Abstract
Objective To understand belief in a specific scientific claim by studying the pattern of citations among papers stating it.
Design A complete citation network was constructed from all PubMed indexed English literature papers addressing the belief that β amyloid, a protein accumulated in the brain in Alzheimer’s disease, is produced by and injures skeletal muscle of patients with inclusion body myositis. Social network theory and graph theory were used to analyse this network.
Main outcome measures Citation bias, amplification, and invention, and their effects on determining authority.
Results The network contained 242 papers and 675 citations addressing the belief, with 220 553 citation paths supporting it. Unfounded authority was established by citation bias against papers that refuted or weakened the belief; amplification, the marked expansion of the belief system by papers presenting no data addressing it; and forms of invention such as the conversion of hypothesis into fact through citation alone. Extension of this network into text within grants funded by the National Institutes of Health and obtained through the Freedom of Information Act showed the same phenomena present and sometimes used to justify requests for funding.
Conclusion Citation is both an impartial scholarly method and a powerful form of social communication. Through distortions in its social use that include bias, amplification, and invention, citation can be used to generate information cascades resulting in unfounded authority of claims. Construction and analysis of a claim specific citation network may clarify the nature of a published belief system and expose distorted methods of social citation.
Introduction
Biomedical knowledge arises from scientific data. The means by which this occurs within individual scientific papers is a generally accepted process whereby papers report rationale, methods, results, and conclusions. How an entire belief system shared by a scientific community ultimately evolves from data across all papers within a specialty is less well understood. I describe and apply methods for the analysis of such belief systems using a specific example.
The belief system studied is that a protein, β amyloid, known for its role in injuring brain in Alzheimer’s disease, is also produced by and injures skeletal muscle fibres in the muscle disease sporadic inclusion body myositis. This belief system was chosen for analysis because of its importance to the care of patients with inclusion body myositis, as this view seems to be accepted by many as likely or established fact (at least 200 different journal articles have stated such), with β amyloid production often reported to be a central element in the pathogenesis of the disease (see web extra note 1), and directs research and treatment trials in the specialty. The approach taken here was simply to collect all statements in the medical literature on this belief system and to study the pattern of citation among them—that is, how each statement is supported by reference to other papers.
Methods
The methods are fully described in web extra note 2. Briefly, queries identified all English language PubMed indexed articles potentially containing statements pertaining to any of three related molecules (β amyloid precursor protein, its transcript, or one of its potential cleaved protein products, β amyloid) and muscle disease. These 766 papers (see web extra table 1) were searched for statements addressing the belief that these molecules are abnormally and specifically present in muscle fibres of patients with inclusion body myositis among many other muscle diseases, identifying 302 papers1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291 292 293 294 295 296 297 298 299 300 301 302 addressing the broad category of “amyloid” and inclusion body myositis of which 242 papers discussed these specific molecules (see web extra table 2). I collected all statements addressing the belief and citations supporting these statements. Each paper was classified as primary data (containing experimental data addressing the specific and abnormal presence of these molecules in inclusion body myositis muscle), myositis review (review papers with the term myositis or the equivalent in their title), model (reporting cell culture or animal model experiments), or other (all other papers). I classified each citation as supportive, neutral, or critical according to how its underlying statement supported the belief. A network was then constructed representing papers as nodes and citations as links from one node to another. Another investigator (Anthony Amato) validated text and citation extraction for 17% of the papers, including all primary data papers.
This citation network was further extended into research proposals funded by the US National Institutes of Health, obtained through the Freedom of Information Act in accordance with National Institutes of Health policy.
This claim specific citation network was then analysed using graph theory303 (see methods in web extra). Briefly, custom MATLAB software (MathWorks; Natick, MA) and the MatlabBGL package (written by David Gleich) were used for the analysis of adjacency matrices representing these networks. A centrality measure304 on the papers was defined (called the citation path index; similar to other variants of centrality measure305). Authority was measured according to the method of Kleinberg.306 Visualisation of networks was carried out using Pajek (http://vlado.fmf.uni-lj.si/pub/networks/pajek/). The maximum likelihood estimate method307 was implemented in MATLAB, with code available from www.santafe.edu/∼aaronc/powerlaws/.
Results
Authority and belief in a claim specific citation network
The claim that β amyloid and its precursors are abnormally and specifically present in inclusion body myositis muscle fibres among many other muscle diseases was studied. The 242 papers containing statements addressing it (all exact text provided in web extra table 3) and the 675 citations (not counting duplicates from one paper to another; see web extra table 4) supporting these statements were used to construct a claim specific citation network (fig 1). This network contained 220 609 citation paths, with chains of citations flowing from one paper to the next representing the entire National Library of Medicine PubMed indexed discourse on the claim as of 26 October 2007. The historical growth and various mathematical properties308 of this network are discussed in web extra note 3.
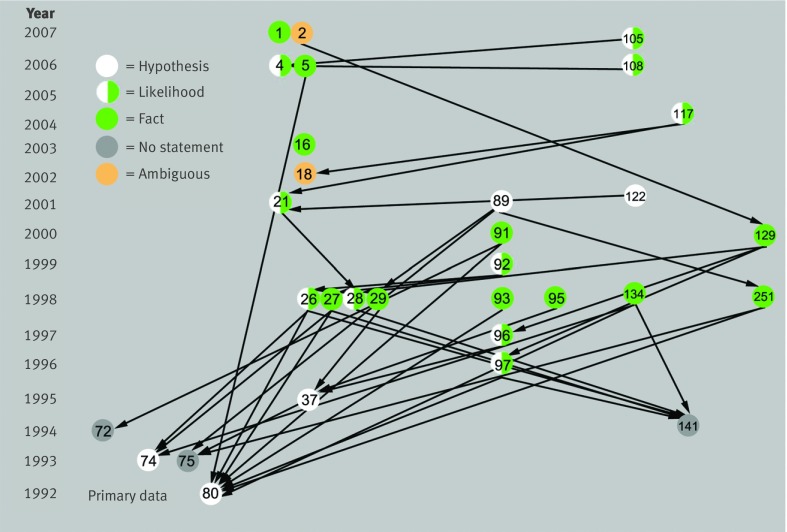
Fig 1 Claim specific citation network. Citations regarding claim that β amyloid precursor protein mRNA or protein, or β amyloid protein, is abnormally present in inclusion body myositis muscle. The network is organised according to paper category and year of publication. Authority status (yellow) was defined computationally by network theory. Many citations flow to supportive primary data but not critical data. Papers are represented as nodes (n=218) and citations as directed edges (supportive n=636, neutral n=18, critical n=21, diversion n=3). Twenty four papers contain statements pertaining to claim but do not make or receive citations about it (not shown)
Within networks certain nodes may be recognised as “authorities,”306 receiving large amounts of network traffic. Such authorities can be identified by computational methods alone through examining the patterns of connections among the nodes; this is how many internet search engines identify authoritative web pages. Because citation is in part an act of communication within a community of people, social network theory309 in particular can be used to analyse it. Under social network theory, authority of a claim indicates the community’s net belief about it. Using these computational methods,306 four primary data papers, five model papers, and one review paper constituted the 10 most authoritative papers. All these papers expressed the view that the claim was true.
Citation bias against critical primary data
Of the 10 most authoritative papers, four provided experimental data addressing the claim, reporting the presence of these molecules in inclusion body myositis muscle fibres.74 75 79 80 All four papers were from the same laboratory, two of which79 80 probably reported mostly the same data without citing each other, a practice currently viewed as one that distorts available evidence (see web extra note 4). Major technical weaknesses were present in these papers, most notably a lack of quantitative data as to how many affected muscle fibres were seen and a lack of specificity of reagents for distinguishing β amyloid protein from β amyloid precursor protein (see web extra note 5).
Inspection of the network disclosed six primary data papers that were relatively isolated, receiving no or few citations (fig 1). These papers contained data that refuted or weakened the claim. Three papers71 73 77 from independent laboratories reported that in a combined 35 patients with inclusion body myositis studied, 28 had no affected muscle fibres while the remaining seven had five or fewer affected muscle fibres (typical biopsy sections contain thousands of muscle fibres). Two papers70 72 by the laboratory that wrote the four authority papers reported that two of these molecules (β amyloid precursor protein transcript and protein) were not specific to inclusion body myositis but were present in muscle fibres during regeneration in all diseased controls (up to 43 patients in seven disease categories, including polymyositis, dermatomyositis, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis). These findings weaken the view that abnormal amounts of these molecules have any specificity to inclusion body myositis and that they cause degeneration of myofibre in patients with inclusion body myositis. One of these papers reported that all three molecules, including β amyloid, were produced by muscle invading macrophages in inclusion body myositis and all other inflammatory myopathies,70 offering an alternative source than myofibre production for them and indicating that β amyloid was non-specifically present in other inflammatory myopathy muscle (see web extra note 6 for a detailed discussion of these papers).
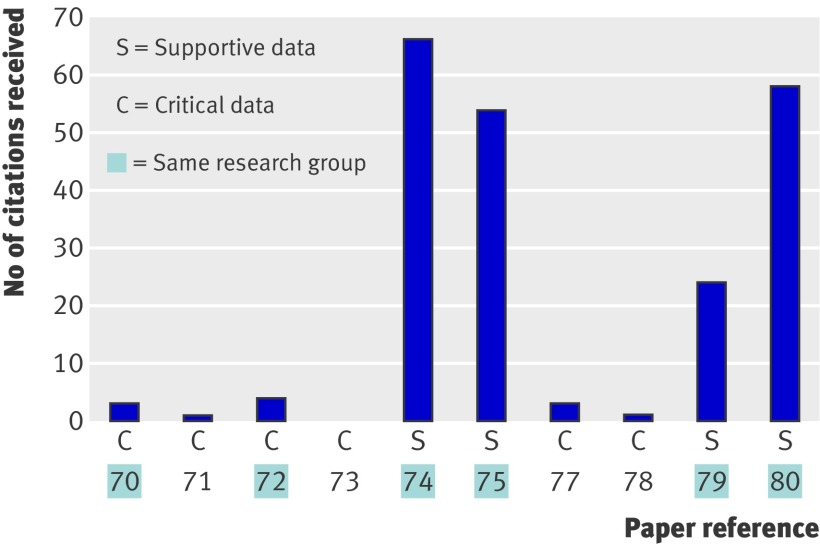
To understand why supportive but not critical data achieved authority over the ensuing 12 years since publication of all of these data, the number of citations received by each paper was analysed (fig 2). The supportive papers received 94% of the 214 citations to these primary data, whereas the six papers containing data that weakened or refuted the claim received only 6% of these citations (differing citation frequency, P=0.01). Citation bias, here defined as statistically significant differences in the number of citations received among primary data papers, seemed to be specifically against critical data not the laboratory producing it, as two papers70 72 that were biased against were written by the same research group that wrote four of the highly cited supportive papers. For example, one of the papers70 addresses a crucial question in the specialty, the relation between inflammation and degeneration,1 2 3 9 but reported data that potentially conflicted with the belief that β amyloid is produced by inclusion body myositis myofibres or is uniquely present in inclusion body myositis muscle (reporting that β amyloid is produced by muscle invading macrophages in all inflammatory myopathies). These data have never been cited by their authors despite them having made 104 citations about β amyloid to other primary data papers.
Fig 2 Citation bias against content critical of claim. Shown are citation frequencies to four authoritative supportive primary data papers and six primary data papers70 71 72 73 74 75 77 78 79 80 containing data critical of claim
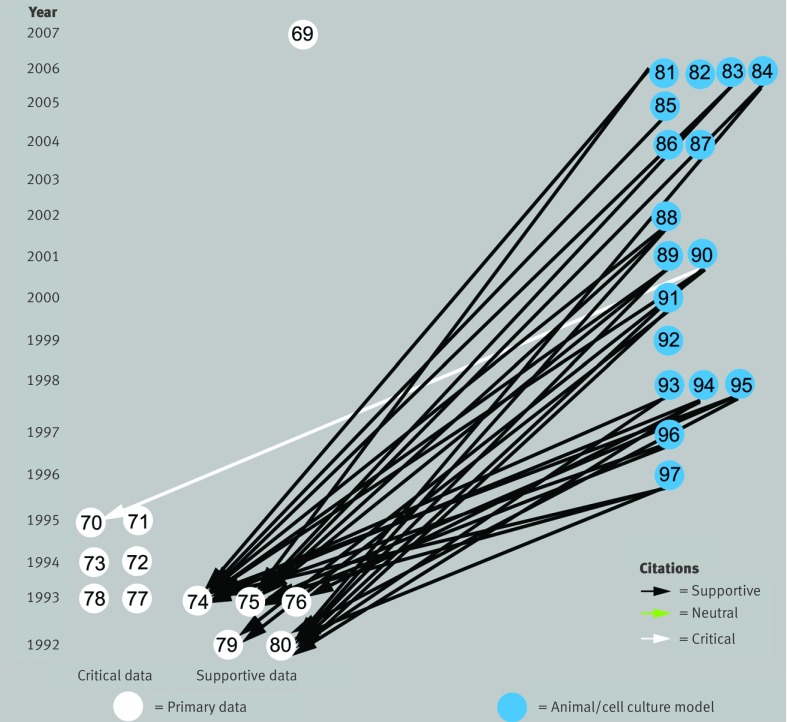
Citation bias to justify models
Citation bias has also been used to claim that animal and cell culture experiments are valid models of inclusion body myositis, in 17 papers.81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 Of the 32 citations to primary data from these papers, 31 (97%) flowed to the four highly supportive papers,74 75 79 80 whereas only one citation (3%) was made to any of the six papers that presented data weakening or refuting these as valid models for inclusion body myositis (fig 3). For example, one paper83 cited another74 in support of “abnormal accumulation of Aβ-containing inclusions are present in skeletal muscle of IBM patients” but not papers that found no71 or little73 77 β amyloid protein. Similarly, the same paper83 cited a paper75 in support of “there is evidence that APP [amyloid precursor protein] mRNA levels are selectively enhanced in human IBM [inclusion body myositis] samples thereby providing physiological justification for the overexpression of this protein in transgenic mice,” but not the paper73 that found no β amyloid precursor protein mRNA or the paper,72 by the same authors as the paper,75 that found β amyloid precursor protein mRNA not “selectively enhanced” in inclusion body myositis but present in muscle fibres in all other muscle diseases examined. The uncited data72 suggest that the animal and cell culture experiments are no more models of inclusion body myositis than any other neuromuscular disease in which muscle regeneration occurs.
