Abstract
Taxanes are first line drugs for treating prostate cancer recurrence after the failure of anti-androgen therapy. There is a need to make taxanes more effective since they only provide palliative benefit. Exploiting endoplasmic reticulum (ER) stress death signaling to enhance drug efficacy has not been delineated. Human PC-3 cells were used as a model of hormone refractory prostate cancer. Thapsigargin and methylseleninic acid (MSA) were examined as sensitizers. Thapsigargin is a classic ER stress inducer. The activity of MSA in inducing ER stress has recently been studied by our group. The efficacy of single drug and the various combinations was evaluated by measuring apoptosis with a cell death ELISA kit. Thapsigargin increased the cell killing potency of paclitaxel or docetaxel by 10- to 12-fold, while MSA caused a 5- to 8-fold increase. Since thapsigargin is not used clinically because of its toxicity, the follow-up experiments were done with MSA. To test the hypothesis that a threshold level of ER stress is crucial to chemotherapeutic sensitization, three different approaches designed to dampen the severity of ER stress induced by MSA were examined. Lowering ER stress consistently attenuated the efficacy of MSA/taxane. GADD153 is a pro-apoptotic transcription factor which is up-regulated during ER stress. Knocking down GADD153 by siRNA also reduced the cell killing effect of MSA/taxane. Both the intrinsic and extrinsic apoptotic pathways were involved in the sensitization mechanism. Our study supports the idea that marshalling ER stress apoptotic response is conducive to chemotherapeutic sensitization.
Keywords: selenium, endoplasmic reticulum stress, taxanes, chemosensitization, prostate cancer
Introduction
Endoplasmic reticulum (ER) stress is caused by the accumulation of unfolded or misfolded proteins in the lumen of the ER. The phenomenon unleashes a torrent of molecular and cellular events known collectively as the unfolded protein response or UPR. Depending on the severity or duration of ER stress, pro-survival or pro-apoptotic UPR is activated to either alleviate the damage or eliminate the stressed cells.1 When ER stress is moderate, the pro-survival machinery is mobilized first in order to (i) increase the transcription of chaperones such as GRP78 and GRP94, (ii) block protein translation in general, and (iii) degrade the malfolded proteins. However, if the rescue effort is unable to keep the burden of malfolded proteins in check, the balance will gradually tip towards apoptosis. Increasing attention is being focused on the role of UPR in cancer biology.2–5 At present, the jury is still out regarding the question of how chemotherapeutic sensitivity might be influenced by UPR. In studies with cancer cell lines, some described a sensitization effect,6,7 while others argued for the evidence of increased resistance.8,9 Cell context and drug action are undoubtedly among a host of factors which are likely to impinge on the outcome.3,5 Despite the controversy, there is little disagreement that powerful ER stress inducers, e.g. thapsigargin, tunicamycin and calcium inophore, are able to stimulate apoptosis in a variety of cancer cells.10–13 Given this backdrop, our research was designed to validate a clinically feasible approach in which the pro-apoptotic arm of UPR can be successfully incorporated in a strategy aimed at improving chemotherapeutic efficacy.
Previously we reported that a physiologically achievable dose of selenium has the propensity to induce an acute and robust UPR in prostate cancer cells.14,15 Furthermore, the stress level is strong enough to activate pro-apoptotic signals including GADD153 and the elevation of cytosolic calcium. Chemotherapeutic sensitization by selenium has recently been demonstrated with CPT-11 in vivo,16 and with SN38 (a metabolite of CPT-11) and taxane in vitro.17–19 Scanty information, however, is available on the underlying mechanism. We are taking a fresh look into the sensitization effect of selenium through the lens of UPR. Our current interest is focused on taxanes because they are first line drugs for treating androgen refractory prostate cancer,20 and our extensive selenium ER stress data were generated with the use of human PC-3 cells which are androgen non-responsive. Although taxanes provide palliative benefit against prostate cancer, the survival advantage has not been impressive.21 There is a need to make them more effective. Our research plan consisted of four sequential steps: (i) to compare the activity of selenium with that of thapsigargin in sensitizing PC-3 prostate cancer cells to taxanes; (ii) to test the hypothesis that a threshold level of ER stress is required to produce optimal sensitization by selenium; (iii) to show that knocking down GADD153, a signature pro-apoptotic transcription factor of UPR, affects sensitization; and (iv) to validate the involvement of pathway-specific caspases in apoptotic death caused by the selenium/taxane combination.
