Abstract
Purpose
To determine the effect of intraocular pressure (IOP) lowering on the optic disc in patients of the Collaborative Initial Glaucoma Treatment Study (CIGTS) after five years.
Design
Randomized clinical trial
Methods
The baseline and five-year stereoscopic optic disc photographs of 348 eyes (patients) randomized to medical or surgical treatment of open-angle glaucoma were assessed by two independent readers for change in a masked side-by-side comparison, and confirmed by an independent committee.
Results
303 (87.1%) eyes showed no change, 22 (6.3%) showed enlargement of the cup along any meridian (progression), and 23 (6.6%) showed a reduction in the cup along any meridian (reversal of cupping). Incidence of optic disc progression was higher (p=0.007) in the medicine group, 18/185 (10%) than in the surgical group 4/163 (3%); and the incidence of reversal of cupping was higher (p<0.001) in the surgical group, 21/163 (13%), than the medicine group, 2/185 (1%), (P<0.001). Visual field worsening (mean deviation) was significantly associated with progression of optic disc cupping (P<0.001). Reversal of cupping was also associated with lower postoperative IOP (P<0.001). Reversal of cupping was not associated with improvement of either visual acuity or central visual fields.
Conclusions
Surgery prevents or delays glaucomatous progression as measured by optic disc criteria in patients with early open-angle glaucoma. Reversal of cupping occurs more frequently in the surgical group than in the medical treatment group. Reversal is associated with lower IOP, but is not associated with improved visual function.
The Collaborative Initial Glaucoma Treatment Study (CIGTS), a randomized clinical trial sponsored by the National Institutes of Health, National Eye Institute, Bethesda, Maryland, was designed to determine the effect of medical and surgical intraocular pressure (IOP) reduction in patients with newly-diagnosed, previously untreated open-angle glaucoma.1 Published interim results demonstrated statistically greater IOP reduction among patients in the surgical group than the medical group, but no statistically significant difference in the primary outcome variable, the CIGTS visual field score.2 Baseline stereoscopic photographs of the optic disc were taken in both eyes of all study participants, and follow-up photographs were obtained on the subset of patients who returned for the five-year follow-up visit. However, these photographs were not examined as an outcome measure of glaucomatous progression. Therefore, we undertook this study to determine the incidence of, and risk factors for glaucomatous optic disc progression in the study eyes of CIGTS patients at five years after treatment.
Methodology
Stereoscopic optic disc photographs were evaluated by the CIGTS Optic Disc Reading Center (ODRC) at the Department of Ophthalmology at the University of Miami Miller School of Medicine. Two independent and experienced optic disc readers, who had graded optic nerve stereoscopic photographs at the Ocular Hypertension Treatment Study Optic Disc Reading Center from 1994 to present, evaluated these photographs. Following the CIGTS-ODRC reading protocol, the two primary readers, who were not physicians, examined each baseline optic disc stereoscopic photograph (right and left stereo-pair) and assessed the quality of the stereoscopic separation and clarity. If photographic quality was adequate, each reader independently evaluated the following stereoscopic disc characteristics: vertical and horizontal central cup-disc (C/D) ratio, focal and diffuse thinning of the neuroretinal rim, presence of a disc hemorrhage, and extent of peripapillary atrophy, and recorded this information on a baseline form. If the two primary readers disagreed with respect to photographic quality, or the adequacy of stereoscopic separation, or any of the optic disc characteristics, a third, senior reader (RKP), who is a glaucoma specialist, adjudicated the assessment.
The CIGTS-ODRC also evaluated all available five-year follow-up stereophotographs of both eyes that were obtained within a 6-month window around the five-year follow-up visit. The two primary readers independently assessed baseline and five-year follow-up stereoscopic photographs in a side-by-side masked fashion to determine clarity and stereoscopic quality, and to determine occurrence of optic disc progression, the main outcome of this report. They were unaware of the dates (sequence) of the stereoscopic photographs, i.e., baseline or follow-up; or the randomization status to either medical or surgical treatment. They recorded change as a dichotomous outcome, i.e., as no change in central C/D ratio in any meridian, or as change, enlargement of the central C/D ratio in any meridian.
