Abstract
Objective
Among women with breast cancer, hot flashes are frequent, severe and bothersome symptoms that can negatively impact quality of life and compromise compliance with life-saving medications (e.g., tamoxifen, aromatase inhibitors). Clinicians' abilities to treat hot flashes are limited due to inadequate understanding of physiological mechanisms involved in hot flashes. Using an acute tryptophan depletion paradigm, we tested whether alterations in central serotonin levels were involved in the induction of hot flashes in women with breast cancer.
Design
This was a within subjects, double-blind, controlled, balanced, crossover study. Twenty-seven women completed two 9-hour test days. On one test day, women ingested a concentrated amino acid drink and encapsulated amino acids (no tryptophan) according to published procedures that have been shown to have specific effects on serotonin within 4.5 to 7 hours. On the other test day, women ingested a control drink. Serial venous blood sampling and objective hot flash monitoring were used to evaluate response to each condition.
Results
Response to acute tryptophan depletion was variable and unexplained by use of selective serotonin reuptake inhibitors, anti-estrogens, breast cancer disease and treatment variables, or genetic polymorphisms in serotonin receptor and transporter genes. Contrary to our hypothesis, hot flashes were not worsened with acute tryptophan depletion.
Conclusions
Physiologically-documented and self-reported hot flashes were not exacerbated by tryptophan depletion. Additional mechanistic research is needed to better understand the etiology of hot flashes.
Keywords: hot flashes, menopause, tryptophan, serotonin, breast cancer
INTRODUCTION
For breast cancer survivors, the hot flash is a frequent, severe, bothersome, and persistent problem 1–5 that negatively impacts daily activities, mood, sleep, and overall quality of life.6 Abrupt withdrawal of hormone therapy at the time of breast cancer diagnosis and treatment with selective estrogen receptor modulators,7–10 aromatase inhibitors,11 or chemotherapy10, 12 can precipitate and/or exacerbate hot flashes. Treating hot flashes in this group is difficult due to contraindications against hormone therapy13, 14 and limited understanding of hot flash etiology. Although there is strong evidence implicating reduced serotonin in the etiology of hot flashes,15, 16 the mechanism underlying serotonin involvement is unclear. Low serotonin has been observed in women after spontaneous or surgical menopause.17, 18 Estrogen replacement alleviates hot flashes and restores serotonin concentrations.17, 18 Estradiol also augments serotonergic activity in postmenopausal women.19 Estrogen appears to affect serotonin metabolism through direct effects on serotonin neurons, which regulate genes involved in serotonin synthesis, transport, and signaling.20, 21 Patients with carcinoid tumors also experience hot flashes.22, 23 Although carcinoid tumors are associated with high peripheral serotonin levels, presumably, central serotonin levels are low.22, 23 Similarly, elevated peripheral serotonin concentrations in perimenopausal women that were positively correlated with hot flashes likely reflect low central levels of serotonin.24 Based on these and other data, selective serotonin reuptake inhibitors (SSRI) or selective norepinephrine reuptake inhibitors (SNRI) are frequently used to treat hot flashes.25, 26 One method for evaluating the role of central serotonin in hot flashes is the acute tryptophan depletion paradigm. This paradigm has been widely used and accepted within the field of psychiatry to evaluate the role of central serotonin neurotransmission in various disorders such as depression, panic disorder, and premenstrual syndrome.27–30 It can be used without serious medical or psychological complications even in patients with such disorders.28, 30–33 This paradigm alters serotonin function in humans, as confirmed with cerebral spinal fluid sampling,34 without the side effects associated with pharmacological agents. In addition, effects are specific for serotonin. There are no direct effects on other neurotransmitters.32
Tryptophan is the precursor for serotonin synthesis and is a naturally occurring amino acid found in foods such as turkey, cheese, and nuts. Since tryptophan is transported into the brain and can be rate limiting for serotonin synthesis, decreasing circulating tryptophan causes a temporary suppression of central serotonin synthesis and concentrations. Acute tryptophan depletion is accomplished by administering a 80–100 gram amino acid drink that contains no tryptophan. Central tryptophan becomes temporarily depleted as (1) hepatic protein synthesis uses up the existing tryptophan and (2) tryptophan competes at a relative disadvantage with the other large neutral amino acids supplied in the drink for the amino acid transporters that transport tryptophan into the brain.31, 32 Acute tryptophan depletion results in temporary lowering of central serotonin neurotransmission within 5 to 7 hours.28, 32 Side effects (nausea, mood changes) are mild and subside after ingestion of a tryptophan-containing meal.
The purpose of this study was to directly reduce central serotonin via acute tryptophan depletion and study acute effects on hot flashes in breast cancer survivors. Our main hypothesis was that deficits in central serotonin levels were involved in the induction of hot flashes in these women who are at heightened vulnerability to hot flashes due to their cancer treatments. Because genetic variations in serotonin receptor and transporter genes have been documented,35–38 we also hypothesized that variability in response to serotonin manipulation could be partly explained by genetic variations.
