Abstract
Recent evidence suggests that genetic and biochemical factors associated with psychoses may also provide an increased propensity to think creatively. The evolutionary theories linking brain growth and diet to the appearance of creative endeavors have been made recently, but they lack a direct link to research on the biological correlates of divergent and creative thought. Expanding upon Horrobin’s theory that changes in brain size and in neural microconnectivity came about as a result of changes in dietary fat and phospholipid incorporation of highly unsaturated fatty acids, we propose a theory relating phospholipase A2 (PLA2) activity to the neuromodulatory effects of the noradrenergic system. This theory offers probable links between attention, divergent thinking, and arousal through a mechanism that emphasizes optimal individual functioning of the PLA2 and NE systems as they interact with structural and biochemical states of the brain. We hope that this theory will stimulate new research in the neural basis of creativity and its connection to psychoses.
Keywords: Creativity, Norepinephrine, Fatty acids, Schizophrenia, Psychoses
1. Introduction
Psychoses have been linked to creativity for as long as humans have been leaving written records. More recently, both Jami son [1] and Andreasen [2] have proposed that mood disorders and artistic creativity are correlated, using mostly biographical and historical records as well as interviews. While these studies offer solid evidence and tantalizing glimpse into the family histories of artistic individuals, there are several limitations. First, although historical studies provide a great impetus for investigating the connection between the psychoses and creativity, they are limited to those who reached public eminence and therefore miss those individuals who slipped quietly through the rough sieve of mainstream written history. Second, biographical and interview-based studies do not inform us about the creative processes themselves. Third, both Andreasen and Jamison postulate that mood disorders but not schizophrenia hold the key to creativity. We argue that their studies were limited mostly to verbal creativity and also to those who are successful enough to be recognized. Creativity is a multifaceted creature. While it can be detected perhaps more easily in language domains, creative output in non-verbal areas must also be examined.
It is our hypothesis that psychosis and creative process are connected via specific brain activity that can be empirically investigated and that this crucial link is provided by the membrane phospholipid metabolism and the norepinephrine (NE) system. Ample evidence indicate that schizophrenia is a disorder of phospholipid membrane metabolism which results in a loss of highly polyunsaturated fatty acids (PUFA) from the membranes owing to increased activity of the enzyme phospholipase A2 (PLA2) [3]. Reduced essential fatty acids such as arachidonic acid (AA) and docosahexaenoic acid (DHA) in the membranes can result from reduced incorporation of these fatty acids into the membrane and/or an excessive removal from the membranes [4,5]. In schizophrenia both processes seem to be at work. One consequence of reduced PUFA is that it is expressed throughout the body and can be detected via changes in vasodilation responses to niacin. Schizophrenia patients do not tend to flush when topical niacin solution is applied to skin [6]. Horrobin has proposed that the membrane lipid abnormalities that can result in schizophrenia can also result in dyslexia, bipolar disorder and creativity [3], but it still remains to be seen exactly how phospholipid metabolism abnormalities may lead to creative output. The goal of this paper is to propose a possible biochemical link that may explain why psychoses and creativity go hand in hand. We believe that although our hypothesis may be overly simplistic and possibly incorrect, but there is enough researchto support at least a tentative link between fatty acid metabolism, NE and creativity. Most importantly, we hope that the connections that we have made will encourage future investigations of these systems in relation to creativity.
The starting point of our hypothesis is the observation that PLA2 inhibitors attenuate the regeneration of NE axons [7,8]. In turn, fluctuating NE levels are associated with several of the physiological states that have been found to enhance creativity such as decreased attention [9], vigilance [10,11], and arousal [12]. Several of the structural and neurochemical changes found in brain tissue related to NE, lipid, and protein changes are overrepresented in conditions where divergent thinking and remote associations are more common [13–16]. It seems that PLA2 inhibitors reduce AA release, causing a reduction in regeneration of NE axons in locus coeruleus, and we will present data from various sources in the subsequent sections to support our assertion. Thus, PLA2 activators should increase arachidonic acids levels, and increase brain microconnectivity through cyclooxygenase and nitric oxide synthetase activity. Our argument, that creative thinking and behavior could be at least partially linked to the noradrenergic neurotransmitter system via specific fatty acid metabolic processes, is based upon research in attention, vigilance, mood, sleep, and neurotransmitter dysfunction in psychopathology. Although creative behavior is as elusive as the different definitions that have come to define it, there have been several attepts made to link creativity to specific biological processes in the brain.
Understandably, a single neural mechanism has not been uncovered, nor is it a common belief that a single system could be responsible for such a metacognitive phenomenon. Since Guilford’s seminal presentation of the behavior and thought processes that underlie creativity [17], several theories of its psychological mechanisms have been developed. Although the concept of creativity has engendered many definitions and heuristic concepts, behavioral scientists have been fairly specific in defining its characteristic components. Creativity can be defined as an ability to create unique ideas that are remote, unusual, removed from stereotyped concepts, and that serve to solve problems in new ways [17]. Creative individuals have the ability to think in these terms (divergent thinking), but they are also able to apply their novel concepts to acts in their lives that produce creative output. That is to say that as much as creativity encompasses a specific cognitive realm in divergence of thought, it also includes the ability to behave in creative ways and to apply new ideas to everyday behavior [18]. In our continuing argument, we will posit several different ways the NE system could mediate the elements of thought that contribute to creative output.
2. The role of the NE system
The locus coeruleus (LC) is the site of NE synthesis the brain, in addition to a smaller level of NE production occurring in the lateral tegmental regions. From the production site in the caudal region of the pons, NE neurons innervate each of the brain cortices in addition to the hypothalamus, cerebellum, and spinal cord [19]. This widely distributed network of fibers has been traditionally studied behaviorally in terms of attention, arousal, and mood. Broadly speaking, each of these psychological components associated with NE activity has also been given attention in the creativity literature. Moderate levels of affective states such as positive feelings achieved after exercise do enhance creative thinking skills [20,10], and moderate (compared to extremely high or low) levels of vigilance and attention either improves creative production or is associated with better performance in creative versus non-creative thinkers [9,12]. Although data linking each of these findings directly to the NE system are unavailable, comparing the analogous neural mechanisms that underlie these processes could elucidate individual mechanisms that contribute to creativity.
The noradrenergic system has been extensively studied for its effects on emotion, behavior, cognition, and physiological states. The system’s most general, overall salient role, can be seen as addressing vigilance and arousal and regulating attention resources. Unlike other behavioral effects of transmitter systems which tend to respond to more noxious stimuli, the NE system, acting as a neuromodulator, responds to general alertness needs, especially those that are due to novel environmental stimuli [21]. In making a link to creativity, it is important to show that the NE system is also involved in modulating more specific cognitive elements. In monkeys, neurons in the LC have been shown to respond preferentially to stimuli associated with specific reward predictors [22] and to specific target stimuli requiring rapid attention compared to non-target distractors [23]. More recent evidence from a computational model addressing the NE system’s involvement in cognitive, as opposed to general motivational, states has shown that LC neurons respond differently to general motivational and exploratory states compared to more specific demands that require goal attainment and specific attention [24]. Although these studies do not predict NE involvement in specific higher cognitive domains related to creativity such as intelligence, flexibility, and task switching, they do provide evidence that what was once thought of as a general arousal system is actually involved in mediating thought processes [25].