The senior reader (RKP) also evaluated many of these photographic sets, including all those baseline and follow-up stereoscopic photographic sets where either (or both) primary reader(s) suspected optic disc change. Copies of baseline and five-year follow-up optic disc stereoscopic photographs that were determined to have changed by both the primary readers and by the senior reader were sent to each member of the CIGTS-ODRC Endpoint Committee (DK Heuer, E Higginbotham, and DR Anderson). Each member independently evaluated the set of stereoscopic photographs in a manner identical to that used by the CIGTS-ODRC. No member of the CIGTS-ODRC Endpoint Committee was aware of the date of the photographs or the randomization status. After their determinations, each member was unmasked to the date/sequence of the photographs, to ensure that each committee member was so secure in the judgment of disc change that s/he would not alter it given this additional information. Indeed, each member confirmed his/her initial assessment. Differences in assessment among Committee members were resolved with phone conferences and consensus was reached for all eyes
The ODRC received 2663 stereoscopic photograph pairs from the CIGTS Coordinating Center at the University of Michigan, Kellogg Eye Center. Of these, 1169 were baseline disc photographs of both eyes of CIGTS patients; 823 were follow-up photographs within the five-year window; and 671 were follow-up photographs not within the five-year window. One eye per patient was included in this report; this study eye was determined by the CIGTS Coordinating Center as the first eye of the 607 patients randomized in CIGTS to undergo treatment.2 The CIGTS-ODRC evaluated the 607 baseline stereoscopic photographs of the study eye, Of these 607 patients, 411 (68%) had five-year follow-up stereoscopic photographs available for comparison; of these 411 photographs, 63 were judged to be of insufficient clarity or inadequate stereoscopic separation to permit assessment. Thus this report analyzes 348 study eye photographs for which both baseline and five-year follow-up photographs were able to be graded.
Of these 348 eyes, 215 (62%) had sequential 35 mm stereoscopic optic disc photographs both at baseline and at five-year follow-up; 94 (27%) had simultaneous stereophotographs both at baseline and follow-up; 20 (5.7%) had sequential stereophotographs at baseline and simultaneous stereophotographs at follow-up; and 19 (5.5%) had simultaneous stereophotographs at baseline and sequential stereophotographs at follow-up.
Interval level variables, including age, visual field parameters, measurement of refractive error, IOP, ETDRS acuity, and vertical and horizontal C/D ratio were expressed as means (standard deviations) and compared with analysis of variance with post hoc least significant difference tests. Visual field parameters included the mean deviation (md), the pattern standard deviation (psd) and the CIGTS visual field score.1 Analysis of 2×2 contingency tables was conducted with Yates’ corrected chi-square or Fisher’s exact test as appropriate. Chi-square analysis was used for higher order tables and the exact permutation form of chi-square was used when small expected values were a consideration. Other analyses are documented as they appear in the results section. All analyses were performed either with SPSS version 15.0 (SPSS, Inc. www.spss.com) or StatXact (Cytel software, www.cytel.com).
Results
Of the 348 eyes with adequate baseline and five-year follow-up stereoscopic photographs, the ODRC judged that 66 demonstrated a change in disc appearance. The baseline and follow-up stereoscopic optic disc photographs of these eyes were sent to the members of the CIGTS-ODRC Endpoint Committee for evaluation. The CIGTS-ODRC Endpoint Committee confirmed a change that had been identified by the ODRC in 45 of 66 eyes between the baseline and five-year follow-up visits. Of these 45 eyes, 22 (49%) were judged to have the larger cup at the five-year follow up visit compared to the baseline. The other 23 eyes (51%) showed a reduction in the size of the central cup at the five-year follow-up visit. By definition, optic disc progression was judged to have occurred in the stereoscopic disc photograph with the larger cup. With this criterion, the follow-up optic disc status can be divided into three groups, 303 (87.1%) with no change, 22 (6.3%) with glaucomatous progression, and 23 (6.6%) with reversal of cupping. (See Figure 1).
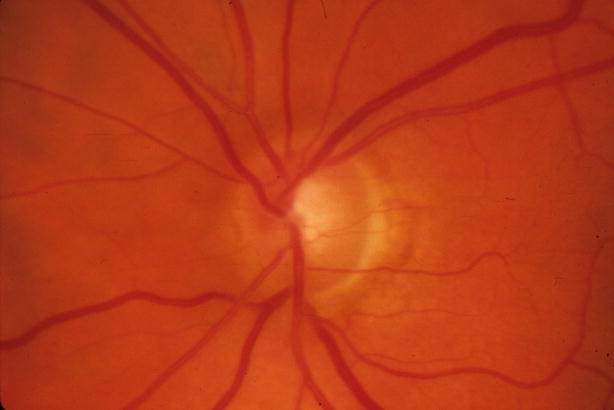
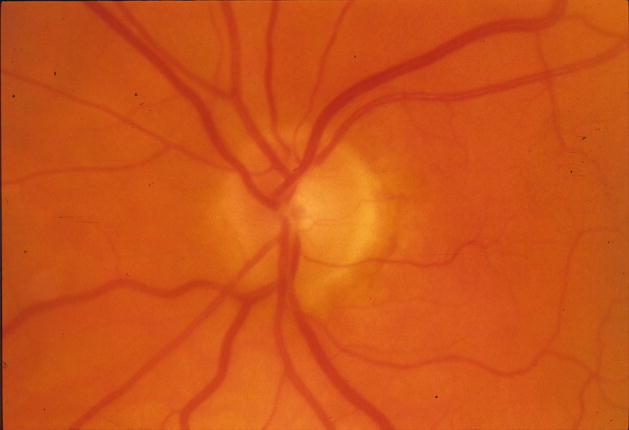
Figure 1.