Fig 3 Citations from animal and cell culture model papers to primary data papers supporting rationale for overproduction of β amyloid precursor protein mRNA as a valid model of inclusion body myositis. Only one of 32 citations flows to papers70 71 72 73 77 78 that present data that conflict with the validity of these models
Citation diversion
Some papers cited content but distorted it. This is not citation bias, as papers are cited, but rather a different process called here “citation diversion”—that is, the citing of content but the altering of its meaning in a manner that diverts its implications.
One primary data paper77 reported no β amyloid precursor protein or β amyloid in three of five patients with inclusion body myositis and its presence in only a “few fibres” in the remaining two patients. Three papers28 37 38 cited these data (fig 1) reporting that they “confirmed” the claim (for example, one paper38 said “βAPP76 77 in s-IBM fibers has been confirmed by others”). Whether such data confirm the claim is perhaps open to interpretation. At the least these data are exaggerated and generalised into a view that β amyloid precursor protein is “accumulated in vacuolated muscle fibers of s-IBM patients77 [other]” as stated by one paper,28 supported by an erroneous citation because three patients in the paper77 had 1.4% to 5% of their myofibres vacuolated but all lacked β amyloid precursor protein. Over the ensuing 10 years, these three supportive citations developed into 7848 supportive citation paths—chains of false claim in the network created by citation diversion.
In another example of citation diversion one paper81 stated “Thus, it has been widely accepted that intracellular accumulation of βAPP, Aβ [β amyloid] and other βAPP proteolytic fragments play an important role in the pathogenesis of IBM,86 89” although one of the papers89 had not widely accepted this claim, stating “Aβ-intracellular deposition may be an epiphenomenon unrelated to myofiber death.”
Amplification through influential papers and citations
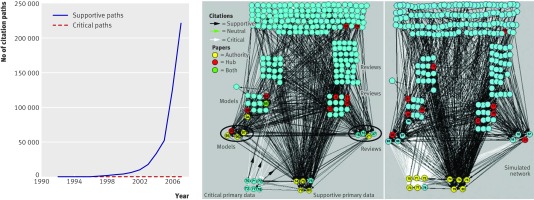
Between 1996 and 2007 support for the claim grew exponentially, with the number of supportive citations and citation paths increasing sevenfold and 777-fold, to 636 citations and 220 553 citation paths. In contrast, the critical view grew to only 21 citations and 28 citation paths (fig 4). No papers refuted or critiqued the critical data, but instead the data were just ignored. Analysis of a claim specific citation network can identify exactly which papers and citations have been most influential in pushing forward belief304 305 (see web extra note 7). The increased support was facilitated by a small number of papers, not reporting any primary data, through which large amounts of traffic (citation paths) flow in the network. For example, 63% of all citation paths (n=139 391) flow through one review paper21 (compared with 2% of citation paths flowing through randomly selected other papers); 95% of all citation paths flow through four review papers16 18 21 37 by the same research group (8% through four randomly selected other papers).
Fig 4 Amplification and authority of claim. (Left) Historical growth of supportive and critical citations in network. (Middle) Rearrangement of network (fig 1) to visualise a lens effect in which eight key papers (surrounded by ovals; seven by same research group; 97% of all network traffic passes through them) create citation flows among each other, and both amplify claim and focus citations to supportive data papers. Net effect results in network authority status306 (yellow) of supportive data papers. (Right) Computational elimination of citation bias results in balanced authority of both support for claim and its refutation through additional recognition of critical data papers70 71 72 73 77 as authorities
A lens effect was present in which a small number of these influential review papers and model papers containing no data on claim validity collected and focused citation (similar to a magnifying lens collecting light) on particular primary data papers supportive of the belief, while isolating others that weakened it (fig 4). Such papers have a network property known as high betweenness centrality.304
The term amplification can be used to describe the expansion of a claim’s belief system by citation to papers lacking any data addressing it, the phenomenon observed here. Amplification is not inherent to published belief systems. Authors could choose to cite only primary data when making claims, resulting in amplification minimal networks. Amplification of a claim is instead introduced into belief systems through the citing of review papers and other papers that lack data addressing the claim. Certainly such papers may be cited for other reasons; amplification only arises when they are cited to support claims of experimental results reported elsewhere. (See web extra note 8 for further discussion of amplification and methods for quantifying it.)
Network authority emerges through bias against critical content and amplification
Papers may be biased against for many potential reasons. To examine the role of bias exclusively against critical content in establishing authority, a simulated network was constructed in which all statements making a supportive claim were amended to recognise critical views of equivalent content and temporal availability. Removing bias against critical content was sufficient to result in authority status for five of the six infrequently cited primary data papers (fig 4), indicating that authority status of the claim emerges from the citation bias against critical content. The claim cannot be both true and false; the resulting balanced authority of supportive and refuting papers indicates that without citation bias there would be balanced belief in its truth and falseness (see web extra note 9).
Invention
Distinct from citation bias and amplification, certain types of fact developed and spread through the belief system. These particular facts were not those that arose from restatement of published claims, but rather involved different mechanisms either deliberate or through scholarly negligence, herein called invention. For example, a subclaim (that the accumulation of β amyloid occurs early and precedes other abnormalities) has variously been stated as hypothesis, likelihood, or fact in 27 papers supported by 37 citations (see web extra note 10). Nine of these citations (24%), used to support text making these claims, in fact flowed to papers that contained no statement on the temporal relation of β amyloid to other abnormalities in inclusion body myositis muscle (dead end citations). This subclaim had transformed from hypothesis to “fact” through citation alone, a process that might be called citation transmutation (fig 5). Thus one paper5 contained it as fact (“The appearance of Aβ-positive, noncongophilic deposits precedes vacuolization in IBM muscle fibers80”) supporting this statement by citing the paper80 where it had only been proposed as hypothesis (“may represent early changes of IBM”). Similarly, another paper134 reported this as fact (“our previous studies demonstrated that abnormalities of βAPP precede other changes including congophilia74 80 141”) even though the cited papers stated it only as hypothesis74 80 or made no statement at all141 about the accumulation of β amyloid precursor protein preceding other abnormalities.
Fig 5 Conversion of hypothesis to fact through citation alone. Citations on statement that accumulation of β amyloid “precedes” other abnormalities in inclusion body myositis muscle. Statement as fact is supported through citation to papers that only state it as hypothesis (for example, references 5 to 80, 91 to 80, 134 to 74) or sometimes supported by citation to papers that contain no statements addressing it (for example, references 91 to 72, 251 to 75; dead end citations). This phenomenon might be called citation transmutation (see web extra note 10 for statements)
In another form of invention, claims are introduced as fact through a “back door” that bypasses peer review and publication of methods and data. This is accomplished by repeated misrepresentation of abstracts as papers (seven different papers, 17 citations to 12 different misrepresented abstracts; for example, citation to Neurol 2003;60:333-334, an abstract with correct listing Neurol 2003;60(suppl 1):A333-4; see web extra note 11). The claim that “β-amyloid42 isoform [is] more common than β-amyloid40”4 is supported in this manner and accepted by peers as fact (paper 2 states this citing paper 4) (see web extra note 12 for another form of invention called title invention).
Bias and invention in National Institutes of Health funded research proposals
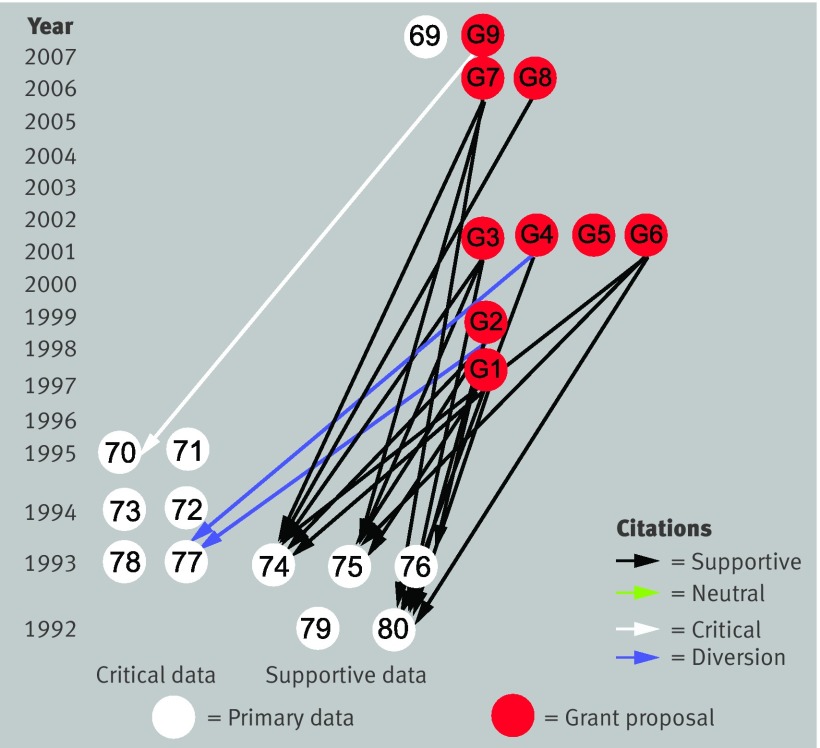
Through the publication of scientific papers and the demonstration of these publications as evidence of productivity, the elements of bias, amplification, and invention can be used indirectly to support requests for research funding. To determine if these mechanisms were used directly to support such requests, the claim specific citation network was extended from the PubMed indexed literature into the research sections and bibliographies of National Institutes of Health funded grant proposals containing text addressing the claim, obtained under the Freedom of Information Act according to National Institutes of Health policy.310 Of 27 grant proposals requested (identified through searches of the National Institutes of Health CRISP database as described in web extra note 13), nine were released by the National Institutes of Health. These seemed to be the proposals most pertinent to the belief system.
Citation bias or invention was present in eight of nine of these proposals (fig 6). Of 23 citations to primary data (not counting multiple citations from one proposal to a single paper) addressing the claim’s validity, 20 were made to supportive primary data (19 supportive citations and one neutral citation), two were instances of citation diversion (one paper77 again cited for supporting the claim when it weakens it), and one was made to critical content. Invention of fact supported through citation to hypothesis, dead end citation, and abstracts misrepresented as papers were similarly present in these funded proposals. These were sometimes used directly to justify requests for funding of the proposed studies (for example, “The accumulation of epitopes of βAPP is an early event in the disease relative to the other changes,37 96 justifying our focused investigation of Aβ”; one paper37 stated this only as hypothesis; the other paper96 stated this as likelihood not fact, supporting that view also through citation to the other paper,37 stated as hypothesis (see web extra note 13 for further discussion).
Fig 6 Extension of PubMed claim specific citation network into National Institutes of Health funded research proposals. Nine funded research grants (G1-G9; see web extra note 13) contain statements and citations addressing claim; their citations to primary data are shown. Citation bias and citation diversion are present
Discussion
Citation, the act of connecting text statements through reference to the broader literature, is not simply an impartial scholarly method for joining related published knowledge. Citation may be used for self serving purposes311 or as a tool for persuasion312 (see web extra note 14). These aspects of citation might be called social citation. I studied how distortions of the persuasive aspect of social citation may result in broad acceptance of unfounded claims as fact. These distortions can be detected and interpreted through social network theory309 because citation as persuasion is a social behaviour. Network theory applied to citation networks constructed from entire paper bibliographies, such as the science citation network,313 can disclose societal attitudes to journals and specific papers (for example, impact factors), but these networks are not suitable for understanding the foundation for belief in specific claims. When networks are instead confined to citation pertaining to one set of related claims (a claim specific citation network), they become sharply focused tools for understanding social communication pertaining to the claims—what is in effect the published record of a belief system shared by a community. These allow for study of not just what is said about a belief (the traditional scope of review papers), but also who hears it and how it is retold.