MATERIALS AND METHODS
Sample and setting
Subjects were recruited from a Midwestern outpatient cancer clinic from 2005 to 2007. All procedures were approved by a local institutional review board, a cancer center scientific review committee, and the funding agency human subjects review board. All subjects were: (a) adults at least 18 years of age (actual age range 30 to 71 years old); (b) willing and able to provide informed consent; (c) reporting daily hot flashes; (d) able to read, write, and speak English; (e) postmenopausal (≥ 12 months amenorrhea39, 40); and (f) >1 month but < 5 years post-treatment (surgery, radiation, chemotherapy) for non-metastatic breast cancer. These criteria allowed inclusion of women successfully treated for recurrent breast cancer since there was no known reason to exclude them.10 Exclusion criterion were: (a) current depression, (b) history of migraines, (c) history of hepatitis, and (d) abnormal chemistry profile (e.g., sodium, potassium, glucose). Women taking SSRI/SNRI were not excluded for two reasons: (1) acute tryptophan depletion was expected to produce a relapse of hot flash symptoms in SSRI/SNRI users31, 32 and (2) we did not want to bias the sample towards those who may not be taking SSRI/SNRI for hot flashes due to lack of efficacy.
Design and intervention
The study was a within subjects, double-blind, controlled, balanced, crossover trial. Subjects took part in two 9-hour clinic visits seven days apart. They received in random order either acute tryptophan depletion drink and capsules or a one-quarter strength control drink and capsules. Acute tryptophan depletion was achieved with a 300 ml amino acid drink and encapsulated amino acids containing 100g total amino acids in the following ratio: L-alanine (5.5g), L-arginine (4.9g), L-cysteine (2.7g), glycine (3.2g), L-histidine (3.2g), L-isoleucine (8.0g), L-leucine (13.5g), L-lysine (11.0g), L-methionine (3.0g), L-phenylalanine (5.7g), L-proline (12.2g), L-serine (6.9g), L-threonine (6.9g), L-tyrosine (6.9g), L-valine (8.9g). 41–43 Orange or chocolate mint flavoring was used to improve palatability of the drink. Cysteine, methionine, and arginine were encapsulated due to their unpleasant taste.34, 41, 43 We used a 100gm drink rather than the 80gm drink used in some studies in order to produce the greatest effect. The 100 gm drink was expected to produce acute tryptophan depletion within 4.5 to 7 hours, with a 80%–90% drop in plasma tryptophan31, 33, 34 and concomitant drop in central tryptophan.34
The control arm was a 300ml drink and encapsulated amino acids but in one-fourth strength: L-alanine (1.4g), L-arginine (1.2g), L-cysteine (0.7g), glycine (0.8g), L-histidine (0.8g), L-isoleucine (2.0g), L-leucine (3.4g), L-lysine (2.8g), L-methionine (0.8g), L-phenylalanine (1.4g), L-proline (3.1g), L-serine (1.7g), L-threonine (1.7g), L-tyrosine (1.7g), L-valine (2.2g), and fillers (7.95g).32, 44 The control drink was expected to lower tryptophan only 25%. Other studies have used tryptophan supplementation as a control condition, however, this practice has been criticized because changes in tryptophan and serotonin can be highly variable.32 We did not compare depletion to both the control and supplementation as this would have required subjects to take part in three all-day study visits.
Randomization and blinding
Biostatisticians created a computer-generated randomization sequence, with randomization done in blocks of four without stratification. After informed consent was obtained, the subject's study number was sent to the study dietician who randomized the subject to one of two sequence groups: acute tryptophan depletion/control or control/acute tryptophan depletion. The study dietician carried out the randomization using amino acids supplied by the investigative team. The dietician dispensed depletion and control drinks in metal cups with black opaque lids and straws to prevent patients, nurses, or study staff from seeing the drink contents. The project manager checked each drink outside of the subject's room to ensure that the dietician had carried out the randomization appropriately. Thus, the dietician and the project manager were not blinded. Subjects and specified team members (nurses, data collectors, hot flash analysts, data entry personnel, lab technicians) were blinded. In the consent form, subjects were told they would receive two different amino acid drinks in random order. The notion of an active drink and a control or placebo drink was never introduced to them. Thus, subjects were not aware that an active drink was being compared to a control drink and subjects who noticed a difference in the viscosity of the drinks were not able to link the more viscous drink to the active condition. In addition, the number of capsules for the unpleasant tasting amino acids was identical between arms.
Study procedures
Women were recruited in the cancer clinic or by telephone after being referred to the study by clinic staff. They were told about the study, screened for eligibility, and if eligible and interested, were mailed a packet of study materials and asked to return signed consent forms in pre-paid envelopes. After consenting, women were interviewed by the study psychologist who verified their eligibility as non-depressed using the Hamilton Rating Scale-Depression. Women were then scheduled for their first study visit.
