Abstract
The signal loss susceptibility artifact is a major limitation in gradient-echo MRI applications. Various methods, including z-shim techniques and multidimensional tailored radio frequency (RF) pulses, have been proposed to mitigate the through-plane signal loss artifact, which is dominant in axial slices above the sinus region. Unfortunately, z-shim techniques require multiple steps and multidimensional RF methods are complex, with long pulse lengths. Parallel transmission methods were recently shown to be promising for improving B1 inhomogeneity and reducing the specific absorption rate. In this work, a novel method using time-shifted slice-select RF pulses is presented for reducing the through-plane signal loss artifact in parallel transmission applications. A simultaneous z-shim is obtained by concurrently applying unique time-shifted pulses on each transmitter. The method is shown to reduce the signal loss susceptibility artifact in gradient-echo images using a four-channel parallel transmission system at 3T.
Keywords: parallel transmission, susceptibility artifacts, z-shim, functional MRI, brain imaging
Susceptibility artifacts are a major limitation in gradient-echo MRI, especially at long echo times (TEs) and high field. For example, functional MRI (fMRI) using blood oxygen level-dependent (BOLD) contrast suffers from susceptibility artifacts (1,2). This is a result of the long TE values required for the -weighted BOLD contrast and the close proximity of air/tissue boundaries to the inferior brain areas. The fMRI data in many important brain regions such as the orbital frontal cortex remain suboptimal as a result. Therefore the development of methods for reducing signal loss artifact in gradient-echo applications is of importance.
The signal loss susceptibility artifact appears as large voids in the images. The through-plane component to the signal loss is particularly problematic in axial brain images due to the presence of large air cavities inferior to the lower slices. Numerous methods have been proposed to mitigate the signal loss artifact, including z-shim methods (3–5), thin slice averaging (6), passive and active shim coils (7,8), and tailored radio frequency (RF) pulses (9–12). All techniques have advantages and disadvantages. For example, z-shim techniques are very simple to implement but require multiple gradient steps to achieve an adequate correction. The gradient steps can be performed as separate scans or in a single shot using multiecho sequences that allow for each echo to have a unique z-shim (13–16). The drawback of the z-shim approach is increased imaging time due to the extra scans or the extended data readouts. Tailored RF pulse methods can also produce a single-shot implementation; however, the pulses are impractically long and require complex design algorithms. It is ultimately desirable to have a signal loss correction method that is easy to implement and incurs no time penalty.
In this work we propose a novel method using standard slice-select RF pulses to achieve signal loss artifact reduction with parallel transmission (17,18). Parallel transmission has been shown to be promising for reducing transmitter B1 inhomogeneity and managing the specific absorption rate (SAR) (19–21). The z-shim is created by uniquely time shifting the RF pulse played out on each transmitter channel. The simultaneous summation of several z-shims results from the parallel excitation process. We demonstrate that the technique successfully reduces much of the through-plane signal loss artifact in gradient-echo images using a four-channel parallel transmission system at 3T.
THEORY
Magnetic susceptibility effects can be modeled using a susceptibility gradient term. If the susceptibility gradient is primarily in the through-plane direction, as is the case for axial slices superior to the air cavities in the brain, the image I(x,y) generated with a slice-select RF pulse at TE can be written as
| [1] |
Here is the spatially varying through-plane susceptibility gradient and p(z) is the slice profile. Phase cancellation from integrating across the slice will produce signal loss in I(x,y).
The standard z-shim method reduces the signal loss artifact by applying an additional compensation gradient along the slice-select direction. If , where T is the length of , the dephasing at (x0,y0) produced by the susceptibility gradient at TE will be canceled. Due to the spatial variation of , the compensation gradient has to be incremented using multiple steps such that a series of images are acquired with varying degree of z-shim. The final corrected image is a combination of all of the individual shimmed images. The gradient steps can be incremented using separate excitations or with multiple echoes. Both approaches lead to increased scan times.