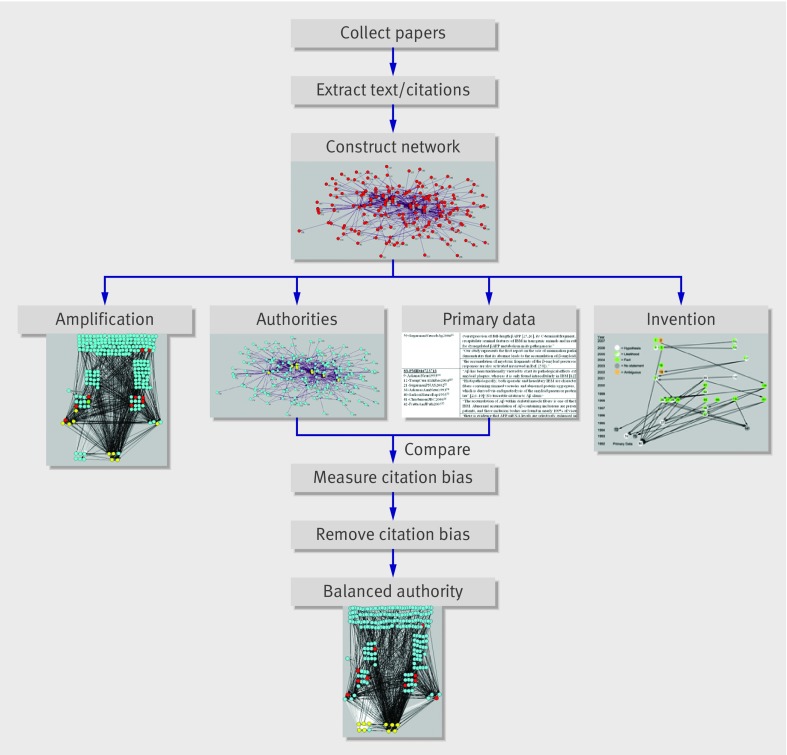
The general approach taken here (fig 7) addressed belief in claims; no experiments were done addressing their truth. The computational analysis of the claim specific citation network representing this belief system detected certain distortions in the patterns of citation that would not have been expected had only scholarly citation been used. Primary data that weakened or refuted claims on which the belief was based were ignored (citation bias) and a small number of influential papers and citations exponentially amplified supportive claim over time without presenting new primary data (amplification). Certain related claims were invented as fact. The combined effects of these citation distortions resulted in authority of the belief (acceptance of it) according to social network theory.
Fig 7 Overview of approach. After construction of the claim specific citation network, a combined manual and computational endeavour, steps on left (determination of authorities [yellow papers] and identification of amplification) require only computational algorithms; right half (identifying which papers contain actual data addressing claim validity and identifying invention) requires careful reading of paper content. Combining results of authority identification with data identification allows for recognition of citation bias and subsequent steps for its simulated removal and assessment of effects on network
There are varied forms and consequences of distorted persuasive citation seen in this study (see box). Citation bias against critical content can be used for the systematic support of claim,314 results in the loss of implications of isolated data (see web extra note 15), and can be used to justify construction of animal models, which can then be circularly used to amplify claim (see web extra note 16). Such animal models have enormous appeal, and some publications describing them achieved authority status in this network (fig 1) despite reporting no data addressing the claim—that is, whether these β amyloid related molecules are present in human inclusion body myositis muscle. Amplification involves repetitive citation of review papers or other papers lacking data, often through self citation, features noted previously in a variation of a claim specific citation network.315 Invention has multiple variations.
Vocabulary of citation distortions
Citation
Both scholarly and social forms: the scholarly form connects statements to the broader medical literature, the social form (social citation) includes self serving and persuasive subtypes
Citation distortions
Self serving citation is always a distortion
Persuasive citation may be necessary to communicate new, sound claims to the scientific community; it may, however, have distorted uses—citation bias, amplification, and invention
Citation bias
Systematic ignoring of papers that contain content conflicting with a claim
Bolster claim; justifying animal models to provide opportunities to amplify claim
Amplification
Expansion of a belief system without data
Citation made to papers that don’t contain primary data, increasing the number of citations supporting the claim without presenting data addressing it
Invention
Citation diversion—citing content but claiming it has a different meaning, thereby diverting its implications
Citation transmutation—the conversion of hypothesis into fact through the act of citation alone
Back door invention—repeated misrepresentation of abstracts as peer reviewed papers to fool readers into believing that claims are based on peer reviewed published methods and data
Dead end citation—support of a claim with citation to papers that do not contain content addressing the claim
Title invention—reporting of “experimental results” in a paper’s title, even though the paper does not report the performance or results of any such experiments
Three factors may account for how citation distortions created authority in this belief system. Foremost is the power of citation through the choice of which papers to cite and which to ignore (citation bias), by citing but distorting content (citation diversion), and by using citation to invent fact (citation transmutation, dead end citation, and back door invention).
Second is an inherent property of negative results, which failed to spread through the network. These were not repeatedly cited by their authors in subsequent papers (only one instance was present274) as perhaps there was simply nothing further to say about them. Unlike “positive results” there is nothing exciting to be repeatedly written about how something was not found in an experiment. Thus the progression from data to accepted claim is different within a single paper compared with across many papers in a specialty. Within a single paper readers generally view new claims as false until proved true through convincing methods and results. Across a network of papers, however, the barrier to the propagation of negative results biases claims as being viewed as true until proved false.
Thirdly, this belief system is possibly an information cascade (also called an informational cascade),316 317 an entity resulting when people perceive advantage in accepting the prevailing view over any private information they may have when making choices. Indeed certain mathematical properties of information cascades (preferential attachment) would be expected to produce a network with properties seen here (a biased network with a power law distribution of node degrees; see web extra note 3). Many authors may just not be aware of the critical data, as these data are effectively isolated from the discourse about this claim and not mentioned in any review articles. Although unsound information cascades are in theory fragile and fall apart quickly when exposed,316 this may not occur in biomedical belief systems, where contradicted claims may persist.318
Many published biomedical belief systems may be information cascades because repetition of claims is ubiquitous in the biomedical literature. Many are built on sound data, with authors repeating claims after trusting the published expert opinion of their colleagues. However, there are incentives for generating and joining information cascades regardless of their soundness. Joining an information cascade aids publication as articles have to say something and negative results are biased against.319 Generating and joining an information cascade may improve the likelihood of obtaining research funding because hypothesis driven research is an essential requirement320 at many research funding agencies such as the National Institutes of Health, and successful funding generally requires a “strong hypothesis . . . based on current scientific literature”320—that is, the published belief system of a claim. Chances for successful funding may therefore be increased through joining the cascade (repeating the claim and proposing experimental plans around it). In the extension of this citation network into text within grant proposals that have been funded by the National Institutes of Health, citation bias, diversion, or invention were often present. Once research funding has been used to join a cascade there are further incentives to interpret results through confirmation bias (“in a way that confirms one’s preconceptions and to avoid information and interpretations which contradict prior beliefs”321) to demonstrate success of the research for subsequent funding. Although joining an information cascade may be an optimal behaviour for some people, it reduces the likelihood that future investigators can discover whether it is sound.317
Methods for the construction and analysis of comprehensive claim specific citation networks present challenges and limitations. These include interpreting meaning of text, as people may reasonably interpret text differently, and understanding the distinct phenomena observed (see web extra note 17 for a discussion of these issues). In principle many biomedical claims have an associated citation network, the study of which provides a powerful approach to detecting citation bias, amplification, and invention, and understanding the nature of the authority of the claim.
What is already known on this topic
In addition to its scholarly use, citation has social uses, both self serving and as a tool for persuasion
One distortion of this persuasive aspect of citation, citation bias, has been recognised in clinical trial reporting where it may lead to false belief about a therapy’s efficacy
What this study adds
Distortions in the persuasive use of citation—bias, amplification, and invention—can be used to establish unfounded scientific claims as fact
Categorising these distorted uses of citation and having vocabulary for them aids in their recognition
How scientific data evolve into entire published biomedical belief systems around specific claims can be studied through a device called a claim specific citation network and the use of social network theory
I thank Daniel Rockmore (Department of Mathematics, Dartmouth College); Daniel Jonah Goldhagen; Peter Park (Harvard Medical School); and Einer Elhauge (Harvard Law School), for thoughtful discussions. Anthony Amato (Harvard Medical School) carried out validation of extracted text and citations.
Funding: SAG is in part supported by National Institutes of Health grants R01NS43471 and R21NS057225, and Muscular Dystrophy Association grant MDA4353. These grants did not contain specific aims directly encompassing this research.
Competing interests: SAG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical approval: Not required.
Cite this as: BMJ 2009;339:b2680
References
- 1.Needham M, Mastaglia FL. Inclusion body myositis: current pathogenetic concepts and diagnostic and therapeutic approaches. Lancet Neurol 2007;6:620-631. [DOI] [PubMed] [Google Scholar]
- 2.Needham M, Mastaglia FL, Garlepp MJ. Genetics of inclusion-body myositis. Muscle Nerve 2007;35:549-561. [DOI] [PubMed] [Google Scholar]
- 3.Askanas V, Engel WK. Inclusion-body myositis, a multifactorial muscle disease associated with aging: current concepts of pathogenesis. Curr Opin Rheumatol 2007;19:550-559. [DOI] [PubMed] [Google Scholar]
- 4.Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology 2006;66(2 Suppl 1):S39-48. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MP, Golde TE. Inclusion-body myositis and Alzheimer disease: two sides of the same coin, or different currencies altogether? Neurology 2006;66(2 Suppl 1):S65-68. [DOI] [PubMed] [Google Scholar]
- 6.Buxbaum JN. Treatment and prevention of the amyloidoses: can the lessons learned be applied to sporadic inclusion-body myositis? Neurology 2006;66(2 Suppl 1):S110-113. [DOI] [PubMed] [Google Scholar]
- 7.Engel WK, Askanas V. Inclusion-body myositis: clinical, diagnostic, and pathologic aspects. Neurology 2006;66(2 Suppl 1):S20-29. [DOI] [PubMed] [Google Scholar]
- 8.Dalakas MC. Inflammatory, immune, and viral aspects of inclusion-body myositis. Neurology 2006;66(2 Suppl 1):S33-38. [DOI] [PubMed] [Google Scholar]
- 9.Dalakas MC. Sporadic inclusion body myositis--diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol 2006;2:437-447. [DOI] [PubMed] [Google Scholar]
- 10.Munshi SK, Thanvi B, Jonnalagadda SJ et al. Inclusion body myositis: an underdiagnosed myopathy of older people. Age Ageing 2006;35:91-94. [DOI] [PubMed] [Google Scholar]
- 11.Askanas V, Engel WK. Molecular pathology and pathogenesis of inclusion-body myositis. Microsc Res Tech 2005;67:114-120. [DOI] [PubMed] [Google Scholar]
- 12.Askanas V, Engel WK. Sporadic inclusion-body myositis: a proposed key pathogenetic role of the abnormalities of the ubiquitin-proteasome system, and protein misfolding and aggregation. Acta Myol 2005;24:17-24. [PubMed] [Google Scholar]