3. The neurobiology of creativity
3.1. NE and arousal mechanisms related to creative ability
Because the NE system arising from projections in the LC has been substantially implicated in mediating changes in arousal and vigilance as a consequence of novelty in the environment [26,27], the most substantial link to creative cognition from NE systems may be through neural mechanisms of arousal. Electroencephalogram (EEG) investigations of divergent thinking tasks have provided evidence that creative thinkers are less aroused than non-creative thinkers when determining creative solutions to problems [28]. EEG measures the electrical potential that is created across nerve cell membranes by placing electrodes on the scalp and amplifying the signal from underlying neuronal clusters. This method of neurophysiological measurement has superior temporal resolution, and it is an excellent tool for distinguishing patterns of neuronal firing related to different underlying brain regions that fire in close temporal proximity. In a series of several tasks testing creative and intellectual abilities, Martindale and Hines [29] showed that EEG α activity (arising from the thalamus and occurring at approximately 8–14 Hz.) increased in a group of creative subjects compared to subjects exhibiting low to medium levels of creative ability. The highest increase in α wave activity occurred while solving items in the Alternate Uses Task which required subjects to determine alternate uses for common objects. This is a more pure measure of creativity than either the Remote Associations Test (which requires subjects to determine a single common associate to three loosely connected words) or general intellectual ability tests that were used in addition to assess differences across abilities. Interestingly, EEG α wave activity is associated with decreased arousal, as it is an inverse measure of the general cortical arousal response. It has also been shown that EEG α relative activity is directly correlated with changes in cerebrospinal levels of NE. As NE levels increase, α relative activity decreases [16].
In a different study, creative individuals showed higher α activity compared to non-creative subjects when asked to create a plot for a fantasy story. When subjects were then asked to free-associate words which would later be used to formulate a story, EEG α was decreased for those creative subjects who were explicitly told to be original compared to those who were not given similar instructions [30]. Hudspith [31] also found slight increases in frontal lobe EEG α (4–8 Hz.) activity for creative individuals in mental imagery tasks, implicating decreased frontal lobe arousal when highly creative subjects think creatively. Studies of general attentive capabilities between creative and non-creative subjects do show that subjects who exhibit fewer creative traits do focus their attention more narrowly [32]. These results support the idea that creative people have a broad focus of attention and greater attentive capacity, which is related to specific mechanisms of cortical activation. In addition to levels of arousal being decreased in divergent thinking, EEG complexity increases during the same type of thinking [33]. This indicates that although general cortical arousal is lower, more neuronal elements are activated.
Evidence also suggests that direct manipulation of the noradrenergic system produces changes in brain EEG waves. The introduction of clonidine, an NE α2 receptor subtype agonist, increased frontal lobe θ activity in subjects who performed arithmetic tasks, although these subjects did not tend to show general frontal θ activity during similar tasks without clonidine [34]. In rats, administration of α2 antagonists increased EEG α and β activity, and α2 agonist administration increased slow wave activity [35]. In humans, administration of α1 agonists decreases the synchrony in EEG tracings, while lesions in structures associated with NE and dopaminergic activity induce decrements in waking arousal levels, and they produce enduring effects on a variety of EEG measures [36]. Interestingly, the effects of manipulating the NE system with either agonists or antagonists produce changes similar to the differences noted in creative versus non-creative subjects with. Both increased theta and alpha desynchronization have been reported in behavioral tasks of creativity, and these results can also be achieved by inducing changes in the neurotransmitter systems.
3.2. Endogenous and exogenous changes in NE levels modulate creativity
The proposed connection between creativity, arousal, and the NE system has been substantiated further by either directly manipulating the NE system pharmacologically, or by investigating the effects on behavior and cognition after predictable endogenous changes in the NE system have occurred (such as in sleep and waking states). One study investigated the direct effects of noradrenergic changes during creative problem solving by administering either an agonist (ephedrine) or an antagonist (propranolol) to subjects while they solved each of three cognitive tasks: number series problems, shape rearrangement tasks, and anagram word arrangement tasks. For subjects who were able to solve the problems, response time was lower in the group that received propranolol compared to the groups that received either ephedrine or placebo [37]. An important implication of this study is that the effect of propranolol was seen with the anagram task, and not with the tasks that required less cognitive flexibility. Further evidence for the effects of adrenergic manipulation on cognitive performance comes from a study of SAT performance in adolescents who received propranolol before taking the SAT [38]. Although the SAT is certainly not a measure of creativity or direct cognitive flexibility, administration of propranolol before taking the test improved total scores with an average range of 130 points. The effects of both of these findings may be directly related to the noradrenergic response to stressful situations. As stress increases, so does NE activity, although performance on cognitive tasks tends to decline [12,39].
3.3. Physiological states associated with NE fluctuations and increased creativity
Variations in endogenous NE levels with exercise and sleep may also be used to assess noradrenergic effects on creative thought. Increases in alpha-amylase can be shown after subjects are exposed to rigorous periods of exercise, and this increase in salivary amylase has been shown to correlate directly with increases in plasma NE [40]. Strobel et al. [41] have indicated that this exercise dependent increase in NE levels may be an effect of anaerobic rather than aerobic exercise, due to the greater demand on stored energy sources in anaerobic exercise. Accordingly, exercise has also been shown to enhance creative thought. When subjects solve creative tests after periods of exercise, scores on specific measures of creativity that require flexibility (an ability to overcome overlearned responses) increase independently of mood ratings [10]. Endogenous regulation of NE can also occur as a result of the sleep/waking cycles. When noradrenergic activation is low, just before falling asleep, subjects are more likely to have a moment of insight, or an “aha” phenomenon [42]. The solutions that occur during moments of insight are arrived at holistically, rather than gradually in a stepwise process and are more associated with truly creative solutions than are piecemeal solutions. The solutions that are impending are more likely to be resolved while the NE system is less activated, and while general cortical arousal is low.
Due to the natural fluctuations in the NE system that occur during stages of sleep, cognition and behavior produced following specific sleep stages can be assessed for creative process and content. Because the effects of sleep manipulation on the NE system are not as “clean” as those derived from directly manipulating the NT systems pharmacologically, it is best to assess behavior when the NE system is either “on” or “off”. During REM sleep, NE neurons in the LC become silent, as REM sleep cannot occur in the presence of noradrenergic arousal and activity. Instead, acetylcholinergic neurons in the pons become active. Waking subjects after periods of sustained REM sleep in order to assess creativity or behavior assures that the NE system has been constrained. If subjects are awoken following different stages of sleep and asked to solve anagrams that require them to rearrange letters to form words, performance is enhanced following REM waking compared to either wakefulness or other stages of sleep [11]. As in Mednick’s Remote Associations Test where appropriate use of a wider semantic network enhances creative responses [43], these results imply that REM sleep either enhances semantic associations, or that it makes navigation through the network more efficient in order to arrive at appropriate responses. There results also imply that decreased NE functioning contributes to enhancing creativity. Unlike convergent tasks, which have been shown to be resistant to sleep loss, divergent thinking abilities suffer as a result of sustained sleep loss [44]. Performance on tests of mental flexibility and originality is impaired after 32 hof sleep loss, even when motivating factors are used in an effort to boost performance. During REM sleep, EEG recordings are desynchronized and they are characterized by low amplitude waves in the absence of NE. The increase in α wave activity seen in creative problem solving are also low amplitude, both indicating a decreased level of cortical arousal.