Reversal of optic disc cupping in a patient in the Collaborative Initial Glaucoma Treatment Study (CIGTS) five-year optic disc study with IOP reduction from 22 mm Hg to 7 mm Hg 61 months after trabeculectomy
Left: Before surgery
Right: 61 months after trabeculectomy
Association of Glaucomatous Disc Changes and Visual Field Changes
The baseline visual field parameters by optic disc change classification are displayed in Table 1, and the changes in visual field parameters from baseline to five-year follow-up in Table 2. An analysis of variance determined significant differences among the three disc change classification groups for each of the measures of follow-up minus baseline visual field differences. (Table 2) Post-hoc least significant difference tests demonstrated that eyes with glaucomatous progression by optic disc criteria had significantly more visual field worsening than those in either the no change group or in the reversal of cupping group for three visual field variables: mean deviation (MD), corrected pattern deviation (PSD) or CIGTS Visual Field Score. The difference in worsening of mean deviation between the progression and no change groups was 3.5 dB (95% confidence interval=1.9, 5.0dB). No differences were determined in visual field parameter change between the reversal of cupping group and the no disc change group. The difference in worsening of mean deviation between the reversal of cupping and no change groups was −0.04dB (95% confidence interval= −1.6, 1.5dB).
Table 1.
Mean (SD) [range] of baseline visual field parameters by disc change classification in the Collaborative Initial Glaucoma Treatment Study (CIGTS) five-year optic disc study
| Baseline Visual Field Parameter | Disc Change Classification | P-valuea | Total N=348 | ||
|---|---|---|---|---|---|
| Reversal of cupping N=23 | No Change N=303 | Glaucomatous Progression N=22 | |||
| Mean Deviation | −5.69 (3.85) [−14.46, 0.03] | −5.13 (4.07) [−19.63,1.54] | −4.45 (2.92) [−10.34, −0.24] | 0.58 | −5.13 (3.99) [−19.63, 1.54] |
| Pattern Standard Deviation | 6.89 (3.94) [1.90, 14.61] | 5.56 (3.45) [1.22, 15.92] | 4.72 (2.49) [2.11, 11.87] | 0.094 | 5.59 (3.45) [1.22, 15.92] |
| CIGTS Visual Field Score | 4.56 (3.81) [0, 14] | 4.43 (4.07) [0, 16] | 4.48 (4.07) [0, 13] | 0.99 | 4.44 (4.05) [0, 16] |
One way analysis of variance
Table 2.
Mean (SD) [range] of follow-up minus baseline changes in visual field parameters by disc change classification in the Collaborative Initial Glaucoma Treatment Study (CIGTS) five-year optic disc study
| Change in Visual Field Parameter | Disc Change Classification | P-valuea | Total N=342b | ||
|---|---|---|---|---|---|
| Reversal of Cupping N=22 | No Change N=298 | Glaucomatous Progression N=22 | |||
| Mean Deviation | 0.33 (3.27) [−7.7, 4.9] | 0.29 (3.35) [−20.3, 10.58] | −3.21 (5.96) [−19.1, 4.36] | <0.001 | 0.06 (3.66) [−20.3, 10.58] |
| Pattern Standard Deviation | 0.01 (2.17) [−3.80, 5.29] | −0.11 (2.10) [−8.84, 8.92] | 1.35 (3.37) [−4.01, 9.22] | 0.012 | −0.01 (2.23) [−8.84, 9.22] |
| CIGTS Visual Field Score | 0.15 (4.02) [−6.90, 9.50] | −0.21 (3.49) [−14.7, 15.00] | 2.20 (3.80) [−3.80, 11.90] | 0.009 | −0.03 (3.58) [−14.7, 15.00] |
One-way analysis of variance
Six eyes lacked follow-up visual field data
Disc Changes and Randomized Treatment Groups
A statistically significant difference in disc classification was confirmed between the medical treatment and surgical treat groups (P<0.001, chi-square), Table 3. Each of the three possible pair-wise associations of optic disc change (baseline to five-year follow-up) classification groups with treatment assignment are also statistically significant (p≤0.014, Fisher’s exact test). A lower proportion of eyes (p=0.007) in the surgical treatment group developed glaucomatous optic disc progression (3%) than did those in the medical treatment group (10%), a seven percent difference (95% confidence interval=1% to 14%). A higher proportion of eyes (p<0.001) in the surgical treatment group also developed reversal of cupping (13%) compared to those in the medical group (1%), a difference of 12% (95% confidence interval=6% to 20%).
Table 3.