Results
Thapsigargin amplifies the cell killing activity of taxanes
Thapsigargin is a prototypical ER stress inducer by acting via the mechanism of calcium release from the ER. To verify that a known ER stressor is able to cause chemosensitization in the PC-3 model, we treated cells with paclitaxel/docetaxel, either alone or in the presence of thapsigargin. In our protocol, cells were exposed to 50 nM of thapsigargin for 6 hours before the addition of paclitaxel or docetaxel. The cells were incubated in the presence of taxane for 16 hours and then processed for the Cell Death ELISA assay. The cell death results are shown in Fig. 1A. Thapsigargin alone produced a modest increase of cell death. Paclitaxel at 30 nM or docetaxel at 20 nM also produced a low level of cell death. However, the combination of thapsigargin/paclitaxel or thapsigargin/docetaxel was found to be much more potent. We also used Western blotting to verify the induction of ER stress markers by thapsigargin. The results of the increases of GRP78, phospho-eIF2α and GADD153 as a function of time are shown in Fig. 1B. Thus there appears to be a good correlation between the ability of thapsigargin to cause ER stress and its efficacy to enhance taxane cell killing.
Figure 1.
A. Induction of cell death by paclitaxel or docetaxel, either in the presence or absence of thapsigargin. *Value statistically greater than the sum of taxane effect plus thapsigargin effect (P<0.05). B. Induction of ER stress response markers by thapsigargin.
The dose of paclitaxel or docetaxel used in this experiment is equivalent to the blood concentration found in patients at 24 hours after a single infusion of the drug. The Cell Death ELISA method is very sensitive. We report the induction of cell death by a particular treatment as a net increase, i.e. treated value minus untreated value, in order to adjust for the slight day-today background variations of the untreated control. Expressing the results as fold of induction tends to distort the empirical data because this method of calculation may lead to either an over-or under-estimation depending on the background value.
MSA sensitization of PC-3 cells to taxanes
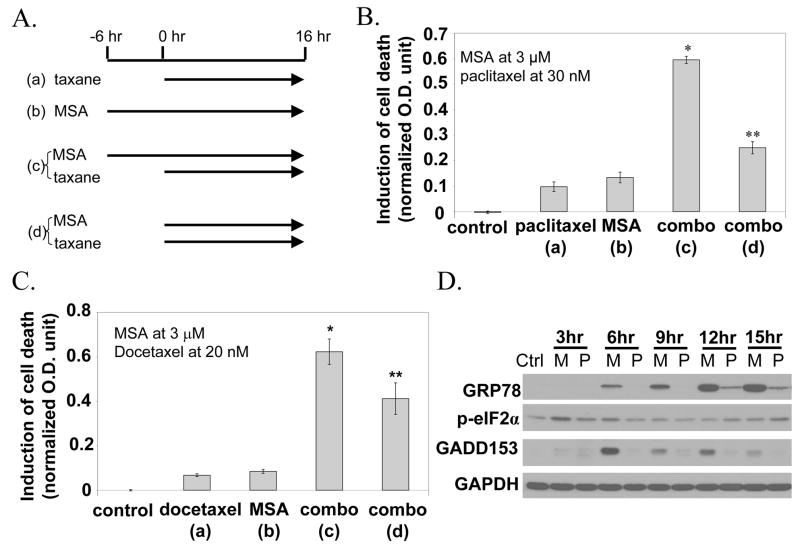
Thapsigargin is not appropriate for clinical use because of its toxicity. Our previous studies showed that methylseleninic acid (MSA) is able to induce ER stress which is severe enough to cause apoptosis.14,15 To study the effect of MSA on taxane sensitization, we evaluated different treatment protocols as illustrated schematically in Fig. 2A. Protocol (a) is taxane alone added to the culture from 0 to 16 hours. Protocol (b) is MSA alone from −6 to 16 hours. The period of −6 to 0 hours is designated as pretreatment with MSA. Protocol (c) is the combination of MSA and taxane, i.e. protocol (a) plus protocol (b). Protocol (d) is also a combination, with the exception that both MSA and taxane are added together from 0 to 16 hours, i.e. there is no pretreatment with MSA. Figure 2B shows the cell death results of the MSA/paclitaxel experiments. The lower case letter in brackets matches the treatment schedule as illustrated in Fig. 2A. Paclitaxel alone at 30 nM or MSA alone at 3 μM produced a very modest increase of cell death. The concentration of MSA was intentionally kept at the lower range of efficacy. The protocol (c) combination produced a robust increase which was substantially greater than the sum of the single drug effect. In contrast, the protocol (d) combination was much less effective, it was only slightly better than the individual agent protocol. The data thus suggest that pretreatment with MSA is necessary for sensitization to paclitaxel. Figure 2C shows essentially the same kind of results with 20 nM docetaxel. In all subsequent experiments, we no longer used protocol (d). Every time we mention a combination protocol, it is protocol (c) that we are referring to.
Figure 2.
A. Treatment schedule of taxane and/or MSA in 4 different protocols. B. Induction of cell death by paclitaxel, MSA, or combination. The lower case letter in brackets matches to a specific protocol illustrated in panel A. C. Induction of cell death by docetaxel, MSA or combination. *Value statistically greater than the sum of taxane effect plus MSA effect (P<0.05). **Statistically different (P<0.05) from protocol (c). D. Induction of ER stress response markers by MSA (M) or paclitaxel (P).