3.4. ApoD may mediate thalamic states present during creative thought
Although relating EEG patterns to creative thought is indirect, an analysis of the relationship between the cellular substrates of EEG and phospholipid abnormalities in schizophrenia provides further evidence of a possible relationship between psychoses, creativity, and fatty acid metabolism. Apolipoprotein D (apoD) is a lipid transporting protein that exists in blood plasma, and brain where it is involved in lipid metabolism and in neuronal growth [45]. apoD has been shown to be elevated in patients with both of the most severe psychotic disorders, schizophrenia and bipolar disorder [14,46]. In particular, it has been shown that levels of apoD are particularly elevated in the amygdala and in the thalamus in schizophrenic patients compared to those with bipolar disorder [15]. Although there has been significant debate concerning the meaning and origin of EEG α; ample evidence suggests that these oscillations arise from the thalamic nuclei [47], especially those arising from the nucleus reticularis [48]. Not surprisingly, one of the NE pathways projecting from the LC arrives in thalamus, and it is our hypothesis that elevated levels of apoD could disturb normal thalamic functioning, arising in changes in EEG α oscillations which are related to creative thought processes at baseline and during active problem solving.
3.5. Endogenous antioxidants correlated with cretive ability are influenced by PUFAs and NE
An additional biochemical substrate related to the neurochemistry of creative thought is serum uric acid (SUA), which acts as a potent antioxidant in protecting cells from free radical damage. When university students were given a divergent thinking test and then divided into groups of extremely high and low scorers, SUA levels were significantly lower in the group that showed higher divergent thinking ability [49]. Although there is some evidence that SUA levels are dependent upon environmental factors, serum levels of uric acid are determined largely by genes [50], suggesting an inherent biochemical pattern related to this maker as well as to the protein, enzyme, and lipid abnormalities noted in schizophrenia and in bipolar disorder. More recently, investigations of the relationship between SUA and PUFAs have elucidated a predictive relationship between n-6 PUFAs and SUA. Serum triglycerides and n-6 PUFAs (measured from red blood cell membranes) predict approximately one-third of the variance in SUA in healthy volunteers [51]. There is also evidence that NE exerts a modulatory effect on SUA, as administration of NE to healthy volunteers decreased SUA levels in urine [52]. In schizophrenics, evidence suggests that SUA is decreased at baseline compared to healthy controls, and that haloperidol (a dopaminergic antipsychotic) raises SUA levels in schizophrenics [53]. Genetic factors that regulate SUA and PUFA metabolism could influence the production of creative thought, and the genes responsible for schizophrenia could be implicated through assertion of effects on lowering SUA.
4. PLA2 and NE function
Levels of fatty acids and fatty acid precursors in the prenatal diet have been shown to directly affect catecholamine levels in the brain. In piglets fed both bioavailable and non-bioavailable trans-α linolenic acid, deficiencies in HUFA incorporation produced an increase in norepinephrine (NE) in both the hippocampus and frontal cortex [54]. However, Owens and Innis [55] found that a lack of linoleic and linolenic acids in the diet of piglets reduced NE levels in the frontal cortex. Takeuchi, Fukumato and Harada found a similar pattern. There was a decrease in NE in cerebral cortex, striatum, and hippocampus in rat pups fed diets deficient in n-3-fatty acid [56]. In rats fed fish oil containing n-3 and n-6 HUFAs, there was a increase in levels of phosphatidyserine [57], which decreases NE levels through increased NE turnover [58].
Other evidence suggests that PLA2 increases NE in a number of other ways. PLA2 hydrolyzes phosphatidyserine, which contains 22:6 fatty acids, or DHA [59]. Phosphatidyserine (22:6) elevates NE turnover in mouse cortex [58], thus increased PLA2 activity would decrease NE turnover caused by phosphatidyserine (22:6). However, evidence also suggests that PLA2 inhibits 3H-NE reuptake [60], implicating optimal functioning of these systems in relation to individual differences rather than simply overall increases or decreases in neurotransmitter modulation. PLA2 inhibition dose-dependently constrains 3H-NE release in cerebral cortical synaptasomes. Moreover, lysophospholipids (LPLs), which are PLA2 metabolites, enhanced 3H-NE release [61]. Intracerebroventricularly administered melittin, a PLA2 activator, and AA dose-dependently increased plasma levels of NE, indicating AA involvement in NE release. Melittin-induced elevation in NE plasma levels was inhibited by mepacrine, a PLA2 inhibitor. Indomethacin, a cycloox-agynase inhibitor, decreased both melattin and AA induced increases in plasma NE, indicating facilitation via AA metabolites [62], and inhibition of cystolic PLA2 (cPLA2) prohibited bradykin-induced NE release [63].
Several bodies of evidence suggest that PLA2-induced NE increase is independent of cellular calcium, as NE-induced PLA2 is independent of cellular calcium activity [64]. This action is mediated by the α1-adrenergic receptor [65]. Neurotoxic PLA2s, crotoxin and taipoxin, inhibit NE reuptake in guinea-pig synaptasomes, and this effect was calcium independent [66]. NE increases PLD activity via cPLA2, generating AA metabolites, which activate the Ras/ERK pathway [67,68]. This pathway is calcium independent in rat cortex [69].
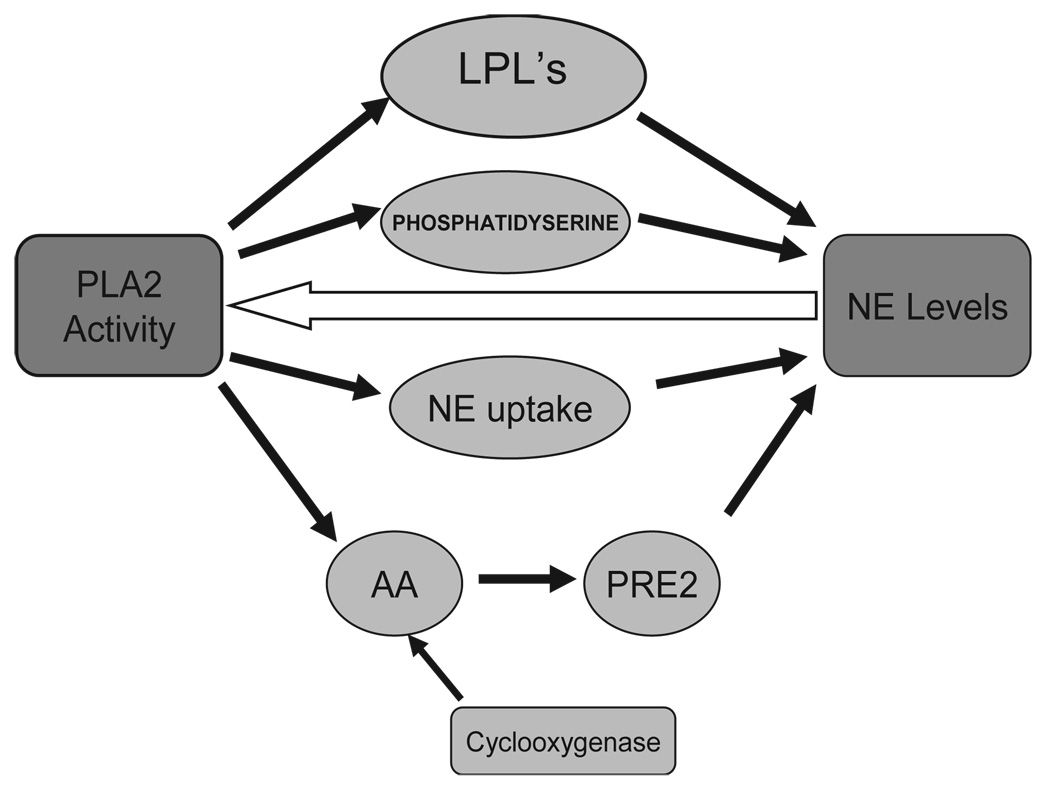
PLA2 activity also asserts control on the density of NE receptors under certain conditions. Melittin, a PLA2 activator, also induced NE axon regeneration. This action was attenuated by p-bromophenacyl bromide (PBPB), which is an inhibitor of PLA2 [7]. PLA2 inhibitors, mepacrine and PBPB attenuated desipramine-induced axonal regeneration [8]. Incubation of rat glioma cells with mepacrine while under chronic treatment with desipramine stopped the down-regulation of β-adrenergic receptors. Fatty acid metabolic pathways do exert a biochemical influence on NE production and activity in the central nervous system that may be attributed to creative thought. For each individual, these optimal levels of NE functioning may be attributable to specific fatty acid metabolic processes, and we would expect to see these processes acting most efficiently at moderate, as opposed to extremely high or low, levels. Fig. 1 shows how this model may be organized in order to show how the precursor effects may influence NE levels.