Optic disc change by treatment assignment in the Collaborative Initial Glaucoma Treatment Study (CIGTS) five-year optic disc study
| Treatment Group | Disc Change Classification | Total | ||
|---|---|---|---|---|
| Reversal of Cupping | No Change | Glaucomatous Progression | ||
| Medicine | 2 (1%) | 165 (89%) | 18 (10%) | 185 |
| Surgery | 21 (13%) | 138 (85%) | 4 (3%) | 163 |
| Total | 23 (7%) | 303 (87%) | 22 (6%) | 348 |
P<0.001, chi-square
Visual Field Changes in Randomized Treatment Groups in Eyes with Gradable Discs
Among the eyes with gradable disc changes the difference in average MD worsening between the medically treated eyes (0.3±3.9 dB worse at five years follow-up) and surgically treated eyes (0.4±3.3 dB improved at follow-up) but was not statistically significant (p=0.08, t-test). Similarly, PSD increased slightly in the medically treated eyes (0.18±2.43) at follow-up and decreased slightly in the surgically treated eyes (−0.20±1.96) at follow-up, but this difference was not statistically significant (p=0.11, t-test). The CIGTS visual field scores also increased (worsened) slightly in the medically treated eyes (0.24±3.47) and decreased (improved) slightly in the surgically treated eyes (−0.34±3.68) and this difference was not statistically significant (p=0.14). These results were unchanged with respect to direction and significance when the analysis was conducted on all 476 eyes for which five-year visual field data were available and not limited to those with gradable discs.
Eyes with reversal of optic disc cupping
The CIGTS-ODRC and CIGTS-ODRC Endpoint Committee independently identified 23 eyes which, by optic disc criteria, had smaller C/D ratios at the five-year follow-up than at baseline (See Figure 1). These eyes were more likely to have been randomized to the surgical than to the medical treatment group. Eyes randomized to surgery and undergoing trabeculectomy with an antifibrotic (5-FU or mitomycin C) were not statistically significantly (p=0.096, Fisher’s exact test) more likely to demonstrate reversal of cupping (17% of 92 eyes) than those undergoing surgery without an antifibrotic (7% of 68 eyes). Refractive error, baseline and five-year IOP, baseline and five-year follow-up ETDRS visual acuity, baseline C/D ratio, age, and ancestry were studied for their possible association with optic disc classification (Table 4). Post-hoc least significant difference tests demonstrate that for two variables: follow-up IOP and change in IOP from baseline to follow-up, the eyes with reversal of cupping are highly significantly different from the other two groups. Study eyes in the no change group and glaucomatous progression group are similar with respect to these variables. Eyes that demonstrated reversal of cupping had an average IOP that was 6 to 7 mm Hg lower than other study eyes at five years (p<0.001, ANOVA), but reversal was not limited to eyes with marked hypotony (IOP≤5mmHg) at follow-up.
Table 4.
Association of disc change classification status and putative risk factors in the Collaborative Initial Glaucoma Treatment Study (CIGTS) five-year optic disc study
| Putative Risk Factors | Disc Change Classification | P-value | Total | ||
|---|---|---|---|---|---|
| Reversal of Cupping N=23 | No Change N=303 | Glaucomatous Progression N=22 | |||
| Mean (SD) Sphere (diopters) | −1.9 (2.7) | −1.2 (2.7) | −1.7 (2.5) | 0.32a | −1.3 (2.7) |
| N (%)Sphere ≤ −6 | 1 (4%) | 27 (9%) | 3 (14%) | 0.58c | 31 (9%) |
| Mean (SD) Follow-up IOP | 10.2 (3.8) | 16.7 (4.5) | 17.4 (3.1) | <0.001a | 16.4 (4.7) |
| N (%) Follow-up IOP ≤5 mmHg | 1 (5%) | 2 (1%) | 0 | 0.34c | 3 (1%) |
| Mean (SD) Baseline minus Follow-up IOP | 17.5 (5.5) | 10.8 (6.2) | 11.5 (6.7) | <0.001a | 11.3 (6.3) |
| Mean (SD) Baseline Acuity (ETDRS letters) | 85.4 (4.8) | 86.2 (5.6) | 86.7 (5.6) | 0.72a | 86.2 (5.5) |
| Mean (SD) Follow-up Acuity (ETDRS letters) | 78.9 (15.1) | 84.1 (9.2) | 83.9 (11.0) | 0.054a | 83.8 (9.8) |
| Mean (SD) Baseline minus Follow-up Acuity (ETDRS letters) | −6.5 (15.0) | −2.1 (8.8) | −2.8 (10.3) | 0.098a | −2.4 (9.5) |
| Mean (SD) Horizontal Cup/Disc Ratio | 0.63 (0.18) | 0.61 (0.22) | 0.60 (0.19) | 0.86a | 0.61 (0.22) |
| Mean (SD) Vertical Cup/Disc Ratio | 0.68 (0.15) | 0.64 (0.22) | 0.66 (0.18) | 0.66a | 0.64 (0.21) |
| Mean (SD) Age at Randomization | 55.6 (11.8) | 57.6 (10.6) | 57.0 (11.1) | 0.66a | 57.4 (10.7) |
| N (%) African American Ancestry | 5 (22%) | 118 (39%) | 9 (41%) | 0.25b | 132 (38%) |
One-way analysis of variance
Chi-square test
Exact permutation chi-square test
To further explore other factors associated with reversal of cupping, the variables in Table 4 were included in a forward stepwise logistic regression model. Variables significant before adjustment included follow-up IOP (p<0.001), change in IOP from baseline to follow-up (p<0.001), follow-up ETDRS acuity (p=0.015), and change in ETDRS acuity from baseline to follow-up (p=0.032). However, after adjustment, only lower follow-up IOP (p<0.001) was significantly associated with an increased risk of reversal of cupping, with odds ratios 7.5 (95% CI:3.8–14.7) for a 5 mmHg decrease in IOP. No other variables in Table 4 were statistically significant after adjustment for follow-up IOP.
