783–793. doi: 10.1152/ajpendo.1993.265.5.E783. [DOI] [PubMed] [Google Scholar]
- Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosenko IR, Kitzmiller JL, Loo SW, Blix P, Rubenstein AH, Gabbay KH. The infant of the diabetic mother: correlation of increased cord C-peptide levels with macrosomia and hypoglycemia. N Engl J Med. 1979;301:859–862. doi: 10.1056/NEJM197910183011603. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt AW, Moore JE, Sharkey JA, et al. Advanced glycation end products in vitreous: Structural and functional implications for diabetic vitreopathy. Invest Ophthalmol Vis Sci. 1998;39:2517–2523. [PubMed] [Google Scholar]
- Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17:3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- Sweet IR, Gilbert M, Maloney E, Hockenbery DM, Schwartz MW, Kim F. Endothelial inflammation induced by excess glucose is associated with cytosolic glucose 6-phosphate but not increased mitochondrial respiration. Diabetologia. 2009;52:921–931. doi: 10.1007/s00125-009-1272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale F. Histomorphometry of the human placenta in class B diabetes mellitus. Placenta. 1983;4:1–12. doi: 10.1016/s0143-4004(83)80012-5. [DOI] [PubMed] [Google Scholar]
- Thornalley P, Wolff S, Crabbe J, Stern A. The autoxidation of glyceraldehyde and other simple monosaccharides under physiological conditions catalysed by buffer ions. Biochim Biophys Acta. 1984;797:276–287. doi: 10.1016/0304-4165(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Tilton RG, Kawamura T, Chang KC, et al. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest. 1997;99:2192–2202. doi: 10.1172/JCI119392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Vareniuk I, Pavlov IA, Obrosova IG. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia. 2008;51:2126–2133. doi: 10.1007/s00125-008-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D. VE-cadherin. The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Throm Vasc Biol. 2008;28:223. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Leach L, Jones P. Dynamics of vascular endothelial-cadherin and β-catenin localisation by vascular endothelial growth factor-induced angiogenesis in human umbilical vein cells. Exp Cell Res. 2002;280:159–168. doi: 10.1006/excr.2002.5636. [DOI] [PubMed] [Google Scholar]
- Xia L, Wang H, Munk S, et al. Reactive oxygen species, PKC-beta1, and PKC-zeta mediate high-glucose-induced vascular endothelial growth factor expression in mesangial cells. Am J Physiol Endocrinol Metab. 2007;293:E1280–1288. doi: 10.1152/ajpendo.00223.2007. [DOI] [PubMed] [Google Scholar]
- Yan SD, Schmidt AM, Anderson GM, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- Yang Z, Laubach VE, French BA, Kron IL. Acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction by activating nicotinamide adenine dinucleotide phosphate oxidase during reperfusion. J Thorac Cardiovasc Surg. 2009;137:723–729. doi: 10.1016/j.jtcvs.2008.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–2634. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]