Fig. 1.
Individual differences in phospholipids metabolism at each step in this model could effectively cause either increases or decreases in NE levels or receptor affinity. We believe that these changes in NE can be dissociated from changes associated with other catecholamines, and that optimal performance of the neurotransmitter system is more important than overall increases or decreases.
5. PLA2 activity in psychoses
A substantial body of evidence finds aberrant PLA2 activity in schizophrenia and bipolar disorder [70–72]. Noponen et al. [73] found increased serum PLA2 levels in schizophrenia patients and psychiatric controls. Several studies suggest that this PLA2 overactivity is calcium independent [74–76]. Evidence also suggests abnormal levels of serum highly unsaturated fatty acids (HUFAs) in schizophrenia [77]. Khan et al. [78] found lower than normal levels of AA and DHA in neuroleptic naive schizophrenia patients, and this finding was correlated with high level s of HUFA by-products. However, similar abnormalities in HUFA levels have not been found in patients with bipolar disorder [79]. Studies have found abnormal membrane phospholipid metabolism in first-degree relatives of patients with schizophrenia [80,81] suggesting heightened PLA2 activity in relatives of schizophrenia. Response to lithium has been shown to follow familial patterns [82]. Given that lithium asserts its action on PLA2 [83,84], this may suggest a possible relation between aberrant PLA2 activity and bipolar relatives. Subjects with schizotypal personality disorder also exhibit overactive phospholipid metabolism in the left temporal lobe [85]. Relatives with schizophrenia and bipolar disorder, as well as subjects with schizotypal personality disorder may exhibit genetically based overactivity of PLA2.
6. Psychoses and the biology of creativity
Noradrenergic systems have been extensively studied in psychopathological conditions related to psychotic thought processes including unipolar and bipolar depression, anxiety and schizophrenia. Accordingly, studies of creativity in these populations could elucidate when fluctuations in noradrenergic systems due to pathological states coincide with enhanced creativity. A relationship between creativity and psychopathology has been theorized since ancient times, however the empirical study of this relationship did not begin until the last century. In one of the first adoption studies that showed a genetic component to the development of schizophrenia, the authors also noted the increased levels of ability and creative interests in children of schizophrenic mothers [86]. Significant relationships between relatives of schizophrenic patients and employment in highly creative professions have been found [87–90], as have relationships directly relating bipolar disorder and depressive episodes to creative productivity [1,91] Horrobin [3] has offered substantial evolutionary and theoretical evidence implicating fatty acid processes in both creativity and psychoses. In terms of noradrenergic modulation of creative processes however, evidence from non-pathological populations suggests that creativity occurs during states when NE levels are decreased. Evidence for a noradrenergic relationship with creativity in these states may be found by looking at correlations between pathological states attributable to low NE levels and high creative productivity.
Depression is associated with low levels of NE [92], and NE levels increase with manic episodes [93]. In schizophrenia, NE and dopamine levels are likewise increased during psychotic episodes [94,95]. In healthy first-degree relatives of schizophrenic patients, however, NE levels are decreased compared to the affected relatives regardless of medication status [96]. Data even suggest that novelty seeking behaviors are correlated with season of birth and relatively lower levels of NE, just as season of birth studies have determined a higher incidence of schizophrenia in those born in winter months [97]. Historical and biographical data do suggest that the relationship between creativity and pathology in schizophrenia is strongest among healthy first degree relatives compared to the affected individuals themselves. Although Jamison provides substantial evidence that periods of extreme creativity are predicted by manic episodes in those affected by bipolar disorder [1,91,98], there is also evidence to suggest that it is the depressive period of bipolar cycling that contributes to the initiation of creative ideas. These ideas are then elaborated upon and combined with other ideas during manic phases, thereby providing the energy to entertain the creative process until the ideas become useful [98].
At this point, however, we cannot estimate the direction of the NE effect as it relates to pathology and creativity. Fluctuations in NE can be seen in pathological conditions, creative thinkers, and in the biological substrates that are purported to underlie both domains. In addition the presence of NE causes fluctuations in levels of other catecholamines, such as dopamine, which have been widely addressed in the schizophrenia literature. Propranolol induces increases in dopamine in the prefrontal cortex, and this has been shown to have a direct effect on improved cognition [99], but we believe that the effects of NE can be dissociated from the effects of other catecholamines. The effects of PLA2 enzymatic activity on fatty acids released from neural and systemic cell membranes have also been shown to directly affect noradrenergic neurons in the central nervous system, and this same activity can be used to differentiate psychotic patients based on niacin skin flushing tests. Direct links between dietary fat and NE have even been made by manipulating HUFAs in food regimens. Future studies that employ several of these variables in an effort to uncover the relationships may find substantial correlations.
7. Discussion
Horrobin has suggested [3] that evolutionary brain changes occurring 2 million years ago coincided with the appearance of both psychoses and creativity, and that creativity and psychoses-proneness have made us truly human. Elements of both psychotic thoughts and creative thinking occur in all humans at some time; indeed there is a continuum attributable to both of these states suggesting that all humans are capable of psychotic thinking and creative problem-solving. If these elements can be related to changes in dietary fat intake, then it is also possible to assume that alterations in catecholamine neurotransmitter function mechanisms may have evolved as well. Although simple hypotheses concerning the pathophysiology of mental disorders are no longer appropriate, it may be important to reexamine the functions of neurotransmitter systems as they relate to both creativity and pathology rather then simply syndromes of disease addressing. As we begin to uncover features associated with the neuropsychology of creativity more specific relationships between creativity, pathology, and human evolution may be unearthed as well. Although creativity research can be elusive, it may represent uncharted territory that has the promise of unearthing significant relationships that have gone unrecognized.
Although the proposal we have offered is a matter of conjecture, it initiates innumerable testable hypotheses that could be employed in elucidating the biology of creative thought and its relationship to psychoses. How does NE activity change during the course of creative problem solving? How do dietary fluctuations in fatty acid ingestion affect creativity? How do the genetic arbiters of PLA2 interact with fat metabolism, NE systems, and creativity? There is substantial need and room for research into these domains. In fact, it is this research that will be able to either identify a concrete link between the mechanisms that we have proposed, identify a third or several other variables that can account for the relationships, or fail to substantially identify a relationship at all.