- 13.Oldfors A, Lindberg C. Diagnosis, pathogenesis and treatment of inclusion body myositis. Curr Opin Neurol 2005;18:497-503. [DOI] [PubMed] [Google Scholar]
- 14.Dalakas MC. Inflammatory disorders of muscle: progress in polymyositis, dermatomyositis and inclusion body myositis. Curr Opin Neurol 2004;17:561-567. [DOI] [PubMed] [Google Scholar]
- 15.Askanas V, Engel WK. Unfolding story of inclusion-body myositis and myopathies: role of misfolded proteins, amyloid-beta, cholesterol, and aging. J Child Neurol 2003;18:185-190. [DOI] [PubMed] [Google Scholar]
- 16.Askanas V, Engel WK. Proposed pathogenetic cascade of inclusion-body myositis: importance of amyloid-beta, misfolded proteins, predisposing genes, and aging. Curr Opin Rheumatol 2003;15:737-744. [DOI] [PubMed] [Google Scholar]
- 17.Dalakas MC. Understanding the immunopathogenesis of inclusion-body myositis: present and future prospects. Rev Neurol (Paris) 2002;158:948-958. [PubMed] [Google Scholar]
- 18.Askanas V, Engel WK. Inclusion-body myositis and myopathies: different etiologies, possibly similar pathogenic mechanisms. Curr Opin Neurol 2002;15:525-531. [DOI] [PubMed] [Google Scholar]
- 19.Tawil R, Griggs RC. Inclusion body myositis. Curr Opin Rheumatol 2002;14:653-657. [DOI] [PubMed] [Google Scholar]
- 20.Askanas V, Engel WK. Newest pathogenetic considerations in inclusion-body myositis: possible role of amyloid-beta, cholesterol, relation to aging and to Alzheimer’s disease. Curr Rheumatol Rep 2002;4:427-433. [DOI] [PubMed] [Google Scholar]
- 21.Askanas V, Engel WK. Inclusion-body myositis: newest concepts of pathogenesis and relation to aging and Alzheimer disease. J Neuropathol Exp Neurol 2001;60:1-14. [DOI] [PubMed] [Google Scholar]
- 22.Oldfors A, Fyhr IM. Inclusion body myositis: genetic factors, aberrant protein expression, and autoimmunity. Curr Opin Rheumatol 2001;13:469-475. [DOI] [PubMed] [Google Scholar]
- 23.Oldfors A, Lindberg C. Inclusion body myositis. Curr Opin Neurol 1999;12:527-533. [DOI] [PubMed] [Google Scholar]
- 24.Cherin P. Treatment of inclusion body myositis. Curr Opin Rheumatol 1999;11:456-461. [PubMed] [Google Scholar]
- 25.Vogel H. Inclusion body myositis--a review. Adv Anat Pathol 1998;5:164-169. [DOI] [PubMed] [Google Scholar]
- 26.Askanas V, Engel WK. Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol 1998;10:530-542. [DOI] [PubMed] [Google Scholar]
- 27.Askanas V, Engel WK. Does overexpression of betaAPP in aging muscle have a pathogenic role and a relevance to Alzheimer’s disease? Clues from inclusion body myositis, cultured human muscle, and transgenic mice. Am J Pathol 1998;153:1673-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askanas V, Engel WK. Sporadic inclusion-body myositis and its similarities to Alzheimer disease brain. Recent approaches to diagnosis and pathogenesis, and relation to aging. Scand J Rheumatol 1998;27:389-405. [DOI] [PubMed] [Google Scholar]
- 29.Askanas V, Engel WK. Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: diseases of oxidative stress and aging? Arch Neurol 1998;55:915-920. [DOI] [PubMed] [Google Scholar]
- 30.Askanas V, Engel WK, Alvarez RB. Fourteen newly recognized proteins at the human neuromuscular junctions--and their nonjunctional accumulation in inclusion-body myositis. Ann N Y Acad Sci 1998;841:28-56. [DOI] [PubMed] [Google Scholar]
- 31.Illa I, Dalakas MC. Dermatomyositis, polymyositis and inclusion body myositis: current concepts. Rev Neurol (Paris) 1998;154:13-16. [PubMed] [Google Scholar]
- 32.Sivakumar K, Dalakas MC. Inclusion body myositis and myopathies. Curr Opin Neurol 1997;10:413-420. [DOI] [PubMed] [Google Scholar]
- 33.Dalakas MC, Sivakumar K. The immunopathologic and inflammatory differences between dermatomyositis, polymyositis and sporadic inclusion body myositis. Curr Opin Neurol 1996;9:235-239. [DOI] [PubMed] [Google Scholar]
- 34.Garlepp MJ, Mastaglia FL. Inclusion body myositis. J Neurol Neurosurg Psychiatry 1996;60:251-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter S. Inclusion body myositis, a review. J Neuropathol Exp Neurol 1996;55:1105-1114. [DOI] [PubMed] [Google Scholar]
- 36.Griggs RC, Askanas V, DiMauro S et al. Inclusion body myositis and myopathies. Ann Neurol 1995;38:705-713. [DOI] [PubMed] [Google Scholar]
- 37.Askanas V, Engel WK. New advances in the understanding of sporadic inclusion-body myositis and hereditary inclusion-body myopathies. Curr Opin Rheumatol 1995;7:486-496. [DOI] [PubMed] [Google Scholar]
- 38.Askanas V, Engel WK, Mirabella M. Idiopathic inflammatory myopathies: inclusion-body myositis, polymyositis, and dermatomyositis. Curr Opin Neurol 1994;7:448-456. [PubMed] [Google Scholar]
- 39.Calabrese LH, Chou SM. Inclusion body myositis. Rheum Dis Clin North Am 1994;20:955-972. [PubMed] [Google Scholar]
- 40.Chou SM. Inclusion body myositis. Baillieres Clin Neurol 1993;2:557-577. [PubMed] [Google Scholar]
- 41.Askanas V, Engel WK. New advances in inclusion-body myositis. Curr Opin Rheumatol 1993;5:732-741. [DOI] [PubMed] [Google Scholar]
- 42.Sekul EA, Dalakas MC. Inclusion body myositis: new concepts. Semin Neurol 1993;13:256-263. [DOI] [PubMed] [Google Scholar]
- 43.Briani C, Doria A, Sarzi-Puttini P, Dalakas MC. Update on idiopathic inflammatory myopathies. Autoimmunity 2006;39:161-170. [DOI] [PubMed] [Google Scholar]
- 44.Dalakas MC. Therapeutic targets in patients with inflammatory myopathies: present approaches and a look to the future. Neuromuscul Disord 2006;16:223-236. [DOI] [PubMed] [Google Scholar]
- 45.Dalakas MC. Mechanisms of disease: signaling pathways and immunobiology of inflammatory myopathies. Nat Clin Pract Rheumatol 2006;2:219-227. [DOI] [PubMed] [Google Scholar]
- 46.Dalakas MC. The future prospects in the classification, diagnosis and therapies of inflammatory myopathies: a view to the future from the “bench-to-bedside”. J Neurol 2004;251:651-657. [DOI] [PubMed] [Google Scholar]
- 47.Chinoy H, Ollier WE, Cooper RG. Have recent immunogenetic investigations increased our understanding of disease mechanisms in the idiopathic inflammatory myopathies? Curr Opin Rheumatol 2004;16:707-713. [DOI] [PubMed] [Google Scholar]
- 48.Christopher-Stine L, Plotz PH. Myositis: an update on pathogenesis. Curr Opin Rheumatol 2004;16:700-706. [DOI] [PubMed] [Google Scholar]
- 49.Christopher-Stine L, Plotz PH. Adult inflammatory myopathies. Best Pract Res Clin Rheumatol 2004;18:331-344. [DOI] [PubMed] [Google Scholar]
- 50.Dalakas MC. The molecular pathophysiology in inflammatory myopathies. Rev Med Interne 2004;25 Suppl 1:S14-16. [DOI] [PubMed]
- 51.Figarella-Branger D, Civatte M, Bartoli C, Pellissier JF. Cytokines, chemokines, and cell adhesion molecules in inflammatory myopathies. Muscle Nerve 2003;28:659-682. [DOI] [PubMed] [Google Scholar]
- 52.Mastaglia FL, Garlepp MJ, Phillips BA, Zilko PJ. Inflammatory myopathies: clinical, diagnostic and therapeutic aspects. Muscle Nerve 2003;27:407-425. [DOI] [PubMed] [Google Scholar]
- 53.Dalakas MC. Therapeutic approaches in patients with inflammatory myopathies. Semin Neurol 2003;23:199-206. [DOI] [PubMed] [Google Scholar]
- 54.Dalakas MC. Muscle biopsy findings in inflammatory myopathies. Rheum Dis Clin North Am 2002;28:779-798. [DOI] [PubMed] [Google Scholar]
- 55.Kissel JT. Misunderstandings, misperceptions, and mistakes in the management of the inflammatory myopathies. Semin Neurol 2002;22:41-51. [DOI] [PubMed] [Google Scholar]
- 56.Hilton-Jones D. Inflammatory muscle diseases. Curr Opin Neurol 2001;14:591-596. [DOI] [PubMed] [Google Scholar]
- 57.Dalakas MC. Molecular immunology and genetics of inflammatory muscle diseases. Arch Neurol 1998;55:1509-1512. [DOI] [PubMed] [Google Scholar]
- 58.Bertorini TE. Inflammatory myopathies [polymyositis, dermatomyositis, inclusion body myositis]. Compr Ther 1998;24:494-502. [PubMed] [Google Scholar]
- 59.Mantegazza R, Bernasconi P, Confalonieri P, Cornelio F. Inflammatory myopathies and systemic disorders: a review of immunopathogenetic mechanisms and clinical features. J Neurol 1997;244:277-287. [DOI] [PubMed] [Google Scholar]
- 60.Amato AA, Barohn RJ. Idiopathic inflammatory myopathies. Neurol Clin 1997;15:615-648. [DOI] [PubMed] [Google Scholar]
- 61.Serratrice G. The three groups of polymyositis. Rev Rhum Engl Ed 1996;63:797-800. [PubMed] [Google Scholar]
- 62.Dalakas MC. Immunopathogenesis of inflammatory myopathies. Ann Neurol 1995;37(suppl 1):S74-S86. [DOI] [PubMed] [Google Scholar]
- 63.Mantegazza R, Bernasconi P. Cellular aspects of myositis. Curr Opin Rheumatol 1994;6:568-574. [DOI] [PubMed] [Google Scholar]
- 64.Dalakas MC. Current treatment of the inflammatory myopathies. Curr Opin Rheumatol 1994;6:595-601. [DOI] [PubMed] [Google Scholar]
- 65.Karpati G, Carpenter S. Pathology of the inflammatory myopathies. Baillieres Clin Neurol 1993;2:527-556. [PubMed] [Google Scholar]
- 66.Dalakas MC. Clinical, immunopathologic, and therapeutic considerations of inflammatory myopathies. Clin Neuropharmacol 1992;15:327-351. [DOI] [PubMed] [Google Scholar]
- 67.Kalovidouris AE. Immune aspects of myositis. Curr Opin Rheumatol 1992;4:809-814. [PubMed] [Google Scholar]
- 68.Dalakas MC. Inflammatory and toxic myopathies. Curr Opin Neurol Neurosurg 1992;5:645-654. [PubMed] [Google Scholar]
- 69.Lunemann JD, Schmidt J, Schmid D et al. Beta-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann Neurol 2007;61:476-483. [DOI] [PubMed] [Google Scholar]
- 70.Askanas V, Sarkozi E, Bilak M et al. Human muscle macrophages express beta-amyloid precursor and prion proteins and their mRNAs. Neuroreport 1995;6:1045-1049. [DOI] [PubMed] [Google Scholar]
- 71.Sherriff FE, Joachim CL, Squier MV, Esiri MM. Ubiquitinated inclusions in inclusion-body myositis patients are immunoreactive for cathepsin D but not beta-amyloid. Neurosci Lett 1995;194:37-40. [DOI] [PubMed] [Google Scholar]
- 72.Sarkozi E, Askanas V, Johnson SA et al. Expression of beta-amyloid precursor protein gene is developmentally regulated in human muscle fibers in vivo and in vitro. Exp Neurol 1994;128:27-33. [DOI] [PubMed] [Google Scholar]
- 73.Nalbantoglu J, Karpati G, Carpenter S. Conspicuous accumulation of a single-stranded DNA binding protein in skeletal muscle fibers in inclusion body myositis. Am J Pathol 1994;144:874-882. [PMC free article] [PubMed] [Google Scholar]
- 74.Askanas V, Alvarez RB, Engel WK. beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol 1993;34:551-560. [DOI] [PubMed] [Google Scholar]
- 75.Sarkozi E, Askanas V, Johnson SA et al. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport 1993;4:815-818. [DOI] [PubMed] [Google Scholar]
- 76.Villanova M, Kawai M, Lubke U et al. Rimmed vacuoles of inclusion body myositis and oculopharyngeal muscular dystrophy contain amyloid precursor protein and lysosomal markers. Brain Res 1993;603:343-347. [DOI] [PubMed] [Google Scholar]
- 77.Leclerc A, Tome FM, Fardeau M. Ubiquitin and beta-amyloid-protein in inclusion body myositis (IBM), familial IBM-like disorder and oculopharyngeal muscular dystrophy: an immunocytochemical study. Neuromuscul Disord 1993;3:283-291. [DOI] [PubMed] [Google Scholar]
- 78.Schubert W, Masters CL, Beyreuther K. APP+ T lymphocytes selectively sorted to endomysial tubes in polymyositis displace NCAM-expressing muscle fibers. Eur J Cell Biol 1993;62:333-342. [PubMed] [Google Scholar]
- 79.Askanas V, Engel WK, Alvarez RB, Glenner GG. beta-Amyloid protein immunoreactivity in muscle of patients with inclusion-body myositis. Lancet 1992;339:560-561. [DOI] [PubMed] [Google Scholar]
- 80.Askanas V, Engel WK, Alvarez RB. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol 1992;141:31-36. [PMC free article] [PubMed] [Google Scholar]
- 81.Sugarman MC, Kitazawa M, Baker M et al. Pathogenic accumulation of APP in fast twitch muscle of IBM patients and a transgenic model. Neurobiol Aging 2006;27:423-432. [DOI] [PubMed] [Google Scholar]