Eyes with reversal of cupping demonstrated visual field changes similar to the group with unchanged discs (Table 2). Table 5 demonstrates the relationship between 5 mm Hg increments of follow-up IOP and the development of reversal of cupping. An example of reversal of cupping is demonstrated in Figure 1. This patient underwent trabeculectomy with reduction of IOP from 22 to 7 mm Hg. Neither visual acuity nor visual field function improved. The change in the course of the first order venule and arteriole at the superior temporal disc border suggest thickening of the peripapillary nerve fiber layer.
Table 5.
Relationship between follow-up IOP and eyes with reversal of cupping, N (%) in the Collaborative Initial Glaucoma Treatment Study (CIGTS) five-year optic disc study
| Ranges of IOP at follow-up (mmHg) | Disc Appearance | Total | |
|---|---|---|---|
| Reversal of Cupping | No Change or Glaucomatous Progression | ||
| ≤5 | 1 (33%) | 2 (67%) | 3 |
| 5.5 – 10 | 9 (35%) | 17 (65%) | 26 |
| 10.5 – 15 | 10 (9%) | 98 (91%) | 108 |
| 15.5 – 20 | 2 (1%) | 151 (99%) | 153 |
| ≥21 | 0 (0%) | 54 (100%) | 54 |
| Total | 22 (6%) | 322 (94%) | 344 |
P<0.001 by Armitage’s test of trend in proportions
Eyes gradable for disc changes at follow-up versus those not gradable
There were available for 482 patients (6 without follow-up field data) with a five-year visit. We compared the 348 (72%) study eyes with gradable disc change status to the 134 (28%) not gradable for disc change status due to lack of stereoscopic photographs or due to photographs having insufficient quality. There was no difference in the prevalence of gradable disc photos between the two randomized treatment arms of the study. Patients graded with respect to disc change were more likely to be of African American ancestry, were slightly younger, and averaged more ETDRS letters read at follow-up, than eyes that were not gradable with respect to disc status. (Supplemental table at AJO.com)
Discussion
Our investigation of baseline and five-year follow-up stereoscopic disc photographs demonstrated a highly statistically significant association of eyes with progressive visual field loss and increased optic nerve cupping or thinning of the neuroretinal rim. Patients in the surgical group were less likely to have progressed by optic disc criteria than those in the medical treatment group. In the previously published interim report no statistically significant difference between visual field loss between the two groups had been demonstrated,2 as was true for the subset with optic disc photographs analyzed in this report. Disc change may be a more sensitive indicator of glaucomatous progression at five-years follow-up than is visual field change in the cohort of patients recruited for the CIGTS, as was the case for detecting the onset of primary open-angle glaucoma in the Ocular Hypertension Treatment Study.3
The finding of more eyes with apparent thickening of the neuroretinal rim and reduction of the size of the central cup (reversal of cupping) than eyes with progressive cupping at follow-up was unexpected. Although this observation has been previously reported in eyes of children with infantile glaucoma after trabeculotomy4–10 and in adults after glaucoma filtering surgery,11–22 we do not believe this finding is commonly appreciated. Transient reduction in optic disc cupping has been described in young adults with acute reduction of IOP associated with probable acute angle closure23, corticosteroid glaucoma24, and surgical intervention.25 The nearly even distribution between the eyes with optic nerve stereoscopic photographs that progressed at follow-up versus those with reversal of cupping may suggest an inability of the six expert optic disc stereoscopic photograph readers (two primary readers, one senior reader, and three endpoint committee readers) to uniformly judge disc progression; however, the substantial differences between these two classes of optic nerve change with respect to IOP, visual field parameters, and treatment assignment make this unlikely.