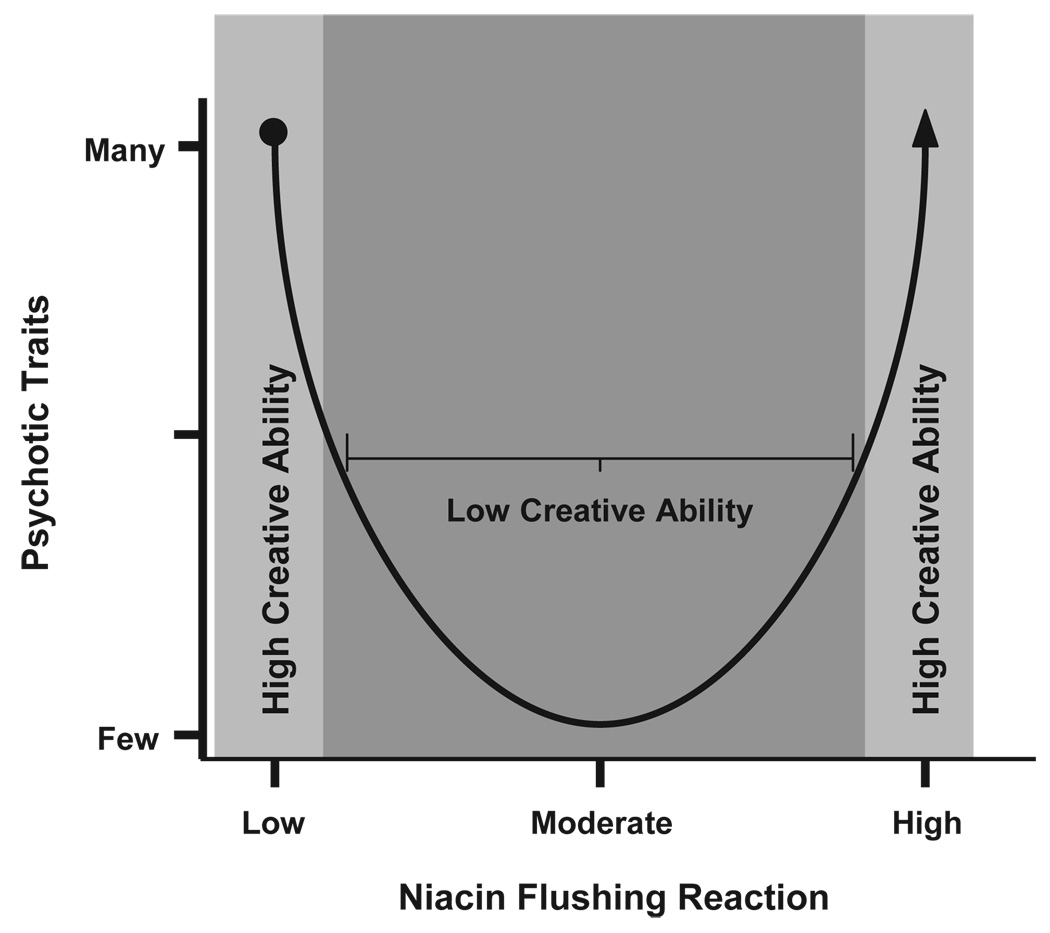
We have begun to address several of the domains that we have posited through research programs in our laboratory. Preliminary unpublished data from our laboratory suggest that prostaglandin release, measured by niacin induced skin flushing, is increased in individuals who report elevated schizotypal traits compared to schizophrenic patients. This is interesting in that medicated schizophrenic subjects may have reduced levels of niacin flushing [6] yet unmedicated, healthy, psychometric schizotypal subjects show an opposite pattern of flushing suggesting that the dysregulation of phospholipids metabolism at either end (either too much or too little) may lead to psychosis-like states and creativity (see Fig. 2). Additionally, our preliminary data indicate that schizotypal individuals do show enhanced performance in select facets of creativity such as generating novel uses for common objects. Within a group of schizophrenic patients, scores on novel tests of creativity are positively correlated with hallmark positive symptoms such as hallucinations and delusions. Although predictions cannot be made from these preliminary results, the findings do suggest differences in populations associated with creativity, levels of schizotypy, and phospholipids metabolism. We are also beginning to test differences in noradrenergic functioning during creative problem solving among these populations using salivary alpha-amylase [40] indicators of that response. The importance of future research in this area cannot be underestimated if we are to uncover the neural bases of creativity and psychopathology, and we hope that these studies will examine the veracity of our predictions.
Fig. 2.
Both schizophrenic (●) and schizotypal (▲) groups show abnormalities in regulating phospholipids assessed by niacin flushing reactions. Schizophrenic patients tend to flush less than controls, and we are finding that schizotypals flush more than controls. At both ends of the continuum, however, these groups show psychotic or psychotic-like traits. It is our assertion that these high levels of psychotic traits are associated with creativity due to a dysregulation in the phospholipid system rather than an overall increase or decrease in available phospholipids.
Acknowledgements
We would like to thank Dr. David Horrobin for inspiring the ideas that are expanded upon in this paper and for his commitment to developing hypotheses in schizophrenia research that have been elucidative and truly creative. We thank Jejoong Kim for the figure. We would also like to thank Drs. Jeffrey Yao, Tomiki Sumiyoshi, Aryeh Routtenberg, and Ruth Condray for their helpful comments.
Footnotes
Dedicated to the memory of David Horrobin, who opened up a vast new intellectual universe, who showed us infinite possibilities, and who inspired us to dream
References
- 1.Jamison KR. Mood disorders and patterns of creativity in British writers and artists. Psychiatry. 1989;52:125–134. doi: 10.1080/00332747.1989.11024436. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC. Creativity and mental illness: prevalence rates in writers and their first-degree relatives. Am. J. Psychiatry. 1987;144:1288–1292. doi: 10.1176/ajp.144.10.1288. [DOI] [PubMed] [Google Scholar]
- 3.Horrobin DF. Schizophrenia: the illness that made us human. Med. Hypotheses. 1998;50:269–288. doi: 10.1016/s0306-9877(98)90000-7. [DOI] [PubMed] [Google Scholar]
- 4.Horrobin DF. The effects of antipsychotic drugs on membrane phospholipids: a possible novel mechanism of action of clozapine. In: Peet M, Glen I, Horrobin DF, editors. Phospholipid Spectrum Disorder in Psychiatry. Carnforth, Lancashire, UK: Marius Press; 1999. pp. 113–117. [Google Scholar]
- 5.Reddy R, Yao JK. Membrane-protective strategies in schizophrenia: conceptual and treatment issues. In: Peet M, Glen I, Horrobin DF, editors. Phospholipid Spectrum Disorder in Psychiatry. Cransforth, Lancashire, UK: Marius Press; 1999. pp. 75–88. [Google Scholar]
- 6.Shah SH, Vankar GK, Peet M, Ramchand CN. Unmedicated schizophrenic patients have a reduced skin flush in response to topical niacin. Schizophr. Res. 2000;43:163–164. [PubMed] [Google Scholar]
- 7.Nakamura S. Involvement of phospholipase A2 in axonal regeneration of brain noradrenergic neurones. Neuroreport. 1993;4:371–374. doi: 10.1097/00001756-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S. Effects of phospholipase A2 inhibitors on the antidepressant-induced axonal regeneration of noradrenergic locus coeruleus neurons. Microsc. Res. Tech. 1994;29:204–210. doi: 10.1002/jemt.1070290305. [DOI] [PubMed] [Google Scholar]
- 9.Martindale C, Armstrong J. The relationship of creativity to cortical activation and its operant control. J. Genet. Psychol. 1974;124:311–320. doi: 10.1080/00221325.1974.10532293. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg H, Sykes EA, Moss T, Lowery S, LeBoutillier N, Dewey A. Exercise enhances creativity independently of mood. Br. J. Sports Med. 1997;31:240–245. doi: 10.1136/bjsm.31.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Brain Res. Cogn. Brain Res. 2002;14:317–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 12.Martindale C, Greenough J. The differential effect of increased arousal on creative and intellectual performance. J. Genet. Psychol. 1973;123:329–335. doi: 10.1080/00221325.1973.10532692. [DOI] [PubMed] [Google Scholar]
- 13.Gattaz WF, Brunner J. Phospholipase A2 and the hypofrontality hypothesis of schizophrenia. Prostaglandins Leukot. Essent. Fatty Acids. 1996;55:109–113. doi: 10.1016/s0952-3278(96)90154-4. [DOI] [PubMed] [Google Scholar]
- 14.Thomas EA, Dean B, Pavey G, Sutcliffe JG. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc. Natl. Acad. Sci. USA. 2001;98:4066–4071. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas EA, Dean B, Scarr E, Copolov D, Sutcliffe JG. Differences in neuroanatomical sites of ApoD elevation discriminate between schizophrenia and bipolar disorder. Mol. Psychiatry. 2003;8:167–175. doi: 10.1038/sj.mp.4001223. [DOI] [PubMed] [Google Scholar]