We also compared the induction of ER stress response by either MSA or paclitaxel using the same markers as described above. The Western blot results are shown in Fig. 2D. MSA caused significant increases of GRP78, phospho-eIF2α and GADD153, especially at the early time points. In contrast, paclitaxel failed to produce much of a change in these markers, with the exception of a slight increase of GRP78, and only at the later time points. The data thus suggests that paclitaxel is not an effective inducer of acute ER stress compared to MSA.
GRP78 over-expression or knockdown on cell death induction by MSA/paclitaxel
GRP78, a resident chaperone in the ER, is a key regulator of UPR.1 Normally, a small amount of GRP78 is present in the free form, while the majority is bound primarily to three UPR transducers, PERK, ATF6 and IRE1, to keep them inactive. In times of ER stress, the free GRP78 associates with the damaged proteins to help correct misfolding. To replenish the free GRP78, the bound GRP78 is released from the transducers. This process activates the transducers. An abundance of GRP78 is thus conducive to lowering the severity of ER stress and negating the need to activate the transducers. Conversely, depleting GRP78 is expected to intensify the stress level in the ER. We have previously described how GRP78 availability affects the induction of ER stress response by MSA, in which the over-expression or knockdown of GRP78 leads to either a reversal or an enhancement of the effect of MSA, respectively.14,15
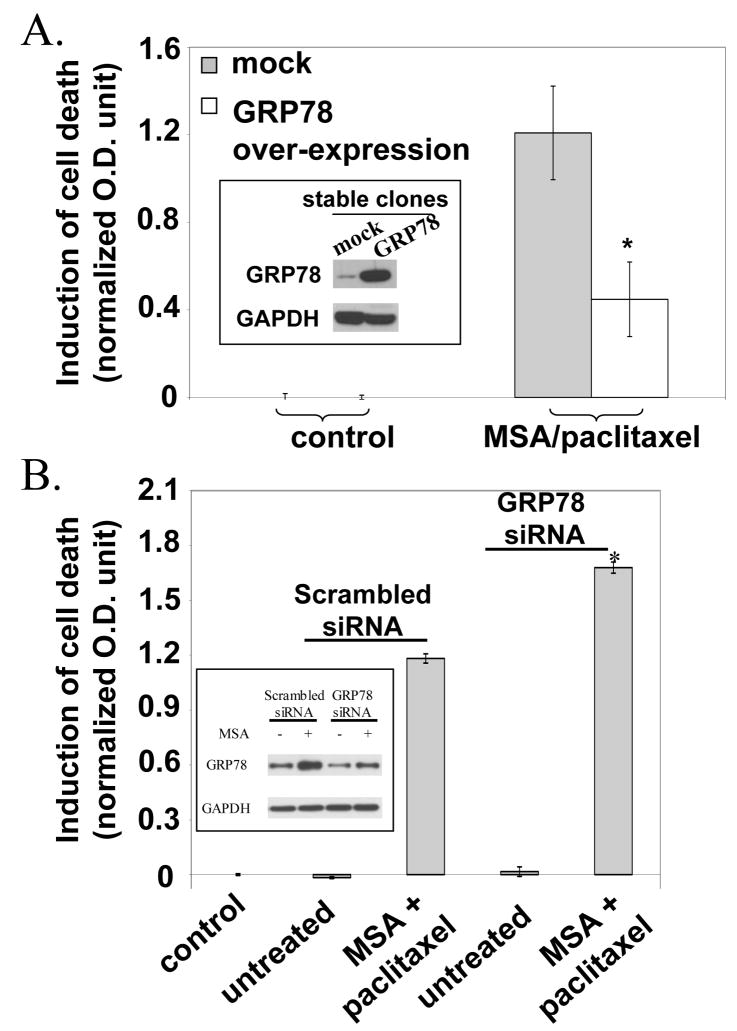
We treated stable GRP78 over-expressing PC-3 cells with MSA and paclitaxel. As shown in Fig. 3A, the combination induced considerably less cell death in the GRP78 over-expressing cells than in the mock transfected cells. The insert shows the Western blot data of GRP78 over-expression. We also performed the opposite experiment in which PC-3 cells were transfected with GRP78 siRNA. The cell death results are shown in Fig. 3B. Transfection with scrambled or GRP78 siRNA alone did not cause cell death in the absence of drugs. As expected, the MSA/paclitaxel combination produced a sizeable increase of cell death in the scrambled siRNA transfected cells. The increase was magnified by about 30% in the GRP78 knockdown cells. The data therefore support the role of ER stress response in paclitaxel sensitization. Since MSA is known to enhance the expression of GRP78 as part of the ER stress response, we carried out Western blot analysis to monitor GRP78 protein level in the scrambled or GRP78 siRNA transfected cells (see insert). The cells were treated with MSA for 6 hours. This time point was chosen to match the length of the pretreatment. In untreated cells, transfection with GRP78 siRNA produced a very small decrease of GRP78 protein level. The reason could be due to the long half-life of GRP78. In contrast, transfection with GRP78 siRNA in MSA-treated cells significantly diminished the induction of newly synthesized GRP78. The Western blot data confirm the high efficiency of GRP78 knockdown, and validate the conclusion that by stripping the cells of GRP78 and henceforth increasing the severity of ER stress, the pro-apoptotic arm of UPR could be recruited to promote paclitaxel sensitization.