Procedures for the two study weeks were identical. Subjects arrived at the General Clinical Research Center after fasting for 8 hours. Outpatient admission procedures included: obtaining a brief medical history, physical exam, vital signs, height, and weight; collecting a urine sample for drug screening; placing an intravenous catheter with heparinized saline for blood draws; and starting objective hot flash monitoring. To obtain a baseline, blood was drawn and the subject was asked to fill out demographic, symptom, and mood questionnaires. The study start (time 0) coincided with ingestion of the amino acids. Subsequent blood draws were completed hourly for 8 hours. Symptom and mood questionnaires were completed 3, 5, and 7 hours later. Subjects engaged in quiet activities while being monitored by nurses. Eight hours later, a meal containing 0.25g of tryptophan was served (the amount of tryptophan in 3 ounces of cooked turkey breast). The study psychologist verified absence of depressive symptoms, the intravenous access was removed, and women were discharged wearing the hot flash monitor. The next morning, a trained nurse phoned subjects with instructions for turning off and disconnecting the hot flash monitor. The nurse verified the absence of depression using the Hamilton Rating Scale-Depression (she was trained by the study psychologist to do this), assessed side effects using a checklist, and reminded subjects to bring the hot flash monitor with them to their week 2 visit. Week 2 was completed 7 days later. The week 2 monitor was returned by mail.
Measures
Demographic information was collected with a questionnaire (birthdates, race, marital status, education, employment status, income, current medications, menopausal status, gynecological and reproductive history).6, 45, 46 Breast cancer information was self-reported by subjects and verified through medical record review (date of diagnosis, disease stage, and dates/types of treatments). In addition, subjects were asked to complete the Co-morbidity Questionnaire to document the presence of medical problems, if the condition caused problems, required medication, and/or limited activities.47
Safety monitoring was completed as follows. First, the Hamilton Rating Scale-Depression48, 49 was used to rule out depressive symptoms at study entry, at the end of each test day, and the day after each test day (e.g., score ≤ 18). This scale has been used to monitor response and/or safety in other acute tryptophan depletion studies.27 Inter-rater reliability between the study psychologist and study nurse exceeded 90%. Second, mood and physical side effects were monitored throughout each test day (baseline and hours 3, 5, 7) and the next morning. The Profile of Mood States-Short Form is a 37-item scale that is based on the 65-item version.50, 51 This list of adjectives is rated on a 0 to 4 point scale and responses are summed to generate a total score and six subscale scores (depression, tension, anger, confusion, vigor, fatigue). This scale has been used in other studies to monitor response to acute tryptophan depletion.27 Reliability and validity in women with breast cancer has been supported.52 The Side Effects Report consisted of a list of 24 physical symptoms, including symptoms previously associated with acute tryptophan depletion (nausea, vomiting, nervousness, loss of concentration).53, 54 Respondents indicated if they were having each symptom (no, yes), and if so, rated severity using a 0 (not at all) to 4 point (extremely) scale. Third, serum glucose was assessed at each hourly blood draw (YSI Life Sciences Instrument, Yellow Springs, OH). Glucose tablets were administered by mouth for glucose values ≤ 70 mg/dl. All safety monitoring data was reviewed routinely by the investigative team and a data safety monitoring board.
Hot flash response was monitored as follows. Physiological hot flash frequency was assessed using sternal skin conductance monitoring.45, 46, 55–57 Subjects wore the monitor during the test day and during the nighttime at home for a total of 24-hours. At the end of the monitoring session, the monitor was downloaded and scored using customized software and established procedures.45, 46, 55–57 Scoring was done by trained raters with inter-rater reliability exceeding 90%. Sternal skin conductance monitoring is the gold standard measure of physiologic or objective hot flash frequency only.46, 58 Each hot flash is defined as an increase of 2 units within a 20 second period. Skin conductance magnitude was the sum total change in skin conductance with each physiologic or self-reported hot flash. Self-reported hot flash frequency, severity, and bother were assessed using written diaries and electronic event markers during the 24-hour period. When the subject experienced a hot flash they were instructed to push the two red buttons on the hot flash monitor, write down the time of the hot flash, and rate severity and bother in a paper diary (0=not at all and 10=extremely severe or 10=extremely bothersome).
Laboratory assessments included (1) hourly measurements of circulating tryptophan, serotonin, and kynurenine during each test day and (2) assessment of tryptophan/large neutral amino acid (TRP/LNAA) ratios at the presumed 5-hour tryptophan nadir each week. Whole blood tryptophan, hydroxyl-tryptophan, serotonin, and kynurenine were assayed with HPLC using procedures similar to that described by others.59 The large neutral amino acids concentrations were determined by the Waters Pico-Tag methods. Briefly, the amino acids were derivitized with phenylisothiocyanate forming phenylthisocarbamyl derivatives. The resulting samples were then analyzed by reversed phase HPLC separation and UV detections, using a Waters Alliance HPLC system.