We determined that eyes with lower postoperative IOP and those that received surgical treatment were more likely to develop reversal of cupping. Spaeth and coworkers previously published photographs of eyes that underwent glaucoma filtering surgery with marked reduction of IOP that also demonstrated reversal of cupping, and were comparable to what we observed in this study.26 Several authors have hypothesized pathophysiologic mechanisms to explain this finding including the increased elasticity of the optic nerve head in children compared with adults8, distension of the scleral canal in infants7, reduction in the diameter of the scleral canal8,13, anterior movement of the lamina cribrosa27, and proliferation of astroglia.5,28,29 Two experimental studies suggested that eyes with larger central cups are less likely to demonstrate reversal of cupping with reduction of IOP.30,31
We speculate that this unexpected five-year finding is likely related to a mechanical deformation of the lamina cribrosa and prelaminar nerve fiber rather than to repair or regeneration of the retinal nerve fiber layer. The reversal likely follows anterior movement of the lamina cribrosa and peripapillary scleral with the possibility of a thickening of the prelaminar neural tissue as a separate component in a subset of these eyes. The eyes with reversal of cupping may have developed clinically visible prelaminal neural tissue thickening on the basis of several possible etiologies, including: 1) shift of axonal fluid from the peripapillary retinal nerve fiber layer downstream into the prelaminar neural tissues; 2) shift of fluid that had been pushed downstream through the lamina at higher IOP levels and subsequently redistruibted posteriorly into the prelaminar axons at lower IOP; 3) some increase in axoplasmic transport blockade within the lamina cribrosa by the relative constriction of the laminar pores after the taut lima relaxes; and 4) long-term prelaminar glial proliferation and connective tissue synthesis in response to axonal loss during the time when the optic nerve head tissues conformed to a new architecture. (Personal communication, Claude F. Burgoyne, MD, March 3, 2008)
The eyes with reversal of cupping did not demonstrate an improvement in either visual acuity or visual field function at five years. Because stereoscopic photographs were not available for study during the interval between the baseline and five-year follow-up, we cannot determine precisely when the reversal of cupping developed. We speculate that if this change is directly related to the acuteness and magnitude of the IOP reduction, then it would have been observed in the early postoperative period. We cannot exclude the possibility that subtle disc damage occurred in eyes that also demonstrated reversal of cupping. In principle, disc damage masked by anterior movement of the lamina and resultant reduction of the central cup, could lessen the magnitude of some of the associations that were significant in our analyses. However, we believe this is unlikely as eyes with disc reversal had reduced IOP and no visual field worsening on average.
As 134 (28%) of study eyes with five-year follow-up data available could not be evaluated for disc changes, either due to lack of stereoscopic photographs or due to photographs having insufficient quality, we investigated possible selection bias by comparing them to those eyes having gradable discs. Neither randomized treatment arm was significantly more likely to have contributed gradable disc photographs. Individuals of African American ancestry were more likely to contribute gradable disc photographs than were individuals of other ethnicities. The highly significant difference (p<0.001) in age between those with gradable photographs and those without was small, only 3.6 years (95% confidence interval: 1.5, 5.7 years). There was a highly significant difference in visual acuity at follow-up, with the ungradable group seeing 4.2 fewer ETDRS letters than those with gradable photographs. If this were due to the effects of unoperated cataracts, we would expect more cataract extractions to have been performed in the group with gradable photographs; however, fewer cataract extractions were performed in this group. (p=0.038). Modest differences were also observed in MD and IOP at five years between these two groups. All differences between those graded with respect to disc change and those not graded were either of relatively low statistical significance or of small size and seem unlikely to account for the observed relationship between treatment and disc change. Most importantly neither randomized treatment group was significantly more likely to contribute gradable photographs.
This study has shown that surgery prevented or delayed glaucomatous progression in CITGS patients with early open-angle glaucoma; the surgical group showed less glaucomatous optic disc progression than the medical treatment group. The study has also documented the phenomenon, reversal of cupping. This reversal of cupping occurred more frequently in the surgical group than in the medical treatment group. Reversal was associated with lower IOP, but was not associated with improved visual function. Future clinical trialists may want to consider the possibility of reversal when measuring other structures that are currently used to assess glaucomatous damage, such as peripapillary retinal nerve layer thickness, as determined by scanning laser polarimety or optical coherence tomography, and optic disc topography, as evaluated with scanning laser ophthalmoscopy.32 This study further supports investigating the role of IOP-lowering with both medical and surgical treatment in altering the characteristics of the anterior optic nerve and retinal nerve fiber layer.