Figure 3.
Effect of GRP78 over-expression (panel A) or knockdown (panel B) on cell death induction by MSA/paclitaxel. *Statistically different (P<0.05) from mock or scrambled siRNA transfected cells.
Treatment with chemical chaperone protects cells from MSA/paclitaxel-induced cell death
In an earlier publication, we reported that MSA causes ER stress by a protein thiol-disulfide redox mechanism.22 If ER stress is the spark that ignites the sensitization effect of MSA, then protecting proteins from misfolding would be expected to reduce cell death by MSA/paclitaxel. To test this hypothesis, we treated cells with tauroursodeoxycholic acid (TUDCA), the latter is a chemical chaperone known to stabilize proteins.23 TUDCA was present in the medium at a concentration of 500 μg/ml, and was added to the culture at the same time as MSA. The results in Fig. 4 show that TUDCA significantly attenuated the cell killing potency of MSA/paclitaxel by 50%. We also have evidence that TUDCA markedly decreased the ability of MSA to activate the UPR transducers (data not shown).
Figure 4.
Effect of tauroursodeoxycholic acid (TUDCA) on cell death induction by MSA/paclitaxel. *Sstatistically different (P<0.05) from MSA/paclitaxel without TUDCA.
Blockage of calcium elevation diminishes MSA/paclitaxel-induced cell death
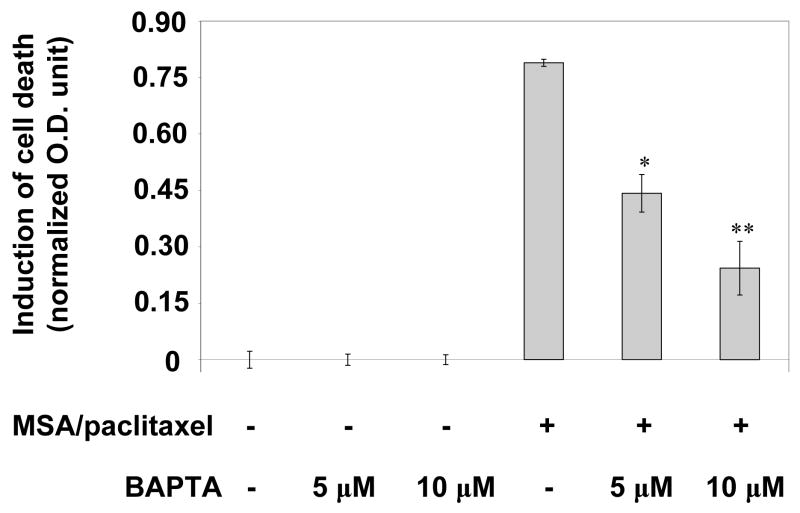
Previously we demonstrated rising calcium in the cytosol after treatment with MSA.24 Calcium is released from the ER to the cytosol in times of ER stress.1,25 The elevation of cytosolic calcium is an apoptotic signal of UPR. To define the role of calcium in chemosensitization, we treated cells with BAPTA-AM, the latter is a cell permeable calcium chelator. We had used this approach to study the modulation of calcium-dependent processes associated with ER stress response.24 BAPTA-AM was added to the culture at the same time as MSA. Figure 5 shows the cell death results following treatment with MSA/paclitaxel, either in the presence or absence of BAPTA-AM. The data indicate that BAPTA-AM, at a concentration of 5 or 10 μM, reversed the cell killing activity of MSA/paclitaxel in a dose-dependent manner.
Figure 5.
Effect of BAPTA-AM on cell death induction by MSA/paclitaxel. *Statistically different (P<0.05) from MSA/paclitaxel without BAPTA-AM. **Statistically different than one asterisk.
GADD153 knockdown attenuates MSA/paclitaxel-induced cell death
GADD153 is a signature pro-apoptotic transcription factor of UPR.26 We reported previously that MSA is able to up-regulate the expression of GADD153.14 We were interested to see whether GADD153 knockdown would reduce the cell killing activity of MSA/paclitaxel. Cells were transfected with scrambled or GADD153 siRNA, and were then treated with MSA alone, paclitaxel alone, or the combination. The efficiency of siRNA knockdown was confirmed by Western blotting as shown on the right side of Fig. 6. These samples were collected after 6 hours of MSA treatment, when the induction of GADD153 was strongly manifested. The cell death results are shown on the left side of Fig. 6. In the scrambled siRNA transfected cells, MSA or paclitaxel by itself caused only small increases of cell death. On the other hand, the MSA/paclitaxel combination produced a much larger increase. In the GADD153 siRNA transfected cells, the single drug effect was similar to that observed in the scrambled siRNA transfected cells. However, the combination effect was significantly reduced by the knockdown of GADD153 (see insert for the Western blot data of GADD153). Granted the decrease was relatively modest. This could be due to two reasons: first, only a fraction of the cell population was successfully transfected; and second, GADD153 is not the only death signaling molecule to contribute to the sensitization effect (this point will be elaborated in greater detail in the Discussion). The fact that the change was in the right direction is more meaningful than the magnitude of the change.