A compensation gradient can also be created by time shifting the RF pulse relative to the slice-select gradient. Following the excitation k-space formalism (22), in the presence of a slice-select gradient Gz a shift in time Δt applied to the RF waveform B1(t) creates a phase term:
| [2] |
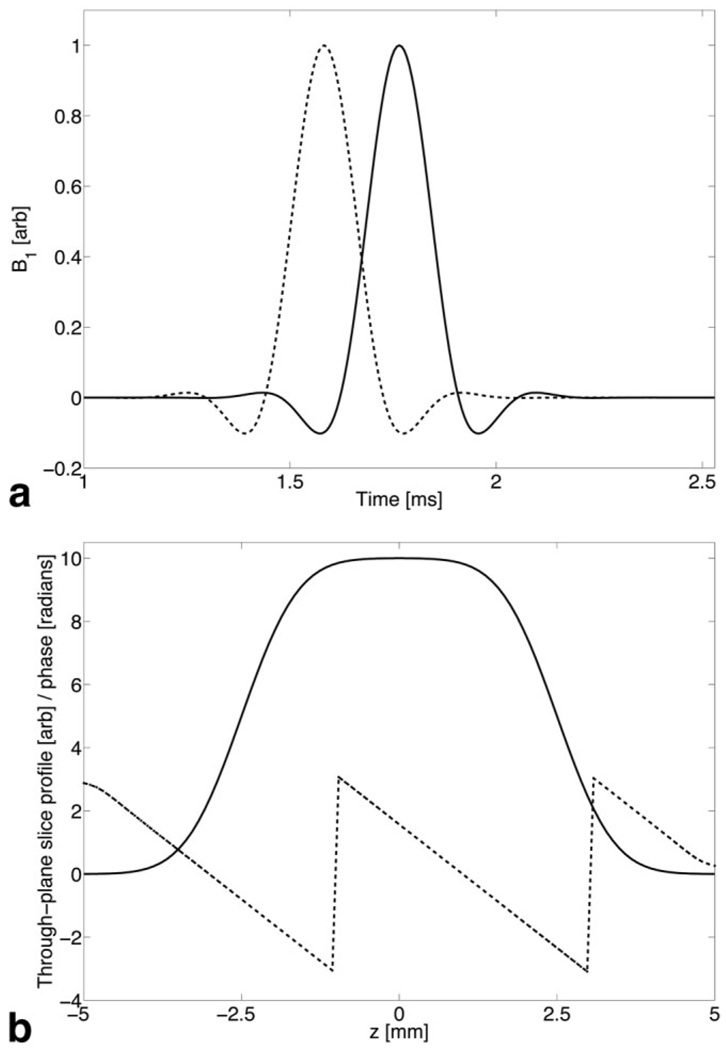
For example, Fig. 1a shows plots of a centered RF wave-form and the same pulse shifted 300 µs back in time. This pulse produces a Δz = 5-mm-thick slice with a Gz = 20 mT/m slice-select gradient. The through-plane phase produced by this pulse is γGzΔzΔt = 8 radians. Figure 1b shows the simulated slice profile and through-plane phase of the shifted pulse. Theoretically, one could duplicate the standard gradient-compensated z-shim method by applying several scans using RF pulses with a range of time shifts. However, this would incur an increase in scan time to obtain a combination of z-shims.
Fig. 1.
a: A centered slice-select RF pulse (solid line) and the same pulse shifted 300 µs back in time (dashed line). b: The slice profile (solid line) and through-plane phase (dashed) created by the shifted RF pulse. The slice-select gradient (not shown) had amplitude 20 mT/m.
We propose a simultaneous z-shim method for multiple transmitter applications. Using this approach, each of j = 1,…,N transmitters applies a pulse with a unique Δtj. The summation of z-shims occurs automatically with the parallel transmission. Assuming linearity, the resultant image can be written by
| [3] |
where sj(x,y) is the transmitter sensitivity. The efficacy of the technique will in part be a function of the locations of the susceptibility artifacts, coil positions, overlap in sensitivities, and pulse superposition effects.