Supplementary Material
Acknowledgments
a. This study was supported by National Institutes of Health, National Eye Institute, Bethesda, Maryland Grants 5R01EY015003-02 and P30EY014801. Allergan Co, Irvine, California provided financial support during years 2004 and 2005 to the CIGTS Administrative, Coordinating, and Quality of Life Centers in Ann Arbor, Michigan as well as to the CIGTS Clinical Centers to support activities related to patient follow-up.
Footnotes
Presented as the American Journal of Ophthalmology Lecture at the XXVII Pan-American Congress of Ophthalmology in Cancun, Mexico, on June 2, 2007
Supplemental material available at AJO.com
b. Financial disclosures: None
c. Contribution of authors: Design of study (RP, WF, JS); Conduct of study (RP, WF, JS); Data Collection (RP, PL, DM); Data management and analysis (RP, WF, JS, PL, DM); Interpretation of data (RP, WF, JS, PL, DM); Manuscript preparation, review, approval (RP, WF, JS, PL, DM)
d. Statement about conformity with Author: Western Institutional Review Board # 20051060; CIGTS is registered with www.clinicaltrials.gov identifier NCT00000149
e. Appendix: The CIGTS Optic Disc Study Group by Center
Optic Disc Reading Center: University of Miami, Miami, FL: RK Parrish, MD (Principal Investigator); RE Vandenbrouke (Reading Center Director); J Beauperthuy (Coordinator); A James (Reader); WJ Feuer, MS (Biostatistician); JC Schiffman, MS (Biostatistician)
Administrative Center: University of Michigan, Ann Arbor, Michigan: PR. Lichter, MD (Study Chairman)
Coordinating Center: University of Michigan, Ann Arbor, Michigan: DC Musch, PhD, MPH (Director); BW Gillespie, PhD (Biostatistician); LM Niziol, MS (Biostatistician)
Endpoint Committee: DR Anderson, MD; DK Heuer, MD; EJ Higginbotham, MD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Musch DC, Lichter PR, Guire KE, Standardi CL CIGTS Study Group. The Collaborative Initial Glaucoma Treatment Study: Study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–662. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 2.Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;11:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 3.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study. A Randomized Trial Determines That Topical Ocular Hypotensive Medication Delays or Prevents the Onset of Primary Open-Angle Glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 4.Chandler PA, Grant WM. Lectures on glaucoma. Philadelphia: Lea & Feibiger; 1965. p. 327. [Google Scholar]
- 5.Shaffer RN, Hetherington J. The glaucomatous disc in infants. A suggested hypothesis for disc cupping. Trans Am Acad Ophthalmol Otolaryngol. 1968;73:929–935. [PubMed] [Google Scholar]
- 6.Armaly M. In discussion of Hetherington J, Shaffer RN, Hoskins HD. The disc in congenital glaucoma. In: Etienne R, Paterson GD, editors. XXII Congres D′ International Ophthalmologie; International Glaucoma Symposium in Albi; France, Marseille, France. 1975. pp. 129–151. Diffusion-General de Librairie. [Google Scholar]
- 7.Quigley HA. The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol. 1977;84:358–370. doi: 10.1016/0002-9394(77)90680-8. [DOI] [PubMed] [Google Scholar]
- 8.Kessing SV, Gregersen E. The distended disc in early stages of congenital glaucoma. Acta Ophthalmol. 1977;55:431–435. doi: 10.1111/j.1755-3768.1977.tb06119.x. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA. Childhood glaucoma results with trabeculotomy and study of reversible cupping. Ophthalmology. 1982;89:219–226. doi: 10.1016/s0161-6420(82)34803-4. [DOI] [PubMed] [Google Scholar]
- 10.Wu SC, Huang SCM, Kuo CL, Lin KK, Lin SM. Reversal of optic disc cupping after trabeculotomy in primary congenital glaucoma. Can J Ophthalmol. 2002;37:337–341. doi: 10.1016/s0008-4182(02)80003-5. [DOI] [PubMed] [Google Scholar]
- 11.Spaeth GL, Fernandes E, Hitchings RA. The pathogenesis of transient or permanent improvement in the appearance of the optic disc following glaucoma surgery. Docum Ophthalmol Proc Series. 1980;22:111–126. [Google Scholar]
- 12.Pederson JE, Herschler J. Reversal of glaucomatous cupping adults. Arch Ophthalmol. 1982;100:426–431. doi: 10.1001/archopht.1982.01030030428008. [DOI] [PubMed] [Google Scholar]