Figure 6.
Effect of GADD153 knockdown on cell death induction by MSA/paclitaxel. *Value statistically greater (P<0.05) than sum of paclitaxel effect plus MSA effect. **Statistically different from one asterisk.
MSA and paclitaxel synergistically activate caspase-8 and caspase-9
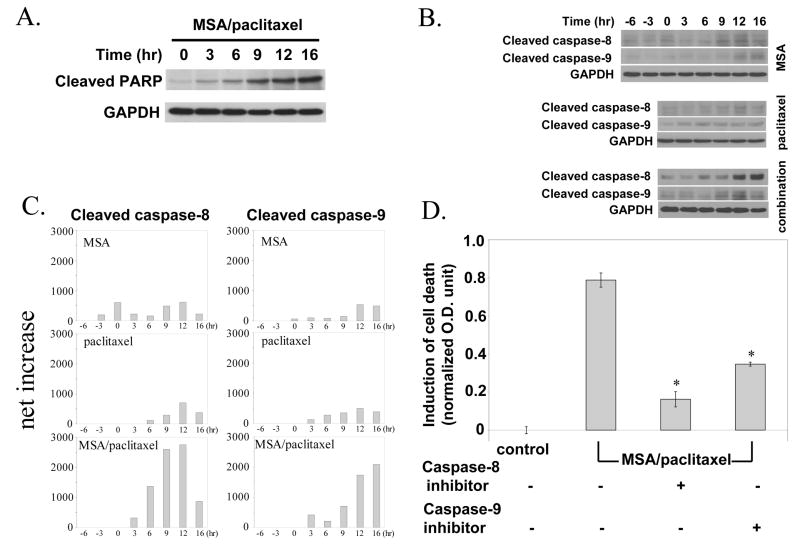
A hallmark of caspase-dependent apoptotic death is the proteolytic cleavage of poly (ADP-ribose) polymerase or PARP, the latter is an enzyme involved in DNA damage repair and maintenance of genome stability. The ability of MSA/paclitaxel to induce PARP cleavage was investigated in a time course experiment. The results in Fig. 7A show that PARP cleavage first became noticeable at 6 hours and progressed steadily as a function of time.
Figure 7.
Role of caspases in cell death induction by MSA/paclitaxel. A. Western blot of PARP cleavage by MSA/paclitaxel. B. Western blot of cleaved caspase-8 and -9 in the presence of MSA or paclitaxel, or the combination. C. Increase of cleaved caspase-8 and caspase-9 by MSA, paclitaxel, or combination. D. Effect of caspase-8 inhibitor or caspase-9 inhibitor on cell death induction by MSA/paclitaxel. *Statistically different (P<0.05) from MSA/paclitaxel without inhibitor.
Caspase-9 and caspase-8 are indicator caspases for the mitochondrial (intrinsic) and death receptor (extrinsic) apoptotic pathways, respectively. With the use of Western blotting, we examined the cleaved form of caspase-8 and -9 by MSA alone, paclitaxel alone, or the combination. Cleavage of the caspases indicates their activation. Representative Western blots are shown in Fig. 7B. In order to make a valid comparison of the different blots, the densitometry reading of each immunoreactive band was first normalized against the reading of GAPDH. The control value was then subtracted from the normalized reading. The net increase by MSA, paclitaxel, or the combination was then expressed quantitatively in arbitrary units, as shown in Fig. 7C. It should be noted that the scale of caspase expression is identical across all 6 panels. MSA or paclitaxel by itself produced relatively small increases of cleaved caspase-8 and caspase-9. In contrast, the combination generated a very robust increase of both caspases. The net increase by the combination was substantially greater than the sum of the net increase by the single drug.
The role of these two caspases in apoptosis was further investigated in cells treated with the combination. We incubated cells with a specific inhibitor to either caspase-8 (z-IETD-FMK) or caspase-9 (z-LEHD-FMK). The results in Fig. 7D show that both caspase inhibitors significantly reduced the activity of MSA/paclitaxel to induce death. The data thus suggest that the mitochondrial and death receptor pathways are equally important to the cell killing effect of the combination.
Discussion
Our study demonstrated that pro-apoptotic ER stress response could be exploited as a mechanism to sensitize cancer cells to chemotherapeutic drugs. Finding a suitable inducer of ER stress is key to the approach. Compared to thapsigargin, tunicamycin and calcium ionophore, all of which are widely used to study UPR but are too toxic for clinical application, organic selenium compounds are well tolerated by humans. A recent phase I study showed that daily administration of selenomethionine to colon cancer patients (also treated with irinotecan) achieved plasma selenium concentration in excess of 25 μM without any symptom of toxicity.27 Although speciation of selenium metabolites in biological samples is not possible at the present time, it is reasonable to assume that a significant portion of selenium in the tumor is in a form that has comparable activity to that produced by 3 μM MSA. Note that both selenomethionine and MSA generate the same active metabolite (see explanation in Methods).