Genetic polymorphisms in three serotonin-related candidate genes were assessed for association with the hot flash response. We chose these specific polymorphisms because previous publications have reported that they were associated with clinical phenotypes. The following single nucleotide polymorphisms were genotyped: rs#6313 and rs#799701 in the serotonin receptor 2A gene (HTR2A); rs#1800532 from the tryptophan hydroxylase gene (TPH1); and the rs#rs6295 from the serotonin receptor 1A (HTR1A) gene. DNA was extracted from whole blood using the Gentra DNA extraction kit. The HTR1A and HTR2A SNPs were genotyped using Taqman assays from Applied Biosystems, Inc (assay id#’s c__11904666_10 (rs6295); c__3042197_1_ (rs6313); and c___1619749_10 (rs7997012)). Genotyping for the TPH1 SNP was conducted using an allele specific PCR assay as follows: We used the iCycler system (Bio-Rad) with allele-specific primers (common forward primer: 5’-AGA ATG GTA CCT GGC ATG AAA-3’, reverse primer for the allele containing A: 5’-C CTA TGC TCA GAA TAG CAG CTC T-3’, reverse primer for the allele containing C: 5’-CTA TGC TCA GAA TAG CAG CTC G-3’) and with the SYBR green Supermix (Bio-Rad). The allele-specific real-time PCR was running for 45 cycles at 95°C for 10 sec and 55°C for 45 sec. Alleles were discriminated based on their Ct values.
Statistical analysis
A preliminary analysis of the first 4 subjects indicated that 25 subjects were needed to provide 80% power to detect a modest and significant difference between depletion and control arms using a paired t-test and alpha of 0.05.
The following analyses were done to verify experimental procedures. Sample characteristics were examined using descriptive statistics. Two-sided t-tests and Fisher's exact tests were used to compare characteristics of subjects who received depletion/control and control/depletion. To verify that adequate depletion was reached, we used (1) repeated measures analysis to compare the percentage change in serum tryptophan over time between randomized groups and (2) Fisher's exact tests to compare tryptophan values and tryptophan/large neutral amino acid (TRP/LNAA) ratios at the nadir time point (5 hours following drink ingestion) between groups. After examining correlations among hot flash measures using Pearson's correlations, the main hypothesis was tested using two sets of hot flash variables: (1) those based on data from the total 8-hour test day (from drink ingestion to 8 hours later [hours 0–8]) and (2) those based on a 3-hour tryptophan nadir period (from 5 hours after drink ingestion to 3 hours later [hours 5–8]). We tested both week 1 to week 2 carry-over effects and week effects using a mixed linear model. Because neither of the effects were significant, we were able to ignore the order effect and collapse data for paired t-tests comparing depletion to control using all subjects as their own controls. Because 5 subjects vomited at some point during the acute tryptophan depletion clinic day, and that could potentially confound effects of the tryptophan depletion, we also compared hot flashes between those who achieved greatest depletion compared to those with a less effective depletion based on (1) tryptophan concentrations < 10 µM at hour 5 vs ≥ 10 at hour 5) and (2) TRP/LNAA <.007 vs. ≥ .007). These cutoffs were determined empirically based on visual inspection of the data. For both measurements, the values appeared in one of two groups that were separated by a region of concentrations where no samples were observed. We also tested whether response to the acute tryptophan depletion vs. control conditions varied by the following: use of SSRI/SNRI, anti-estrogens (such as Tamoxifen or aromatase inhibitors), breast cancer disease and treatment variables (time since diagnosis, type of treatment), and genetics. Due to small sample sizes, genetic analyses were conducted by descriptive methods.
RESULTS
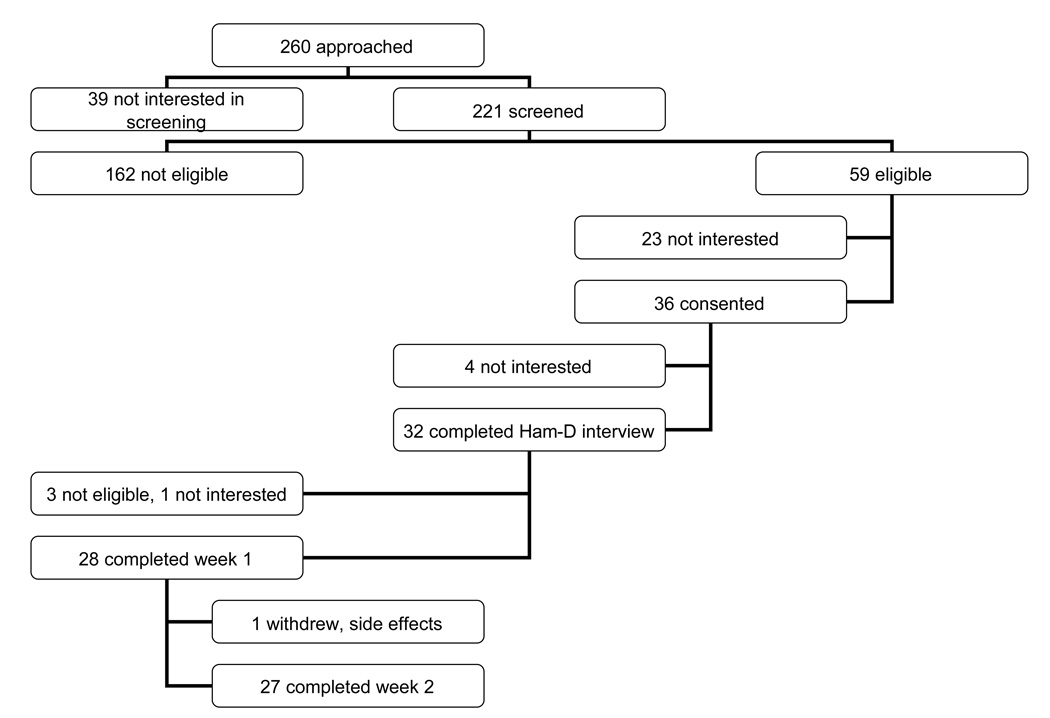
The accrual flow is shown in Figure 1. Of the 36 consenting women, 5 expressed lack of interest and withdrew and 3 were withdrawn due to ineligibility. Of the remaining 28 women, 28 completed week 1 and 27 completed week 2 (one withdrew after week 1 due to dizziness occurring 3 days after the study visit). Genetic analyses were available for all women. However, due to difficulties in sustaining venous access, only 24 women had serial tryptophan and metabolites data during the depletion week and only 23 during the control week.