- 13.Greenidge KC, Spaeth GL, Traverso CE. Change in appearance of the optic disc associated with lowering of intraocular pressure. Ophthalmology. 1985;92:897–903. doi: 10.1016/s0161-6420(85)33937-4. [DOI] [PubMed] [Google Scholar]
- 14.Katz LJ, Spaeth GL, Cantor LB, Poryzees EM, Steinmann WC. Reversible optic disk cupping and visual field improvement in adults with glaucoma. Am J Ophthalmol. 1989;107:485–492. doi: 10.1016/0002-9394(89)90492-3. [DOI] [PubMed] [Google Scholar]
- 15.Funk J. Increase in neuroretinal rim area after surgical intraocular pressure reduction. Ophthalmic Surg. 1990;21:585–588. [PubMed] [Google Scholar]
- 16.Matsubara K, Fujitsuka, Tomita G, Kitazawa Y. Measurements of reversibility of optic disc cupping in glaucoma using a computerized videographic image analyzer. Nippon Ganka Gakkai Zasshi. 1990;94:604–609. [PubMed] [Google Scholar]
- 17.Parrow KA, Shin DH, Tsai CS, Hong YJ, Juzych MS, Shi DX. Intraocular pressure-dependent dynamic changes of optic disc cupping in adult glaucoma patients. Ophthalmology. 1992;99:36–40. doi: 10.1016/s0161-6420(92)32015-9. [DOI] [PubMed] [Google Scholar]
- 18.Irak I, Zangwill L, Garden V, Shakiba S, Weinreb RN. Change in optic disc topography after trabeculectomy. Am J Ophthalmol. 1996;122:690–695. doi: 10.1016/s0002-9394(14)70488-x. [DOI] [PubMed] [Google Scholar]
- 19.Raitta C, Tomita G, Vesti E, Harju M, Nakao H. Optic disc topography before and after trabeculectomy in advanced glaucoma. Ophthalmic Surg Lasers. 1996;27:349–354. [PubMed] [Google Scholar]
- 20.Yoshikawa K, Inoue Y. Changes in optic disc parameters after intraocular pressure reduction in adult glaucoma patients. Jpn J Ophthalmol. 1999;43:225–231. doi: 10.1016/s0021-5155(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 21.Lesk MR, Spaeth GL, Azuara-Blanco A, et al. Reversal of optic disc cupping after glaucoma surgery analyzed with scanning laser tomograph. Ophthalmology. 1999;106:1013–1018. doi: 10.1016/S0161-6420(99)00526-6. [DOI] [PubMed] [Google Scholar]
- 22.Topouzis F, Peng F, Kotas-Neumann R, et al. Longitudinal changes in optic disc topography of adults patients after trabeculectomy. Ophthalmology. 1999;106:1147–1151. doi: 10.1016/S0161-6420(99)90248-8. [DOI] [PubMed] [Google Scholar]
- 23.Von Jaeger E. Ophthalmoskopischer Hand-atlas. Druck und Verlag der K. K. Hofund Staatsdruckerei; Vienna: 1869. [Google Scholar]
- 24.Neuman E, Hyams SW. Intermittent glaucomatous excavation. Arch Ophthalmol. 1973;90:64–66. doi: 10.1001/archopht.1973.01000050066014. [DOI] [PubMed] [Google Scholar]
- 25.Robin AL, Quigley HA. Transient reversible cupping in juvenile-onset glaucoma. Am J Ophthalmol. 1979;88:580–584. doi: 10.1016/0002-9394(79)90518-x. [DOI] [PubMed] [Google Scholar]
- 26.Spaeth GL, Fernandes E, Hitchings RA. The pathogenesis of transient or permanent improvement in the appearance of the optic disc following glaucoma surgery. Docum Ophthal Proc Series. 1980;22:111–125. [Google Scholar]
- 27.Levy NS, Crapps EE. Displacement of optic nerve in response to short-term intraocular pressure elevation in human eyes. Arch Ophthalmol. 1984;102:782–786. doi: 10.1001/archopht.1984.01040030630037. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer RN. The role of astroglial cells in glaucomatous disc cupping. Doc Ophthalmol. 1969;26:516–525. doi: 10.1007/BF00944007. [DOI] [PubMed] [Google Scholar]
- 29.Henkind P, Charles NC, Pearson J. Histopathology of ischemic optic neuropathy. Am J Ophthalmol. 1970;69:78–90. doi: 10.1016/0002-9394(70)91859-3. [DOI] [PubMed] [Google Scholar]
- 30.Shirakashi M, Nanba K, Iwata K. Changes in reversal of cupping in experimental glaucoma. Ophthalmology. 1992;99:1104–1110. doi: 10.1016/s0161-6420(92)31844-5. [DOI] [PubMed] [Google Scholar]
- 31.Coleman AL, Quigley HA, Vitale S, Dunkelberger G. Displacement of the optic nerve head by acute changes in intraocular pressure in monkey eyes. Ophthalmology. 1991;98:35–40. doi: 10.1016/s0161-6420(91)32345-5. [DOI] [PubMed] [Google Scholar]
- 32.Lin SC, Singh K, Jampel HD, Hodapp EA, et al. Optic nerve head and retinal nerve fiber layer analysis. A report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1937–1949. doi: 10.1016/j.ophtha.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.