We believe that MSA serves as a modulator to sensitize cells to taxane, even though each drug by itself produces a low level of cell death. In our protocol, the cells were treated with 3 μM MSA for a total of 22 hours, i.e. 6 hours before plus 16 hours after the addition of taxane. This level of MSA will not lead to significantly more cell death if the incubation time is extended to 48 hours. On the other hand, 30 nM paclitaxel or 20 nM docetaxel will produce more cell death at 48 hours than at 16 hours (data not shown). When cells are primed by MSA first before taxane treatment, the rate of cell death is hastened considerably. This is fairly good evidence that MSA is helping taxane instead of the other way around. Why is the 6-hour MSA pretreatment period critical to the sensitization effect? It seems that this is the time required by MSA to fully mobilize the arsenal of ER stress response that is suited to enhance the cell killing action of taxanes.
How does ER stress response commit cells to apoptotic death? The direct activation of caspase-12, -7, and possibly -4, has been implicated.28,29 In our study, we touched upon two pro-apoptotic UPR signals: calcium and GADD153. There are multiple ways in which calcium is engaged in cell death. Free cystolic calcium could be taken up by the mitochondria, thus leading to a decrease of transmembrane potential and the release of pro-apoptotic molecules such as cytochrome c to activate caspase-9.30 Elevated calcium is known to activate calpains, these are calcium-dependent proteases involved in the destruction of the cytoskeleton a characteristic feature of apoptosis,31 as well as in the proteolytic cleavage of Bcl-2 32 and Bid.33 Previously we reported the dephosphorylation of Akt by calcineurin (a calcium-dependent phosphatase), the latter is activated through the induction of ER stress response by MSA.24 The dephosphorylation of Akt may in turn stimulate the activity of pro-apoptotic molecules such as Bad and FOXO.34 Bad is a regulatory component of the mitochondrial pathway,35 while FOXO is known to up-regulate the expression of death receptor ligands.36
GADD153 is a pro-apoptotic transcription factor which is historically associated with ER stress response. It has been shown that GADD153 down-regulates the expression of Bcl-2 37 and up-regulates the expression of death receptors.38,39 In our GADD153 knockdown experiment, we observed only a 30% reversal of the cell killing activity of MSA/paclitaxel. This is not surprising considering that GADD153 is but one of many apoptotic signaling molecules of UPR. Aside from those mentioned above, JNK is another major pathway which is activated by one of the UPR transducers, IRE1. The same IRE1 also regulates the expression of GADD153 via XBP1. Thus knocking down one single mediator which operates part way down the signaling cascade is unlikely to produce a dramatic effect.
As alluded to in the Introduction, the impact of ER stress on cancer biology still remains to be clarified. Before the dust is settled, it may be timely to make a distinction between chronic and acute ER stress. Tumor tissues are generally more prone to ER stress. Due to an inadequate or unstable vasculature in solid tumors, cancer cells are constantly facing a multitude of challenges including nutrient deprivation, low pH and hypoxia. All of these perturbations contribute to ER stress.4,5 GRP78 is often found to be up-regulated in tumors.40,41 The interpretation usually hinges on the idea that tumor cells desire GRP78 to adapt to the hostile microenvironment. High GRP78 expression has also been correlated with poor prognosis and drug resistance.42–44 Thus chronic ER stress in tumors may actually confer an advantage to survival. Consequently, new therapeutic strategies are being developed to reduce GRP78.45,46 The situation we have created in this study is acute ER stress induction by an exogenous agent. Cancer cells may be genetically programmed to fight chronic ER stress, but this does not necessarily mean that they are prepared to meet the challenge of acute ER stress. The need to cope with chronic physiological stress might have nearly exhausted the capacity to mount a survival response. When confronted by an acute exacerbating insult, the pro-apoptotic machinery becomes the next option to be called into play. It is our contention that this idiosyncrasy could be exploited for chemotherapeutic sensitization.
Materials and Methods
Cell culture
The PC-3 human prostate caner cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA). PC-3 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin/streptomycin and 2 mM glutamine. Cells were maintained at 37 °C in an atmosphere of 5% CO2 and 95% air.
Selenium reagent
Methylseleninic acid (MSA) was used in all the cell culture experiments. It was purchased from PharmSe, Inc. (Lubbock, TX). MSA was developed specifically for in vitro studies because of certain unique attributes.47,48 Selenoamino acids are generally not suitable for cell culture experiments since many epithelial cells, including prostate cells, have a low capacity in converting selenoamino acids to the active metabolite.
Other chemicals
Paclitaxel, docetaxel, and tauroursodeoxycholic acid were purchased from Sigma (St. Louis, MO). Thapsigargin and BAPTA-AM were purchased from Calbiochem (San Diego, CA). Caspase-8 and caspase-9 inhibitors, z-IETD-FMK and z-LEHD-FMK, were purchased from Biovision (Mountain View, CA).