Figure 1. Study Accrual and Retention.
Legend: This figure shows the participant flow with attrition throughout the study. The Ham-D phone interview refers to the study psychologist verifying eligibility as non-depressed using the Hamilton Rating Scale-Depression (score ≤ 18) .
The 27 subjects were mostly Caucasian (93%), married or living with a partner (82%), and with household incomes above $60K (59%). Mean age was 53.4 years old (SD= 9.6, range 30–71). All had been successfully treated for non-metastatic breast cancer (e.g., stage III or less) and were considered to be free of cancer at the time of the study. Mean time post-completion of primary treatment (e.g., surgery, chemotherapy, radiation) was 37.8 months (SD=18.6, range 13–77). Breast cancer treatments received included surgery alone (15%), surgery with radiation therapy (15%), surgery with chemotherapy (19%), and surgery with radiation and chemotherapy (52%). No subjects were taking hormone replacement therapy, 37% were taking tamoxifen, 42% were taking an aromatase inhibitor, and 33% were taking an SSRI/SNRI. No significant differences were found between those randomized to depletion/control versus control/depletion for age, race, marital status, education, employment, income, or SSRI/SNRI use (p > .10).
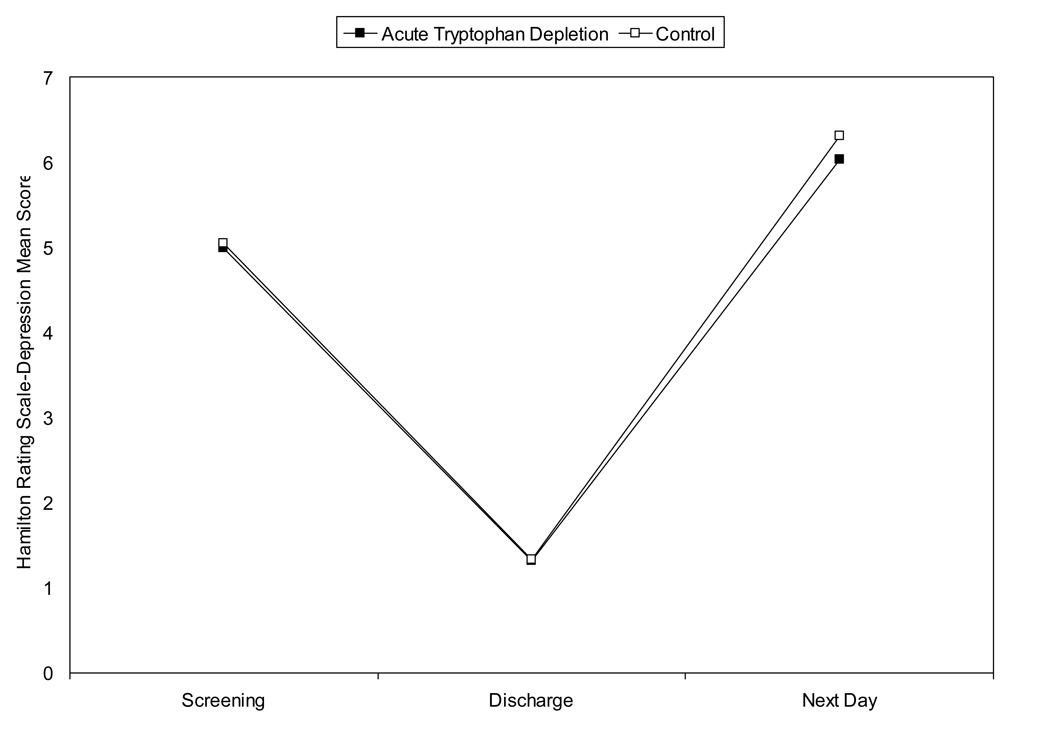
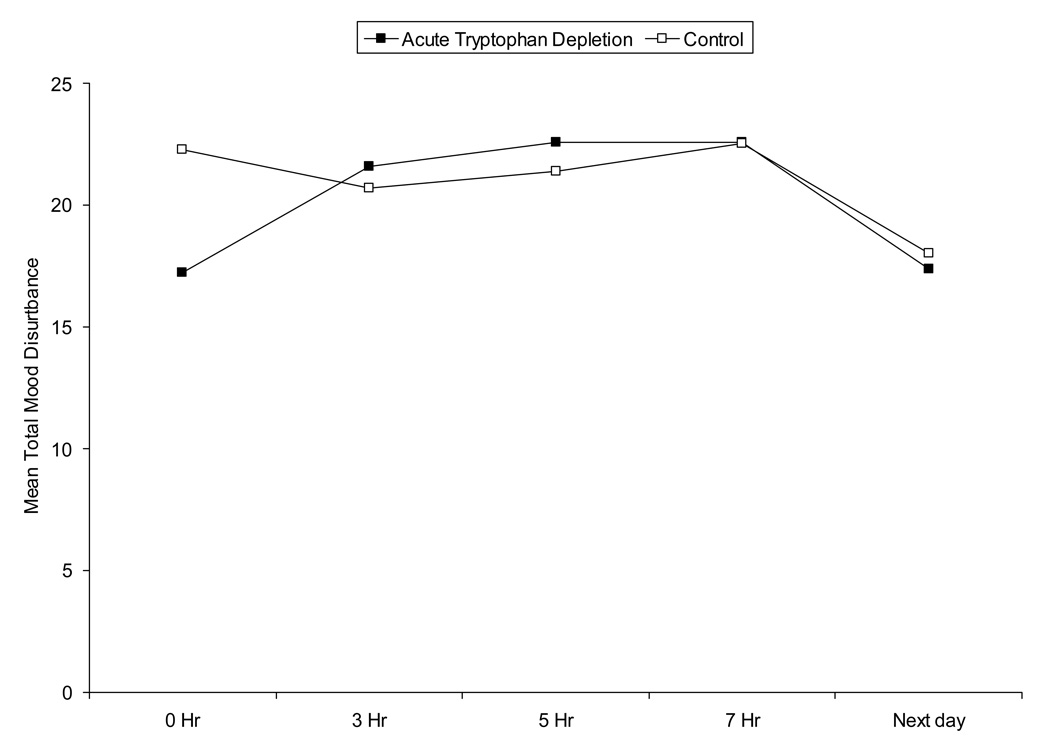