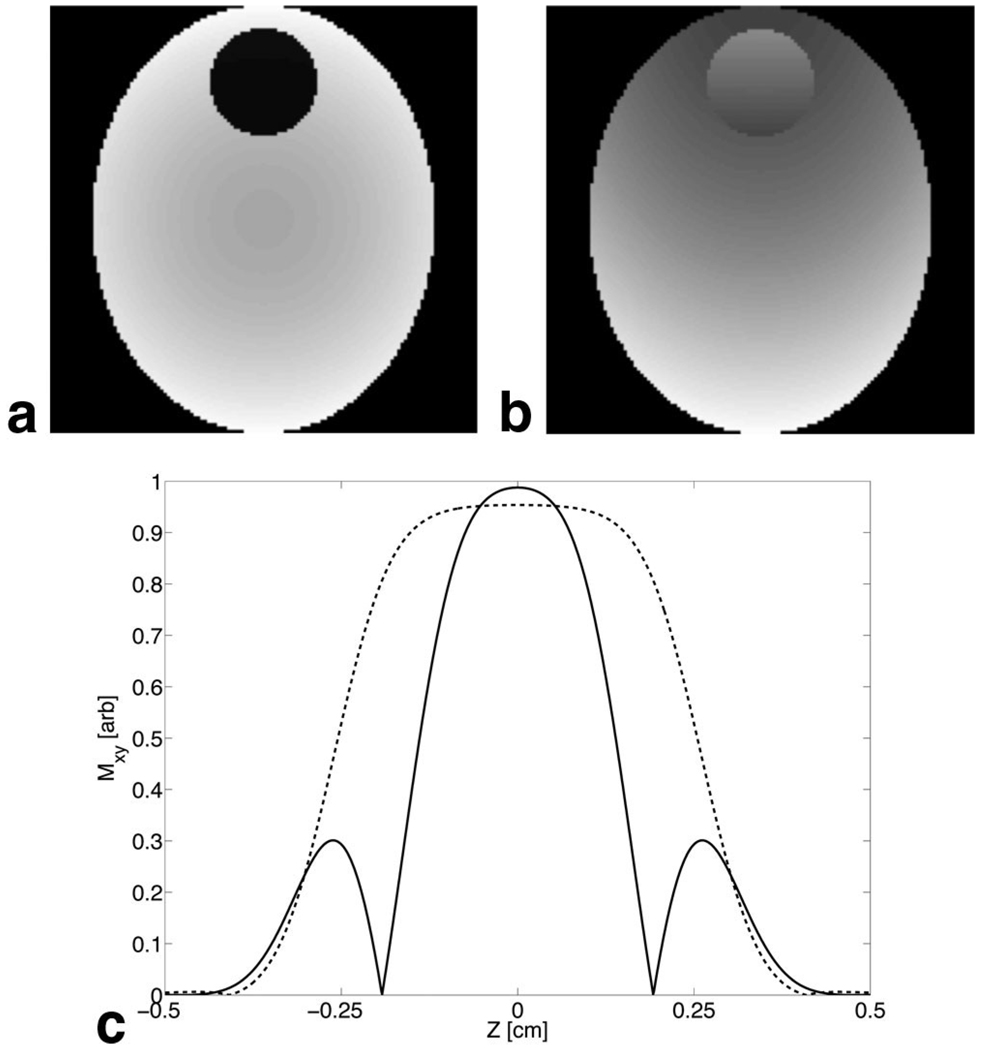
As a simple demonstration, a Bloch equation simulation of an oval-shaped object I(x,y) with a circular region of through-plane susceptibility gradient is shown in Fig. 2. Four transmitters with sensitivities that varied linearly from 1 to 0, successively rotated 90°, were used. Figure 2a shows I(x,y) excited with unshifted pulses on all four transmitters. Note the signal loss artifact in the circular region. Figure 2b shows the same slice excited by four transmitters with the pulse on the top transmitter time shifted to cancel the through-plane gradient. The circular region now has recovered magnitude; however, there is reduced magnitude in the surrounding areas. This is due to the fall-off in sensitivity from the other transmitters as well from a degradation of the slice profile when shifted and unshifted pulses are superimposed. Figure 2c shows a simulated slice profile from overlapping pulses with different time shifts. Note the zeros in the profile due to phase cancellation, as well as the narrower profile. In the limit of uniform sensitivities, all pixels will have similar slice profile issues as well as increased SAR.
Fig. 2.
a: Bloch equation simulation of an image excited by four transmitters (one on each side) using unshifted RF pulses. A through-plane gradient added to a circular region produces a void in the image. b: The same image excited by four transmitters with the RF on the top transmitter shifted. Note the recovered magnitude in the circular region. c: The solid line shows the simulated slice profile from region of equal overlap between unshifted and shifted (300 µs) pulses. The dashed line shows the slice profile from one pulse.