Western blotting
The method of Western blotting has been described in our previous publication.14 GRP78 polyclonal antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies to uncleaved caspase-8 and -9, cleaved caspase-8 and -9, PARP, and cleaved PARP were purchased from Cell Signaling Technology (Beverly, MA). GAPDH monoclonal antibody (Chemicon, Temecula, CA) was used as the loading control. Immunoreactive bands were quantified by volume densitometry with the ImageQuant software (Molecular Dynamics, Sunnyvale, CA).
Cell death analysis
The analysis was performed by using a Cell Death Detection ELISA Kit (Roche Applied Science, Indianapolis, IN). This method quantifies apoptotic death by determining the presence of cytoplasmic histone-associated-DNA-fragments. Cell death analysis was carried out in 96-well plates. For each treatment, 6 wells of cells were used: 3 for the cell death assay, and the remaining 3 for the MTT cell number assay.49 The cell death reading was then normalized against the MTT reading. Cell death values were measured in O.D. units. The data are expressed as induction of cell death, i.e. the net increase due to treatment. Untreated cells served as the control in every experiment.
Stable GRP78-overexpressing PC-3 cells
The expression vector for human GRP78 over-expression was kindly provided by Dr. Richard C. Austin.50 The parental empty vector pcDNA3.1 was used for mock stable transfected clone. Briefly, PC-3 cells were transfected by using FuGene6 (Roche Applied Science, Indianapolis, IN). Clone selection was carried out by culturing in the presence of G418 (Calbiochem, Mountain View, CA) at 50 μg/ml for two weeks. The medium was changed every 4 days. G418 resistant clones were confirmed for the adequate over-expression of GRP78 by Western blot before expanding for the designed experiments.
Small interference RNA (siRNA) transfection
GRP78 or GADD153 siRNA, the control scrambled siRNA, and siRNA transfection agents were purchased from Santa Cruz (Santa Cruz, CA). Cells were transfected in 6-well plates according to the product instructions. Transfected cells were replated in 96-well plates in regular medium after 12 hours of transfection. Replated cells were treated with MSA/paclitaxel after 12 hours of culture in regular medium.
Statistical analysis
The Student t-test was used to determine statistical differences between treatment and control values, and p<0.05 was considered significant.
Acknowledgments
Supported by Grant R01-CA09796 from the National Cancer Institute; Grant 62-2378-01 from the Roswell Park Alliance Foundation (CI); and partially supported by the Roswell Park Cancer Center Support Grant 1P30-CA016056 from the National Cancer Institute.
Footnotes
There are no conflicts of interest or financial disclosure to report.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–29. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Lee AS, Hendershot LM. ER stress and cancer. Cancer Biol Ther. 2006;5:721–2. doi: 10.4161/cbt.5.7.3120. [DOI] [PubMed] [Google Scholar]
- 3.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–77. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 4.Mann MJ, Hendershot LM. UPR activation alters chemosensitivity of tumor cells. Cancer Biol Ther. 2006;5:736–40. doi: 10.4161/cbt.5.7.2969. [DOI] [PubMed] [Google Scholar]
- 5.Scriven P, Brown NJ, Pockley AG, Wyld L. The unfolded protein response and cancer: a brighter future unfolding? J Mol Med. 2007;85:331–41. doi: 10.1007/s00109-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 6.Jiang CC, Chen LH, Gillespie S, et al. Tunicamycin sensitizes human melanoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response. Cancer Res. 2007;67:5880–8. doi: 10.1158/0008-5472.CAN-07-0213. [DOI] [PubMed] [Google Scholar]
- 7.Shiraishi T, Yoshida T, Nakata S, et al. Tunicamycin enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human prostate cancer cells. Cancer Res. 2005;65:6364–70. doi: 10.1158/0008-5472.CAN-05-0312. [DOI] [PubMed] [Google Scholar]
- 8.Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003;63:7284–90. [PubMed] [Google Scholar]
- 9.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–11. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Q, Lee DI, Rong R, et al. Endoplasmic reticulum calcium pool depletion-induced apoptosis is coupled with activation of the death receptor 5 pathway. Oncogene. 2002;21:2623–33. doi: 10.1038/sj.onc.1205345. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs JT. New strategies for the medical treatment of prostate cancer. BJU Int. 2005;96 (Suppl 2):35–40. doi: 10.1111/j.1464-410X.2005.05945.x. [DOI] [PubMed] [Google Scholar]
- 12.Park HJ, Makepeace CM, Lyons JC, Song CW. Effect of intracellular acidity and ionomycin on apoptosis in HL-60 cells. Eur J Cancer. 1996;32A:540–6. doi: 10.1016/0959-8049(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Shiraishi T, Horinaka M, Wakada M, Sakai T. Glycosylation modulates TRAIL-R1/death receptor 4 protein: different regulations of two pro-apoptotic receptors for TRAIL by tunicamycin. Oncol Rep. 2007;18:1239–42. [PubMed] [Google Scholar]
- 14.Wu Y, Zhang H, Dong Y, Park YM, Ip C. Endoplasmic reticulum stress signal mediators are targets of selenium action. Cancer Res. 2005;65:9073–9. doi: 10.1158/0008-5472.CAN-05-2016. [DOI] [PubMed] [Google Scholar]