Adverse events included the following. During acute tryptophan depletion, five subjects vomited. Vomiting occurred within 20 minutes after ingesting amino acids (n=1) or between 4 and 6.25 hours later (n=4). One patient experienced hypoglycemia 3 hours after drink ingestion both study visits that was resolved with glucose tablets. Mood was not significantly impacted. Hamilton Rating Scale-Depression scores remained < 18 at all time points and for both study arms. Similarly, total mood disturbance and symptom rating scores were not significantly different between study arms over time (p > .20) (see Figure 2).
Figure 2. Safety Monitoring During Acute Tryptophan Depletion (ATD) and Control Arm.
(2a) Hamilton Rating Scale-Depression Scores
Legend: This figure denotes the mean Hamilton Rating Scale-Depression score at each time point for the study sample (n=27): initial screening to verify eligibility (as assessed by the study psychologist), at discharge from the study visit (as assessed by the study psychologist), and the following day (as assessed by a trained nurse). The solid square refers to the active amino acid drink and the open square to the control drink. Scores remained below 18 (cutoff for depression) at all time points and for both study arms.
(2b) Profile of Mood States Total Mood Disturbance Scores
Legend: This figure denotes the Profile of Mood Disturbance Total Mood Disturbance Score at each time point for the study sample (n=27). The solid square refers to the active amino acid drink and the open square to the control drink. Hours 0 to 7 were during the test day for each condition. Total mood disturbance scores were not significantly different over time (p > .20).