- 82.Rosen KM, Veereshwarayya V, Moussa CE et al. Parkin protects against mitochondrial toxins and beta-amyloid accumulation in skeletal muscle cells. J Biol Chem 2006;281:12809-12816. [DOI] [PubMed] [Google Scholar]
- 83.Kitazawa M, Green KN, Caccamo A, LaFerla FM. Genetically augmenting Abeta42 levels in skeletal muscle exacerbates inclusion body myositis-like pathology and motor deficits in transgenic mice. Am J Pathol 2006;168:1986-1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moussa CE, Fu Q, Kumar P et al. Transgenic expression of beta-APP in fast-twitch skeletal muscle leads to calcium dyshomeostasis and IBM-like pathology. Faseb J 2006;20:2165-2167. [DOI] [PubMed] [Google Scholar]
- 85.Fratta P, Engel WK, McFerrin J et al. Proteasome inhibition and aggresome formation in sporadic inclusion-body myositis and in amyloid-beta precursor protein-overexpressing cultured human muscle fibers. Am J Pathol 2005;167:517-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Christensen RA, Shtifman A, Allen PD et al. Calcium dyshomeostasis in beta-amyloid and tau-bearing skeletal myotubes. J Biol Chem 2004;279:53524-53532. [DOI] [PubMed] [Google Scholar]
- 87.Strazielle C, Dumont M, Fukuchi K, Lalonde R. Transgenic mice expressing the human C99 terminal fragment of betaAPP: effects on cytochrome oxidase activity in skeletal muscle and brain. J Chem Neuroanat 2004;27:237-246. [DOI] [PubMed] [Google Scholar]
- 88.Sugarman MC, Yamasaki TR, Oddo S et al. Inclusion body myositis-like phenotype induced by transgenic overexpression of beta APP in skeletal muscle. Proc Natl Acad Sci U S A 2002;99:6334-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Querfurth HW, Suhara T, Rosen KM et al. Beta-amyloid peptide expression is sufficient for myotube death: implications for human inclusion body myopathy. Mol Cell Neurosci 2001;17:793-810. [DOI] [PubMed] [Google Scholar]
- 90.Baron P, Galimberti D, Meda L et al. Production of IL-6 by human myoblasts stimulated with Abeta: relevance in the pathogenesis of IBM. Neurology 2001;57:1561-1565. [DOI] [PubMed] [Google Scholar]
- 91.Baron P, Galimberti D, Meda L et al. Synergistic effect of beta-amyloid protein and interferon gamma on nitric oxide production by C2C12 muscle cells. Brain 2000;123:374-379. [DOI] [PubMed] [Google Scholar]
- 92.McFerrin J, Engel WK, Askanas V. Cultured inclusion-body myositis muscle fibers do not accumulate beta-amyloid precursor protein and can be innervated. Neurology 1999;53:2184-2187. [DOI] [PubMed] [Google Scholar]
- 93.Fukuchi K, Pham D, Hart M et al. Amyloid-beta deposition in skeletal muscle of transgenic mice: possible model of inclusion body myopathy. Am J Pathol 1998;153:1687-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin LW, Hearn MG, Ogburn CE et al. Transgenic mice over-expressing the C-99 fragment of betaPP with an alpha-secretase site mutation develop a myopathy similar to human inclusion body myositis. Am J Pathol 1998;153:1679-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McFerrin J, Engel WK, Askanas V. Impaired innervation of cultured human muscle overexpressing betaAPP experimentally and genetically: relevance to inclusion-body myopathies. Neuroreport 1998;9:3201-3205. [DOI] [PubMed] [Google Scholar]
- 96.Askanas V, McFerrin J, Alvarez RB et al. Beta APP gene transfer into cultured human muscle induces inclusion-body myositis aspects. Neuroreport 1997;8:2155-2158. [DOI] [PubMed] [Google Scholar]
- 97.Askanas V, McFerrin J, Baque S et al. Transfer of beta-amyloid precursor protein gene using adenovirus vector causes mitochondrial abnormalities in cultured normal human muscle. Proc Natl Acad Sci U S A 1996;93:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Banwell BL, Engel AG. AlphaB-crystallin immunolocalization yields new insights into inclusion body myositis. Neurology 2000;54:1033-1041. [DOI] [PubMed] [Google Scholar]
- 99.Hutchinson DO. Inclusion body myositis: abnormal protein accumulation does not trigger apoptosis. Neurology 1998;51:1742-1745. [DOI] [PubMed] [Google Scholar]
- 100.Pruitt JN, 2nd, Showalter CJ, Engel AG. Sporadic inclusion body myositis: counts of different types of abnormal fibers. Ann Neurol 1996;39:139-143. [DOI] [PubMed] [Google Scholar]
- 101.Askanas V, Engel WK, Alvarez RB. Enhanced detection of congo-red-positive amyloid deposits in muscle fibers of inclusion body myositis and brain of Alzheimer’s disease using fluorescence technique. Neurology 1993;43:1265-1267. [DOI] [PubMed] [Google Scholar]
- 102.Mendell JR, Sahenk Z. Inclusion body myositis. Neurology 1992;42:2231-2232. [DOI] [PubMed] [Google Scholar]
- 103.Neville HE, Baumbach LL, Ringel SP et al. Familial inclusion body myositis: evidence for autosomal dominant inheritance. Neurology 1992;42:897-902. [DOI] [PubMed] [Google Scholar]
- 104.Mendell JR, Sahenk Z, Gales T, Paul L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol 1991;48:1229-1234. [DOI] [PubMed] [Google Scholar]
- 105.Wojcik S, Nogalska A, McFerrin J et al. Myostatin precursor protein is increased and associates with amyloid-beta precursor protein in inclusion-body myositis culture model. Neuropathol Appl Neurobiol 2007;33:238-242. [DOI] [PubMed] [Google Scholar]
- 106.Wojcik S, Engel WK, Yan R et al. NOGO is increased and binds to BACE1 in sporadic inclusion-body myositis and in AbetaPP-overexpressing cultured human muscle fibers. Acta Neuropathol (Berl) 2007;114:517-526. [DOI] [PubMed] [Google Scholar]
- 107.Salajegheh M, Raju R, Schmidt J, Dalakas MC. Upregulation of thrombospondin-1(TSP-1) and its binding partners, CD36 and CD47, in sporadic inclusion body myositis. J Neuroimmunol 2007;187:166-174. [DOI] [PubMed] [Google Scholar]
- 108.Wojcik S, Engel WK, McFerrin J et al. AbetaPP-overexpression and proteasome inhibition increase alphaB-crystallin in cultured human muscle: relevance to inclusion-body myositis. Neuromuscul Disord 2006;16:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paciello O, Wojcik S, Engel WK et al. Parkin and its association with alpha-synuclein and AbetaPP in inclusion-body myositis and AbetaPP-overexpressing cultured human muscle fibers. Acta Myol 2006;25:13-22. [PubMed] [Google Scholar]
- 110.Nogalska A, Engel WK, McFerrin J et al. Homocysteine-induced endoplasmic reticulum protein (Herp) is up-regulated in sporadic inclusion-body myositis and in endoplasmic reticulum stress-induced cultured human muscle fibers. J Neurochem 2006;96:1491-1499. [DOI] [PubMed] [Google Scholar]
- 111.Broccolini A, Gidaro T, Morosetti R et al. Neprilysin participates in skeletal muscle regeneration and is accumulated in abnormal muscle fibres of inclusion body myositis. J Neurochem 2006;96:777-789. [DOI] [PubMed] [Google Scholar]
- 112.Li J, Yin C, Okamoto H et al. Proteomic analysis of inclusion body myositis. J Neuropathol Exp Neurol 2006;65:826-833. [DOI] [PubMed] [Google Scholar]
- 113.Wojcik S, Engel WK, McFerrin J, Askanas V. Myostatin is increased and complexes with amyloid-beta within sporadic inclusion-body myositis muscle fibers. Acta Neuropathol (Berl) 2005;110:173-177. [DOI] [PubMed] [Google Scholar]
- 114.Ferrer I, Carmona M, Blanco R et al. Involvement of clusterin and the aggresome in abnormal protein deposits in myofibrillar myopathies and inclusion body myositis. Brain Pathol 2005;15:101-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fratta P, Engel WK, Van Leeuwen FW et al. Mutant ubiquitin UBB+1 is accumulated in sporadic inclusion-body myositis muscle fibers. Neurology 2004;63:1114-1117. [DOI] [PubMed] [Google Scholar]
- 116.Broccolini A, Ricci E, Pescatori M et al. Insulin-like growth factor I in inclusion-body myositis and human muscle cultures. J Neuropathol Exp Neurol 2004;63:650-659. [DOI] [PubMed] [Google Scholar]
- 117.Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol 2004;164:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Choi YC, Kim TS, Kim SY. Increase in transglutaminase 2 in idiopathic inflammatory myopathies. Eur Neurol 2004;51:10-14. [DOI] [PubMed] [Google Scholar]
- 119.Vattemi G, Engel WK, McFerrin J, Askanas V. Cystatin C colocalizes with amyloid-beta and coimmunoprecipitates with amyloid-beta precursor protein in sporadic inclusion-body myositis muscles. J Neurochem 2003;85:1539-1546. [DOI] [PubMed] [Google Scholar]
- 120.Jaworska-Wilczynska M, Wilczynski GM, Engel WK et al. Three lipoprotein receptors and cholesterol in inclusion-body myositis muscle. Neurology 2002;58:438-445. [DOI] [PubMed] [Google Scholar]
- 121.Greenberg SA, Sanoudou D, Haslett JN et al. Molecular profiles of inflammatory myopathies. Neurology 2002;59:1170-1182. [DOI] [PubMed] [Google Scholar]
- 122.Vattemi G, Engel WK, McFerrin J et al. Presence of BACE1 and BACE2 in muscle fibres of patients with sporadic inclusion-body myositis. Lancet 2001;358:1962-1964. [DOI] [PubMed] [Google Scholar]
- 123.Wilczynski GM, Engel WK, Askanas V. Novel cytoplasmic immunolocalization of RNA polymerase II in inclusion-body myositis muscle. Neuroreport 2001;12:1809-1814. [DOI] [PubMed] [Google Scholar]
- 124.Zanusso G, Vattemi G, Ferrari S et al. Increased expression of the normal cellular isoform of prion protein in inclusion-body myositis, inflammatory myopathies and denervation atrophy. Brain Pathol 2001;11:182-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi Y-C, Park GT, Kim T-S et al. Sporadic inclusion body myositis correlates with increased expression and cross-linking by transglutaminases 1 and 2. J. Biol. Chem 2000;275:8703-8710. [DOI] [PubMed] [Google Scholar]
- 126.Choi YC, Dalakas MC. Expression of matrix metalloproteinases in the muscle of patients with inflammatory myopathies. Neurology 2000;54:65-71. [DOI] [PubMed] [Google Scholar]
- 127.Wilczynski GM, Engel WK, Askanas V. Cyclin-dependent kinase 5 colocalizes with phosphorylated tau in human inclusion-body myositis paired-helical filaments and may play a role in tau phosphorylation. Neurosci Lett 2000;293:33-36. [DOI] [PubMed] [Google Scholar]
- 128.Kok CC, Boyt A, Gaudieri S et al. Mitochondrial DNA variants in inclusion body myositis. Neuromuscul Disord 2000;10:604-611. [DOI] [PubMed] [Google Scholar]
- 129.Askanas V, Engel WK, Alvarez RB et al. Novel immunolocalization of alpha-synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J Neuropathol Exp Neurol 2000;59:592-598. [DOI] [PubMed] [Google Scholar]
- 130.Wilczynski GM, Engel WK, Askanas V. Association of active extracellular signal-regulated protein kinase with paired helical filaments of inclusion-body myositis muscle suggests its role in inclusion-body myositis tau phosphorylation. Am J Pathol 2000;156:1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Broccolini A, Engel WK, Alvarez RB, Askanas V. Redox factor-1 in muscle biopsies of patients with inclusion-body myositis. Neurosci Lett 2000;287:1-4. [DOI] [PubMed] [Google Scholar]
- 132.Li M, Dalakas MC. The muscle mitogen-activated protein kinase is altered in sporadic inclusion body myositis. Neurology 2000;54:1665-1670. [DOI] [PubMed] [Google Scholar]
- 133.Broccolini A, Engel WK, Alvarez RB, Askanas V. Paired helical filaments of inclusion-body myositis muscle contain RNA and survival motor neuron protein. Am J Pathol 2000;156:1151-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Askanas V, Engel WK, Yang CC et al. Light and electron microscopic immunolocalization of presenilin 1 in abnormal muscle fibers of patients with sporadic inclusion-body myositis and autosomal-recessive inclusion-body myopathy. Am J Pathol 1998;152:889-895. [PMC free article] [PubMed] [Google Scholar]
- 135.Yang CC, Askanas V, Engel WK, Alvarez RB. Immunolocalization of transcription factor NF-kappaB in inclusion-body myositis muscle and at normal human neuromuscular junctions. Neurosci Lett 1998;254:77-80. [DOI] [PubMed] [Google Scholar]