- 16.Kemali D, Maj M, Iorio G, Marciano F, Nolfe G, Galderisi S, Salvati A. Relationship between CSF noradrenaline levels, C-EEG indicators of activation and psychosis ratings in drug-free schizophrenic patients. Acta Psychiatr. Scand. 1985;71:19–24. doi: 10.1111/j.1600-0447.1985.tb05046.x. [DOI] [PubMed] [Google Scholar]
- 17.Guilford JP. Traits of creativity. In: Anderson HH, editor. Creativity and its Cultivation, Addresses Presented at the Interdisciplinary Symposia on Creativity. 1st Edition. Harper, New York: Michigan State University, East Lansing, Michigan; 1959. pp. 142–161. [Google Scholar]
- 18.Kinney DK, Richards RL, Lowing PA, LeBlanc D, Zimbalist ME. Creativity in offspring of schizophrenic and control parents: an adoption study. Creativity Res. J. 2001;13:17–25. [Google Scholar]
- 19.Cooper JR, Bloom FE, Roth RR. The Biochemical Basis of Neuropharmacology. 6th Edition. New York: Oxford University Press; 1991. [Google Scholar]
- 20.Heinzen T. On moderate challenge increasing ideational creativity. Creativity Res. J. 1989;2:223–226. [Google Scholar]
- 21.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu. Rev. Neurosci. 1979;2:113. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 22.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 23.Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- 25.Sara SJ, Dyon-Laurent C, Herve A. Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res. Cogn. Brain Res. 1995;2:181–187. doi: 10.1016/0926-6410(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 26.Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog. Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler MG, Lake RCR. Norepinephrine: frontiers of clinical neuroscience. 2nd Edition. Baltimore, MD: Williams & Wilkins; 1984. [Google Scholar]
- 28.Martindale C. Creative imagination and neural activity. In: Kunzendorf R, Sheikh A, editors. Psychophysiology of Mental Imagery: Theory, Research and Application. Amityville, NY: Baywood; 1990. pp. 89–108. [Google Scholar]
- 29.Martindale C, Hines D. Creativity and cortical activation during creative, intellectual and EEG feedback tasks. Biol. Psychol. 1975;3:91–100. doi: 10.1016/0301-0511(75)90011-3. [DOI] [PubMed] [Google Scholar]
- 30.Martindale C, Hasenfus N. EEG differences as a function of creativity, tage of the creative process, and effort to be original. Biol. Psychol. 1978;6:157–167. doi: 10.1016/0301-0511(78)90018-2. [DOI] [PubMed] [Google Scholar]
- 31.Hudspith S. Ph.D. Dissertation. University of Southern California; 1985. The neurological correlates of creative thought. [Google Scholar]
- 32.Dykes M, McGhie A. A comparative study of attentional strategies of schizophrenic and highly creative normal subjects. Br. J. Psychiatry. 1976;50:50–56. doi: 10.1192/bjp.128.1.50. [DOI] [PubMed] [Google Scholar]
- 33.Molle M, Marshall L, Lutzenberger W, Pietrowsky R, Fehm HL, Born J. Enhanced dynamic complexity in the human EEG during creative thinking. Neurosci. Lett. 1996;208:61–64. doi: 10.1016/0304-3940(96)12539-8. [DOI] [PubMed] [Google Scholar]
- 34.Mizuki Y, Suetsugi M, Ushijima I, Yamada M. Differential effects of noradrenergic drugs on anxiety and arousal in healthy volunteers with high and low anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1996;20:1353–1367. doi: 10.1016/s0278-5846(96)00131-5. [DOI] [PubMed] [Google Scholar]
- 35.Riekkinen P, Jr, Sirvio J, Jakala P, Lammintausta R, Riekkinen P. Effect of alpha2 antagonists and an agonist on EEG slowing induced by scopolamine and lesion of the nucleus basalis. Neuropharmacology. 1990;29:993–999. doi: 10.1016/0028-3908(90)90104-y. [DOI] [PubMed] [Google Scholar]
- 36.Monti JM. Catecholamines and the sleep-wake cycle. I. EEG and behavioral arousal. Life Sci. 1982;30:1145–1157. doi: 10.1016/0024-3205(82)90656-7. [DOI] [PubMed] [Google Scholar]
- 37.Beversdorf DQ, Hughes JD, Steinberg BA, Lewis LD, Heilman KM. Noradrenergic modulation of cognitive flexibility in problem solving. Neuroreport. 1999;10:2763–2767. doi: 10.1097/00001756-199909090-00012. [DOI] [PubMed] [Google Scholar]
- 38.Faigel HC. The effect of beta blockade on stress-induced cognitive dysfunction in adolescents. Clin. Pediatr. (Phila. ) 1991;30:441–445. doi: 10.1177/000992289103000706. [DOI] [PubMed] [Google Scholar]
- 39.Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Goldstein DS. Stressor specificity of peripheral catecholaminergic activation. Adv. Pharmacol. 1998;42:556–560. doi: 10.1016/s1054-3589(08)60811-x. [DOI] [PubMed] [Google Scholar]
- 40.Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 41.Strobel G, Friedmann B, Siebold R, Bartsch P. Effect of severe exercise on plasma catecholamines in differently trained athletes. Med. Sci. Sports Exerc. 1999;31:560–565. doi: 10.1097/00005768-199904000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Rajkowski J, Kubiak P, Ivanova S, Aston-Jones G. State-related activity, reactivity of locus ceruleus neurons in behaving monkeys. Adv. Pharmacol. 1998;42:740–744. doi: 10.1016/s1054-3589(08)60854-6. [DOI] [PubMed] [Google Scholar]
- 43.Mednick SA. The associative basis of the creative process. Psychol. Rev. 1962;69:220–232. doi: 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- 44.Horne JA. Sleep loss and “divergent” thinking ability. Sleep. 1988;11:528–536. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 45.Mahadik SP, Khan MM, Evans DR, Parikh VV. Elevated plasma level of apolipoprotein D in schizophrenia and its treatment and outcome. Schizophr. Res. 2002;58:55–62. doi: 10.1016/s0920-9964(01)00378-4. [DOI] [PubMed] [Google Scholar]
- 46.Sutcliffe JG, Thomas EA. The neurobiology of apolipoproteins in psychiatric disorders. Mol. Neurobiol. 2002;26:369–388. doi: 10.1385/mn:26:2-3:369. [DOI] [PubMed] [Google Scholar]
- 47.Steriade M, Jones EG, Llinas RR. Neuroscience Research Foundation, Inc. New York: Wiley; 1990. Thalamic Oscillations and Signaling. [Google Scholar]
- 48.Larson CL, Davidson RJ, Abercrombie HC, Ward RT, Schaefer SM, Jackson DC, Holden JE, Perlman SB. Relations between PET-derived measures of thalamic glucose metabolism and EEG alpha power. Psychophysiology. 1998;35:162–169. [PubMed] [Google Scholar]