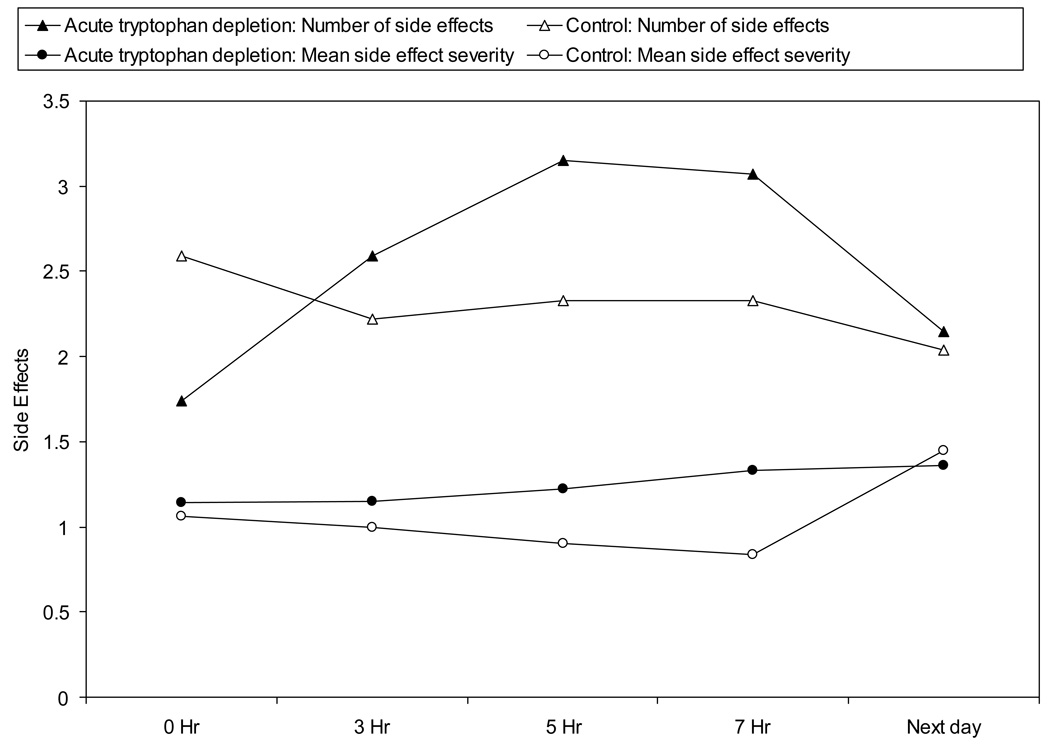
(c) Number and Severity of Side Effects
Legend: This figure denotes the number and mean severity of side effects over time for each condition. Subjects (n=27) indicated if they were having symptoms using a checklist and the number they endorsed was summed to calculate the total number of symptoms. For each symptom that they endorsed, subjects rated severity from 0 (not at all) to 4 (extremely) severe. Mean severity for all symptoms endorsed was calculated (total severity/total number of symptoms). The closed triangle and circle refer to the active amino acid drink and the open triangle and circle to the control drink. Hours 0 to 7 were during the test day for each condition. Number and severity of symptoms were not significantly different between study arms over time (p > .20).
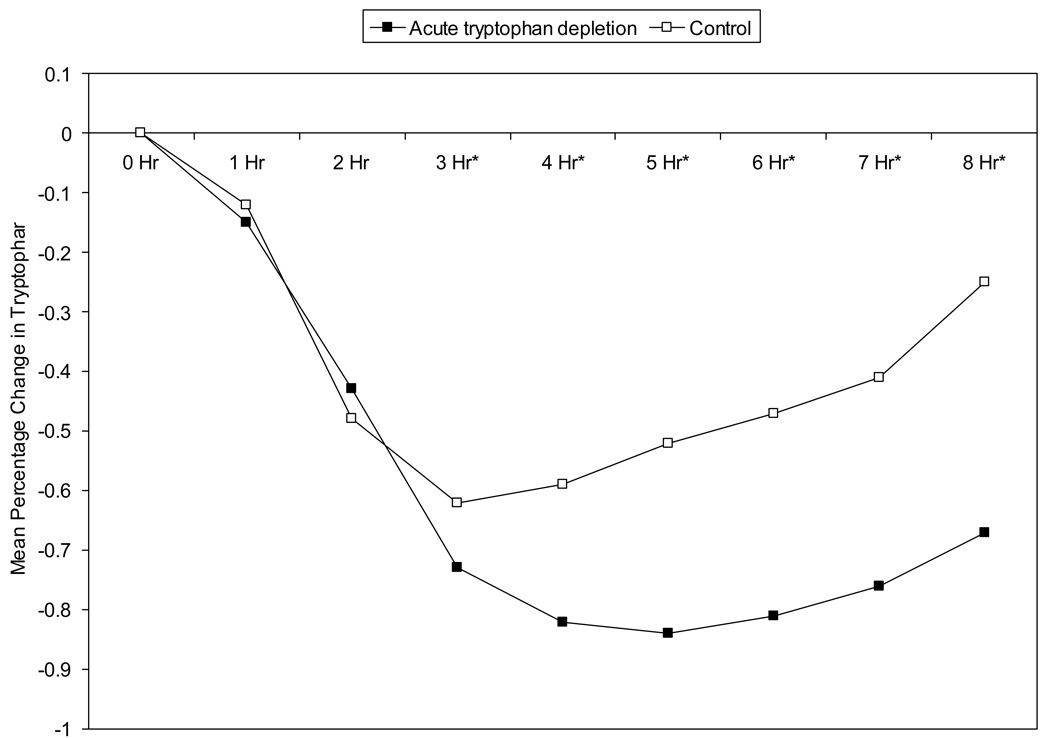
The percentage change in serum tryptophan during depletion and control conditions is shown in Figure 3. Baseline, 1 hour, and 2 hour total tryptophan values were not significantly different (p=.46) between the group randomized to depletion treatment first and the group randomized to the depletion second. However, the tryptophan depletion group had significantly lower total tryptophan than control at hours 3 through 8 indicating that adequate separation between study arms was achieved. As expected, during the nadir period (5 hours after drink ingestion), 83% of women receiving tryptophan depletion had total tryptophan values < 10 µM compared to 35% while on control (p = 0.001). Similarly, nadir TRP-LNAA ratios were significantly lower at hour 5 during tryptophan depletion (M=0.0034, SD=0.0027) compared to control (M=0.02, SD=0.02) (p=< 0.0001).