- 136.Semino-Mora C, Dalakas MC. Rimmed vacuoles with beta-amyloid and ubiquitinated filamentous deposits in the muscles of patients with long-standing denervation (postpoliomyelitis muscular atrophy): similarities with inclusion body myositis. Hum Pathol 1998;29:1128-1133. [DOI] [PubMed] [Google Scholar]
- 137.Yang CC, Alvarez RB, Engel WK, Askanas V. Increase of nitric oxide synthases and nitrotyrosine in inclusion-body myositis. Neuroreport 1996;8:153-158. [DOI] [PubMed] [Google Scholar]
- 138.Mirabella M, Alvarez RB, Engel WK et al. Apolipoprotein E and apolipoprotein E messenger RNA in muscle of inclusion body myositis and myopathies. Ann Neurol 1996;40:864-872. [DOI] [PubMed] [Google Scholar]
- 139.Sarkozi E, Askanas V, Engel WK. Abnormal accumulation of prion protein mRNA in muscle fibers of patients with sporadic inclusion-body myositis and hereditary inclusion-body myopathy. Am J Pathol 1994;145:1280-1284. [PMC free article] [PubMed] [Google Scholar]
- 140.Askanas V, Mirabella M, Engel WK et al. Apolipoprotein E immunoreactive deposits in inclusion-body muscle diseases. Lancet 1994;343:364-365. [DOI] [PubMed] [Google Scholar]
- 141.Askanas V, Engel WK, Bilak M et al. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol 1994;144:177-187. [PMC free article] [PubMed] [Google Scholar]
- 142.Askanas V, Bilak M, Engel WK et al. Prion protein is abnormally accumulated in inclusion-body myositis. Neuroreport 1993;5:25-28. [DOI] [PubMed] [Google Scholar]
- 143.Bilak M, Askanas V, Engel WK. Strong immunoreactivity of alpha 1-antichymotrypsin co-localizes with beta-amyloid protein and ubiquitin in vacuolated muscle fibers of inclusion-body myositis. Acta Neuropathol (Berl) 1993;85:378-382. [DOI] [PubMed] [Google Scholar]
- 144.Greenberg SA. A gene expression approach to study perturbed pathways in myositis. Curr Opin Rheumatol 2007;19:536-541. [DOI] [PubMed] [Google Scholar]
- 145.Kimonis VE, Watts GD. Autosomal dominant inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Alzheimer Dis Assoc Disord 2005;19 Suppl 1:S44-47. [DOI] [PubMed]
- 146.Nogalska A, Wojcik S, Engel WK et al. Endoplasmic reticulum stress induces myostatin precursor protein and NF-kappaB in cultured human muscle fibers: relevance to inclusion body myositis. Exp Neurol 2007;204:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Malicdan MC, Noguchi S, Nonaka I et al. A GNE knockout mouse expressing human V572L mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 2007;16:115-128. [DOI] [PubMed] [Google Scholar]
- 148.Morosetti R, Mirabella M, Gliubizzi C et al. MyoD expression restores defective myogenic differentiation of human mesoangioblasts from inclusion-body myositis muscle. Proc Natl Acad Sci U S A 2006;103:16995-17000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chahin N, Engel AG. Correlation of muscle biopsy, clinical course, and outcome in PM and sporadic IBM. Neurology 2008;70:418-24. [DOI] [PubMed] [Google Scholar]
- 150.Huang S, Liang J, Zheng M et al. Inducible overexpression of wild-type prion protein in the muscles leads to a primary myopathy in transgenic mice. Proc Natl Acad Sci U S A 2007;104:6800-6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dalakas MC, Rakocevic G, Shatunov A et al. Inclusion body myositis with human immunodeficiency virus infection: four cases with clonal expansion of viral-specific T cells. Ann Neurol 2007;61:466-475. [DOI] [PubMed] [Google Scholar]
- 152.Hadjivassiliou M, Chattopadhyay AK, Grunewald RA et al. Myopathy associated with gluten sensitivity. Muscle Nerve 2007;35:443-450. [DOI] [PubMed] [Google Scholar]
- 153.Weihl CC, Miller SE, Hanson PI, Pestronk A. Transgenic expression of inclusion body myopathy associated mutant p97/VCP causes weakness and ubiquitinated protein inclusions in mice. Hum Mol Genet 2007;16:919-928. [DOI] [PubMed] [Google Scholar]
- 154.Hatanaka Y, Oh SJ. Single-fiber electromyography in sporadic inclusion body myopathy. Clin Neurophysiol 2007;118:1563-1568. [DOI] [PubMed] [Google Scholar]
- 155.Finch CE. A perspective on sporadic inclusion-body myositis: the role of aging and inflammatory processes. Neurology 2006;66(2 Suppl 1):S1-6. [DOI] [PubMed] [Google Scholar]
- 156.Glabe CG, Kayed R. Common structure and toxic function of amyloid oligomers implies a common mechanism of pathogenesis. Neurology 2006;66(2 Suppl 1):S74-78. [DOI] [PubMed] [Google Scholar]
- 157.Vetrivel KS, Thinakaran G. Amyloidogenic processing of beta-amyloid precursor protein in intracellular compartments. Neurology 2006;66(2 Suppl 1):S69-73. [DOI] [PubMed] [Google Scholar]
- 158.Moreira PI, Honda K, Zhu X et al. Brain and brawn: parallels in oxidative strength. Neurology 2006;66(2 Suppl 1):S97-101. [DOI] [PubMed] [Google Scholar]
- 159.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology 2006;66(2 Suppl 1):S102-109. [DOI] [PubMed] [Google Scholar]
- 160.Koistinen H, Prinjha R, Soden P et al. Elevated levels of amyloid precursor protein in muscle of patients with amyotrophic lateral sclerosis and a mouse model of the disease. Muscle Nerve 2006;34:444-450. [DOI] [PubMed] [Google Scholar]
- 161.Fidzianska A, Glinka Z. Rimmed vacuoles with beta-amyloid and tau protein deposits in the muscle of children with hereditary myopathy. Acta Neuropathol (Berl) 2006;112:185-193. [DOI] [PubMed] [Google Scholar]
- 162.Dimitri D, Benveniste O, Dubourg O et al. Shared blood and muscle CD8+ T-cell expansions in inclusion body myositis. Brain 2006;129:986-995. [DOI] [PubMed] [Google Scholar]
- 163.McGavern DB. Immunotherapeutic relief from persistent infections and amyloid disorders. Neurology 2006;66(2 Suppl 1):S59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steinman L. Controlling autoimmunity in sporadic inclusion-body myositis. Neurology 2006;66(2 Suppl 1):S56-58. [DOI] [PubMed] [Google Scholar]
- 165.Oldfors A, Moslemi AR, Jonasson L et al. Mitochondrial abnormalities in inclusion-body myositis. Neurology 2006;66(2 Suppl 1):S49-55. [DOI] [PubMed] [Google Scholar]
- 166.Ghetti B, Goebel HH. Frontotemporal dementia: the post-tau era. Neurology 2006;67:560-561. [DOI] [PubMed] [Google Scholar]
- 167.Weihl CC, Dalal S, Pestronk A, Hanson PI. Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet 2006;15:189-199. [DOI] [PubMed] [Google Scholar]
- 168.De Bleecker JL, Creus KK, De Paepe B. Potential therapeutic targets for idiopathic inflammatory myopathies. Drug News Perspect 2006;19:549-557. [DOI] [PubMed] [Google Scholar]
- 169.Authier FJ, Chariot P, Gherardi RK. Skeletal muscle involvement in human immunodeficiency virus (HIV)-infected patients in the era of highly active antiretroviral therapy (HAART). Muscle Nerve 2005;32:247-260. [DOI] [PubMed] [Google Scholar]
- 170.Dalakas MC. Autoimmune muscular pathologies. Neurol Sci 2005;26(Suppl 1):S7-8. [DOI] [PubMed] [Google Scholar]
- 171.Krivickas LS, Amato AA, Krishnan G et al. Preservation of in vitro muscle fiber function in dermatomyositis and inclusion body myositis: a single fiber study. Neuromuscul Disord 2005;15:349-354. [DOI] [PubMed] [Google Scholar]
- 172.Ranque-Francois B, Maisonobe T, Dion E et al. Familial inflammatory inclusion body myositis. Ann Rheum Dis 2005;64:634-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Caccamo A, Oddo S, Sugarman MC et al. Age- and region-dependent alterations in Abeta-degrading enzymes: implications for Abeta-induced disorders. Neurobiol Aging 2005;26:645-654. [DOI] [PubMed] [Google Scholar]
- 174.Koudinov AR, Koudinova NV. Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. J Neurol Sci 2005;229-230:233-240. [DOI] [PubMed]
- 175.Cafforio G, Pistolesi S, D’Avino C et al. Inclusion body myopathy associated with motor neuron syndrome: three case reports. Clin Neuropathol 2005;24:36-41. [PubMed] [Google Scholar]
- 176.Boros S, Kamps B, Wunderink L et al. Transglutaminase catalyzes differential crosslinking of small heat shock proteins and amyloid-beta. FEBS Lett 2004;576:57-62. [DOI] [PubMed] [Google Scholar]
- 177.Gossrau G, Gestrich B, Koch R et al. Apolipoprotein E and alpha-1-antichymotrypsin polymorphisms in sporadic inclusion body myositis. Eur Neurol 2004;51:215-220. [DOI] [PubMed] [Google Scholar]
- 178.Fidzianska A, Rowinska-Marcinska K, Hausmanowa-Petrusewicz I. Coexistence of X-linked recessive Emery-Dreifuss muscular dystrophy with inclusion body myositis-like morphology. Acta Neuropathol (Berl) 2004;107:197-203. [DOI] [PubMed] [Google Scholar]
- 179.Lampe JB, Gossrau G, Kempe A et al. Analysis of HLA class I and II alleles in sporadic inclusion-body myositis. J Neurol 2003;250:1313-1317. [DOI] [PubMed] [Google Scholar]
- 180.Nirmalananthan N, Holton JL, Hanna MG. Is it really myositis? A consideration of the differential diagnosis. Curr Opin Rheumatol 2004;16:684-691. [DOI] [PubMed] [Google Scholar]
- 181.Maurage CA, Bussiere T, Sergeant N et al. Tau aggregates are abnormally phosphorylated in inclusion body myositis and have an immunoelectrophoretic profile distinct from other tauopathies. Neuropathol Appl Neurobiol 2004;30:624-634. [DOI] [PubMed] [Google Scholar]
- 182.Price P, Santoso L, Mastaglia F et al. Two major histocompatibility complex haplotypes influence susceptibility to sporadic inclusion body myositis: critical evaluation of an association with HLA-DR3. Tissue Antigens 2004;64:575-580. [DOI] [PubMed] [Google Scholar]
- 183.Dalakas MC. The use of intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: evidence-based indications and safety profile. Pharmacol Ther 2004;102:177-193. [DOI] [PubMed] [Google Scholar]
- 184.Dalakas MC. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 2004;291:2367-2375. [DOI] [PubMed] [Google Scholar]
- 185.Olive M, Unzeta M, Moreno D, Ferrer I. Overexpression of semicarbazide-sensitive amine oxidase in human myopathies. Muscle Nerve 2004;29:261-266. [DOI] [PubMed] [Google Scholar]
- 186.Bronner IM, Linssen WH, van der Meulen MF et al. Polymyositis: an ongoing discussion about a disease entity. Arch Neurol 2004;61:132-135. [DOI] [PubMed] [Google Scholar]
- 187.Tseng BP, Kitazawa M, LaFerla FM. Amyloid beta-peptide: the inside story. Curr Alzheimer Res 2004;1:231-239. [DOI] [PubMed] [Google Scholar]
- 188.Askanas V, Engel WK. Unicorns, dragons, polymyositis, and other mythical beasts. Neurology 2004;63:403-404, author reply 404. [DOI] [PubMed] [Google Scholar]
- 189.Chitnis T, Khoury SJ. 20. Immunologic neuromuscular disorders. J Allergy Clin Immunol 2003;111:S659-668. [DOI] [PubMed] [Google Scholar]
- 190.Askanas V, Engel WK, McFerrin J, Vattemi G. Transthyretin Val122Ile, accumulated Abeta, and inclusion-body myositis aspects in cultured muscle. Neurology 2003;61:257-260. [DOI] [PubMed] [Google Scholar]
- 191.Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-982. [DOI] [PubMed] [Google Scholar]
- 192.Derk CT, Vivino FB, Kenyon L, Mandel S. Inclusion body myositis in connective tissue disorders: case report and review of the literature. Clin Rheumatol 2003;22:324-328. [DOI] [PubMed] [Google Scholar]
- 193.Massawi G, Hickling P, Hilton D, Patterson C. Inclusion body myositis evolving in systemic lupus erythrematosus? A case report. Rheumatology (Oxford) 2003;42:1012-1014. [DOI] [PubMed] [Google Scholar]
- 194.Muntzing K, Lindberg C, Moslemi AR, Oldfors A. Inclusion body myositis: clonal expansions of muscle-infiltrating T cells persist over time. Scand J Immunol 2003;58:195-200. [DOI] [PubMed] [Google Scholar]
- 195.Parissis D, Karkavelas G, Taskos N, Milonas I. Inclusion body myositis in a patient with a presumed diagnosis of post-polio syndrome. J Neurol 2003;250:619-621. [DOI] [PubMed] [Google Scholar]
- 196.Krause S, Schlotter-Weigel B, Walter MC et al. A novel homozygous missense mutation in the GNE gene of a patient with quadriceps-sparing hereditary inclusion body myopathy associated with muscle inflammation. Neuromuscul Disord 2003;13:830-834. [DOI] [PubMed] [Google Scholar]
- 197.Dalakas MC. High-dose intravenous immunoglobulin in inflammatory myopathies: experience based on controlled clinical trials. Neurol Sci 2003;24(Suppl 4):S256-259. [DOI] [PubMed] [Google Scholar]