- 15.Zu K, Bihani T, Lin A, Park YM, Mori K, Ip C. Enhanced selenium effect on growth arrest by BiP/GRP78 knockdown in p53-null human prostate cancer cells. Oncogene. 2006;25:546–54. doi: 10.1038/sj.onc.1209071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao S, Durrani FA, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–9. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 17.Hu H, Jiang C, Ip C, Rustum YM, Lu J. Methylseleninic acid potentiates apoptosis induced by chemotherapeutic drugs in androgen-independent prostate cancer cells. Clin Cancer Res. 2005;11:2379–88. doi: 10.1158/1078-0432.CCR-04-2084. [DOI] [PubMed] [Google Scholar]
- 18.Azrak RG, Frank CL, Ling X, Li F, Foster BA, Rustum YM. The mechanism of methylselenocysteine (MSeC) and docetaxel synergistic activity in prostate cancer cells. Mol Cancer Ther. 2006;5:2540–8. doi: 10.1158/1535-7163.MCT-05-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vadgama JV, Wu Y, Shen D, Hsia S, Block J. Effect of selenium in combination with Adriamycin or Taxol on several different cancer cells. Anticancer Res. 2000;20:1391–414. [PubMed] [Google Scholar]
- 20.Ryan CJ, Eisenberger M. Chemotherapy for hormone-refractory prostate cancer: now it’s a question of “when?”. J Clin Oncol. 2005;23:8242–6. doi: 10.1200/JCO.2005.03.3092. [DOI] [PubMed] [Google Scholar]
- 21.Kibel AS. An interdisciplinary approach to treating prostate cancer. Urology. 2005;65:13–8. doi: 10.1016/j.urology.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Ma YB, Choi KS, et al. Neural network-based analysis of thiol proteomics data in identifying potential selenium targets. Prep Biochem Biotechnol. 2006;36:37–64. doi: 10.1080/10826060500388512. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Zu K, Warren MA, Wallace PK, Ip C. Delineating the mechanism by which selenium deactivates Akt in prostate cancer cells. Mol Cancer Ther. 2006;5:246–52. doi: 10.1158/1535-7163.MCT-05-0376. [DOI] [PubMed] [Google Scholar]
- 25.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–80. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 26.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–9. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 27.Fakih MG, Pendyala L, Smith PF, et al. A phase I and pharmacokinetic study of fixed-dose selenomethionine and irinotecan in solid tumors. Clin Cancer Res. 2006;12:1237–44. doi: 10.1158/1078-0432.CCR-05-2004. [DOI] [PubMed] [Google Scholar]
- 28.Hitomi J, Katayama T, Eguchi Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–56. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RV, Hermel E, Castro-Obregon S, et al. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem. 2001;276:33869–74. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- 30.Scorrano L. Divide et impera: Ca2+ signals, mitochondrial fission and sensitization to apoptosis. Cell Death Differ. 2003;10:1287–9. doi: 10.1038/sj.cdd.4401310. [DOI] [PubMed] [Google Scholar]
- 31.Kidd VJ, Lahti JM, Teitz T. Proteolytic regulation of apoptosis. Semin Cell Dev Biol. 2000;11:191–201. doi: 10.1006/scdb.2000.0165. [DOI] [PubMed] [Google Scholar]
- 32.Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, et al. Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem. 2002;277:27217–26. doi: 10.1074/jbc.M202945200. [DOI] [PubMed] [Google Scholar]
- 33.Mandic A, Viktorsson K, Strandberg L, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–13. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 35.Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1999;1:E33–E35. doi: 10.1038/10026. [DOI] [PubMed] [Google Scholar]
- 36.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 37.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–59. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He Q, Luo X, Jin W, et al. Celecoxib and a novel COX-2 inhibitor ON09310 upregulate death receptor 5 expression via GADD153/CHOP. Oncogene. 2007 doi: 10.1038/sj.onc.1210894. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 40.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 42.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–4. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 43.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–40. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 44.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–53. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 45.Ermakova SP, Kang BS, Choi BY, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006;66:9260–9. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 46.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BIP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67:9809–16. doi: 10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 47.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–6. [PubMed] [Google Scholar]
- 48.Ip C, Dong Y, Ganther HE. New concepts in selenium chemoprevention. Cancer Metastasis Rev. 2002;21:281–9. doi: 10.1023/a:1021263027659. [DOI] [PubMed] [Google Scholar]
- 49.Li S, Zhou Y, Wang R, Zhang H, Dong Y, Ip C. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Mol Cancer Ther. 2007;6:1031–8. doi: 10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- 50.Werstuck GH, Lentz SR, Dayal S, et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–73. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]