MATERIALS AND METHODS
Imaging experiments were performed on a TIM Trio 3T whole-body scanner (Siemens Medical Systems, Erlangen, Germany) with 150 mT/m/s peak slew rate and 40 mT/m gradient amplitude. A standard 2D fast low-angle shot (FLASH) sequence for spoiled gradient-echo imaging was used for the experiments (40/1000 ms TE/TR, 70° flip angle, six 5-mm-thick slices, 1-mm slice gap, 22-cm FOV, 128 × 128 matrix size, 2:08 scan time). Parallel transmission was achieved using a custom RF transmission system separate from the 3T scanner. The system consisted of a four-transmitter Tecmag Apollo NMR console (Tecmag Inc., Houston, TX, USA) with four 300-W amplifiers and a customized four-channel transmit-receive head coil (23). The head coil was mounted on a cylinder approximately 30 cm long and 30 cm in diameter, and consisted of four 8-cm × 17-cm loops. The Tecmag console was capable of transmitting four unique phase- and frequency-synchronized complex RF waveforms that could be programmed using the Tecmag Windows NT Nuclear Magnetic Resonance (NTNMR) pulse sequence software and triggered by the Siemens scanner. The Siemens console was used to generate all gradients, acquire data, and reconstruct images using the FLASH sequence and related software. Both consoles were synchronized using the Siemens scanner’s 10 MHz clock. Figure 3 shows a diagram of the pulse sequence and Fig. 4 shows pictures of the head coil and the four-transmitter Tecmag console.
Fig. 3.
Diagram of the FLASH sequence with four transmitters. The four pulses were applied simultaneously using the Tecmag console. The Siemens console generated the gradients and received the data.
Fig. 4.
Custom-built parallel transmission system. The system consisted of a four-channel RF transmitter/receiver coil (left) and a four-transmitter Tecmag Apollo NMR console including four RF amplifiers (right).
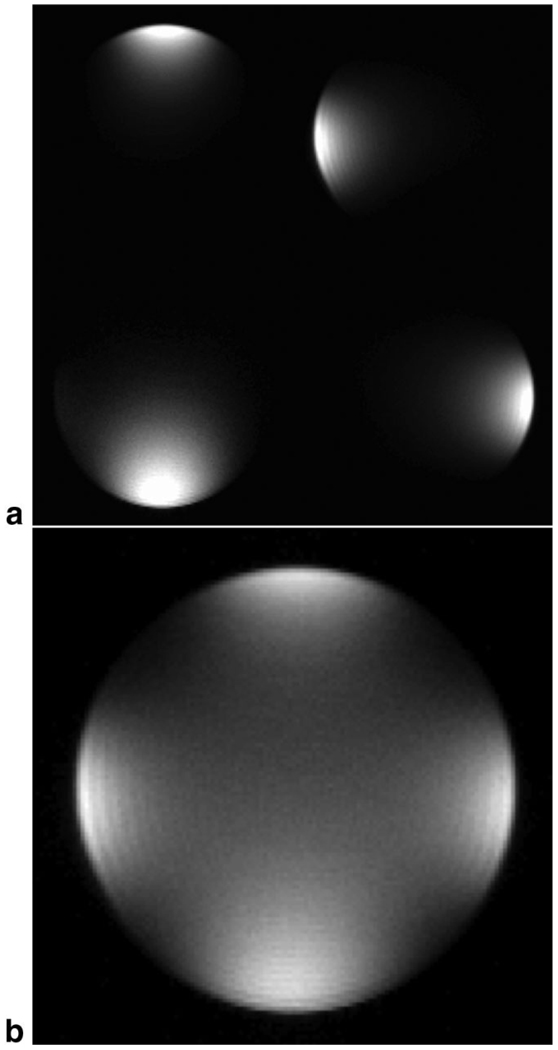
The RF waveform was a Hamming-filtered sinc sampled to 256 points with a sampling time of 10 µs (Fig. 1a). The slice-select gradient had Gz = 20 mT/m, producing a 5-mm slice. The RF waveform was loaded into the Tecmag console and each channel had successive phase increments of 90° to ensure a circularly polarized excitation. The prescan routine was run using the Siemens scanner alone with an identical RF pulse played out on its single transmitter attached to one of the coils. The prescan information, including flip angle (a 70° flip angle corresponded to a coil voltage of approximately 70 V), center frequency, and slice offsets, was passed from the Siemens scanner to the Tecmag NTNMR sequence using a customized Visual Basic program. Figure 5 shows images of a standard MRI phantom from the individual transmitters and the combined excitation from all four channels. The SAR was monitored in separate experiments using the Siemens scanner running the FLASH sequence with its single RF transmitter attached to one of the coils. It was found that the SAR was always well below the safety threshold. The SAR was not expected to be worse during parallel transmission due to the low overlap of coil sensitivities.