- 198.Tateyama M, Saito N, Fujihara K et al. Familial inclusion body myositis: a report on two Japanese sisters. Intern Med 2003;42:1035-1038. [DOI] [PubMed] [Google Scholar]
- 199.Fidzianska A, Kaminska A. Congenital myopathy with abundant ring fibres, rimmed vacuoles and inclusion body myositis-type inclusions. Neuropediatrics 2003;34:40-44. [DOI] [PubMed] [Google Scholar]
- 200.Rutkove SB, Parker RA, Nardin RA et al. A pilot randomized trial of oxandrolone in inclusion body myositis. Neurology 2002;58:1081-1087. [DOI] [PubMed] [Google Scholar]
- 201.Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int 2002;40:85-103. [DOI] [PubMed] [Google Scholar]
- 202.Yazici Y, Kagen LJ. Clinical presentation of the idiopathic inflammatory myopathies. Rheum Dis Clin North Am 2002;28:823-832. [DOI] [PubMed] [Google Scholar]
- 203.van der Meulen MF, Hoogendijk JE, Moons KG et al. Rimmed vacuoles and the added value of SMI-31 staining in diagnosing sporadic inclusion body myositis. Neuromuscul Disord 2001;11:447-451. [DOI] [PubMed] [Google Scholar]
- 204.Phillips BA, Cala LA, Thickbroom GW et al. Patterns of muscle involvement in inclusion body myositis: clinical and magnetic resonance imaging study. Muscle Nerve 2001;24:1526-1534. [DOI] [PubMed] [Google Scholar]
- 205.Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001;80:320-327. [DOI] [PubMed] [Google Scholar]
- 206.Cherin P, Menard D, Mouton P et al. Macrophagic myofasciitis associated with inclusion body myositis: a report of three cases. Neuromuscul Disord 2001;11:452-457. [DOI] [PubMed] [Google Scholar]
- 207.Arnardottir S, Ansved T, Nennesmo I, Borg K. Report of a patient with inclusion body myositis and CD8+ chronic lymphocytic leukaemia--post-mortem analysis of muscle and brain. Acta Neurol Scand 2001;103:131-135. [DOI] [PubMed] [Google Scholar]
- 208.Dalakas MC, Koffman B, Fujii M et al. A controlled study of intravenous immunoglobulin combined with prednisone in the treatment of IBM. Neurology 2001;56:323-327. [DOI] [PubMed] [Google Scholar]
- 209.Ozden S, Gessain A, Gout O, Mikol J. Sporadic inclusion body myositis in a patient with human T cell leukemia virus type 1-associated myelopathy. Clin Infect Dis 2001;32:510-514. [DOI] [PubMed] [Google Scholar]
- 210.Kieseier BC, Schneider C, Clements JM et al. Expression of specific matrix metalloproteinases in inflammatory myopathies. Brain 2001;124:341-351. [DOI] [PubMed] [Google Scholar]
- 211.Lampe JB, Walter MC, Reichmann H. Neurodegeneration-associated proteins and inflammation in sporadic inclusion-body myositis. Adv Exp Med Biol 2001;487:219-228. [DOI] [PubMed] [Google Scholar]
- 212.Kovach MJ, Waggoner B, Leal SM et al. Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab 2001;74:458-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Muller-Felber W, Pongratz D, Reimers C. 64th ENMC International Workshop: therapeutic approaches to dermatomyositis, polymyositis, and inclusion body myositis29-31 January 1999, Naarden, The Netherlands. Neuromuscul Disord 2001;11:88-92. [DOI] [PubMed] [Google Scholar]
- 214.Yamada T, Minohara M, Imaiso Y et al. High-dose vitamin C therapy for inclusion body myositis. Fukuoka Igaku Zasshi 2001;92:99-104. [PubMed] [Google Scholar]
- 215.Tsuruta Y, Yamada T, Yoshimura T et al. Inclusion body myositis associated with hepatitis C virus infection. Fukuoka Igaku Zasshi 2001;92:370-376. [PubMed] [Google Scholar]
- 216.Fam AG. Recent advances in the management of adult myositis. Expert Opin Investig Drugs 2001;10:1265-1277. [DOI] [PubMed] [Google Scholar]
- 217.Fukuchi K, Li L, Hart M, Lindsey JR. Accumulation of amyloid-beta protein in exocrine glands of transgenic mice overexpressing a carboxyl terminal portion of amyloid protein precursor. Int J Exp Pathol 2000;81:231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Capsoni S, Ruberti F, Di Daniel E, Cattaneo A. Muscular dystrophy in adult and aged anti-NGF transgenic mice resembles an inclusion body myopathy. J Neurosci Res 2000;59:553-560. [DOI] [PubMed] [Google Scholar]
- 219.Bedlack RS, Strittmatter WJ, Morgenlander JC. Apolipoprotein E and neuromuscular disease: a critical review of the literature. Arch Neurol 2000;57:1561-1565. [DOI] [PubMed] [Google Scholar]
- 220.Frederikse PH, Zigler SJ, Jr., Farnsworth PN, Carper DA. Prion protein expression in mammalian lenses. Curr Eye Res 2000;20:137-143. [PubMed] [Google Scholar]
- 221.Askanas V, Engel WK, Alvarez RB et al. Inclusion body myositis, muscle blood vessel and cardiac amyloidosis, and transthyretin Val122Ile allele. Ann Neurol 2000;47:544-549. [PubMed] [Google Scholar]
- 222.Gayathri N, Anisya V, Veerendra Kumar M et al. Inclusion body myositis (IBM). Clin Neuropathol 2000;19:13-20. [PubMed] [Google Scholar]
- 223.Amemiya K, Granger RP, Dalakas MC. Clonal restriction of T-cell receptor expression by infiltrating lymphocytes in inclusion body myositis persists over time. Studies in repeated muscle biopsies. Brain 2000;123:2030-2039. [DOI] [PubMed] [Google Scholar]
- 224.Grau JM, Perea M. Dermatomyositis with the features of inclusion body myositis associated with carcinoma of the bladder: a true association? Br J Dermatol 2000;143:671. [DOI] [PubMed] [Google Scholar]
- 225.Boon AJ, Stolp-Smith KA. Inclusion body myositis masquerading as polymyositis: a case study. Arch Phys Med Rehabil 2000;81:1123-1126. [DOI] [PubMed] [Google Scholar]
- 226.Di Blasi C, Mora M, Pareyson D et al. Partial laminin alpha2 chain deficiency in a patient with myopathy resembling inclusion body myositis. Ann Neurol 2000;47:811-816. [PubMed] [Google Scholar]
- 227.Phillips BA, Zilko PJ, Mastaglia FL. Prevalence of sporadic inclusion body myositis in Western Australia. Muscle Nerve 2000;23:970-972. [DOI] [PubMed] [Google Scholar]
- 228.Walter MC, Lochmuller H, Toepfer M et al. High-dose immunoglobulin therapy in sporadic inclusion body myositis: a double-blind, placebo-controlled study. J Neurol 2000;247:22-28. [DOI] [PubMed] [Google Scholar]
- 229.Nakayama T, Horiuchi E, Watanabe T et al. A case of inclusion body myositis with benign monoclonal gammopathy successfully responding to repeated immunoabsorption. J Neurol Neurosurg Psychiatry 2000;68:230-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mirabella M, Christodoulou K, Di Giovanni S et al. An Italian family with autosomal recessive quadriceps-sparing inclusion-body myopathy (ARQS-IBM) linked to chromosome 9p1. Neurol Sci 2000;21:99-102. [DOI] [PubMed] [Google Scholar]
- 231.Kuo YM, Kokjohn TA, Watson MD et al. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol 2000;156:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Limaye V, Scott G, Kwiatek R, Pile K. Inclusion body myositis associated with systemic lupus erythematosus (SLE). Aust N Z J Med 2000;30:275-276. [DOI] [PubMed] [Google Scholar]
- 233.Guyer C. Recent developments in animal models of human neurodegenerative diseases. Toxicol Pathol 2000;28:363-366. [DOI] [PubMed] [Google Scholar]
- 234.Barohn RJ, Amato AA. Inclusion Body Myositis. Curr Treat Options Neurol 2000;2:7-12. [DOI] [PubMed] [Google Scholar]
- 235.Talanin NY, Bushore D, Rasberry R et al. Dermatomyositis with the features of inclusion body myositis associated with carcinoma of the bladder. Br J Dermatol 1999;141:926-930. [DOI] [PubMed] [Google Scholar]
- 236.Nakano S, Akiguchi I, Nakamura S et al. Aberrant expression of cyclin-dependent kinase 5 in inclusion body myositis. Neurology 1999;53:1671-1676. [DOI] [PubMed] [Google Scholar]
- 237.Kok CC, Croager EJ, Witt CS et al. Mapping of a candidate region for susceptibility to inclusion body myositis in the human major histocompatibility complex. Immunogenetics 1999;49:508-516. [DOI] [PubMed] [Google Scholar]
- 238.Lampe J, Kitzler H, Walter MC et al. Methionine homozygosity at prion gene codon 129 may predispose to sporadic inclusion-body myositis. Lancet 1999;353:465-466. [DOI] [PubMed] [Google Scholar]
- 239.Villanova M, Ceuterick C, Dotti MT et al. Detection of beta-A4 amyloid and its precursor protein in the muscle of a patient with juvenile neuronal ceroid lipofuscinosis (Spielmeyer-Vogt-Sjogren). Acta Neuropathol (Berl) 1999;98:78-84. [DOI] [PubMed] [Google Scholar]
- 240.McCoy AL, Bubb MR, Plotz PH, Davis JC. Inclusion body myositis long after dermatomyositis: a report of two cases. Clin Exp Rheumatol 1999;17:235-239. [PubMed] [Google Scholar]
- 241.Mastaglia FL, Phillips BA, Zilko PJ. Inflammatory Myopathy. Curr Treat Options Neurol 1999;1:263-272. [DOI] [PubMed] [Google Scholar]
- 242.Fyhr IM, Moslemi AR, Lindberg C, Oldfors A. T cell receptor beta-chain repertoire in inclusion body myositis. J Neuroimmunol 1998;91:129-134. [DOI] [PubMed] [Google Scholar]
- 243.Levine TD, Pestronk A. Inflammatory myopathy with cytochrome oxidase negative muscle fibers: methotrexate treatment. Muscle Nerve 1998;21:1724-1728. [DOI] [PubMed] [Google Scholar]
- 244.Lodi R, Taylor DJ, Tabrizi SJ et al. Normal in vivo skeletal muscle oxidative metabolism in sporadic inclusion body myositis assessed by 31P-magnetic resonance spectroscopy. Brain 1998;121:2119-2126. [DOI] [PubMed] [Google Scholar]
- 245.Koffman BM, Sivakumar K, Simonis T et al. HLA allele distribution distinguishes sporadic inclusion body myositis from hereditary inclusion body myopathies. J Neuroimmunol 1998;84:139-142. [DOI] [PubMed] [Google Scholar]
- 246.Bender A, Behrens L, Engel AG, Hohlfeld R. T-cell heterogeneity in muscle lesions of inclusion body myositis. J Neuroimmunol 1998;84:86-91. [DOI] [PubMed] [Google Scholar]
- 247.van der Meulen MF, Hoogendijk JE, Jansen GH et al. Absence of characteristic features in two patients with inclusion body myositis. J Neurol Neurosurg Psychiatry 1998;64:396-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Tome FM, Fardeau M. Hereditary inclusion body myopathies. Curr Opin Neurol 1998;11:453-459. [DOI] [PubMed] [Google Scholar]
- 249.Darin N, Kyllerman M, Wahlstrom J et al. Autosomal dominant myopathy with congenital joint contractures, ophthalmoplegia, and rimmed vacuoles. Ann Neurol 1998;44:242-248. [DOI] [PubMed] [Google Scholar]
- 250.Oyama F, Murakami N, Ihara Y. Chloroquine myopathy suggests that tau is degraded in lysosomes: implication for the formation of paired helical filaments in Alzheimer’s disease. Neurosci Res 1998;31:1-8. [DOI] [PubMed] [Google Scholar]
- 251.Yang CC, Alvarez RB, Engel WK et al. Nitric oxide-induced oxidative stress in autosomal recessive and dominant inclusion-body myopathies. Brain 1998;121:1089-1097. [DOI] [PubMed] [Google Scholar]
- 252.Horvath R, Fu K, Johns T et al. Characterization of the mitochondrial DNA abnormalities in the skeletal muscle of patients with inclusion body myositis. J Neuropathol Exp Neurol 1998;57:396-403. [DOI] [PubMed] [Google Scholar]
- 253.Amato AA, Shebert RT. Inclusion body myositis in twins. Neurology 1998;51:598-600. [DOI] [PubMed] [Google Scholar]
- 254.Dalakas MC. Controlled studies with high-dose intravenous immunoglobulin in the treatment of dermatomyositis, inclusion body myositis, and polymyositis. Neurology 1998;51(6 Suppl 5):S37-45. [DOI] [PubMed] [Google Scholar]
- 255.Querfurth HW, Jiang J, Geiger JD, Selkoe DJ. Caffeine stimulates amyloid beta-peptide release from beta-amyloid precursor protein-transfected HEK293 cells. J Neurochem 1997;69:1580-1591. [DOI] [PubMed] [Google Scholar]
- 256.Sivakumar K, Semino-Mora C, Dalakas MC. An inflammatory, familial, inclusion body myositis with autoimmune features and a phenotype identical to sporadic inclusion body myositis. Studies in three families. Brain 1997;120:653-661. [DOI] [PubMed] [Google Scholar]
- 257.Dalakas MC, Sonies B, Dambrosia J et al. Treatment of inclusion-body myositis with IVIg: a double-blind, placebo-controlled study. Neurology 1997;48:712-716. [DOI] [PubMed] [Google Scholar]