- 49.Cropley AJ, Cassell WA, Maslany GW. A biochemical correlate of divergent thinking. Can. J. Behav. Sci. 1970;2:174–180. [Google Scholar]
- 50.Goldbourt U, Medalie JH, Herman JB, Neufeld HN. Serumuric acid: correlation with biochemical, anthropometric, clinical and behavioral parameters in 10,000 Israeli men. J. Chronic Dis. 1980;33:435–443. doi: 10.1016/0021-9681(80)90040-5. [DOI] [PubMed] [Google Scholar]
- 51.Russo C, Olivieri O, Girelli D, Guarini P, Corrocher R. Relationships between serum uric acid and lipids in healthy subjects. Prev. Med. 1996;25:611–616. doi: 10.1006/pmed.1996.0096. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Hada T. Effect of norepinephrine on the urinary excretion of purine bases and oxypurinol. Metabolism. 2001;50:1230–1233. doi: 10.1053/meta.2001.26709. [DOI] [PubMed] [Google Scholar]
- 53.Yao JK, Reddy R, van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 1998;80:29–39. doi: 10.1016/s0165-1781(98)00051-1. [DOI] [PubMed] [Google Scholar]
- 54.Acar N, Chardigny JM, Berdeaux O, Almanza S, Sebedio JL. Modification of the monoaminergic neurotransmitters in frontal cortex and hippocampus by dietary trans alpha-linolenic acid in piglets. Neurosci. Lett. 2002;331:198–202. doi: 10.1016/s0304-3940(02)00879-0. [DOI] [PubMed] [Google Scholar]
- 55.Owens SP, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and [{alpha}]-linolenic acid deficient diet in formula-fed piglets. J. Nutr. 1999;129:2088–2093. doi: 10.1093/jn/129.11.2088. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi T, Fukumoto Y, Harada E. Influence of a dietary n-3 fatty acid deficiency on the cerebral catecholamine contents, EEG and learning ability in rat. Behav. Brain Res. 2002;131:193–203. doi: 10.1016/s0166-4328(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 57.Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet A, Besnard J, Durand G. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J. Nutr. 1998;128:2512–2519. doi: 10.1093/jn/128.12.2512. [DOI] [PubMed] [Google Scholar]
- 58.Toffano G, Leon A, Benvegnu D, Boarato E, Azzone GF. Effect of brain cortex phospholipids on catechol-amine content of mouse brain. Pharmacol. Res. Commun. 1976;8:581–590. doi: 10.1016/0031-6989(76)90050-3. [DOI] [PubMed] [Google Scholar]
- 59.Lloyd T. The effects of phosphatidylinositol on tyrosine hydroxylase, stimulation and inactivation. J. Biol. Chem. 1979;254:7247–7254. [PubMed] [Google Scholar]
- 60.Rotman A. The effect of phospholipase C, phospholipase A2 and neuraminidase on the uptake of [3H]norepinephrine and [3H]serotonin by rat brain synaptosomes. J. Neurochem. 1977;28:1367–1372. doi: 10.1111/j.1471-4159.1977.tb12333.x. [DOI] [PubMed] [Google Scholar]
- 61.Nishikawa T, Tomori Y, Yamashita S, Shimizu S. Inhibition of Na+,K+-ATPase activity by phospholipase A2 and several lysophospholipids: possible role of phospholipase A2 in noradrenaline release from cerebral cortical synaptosomes. J. Pharm. Pharmacol. 1989;41:450–458. doi: 10.1111/j.2042-7158.1989.tb06499.x. [DOI] [PubMed] [Google Scholar]
- 62.Yokotani K, Wang M, Murakami Y, Okada S, Hirata M. Brain phospholipase A2-arachidonic acid cascade is involved in the activation of central sympatho-adrenomedullary outflow in rats. Eur. J. Pharmacol. 2000;398:341–347. doi: 10.1016/s0014-2999(00)00276-4. [DOI] [PubMed] [Google Scholar]
- 63.Kurz T, Tolg R, Richardt G. Bradykinin B2-receptor-mediated stimulation of exocytotic noradrenaline release from cardiac sympathetic neurons. J. Mol. Cell Cardiol. 1997;29:2561–2569. doi: 10.1006/jmcc.1997.0492. [DOI] [PubMed] [Google Scholar]
- 64.Jones AW, Shukla SD, Geisbuhler BB, Jones SB, Smith JM. Altered phospholipase activities related to α1-adrenergic receptor supersensitivity of aortas from aldosterone-salt hypertensive rats. Adv. Exp. Med. Biol. 1991;308:55–69. doi: 10.1007/978-1-4684-6015-5_5. [DOI] [PubMed] [Google Scholar]
- 65.Mier K, Kemken D, Katus HA, Richardt G, Kurz T. Adrenergic activation of cardiac phospholipase D: role of α1-adrenoceptor subtypes. Cardiovasc. Res. 2002;54:133–139. doi: 10.1016/s0008-6363(01)00566-1. [DOI] [PubMed] [Google Scholar]
- 66.Tzeng MC, Yen CH, Hseu MJ, Tseng CC, Tsai MD, Dupureur CM. Binding proteins on synaptic membranes for crotoxin and taipoxin, two phospholipases A2 with neurotoxicity. Toxicon. 1995;33:451–457. doi: 10.1016/0041-0101(94)00189-f. [DOI] [PubMed] [Google Scholar]
- 67.Parmentier JH, Muthalif MM, Saeed AE, Malik KU. Phospholipase D activation by norepinephrine is mediated by 12(s)-, 15(s)-, and 20-hydroxyeicosatetraenoic acids generated by stimulation of cytosolic phospholipase A2. Tyrosine phosphorylation of phospholipase D2 in response to norepinephrine. J. Biol. Chem. 2001;276:15704–15711. doi: 10.1074/jbc.M011473200. [DOI] [PubMed] [Google Scholar]
- 68.Fatima S, Yaghini FA, Ahmed A, Khandekar Z, Malik KU. CaM kinase IIα mediates norepinephrine-induced translocation of cytosolic phospholipase A2 to the nuclear envelope. J. Cell Sci. 2003;116:353–365. doi: 10.1242/jcs.00242. [DOI] [PubMed] [Google Scholar]
- 69.Llahi S, Fain JN. Alpha 1-adrenergic receptor-mediated activation of phospholipase D in rat cerebral cortex. J. Biol. Chem. 1992;267:3679–3685. [PubMed] [Google Scholar]
- 70.Horrobin DF, Bennett CN. New gene targets related to schizophrenia and other psychiatric disorders: enzymes, binding proteins and transport proteins involved in phospholipid and fatty acid metabolism, Prostaglandins Leukot. Essent. Fatty Acids. 1999;60:141–167. doi: 10.1054/plef.1999.0027. [DOI] [PubMed] [Google Scholar]
- 71.Ross BM, Turenne S, Moszczynska A, Warsh JJ, Kish SJ. Differential alteration of phospholipase A2 activities in brain of patients with schizophrenia. Brain Res. 1999;821:407–413. doi: 10.1016/s0006-8993(99)01123-3. [DOI] [PubMed] [Google Scholar]
- 72.Stoll AL, Locke CA, Marangell LB, Severus WE. Omega-3 fatty acids and bipolar disorder: a review, Prostaglandins Leukot. Essent. Fatty Acids. 1999;60:329–337. doi: 10.1016/s0952-3278(99)80008-8. [DOI] [PubMed] [Google Scholar]