Fig. 5.
a: Phantom images from each of the four transmitter/receiver channels alone. b: Phantom image using all four channels simultaneously.
Proof-of-concept data were obtained from five healthy human subjects who were scanned after they provided informed consent as approved by the University of Hawaii and Queens Medical Center Joint Institutional Review Board (IRB). The general protocol was to first apply a three-plane localizer to align the six gradient-echo axial slices above the sinus region. Two sets of images were then acquired with the RF on all four transmitters shifted by Δt = 0 for the first set of images and Δt = −300 µs for the second set. In this manner, the uncorrected images as well as images of the dephased regions could be seen. The knowledge that Δt = −300 µs was adequate to refocus the corrupted regions with these sequence parameters was obtained from prior experiments. The two sets of images were then visually inspected to determine which transmitters for a given slice needed RF shifts. A third set of images was then acquired with RF from each coil having uniquely determined shifts. Typically, the RF from the transmitter closest to the corrupted region was shifted by Δt = −300 µs and the others were shifted by Δt = 0.
RESULTS
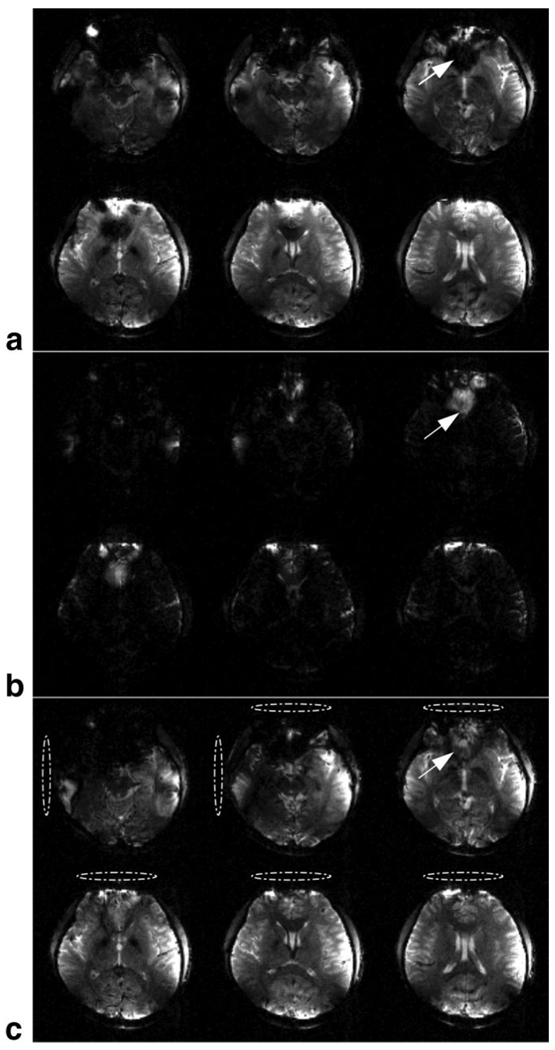
Figure 6 shows the three sets of axial images in one of the volunteers. All of the images are displayed at the same window and level. Figure 6a shows the images when Δt = 0 was applied to the RF on all four channels. These are standard gradient-echo images with several noticeable regions of signal loss artifact, such as above the sinus and auditory canals. The white arrow shows an example of the signal loss artifact above the sinus. Figure 6b shows the same set of images acquired with Δt = −300 µs for the RF on all four transmitters. Only the regions dephased by the through-plane susceptibility gradient can be seen. Figure 6c shows the same set of images acquired with RF on one or two coils (indicated by dashed ellipses) set with Δt = −300 µs and the remaining channels with Δt = 0 (coils not shown). The recovery of missing image magnitude can be clearly seen in all slices compared to Fig. 6a. The image magnitude was measured in the region of signal loss indicated by the arrow in Fig. 6a and c. The average value of the image magnitude in the region in Fig. 6c was 2.42 greater than that in Fig. 6a. The through-plane gradient created by the susceptibility variation in this region was estimated as 0.15 mT/m using . Similar results were found in all subjects.