- 258.Brannagan TH, Hays AP, Lange DJ, Trojaborg W. The role of quantitative electromyography in inclusion body myositis. J Neurol Neurosurg Psychiatry 1997;63:776-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Fyhr IM, Moslemi AR, Mosavi AA et al. Oligoclonal expansion of muscle infiltrating T cells in inclusion body myositis. J Neuroimmunol 1997;79:185-189. [DOI] [PubMed] [Google Scholar]
- 260.Spector SA, Lemmer JT, Koffman BM et al. Safety and efficacy of strength training in patients with sporadic inclusion body myositis. Muscle Nerve 1997;20:1242-1248. [DOI] [PubMed] [Google Scholar]
- 261.Prayson RA, Cohen ML. Ubiquitin immunostaining and inclusion body myositis: study of 30 patients with inclusion body myositis. Hum Pathol 1997;28:887-892. [DOI] [PubMed] [Google Scholar]
- 262.Mastaglia FL, Phillips BA, Zilko P. Treatment of inflammatory myopathies. Muscle Nerve 1997;20:651-664. [DOI] [PubMed] [Google Scholar]
- 263.Jensen ML, Wieting JM, Andary MT et al. Inclusion body myositis and transitional cell carcinoma of the bladder: significant resolution of symptoms after tumor excision. Arch Phys Med Rehabil 1997;78:327-329. [DOI] [PubMed] [Google Scholar]
- 264.Dalakas MC, Illa I, Gallardo E, Juarez C. Inclusion body myositis and paraproteinemia: incidence and immunopathologic correlations. Ann Neurol 1997;41:100-104. [DOI] [PubMed] [Google Scholar]
- 265.Askanas V. New developments in hereditary inclusion body myopathies. Ann Neurol 1997;41:421-422. [DOI] [PubMed] [Google Scholar]
- 266.Authier FJ, Mhiri C, Chazaud B et al. Interleukin-1 expression in inflammatory myopathies: evidence of marked immunoreactivity in sarcoid granulomas and muscle fibres showing ischaemic and regenerative changes. Neuropathol Appl Neurobiol 1997;23:132-140. [PubMed] [Google Scholar]
- 267.Sekul EA, Chow C, Dalakas MC. Magnetic resonance imaging of the forearm as a diagnostic aid in patients with sporadic inclusion body myositis. Neurology 1997;48:863-866. [DOI] [PubMed] [Google Scholar]
- 268.Barohn RJ. The therapeutic dilemma of inclusion body myositis. Neurology 1997;48:567-568. [DOI] [PubMed] [Google Scholar]
- 269.Robertson TA, Dutton NS, Martins RN et al. Beta-amyloid protein-containing inclusions in skeletal muscle of apolipoprotein-E-deficient mice. Am J Pathol 1997;150:417-427. [PMC free article] [PubMed] [Google Scholar]
- 270.Luciano CA, Dalakas MC. Inclusion body myositis: no evidence for a neurogenic component. Neurology 1997;48:29-33. [DOI] [PubMed] [Google Scholar]
- 271.Cupler EJ, Leon-Monzon M, Miller J et al. Inclusion body myositis in HIV-1 and HTLV-1 infected patients. Brain 1996;119:1887-1893. [DOI] [PubMed] [Google Scholar]
- 272.Sivakumar K, Dalakas MC. The spectrum of familial inclusion body myopathies in 13 families and a description of a quadriceps-sparing phenotype in non-Iranian Jews. Neurology 1996;47:977-984. [DOI] [PubMed] [Google Scholar]
- 273.De Bleecker JL, Engel AG, Ertl BB. Myofibrillar myopathy with abnormal foci of desmin positivity. II. Immunocytochemical analysis reveals accumulation of multiple other proteins. J Neuropathol Exp Neurol 1996;55:563-577. [DOI] [PubMed] [Google Scholar]
- 274.Love S, Nicoll JA, Lowe J, Sherriff F. Apolipoprotein E allele frequencies in sporadic inclusion body myositis. Muscle Nerve 1996;19:1605-1607. [DOI] [PubMed] [Google Scholar]
- 275.Amato AA, Gronseth GS, Jackson CE et al. Inclusion body myositis: clinical and pathological boundaries. Ann Neurol 1996;40:581-586. [DOI] [PubMed] [Google Scholar]
- 276.Askanas V, Engel WK, Mirabella M et al. Apolipoprotein E alleles in sporadic inclusion-body myositis and hereditary inclusion-body myopathy. Ann Neurol 1996;40:264-265. [DOI] [PubMed] [Google Scholar]
- 277.Santorelli FM, Sciacco M, Tanji K et al. Multiple mitochondrial DNA deletions in sporadic inclusion body myositis: a study of 56 patients. Ann Neurol 1996;39:789-795. [DOI] [PubMed] [Google Scholar]
- 278.Felice KJ, Grunnet ML. Inclusion body myositis associated with a severe unilateral levodopa-responsive upper extremity tremor. Muscle Nerve 1996;19:787-789. [DOI] [PubMed] [Google Scholar]
- 279.Dalakas MC. Clinical benefits and immunopathological correlates of intravenous immune globulin in the treatment of inflammatory myopathies. Clin Exp Immunol 1996;104(Suppl 1):55-60. [PubMed] [Google Scholar]
- 280.Naumann M, Reichmann H, Goebel HH et al. Glucocorticoid-sensitive hereditary inclusion body myositis. J Neurol 1996;243:126-130. [DOI] [PubMed] [Google Scholar]
- 281.Mirabella M, Alvarez RB, Bilak M et al. Difference in expression of phosphorylated tau epitopes between sporadic inclusion-body myositis and hereditary inclusion-body myopathies. J Neuropathol Exp Neurol 1996;55:774-786. [DOI] [PubMed] [Google Scholar]
- 282.Askanas V, Engel WK. Response to Letter from Rosenblum, W. I.: Two distinct forms of hyperphosphorylated tau in sporatic versus hereditary inclusion myopathy. J Neuropathol Exp Neurol 1996;55:1179-1180. [PubMed] [Google Scholar]
- 283.Fiori MG, Salvi F, Plasmati R, Tassinari CA. Muscle fiber splitting, capillary internalization, and target-like fiber formation in familial amyloidotic polyneuropathy. Clin Neuropathol 1996;15:240-247. [PubMed] [Google Scholar]
- 284.Kagen LJ. New developments in the myositis syndromes. Bull Rheum Dis 1996;45:1-4. [PubMed] [Google Scholar]
- 285.Garlepp MJ, Tabarias H, van Bockxmeer FM et al. Apolipoprotein E epsilon 4 in inclusion body myositis. Ann Neurol 1995;38:957-959. [DOI] [PubMed] [Google Scholar]
- 286.Nadkarni N, Freimer M, Mendell JR. Amyloidosis causing a progressive myopathy. Muscle Nerve 1995;18:1016-1018. [DOI] [PubMed] [Google Scholar]
- 287.Barohn RJ, Amato AA, Sahenk Z et al. Inclusion body myositis: explanation for poor response to immunosuppressive therapy. Neurology 1995;45:1302-1304. [DOI] [PubMed] [Google Scholar]
- 288.Murakami N, Ihara Y, Nonaka I. Muscle fiber degeneration in distal myopathy with rimmed vacuole formation. Acta Neuropathol (Berl) 1995;89:29-34. [DOI] [PubMed] [Google Scholar]
- 289.Cohen AS. Clinical aspects of amyloidosis, including related proteins and central nervous system amyloid. Curr Opin Rheumatol 1994;6:68-77. [DOI] [PubMed] [Google Scholar]
- 290.Harrington CR, Anderson JR, Chan KK. Apolipoprotein E type epsilon 4 allele frequency is not increased in patients with sporadic inclusion-body myositis. Neurosci Lett 1995;183:35-38. [DOI] [PubMed] [Google Scholar]
- 291.Dalakas MC. Update on the use of intravenous immune globulin in the treatment of patients with inflammatory muscle disease. J Clin Immunol 1995;15(6 Suppl):70S-75S. [DOI] [PubMed] [Google Scholar]
- 292.Dalakas MC, Illa I. Common variable immunodeficiency and inclusion body myositis: a distinct myopathy mediated by natural killer cells. Ann Neurol 1995;37:806-810. [DOI] [PubMed] [Google Scholar]
- 293.Dasque F, Laroche M, Marque P et al. Isokinetic strength testing for evaluating the efficacy of intravenous immune globulin therapy for inclusion body myositis. Rev Rhum Engl Ed 1995;62:598-601. [PubMed] [Google Scholar]
- 294.Tsuzuki K, Fukatsu R, Takamaru Y et al. Co-localization of amyloid-associated proteins with amyloid beta in rat soleus muscle in chloroquine-induced myopathy: a possible model for amyloid beta formation in Alzheimer’s disease. Brain Res 1995;699:260-265. [DOI] [PubMed] [Google Scholar]
- 295.Garlepp MJ, Laing B, Zilko PJ et al. HLA associations with inclusion body myositis. Clin Exp Immunol 1994;98:40-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tsuzuki K, Fukatsu R, Takamaru Y et al. Immunohistochemical evidence for amyloid beta in rat soleus muscle in chloroquine-induced myopathy. Neurosci Lett 1994;182:151-154. [DOI] [PubMed] [Google Scholar]
- 297.Gambetti P, Perry G. Alzheimer’s disease and prion proteins: a meeting made in muscle. Am J Pathol 1994;145:1261-1264. [PMC free article] [PubMed] [Google Scholar]
- 298.Akaaboune M, Ma J, Festoff BW et al. Neurotrophic regulation of mouse muscle beta-amyloid protein precursor and alpha 1-antichymotrypsin as revealed by axotomy. J Neurobiol 1994;25:503-514. [DOI] [PubMed] [Google Scholar]
- 299.DeArmond SJ. Alzheimer’s disease and Creutzfeldt-Jakob disease: overlap of pathogenic mechanisms. Curr Opin Neurol 1993;6:872-881. [DOI] [PubMed] [Google Scholar]
- 300.Hopkinson ND, Hunt C, Powell RJ, Lowe J. Inclusion body myositis: an underdiagnosed condition? Ann Rheum Dis 1993;52:147-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Beyenburg S, Zierz S, Jerusalem F. Inclusion body myositis: clinical and histopathological features of 36 patients. Clin Investig 1993;71:351-361. [DOI] [PubMed] [Google Scholar]
- 302.Askanas V, Engel WK, Alvarez RB. Strong immunoreactivity of beta-amyloid precursor protein, including the beta-amyloid protein sequence, at human neuromuscular junctions. Neurosci Lett 1992;143:96-100. [DOI] [PubMed] [Google Scholar]
- 303.Wikipedia, the free encyclopedia. Network theory. http://en.wikipedia.org/wiki/Network_theory (accessed 24 Jan 2009).
- 304.Wikipedia, the free encyclopedia. Centrality. http://en.wikipedia.org/wiki/Centrality (accessed 24 Jan 2009).
- 305.Newman MEJ. A measure of betweenness centrality based on random walks. Social Networks 2005;27:39-54. http://arxiv.org/PS_cache/cond-mat/pdf/0309/0309045v1.pdf (accessed 24 Jan 2009). [Google Scholar]
- 306.Kleinberg JM. Authoritative sources in a hyperlinked environment. Proceedings of the ACM-SIAM symposium on discrete algorithms, 1998:668-77. www.cs.cornell.edu/home/kleinber/auth.pdf (accessed 24 Jan 2009).
- 307.Clauset A, Shalizi CR, Newman MEJ. Power-law distributions in empirical data. http://arxiv.org/PS_cache/arxiv/pdf/0706/0706.1062v1.pdf (accessed 24 Jan 2009).
- 308.Newman MEJ Assortativity mixing in networks. Phys Rev Lett 2002;89:208701 Retrieved January 24, 2009 from http://arxiv.org/PS_cache/cond-mat/pdf/0205/0205405v1.pdf (accessed 24 Jan 2009). [DOI] [PubMed] [Google Scholar]
- 309.Wikipedia, the free encyclopedia. Social networks. http://en.wikipedia.org/wiki/Social_network (accessed 24 Jan 2009).
- 310.National Institutes of Health Freedom of Information Act Office. www.nih.gov/icd/od/foia/index.htm
- 311.Hyland K. Self-citation and self-reference: credibility and promotion in academic publication. J Am Soc Inform Sci Technol 2003;54:251-9. [Google Scholar]
- 312.Gilbert GN. Referencing as persuasion. Soc Stud Sci 1977;7:113-22. www.jstor.org/stable/284636 (accessed 28 Feb 2009). [Google Scholar]
- 313.Garfield E. Citation indexes for science. Science 1955;122:108-11. [DOI] [PubMed] [Google Scholar]
- 314.Dickersin K. Reporting and other biases in studies of Neurontin for migraine, psychiatric/bipolar disorders, nociceptive pain, and neuropathic pain. http://dida.library.ucsf.edu/pdf/oxx18r10 (accessed 28 Feb 2009).
- 315.Hengstman GJD, van Engelen BGM. Polymyositis, invasion of non-necrotic muscle fibres, and the art of repetition. BMJ 2004;329:1464-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bikhchandani S, Hirshleifer D, Welch I. A theory of fads, fashion, custom, and cultural change as informational cascades. J Political Econ 1992;100:992-1026. [Google Scholar]
- 317.Banerjee AH. A simple model of herd behavior. Quart J Econ 1992;107:797-817. [Google Scholar]
- 318.Tatsioni A, Bonitsis NG, Ioannidis JPA. Persistence of contradicted claims in the literature. JAMA 2007;298:2517-26. [DOI] [PubMed] [Google Scholar]
- 319.Wikipedia, the free encyclopedia. Publication bias. http://en.wikipedia.org/wiki/Publication_bias (accessed 24 Jan 2009).
- 320.How to Write a Research Grant. Guidelines from the National Institute of Neurological Disorders and Stroke. www.ninds.nih.gov/funding/write_grant_doc.htm (accessed 24 Jan 2009).
- 321.Wikipedia, the free encyclopedia. Confirmation bias. http://en.wikipedia.org/wiki/Confirmation_bias (accessed 24 Jan 2009).