- 73.Noponen M, Sanfilipo M, Samanich K, Ryer H, Ko G, Angrist B, Wolkin A, Duncan E, Rotrosen J. Elevated PLA2 activity in schizophrenics and other psychiatric patients. Biol. Psychiatry. 1993;34:641–649. doi: 10.1016/0006-3223(93)90157-9. [DOI] [PubMed] [Google Scholar]
- 74.Balsinde J, Dennis EA. Function and inhibition of intracellular calcium-independent phospholipase A2. J. Biol. Chem. 1997;272:16069–16072. doi: 10.1074/jbc.272.26.16069. [DOI] [PubMed] [Google Scholar]
- 75.Nilsson CL, Hellstrand M, Ekman A, Eriksson E. Direct dopamine D2-receptor-mediated modulation of arachidonic acid release in transfected CHO cells without the concomitant administration of a Ca2+-mobilizing agent. Br. J. Pharmacol. 1998;124:1651–1658. doi: 10.1038/sj.bjp.0702025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ. Increased phospholipid breakdown in schizophrenia, Evidence for the involvement of a calcium-independent phospholipase A2. Arch. Gen. Psychiatry. 1997;54:487–494. doi: 10.1001/archpsyc.1997.01830170113015. [DOI] [PubMed] [Google Scholar]
- 77.Ward PE, Sutherland J, Glen EM, Glen AI. Niacin skin flush in schizophrenia: a preliminary report. Schizophr. Res. 1998;29:269–274. doi: 10.1016/s0920-9964(97)00100-x. [DOI] [PubMed] [Google Scholar]
- 78.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr. Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 79.Mahadik SP, Mukherjee S, Horrobin DF, Jenkins K, Correnti EE, Scheffer RE. Plasma membrane phospholipid fatty acid composition of cultured skin fibroblasts from schizophrenic patients: comparison withbipolar patients and normal subjects. Psychiatry Res. 1996;63:133–142. doi: 10.1016/0165-1781(96)02899-5. [DOI] [PubMed] [Google Scholar]
- 80.Klemm S, Rzanny R, Riehemann S, Volz HP, Schmidt B, Gerhard UJ, Filz C, Schonberg A, Mentzel HJ, Kaiser WA, Blanz B. Cerebral phosphate metabolism in first-degree relatives of patients with schizophrenia. Am. J. Psychiatry. 2001;158:958–960. doi: 10.1176/appi.ajp.158.6.958. [DOI] [PubMed] [Google Scholar]
- 81.Peet M, Laugharne JD, Mellor J, Ramchand CN. Essential fatty acid deficiency in erythrocyte membranes from chronic schizophrenic patients, and the clinical effects of dietary supplementation, Prostaglandins Leukot. Essent. Fatty Acids. 1996;55:71–75. doi: 10.1016/s0952-3278(96)90148-9. [DOI] [PubMed] [Google Scholar]
- 82.Grof P, Duffy A, Cavazzoni P, Grof E, Garnham J, MacDougall M, O’Donovan C, Alda M. Is response to prophylactic lithium a familial trait? J. Clin. Psychiatry. 2002;63:942–947. doi: 10.4088/jcp.v63n1013. [DOI] [PubMed] [Google Scholar]
- 83.Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci. Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. [DOI] [PubMed] [Google Scholar]
- 84.Chang MC, Jones CR. Chronic lithium treatment decreases brain phospholipase A2 activity Neurochem. Neurochem. Res. 1998;23:887–892. doi: 10.1023/a:1022415113421. [DOI] [PubMed] [Google Scholar]
- 85.Fukuzako H, Kodama S, Fukuzako T. Phosphorus metabolite changes in temporal lobes of subjects with schizotypal personality disorder. Schizophr. Res. 2002;58:201–203. doi: 10.1016/s0920-9964(02)00157-3. [DOI] [PubMed] [Google Scholar]
- 86.Heston LL. Psychiatric disorders in foster home reared children of schizophrenic mothers. Br. J. Psychiatry. 1966;112:819–825. doi: 10.1192/bjp.112.489.819. [DOI] [PubMed] [Google Scholar]
- 87.Juda A. The relationships between highest mental capacity and psychic abnormalities. Am. J. Psychiatry. 1949;106:296–304. doi: 10.1176/ajp.106.4.296. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson JL. Genetic association of giftedness and creativity with schizophrenia. Hereditas. 1970;66:177–182. doi: 10.1111/j.1601-5223.1970.tb02343.x. [DOI] [PubMed] [Google Scholar]
- 89.Karlsson JL. Creative intelligence in relatives of mental patients. Hereditas. 1984;100:83–86. doi: 10.1111/j.1601-5223.1984.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 90.Karlsson JL. Mental abilities of male relatives of psychotic patients. Acta Psychiatr. Scand. 2001;104:466–468. doi: 10.1034/j.1600-0447.2001.00515.x. [DOI] [PubMed] [Google Scholar]
- 91.Jamison KR. Manic-depressive illness and creativity. Sci. Am. 1995;272:62–67. doi: 10.1038/scientificamerican0295-62. [DOI] [PubMed] [Google Scholar]
- 92.Schatzberg AF, Schildkraut JJ. Recent studies on epinepheine systems in mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. New York: Raven Press; 1995. [Google Scholar]
- 93.Maj M, Ariano MG, Arena F, Kemali D. Plasma cortisol, catecholamine and cyclic AMP levels, response to dexamethasone suppression test and platelet MAO activity in manic-depressive patients. A longitudinal study. Neuropsychobiology. 1984;11:168–173. doi: 10.1159/000118071. [DOI] [PubMed] [Google Scholar]
- 94.Kemali D, Maj M. CSF noradrenaline and schizophrenia. Am. J. Psychiatry. 1986;143:126–127. doi: 10.1176/ajp.143.1.126b. [DOI] [PubMed] [Google Scholar]
- 95.Kendler KS, Davis KL. The genetics and biochemistry of paranoid schizophrenia and other paranoid psychoses. Schizophr. Bull. 1981;7:689–709. doi: 10.1093/schbul/7.4.689. [DOI] [PubMed] [Google Scholar]
- 96.Wei J, Ramchand CN, Hemmings GP. Studies on concentrations of NA and HVA and activity of DBH in the serum from schizophrenic patients, first-degree relatives and normal subjects. Schizophr. Res. 1992;8:103–110. doi: 10.1016/0920-9964(92)90025-z. [DOI] [PubMed] [Google Scholar]
- 97.Chotai J, Adolfsson R. Converging evidence suggests that monoamine neurotransmitter turnover in human adults is associated with their season of birth. Eur. Arch. Psychiatry Clin. Neurosci. 2002;252:130–134. doi: 10.1007/s00406-002-0372-7. [DOI] [PubMed] [Google Scholar]
- 98.Jamison KR. Touched With Fire. New York: Free Press; 1993. [Google Scholar]
- 99.Murphy BL, Arnsten AF, Jentsch JD, Roth RH. Dopamine and spatial working memory in rats and monkeys: pharmacological reversal of stress-induced impairment. J. Neurosci. 1996;16:7768–7775. doi: 10.1523/JNEUROSCI.16-23-07768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]