Fig. 6.
Gradient-echo images acquired in the brain of one of the human subjects. a: Slices acquired with Δt = 0 for the RF on all four transmitters. Note the signal loss artifacts in the slices (the white arrow shows an example). b: The same slices acquired with Δt = −300 µs for the RF on all four transmitters. Images of the dephased regions not seen in part a can now be seen. c: The same slices acquired with Δt = −300 µs for the RF on the transmitters indicated by the dashed ellipses. The remaining transmitters (not shown) had the RF set with no shift.
DISCUSSION AND CONCLUSIONS
Here we present a novel method that uses time-shifted slice-select pulses to create a simultaneous z-shim for parallel transmission applications. The method was successful at reducing much of the signal loss artifact in gradient-echo images at 3T. The method is as easy to implement as the gradient-compensated z-shim approach and does not suffer a time penalty. One could easily combine the proposed technique with multiecho z-shim methods to obtain a larger number of shims. This technique is also computationally less demanding than multidimensional RF methods for reducing signal loss artifact. This is because the intrinsic susceptibility gradients and coil sensitivities perform the in-plane spatial localization, allowing for 1D pulses.
We found that only two z-shims were needed for a proof of concept; however, optimization of the shifts, RF amplitudes, and transmitter sensitivity effects is still needed. There was a noticeable magnitude loss in some of the images, possibly the result of low sensitivity from the other transmitters and pulse superposition effects. For example, the first and second slices in Fig. 6c show reduced image magnitude surrounding the corrected region near the auditory canals. A detailed analysis of the tradeoffs in coil sensitivity coverage, pulse superposition, and SAR is currently under way. A combination of this method with multidimensional RF parallel transmission techniques for B1 shimming is also being pursued.
ACKNOWLEDGMENTS
We thank Bernd Stoeckel (Siemens Medical Systems) and Paul Kanyha (Tecmag Inc.) for assistance with the parallel transmission hardware and interface. We also appreciate useful interactions with Thomas Ernst (University of Hawaii) regarding the RF concepts and manuscript preparation.
Grant sponsor: National Institute on Drug Abuse, National Institutes of Health; Grant numbers: R01 DA019912; K02 DA020569.
REFERENCES
- 1.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. Biophys J. 1993;64:803–812. doi: 10.1016/S0006-3495(93)81441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belliveau J, Kennedy D, McKinstry R, Buchbinder B, Weisskoff R, Cohen M, Vevea J, Brady T, Rosen B. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- 3.Frahm J, Merboldt K-D, Hanicke W. The influence of the slice-selection gradient on functional MRI of human brain activation. J Magn Reson B. 1994;103:91–93. doi: 10.1006/jmrb.1994.1015. [DOI] [PubMed] [Google Scholar]
- 4.Constable R. Functional MR imaging using gradient-echo echo-planar imaging in the presence of large static field inhomogeneities. J Magn Reson Imaging. 1995;5:746–752. doi: 10.1002/jmri.1880050622. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q, Dardzinski B, Li S, Eslinger P, Smith M. Removal of local field gradient artifacts in T2*-weighted images at high fields by gradientecho slice excitation profile imaging. Magn Reson Med. 1998;39:402–409. doi: 10.1002/mrm.1910390310. [DOI] [PubMed] [Google Scholar]
- 6.Merboldt K-D, Finsterbusch J, Frahm J. Reducing inhomogeneity artifacts in functional MRI of human brain activation-thin slices vs gradient compensation. J Magn Reson. 2000;145:184–191. doi: 10.1006/jmre.2000.2105. [DOI] [PubMed] [Google Scholar]
- 7.Wilson JL, Jezzard P. Utilization of an intra-oral diamagnetic passive shim in functional MRI of the inferior frontal cortex. Magn Reson Med. 2003;50:1089–1094. doi: 10.1002/mrm.10626. [DOI] [PubMed] [Google Scholar]
- 8.Hsu JJ, Glover GH. Mitigation of susceptibility-induced signal loss in neuroimaging using localized shim coils. Magn Reson Med. 2005;53:243–248. doi: 10.1002/mrm.20365. [DOI] [PubMed] [Google Scholar]
- 9.Cho Z, Ro Y. Reduction of susceptibility artifact in gradient-echo imaging. Magn Reson Med. 1992;23:193–200. doi: 10.1002/mrm.1910230120. [DOI] [PubMed] [Google Scholar]
- 10.Glover G, Lai S. Reduction of susceptibility effects in BOLD fMRI using tailored RF pulses; Proceedings of the 6th Annual Meeting of ISMRM; Sydney, Australia. 1998. Abstract 298. [Google Scholar]
- 11.Stenger VA, Boada FE, Noll DC. Three-dimensional tailored RF pulses for the reduction of susceptibility artifacts in T2*-weighted functional MRI. Magn Reson Med. 2000;44:525–531. doi: 10.1002/1522-2594(200010)44:4<525::aid-mrm5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yip CY, Fessler JA, Noll DC. Advanced three-dimensional tailored RF pulse for signal recovery in T2*-weighted functional magnetic resonance imaging. Magn Reson Med. 2006;56:1050–1059. doi: 10.1002/mrm.21048. [DOI] [PubMed] [Google Scholar]
- 13.Song AW. Single-shot EPI with signal recovery from the susceptibility-induced losses. Magn Reson Med. 2001;46:407–411. doi: 10.1002/mrm.1205. [DOI] [PubMed] [Google Scholar]
- 14.Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn Reson Med. 2004;51:212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Gu H, Silbersweig DA, Stern E. Simultaneous perfusion and blood-oxygenation-level-dependent measurements using single-shot interleaved z-shim echo-planar imaging. Magn Reson Med. 2005;53:1207–1211. doi: 10.1002/mrm.20431. [DOI] [PubMed] [Google Scholar]
- 16.Truong TK, Song AW. Single-shot dual-z-shimmed sensitivity-encoded spiral-in/out imaging for functional MRI with reduced susceptibility artifacts. Magn Reson Med. 2008;59:221–227. doi: 10.1002/mrm.21473. [DOI] [PubMed] [Google Scholar]
- 17.Katscher U, Bornert P, Leussler C, van den Brink J. Transmit SENSE. Magn Reson Med. 2003;49:144–150. doi: 10.1002/mrm.10353. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y. Parallel excitation with an array of transmit coils. Magn Reson Med. 2004;51:775–784. doi: 10.1002/mrm.20011. [DOI] [PubMed] [Google Scholar]
- 19.Collins CM, Liu W, Swift BJ, Smith MB. Combination of optimized transmit arrays and some receive array reconstruction methods can yield homogeneous images at very high frequencies. Magn Reson Med. 2005;54:1327–1332. doi: 10.1002/mrm.20729. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Yip CY, Grissom W, Noll DC, Boada FE, Stenger VA. Reduction of transmitter B1 inhomogeneity with transmit SENSE slice-select pulses. Magn Reson Med. 2007;57:842–847. doi: 10.1002/mrm.21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y. RF power reduction with parallel excitation; Proceedings of the 12th Annual Meeting of ISMRM; Kyoto, Japan. 2004. Abstract 331. [Google Scholar]
- 22.Pauly JM, Nishimura D, Macovski A. A k-space analysis of small-tip-angle excitation. J Magn Reson. 1989;81:43–56. doi: 10.1016/j.jmr.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Alagappan V, Nistler J, Adalsteinsson E, Setsompop K, Fontius U, Zelinski A, Vester M, Wiggins GC, Hebrank F, Renz W, Schmitt F, Wald LL. Degenerate mode band-pass birdcage coil for accelerated parallel excitation. Magn Reson Med. 2007;57:1148–1158. doi: 10.1002/mrm.21247. [DOI] [PubMed] [Google Scholar]