Figure 3. Percentage Change in Serum Tryptophan Over Time During Acute Tryptophan Depletion (ATD) Versus Control Arms.
Legend: This figure denotes the percentage change in tryptophan from baseline over time for each study arm (n=27). Times 0 to 8 refer to hourly blood draws during each test day. The solid square refers to the active amino acid drink and the open square to the control drink. Repeated measures analysis indicated that tryptophan values were not significantly different (p=.46) between randomized groups at hours 0, 1, or 2 but were significantly lower in the acute tryptophan depletion group compared to control group at hour 3 (p < .01) and hours 4, 5, 6, 7, and 8 (p < .001). Significant differences are shown with an asterisk next to the time point (i.e. hours 3 through 8).
As shown in Table 1, objective hot flash frequency and magnitude were highly correlated. Similarly, subjective hot flash variables (diary frequency, event button frequency, intensity, bother) were highly correlated. Correlations between objective and subjective hot flash variables were modest (.46 < r < .58) in this study, but higher than correlations for similar data collected during ambulatory monitoring outside of a laboratory (.06 < r < .26).60
Table 1.
Pearson Correlations Denoting Relationships Among Hot Flash Variables
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Objective frequency | --- | .96*** | .68** | .71** | .69** | .64** |
| 2. Skin conductance magnitude | .97*** | --- | .69** | .73*** | .69** | .61** |
| 3. Subjective frequency (diary) | .42* | .48* | --- | .94*** | .80*** | .79*** |
| 4. Subjective frequency (event button) | .43* | .49* | .98*** | --- | .85*** | .84*** |
| 5. Subjective intensity | .43* | .46* | .83*** | .77*** | --- | .94*** |
| 6. Subjective bother | .32 | .35 | .77*** | .74*** | .89*** | --- |
The numbers in columns 2 to 7 correspond with the numbered variables listed in column 1, such that 1 is objective frequency, 2 is skin conductance magnitude, etc.
Correlations above the diagonal are total or mean values during entire 8 hour acute tryptophan depletion condition.
Correlations below the diagonal are total or mean values during entire 8 hour control condition.
p < .05
p < .01
p< .001
Table 2 shows no significant differences in any hot flash variables between depletion and control conditions for any of the 8-hour long variables or any of the 3-hour long tryptophan nadir variables. Not shown are t-tests for differences between groups based on tryptophan response and TRP/LNAA response. These t-tests were similar and non-significant. In addition, response did not vary by use of SSRI/SNRI, anti-estrogens, breast cancer disease and treatment variables, or genetic variants in the HTR1A, HTR2A, and TRH1 genes.
Table 2.
Descriptive Statistics for Hot Flash Variables During Acute Tryptophan Depletion (ATD) and Control Arm for 8 Hour Test Day and 3 Hour Nadir Periods
| 8 Hour Test Daya | 3 Hour Nadir Periodb | |||||
|---|---|---|---|---|---|---|
| ATD | Control | ATD | Control | |||
| M(SD) | M(SD) | p | M(SD) | M(SD) | p | |
| Objective frequency | 2.30 (2.92) | 2.62 (3.29) | .71 | 1.22 (1.69) | 1.23 (1.66) | .99 |
| Skin conductance magnitudec | 8.54 (9.17) | 9.20 (11.24) | .82 | 4.29 (4.92) | 4.36 (5.54) | .96 |
| Subjective frequency (diary) | 4.56 (3.11) | 3.65 (3.02) | .29 | 1.85 (1.66) | 1.42 (1.39) | .31 |
| Subjective frequency (event button) | 4.41 (2.93) | 3.54 (3.11) | .30 | 1.85 (1.49) | 1.38 (1.55) | .27 |
| Subjective intensity | 16.81 (15.87) | 13.69 (16.30) | .48 | 7.22 (7.83) | 5.81 (7.30) | .50 |
| Subjective bother | 14.26 (15.29) | 10.23 (12.73) | .30 | 6.33 (7.64) | 4.65 (6.41) | .39 |
From drink ingestion to 8 hours later.
Three hour period spanning 5 to 8 hours after drink ingestion.
Sum of all changes in skin conductance that accompanied objective and subjective hot flashes.
DISCUSSION
The primary aim of this study was to investigate the effects of lowered serotonin synthesis by acute tryptophan depletion on hot flashes in female survivors of breast cancer - a group known to have frequent, severe, and bothersome hot flashes. It was hypothesized that acute tryptophan depletion would exacerbate hot flashes, however, our data did not support this assumption. We did not find any significant differences between study conditions for objective or subjective hot flash measures.
There are two potential explanations for these negative study findings. First, if central serotonin functioning was severely diminished in these postmenopausal women, we may not have accomplished a further reduction in serotonin with acute tryptophan depletion. Although women are generally more susceptible than men to the effects of acute tryptophan depletion on memory,61 estrogen loss at menopause may affect how postmenopausal women respond to acute tryptophan depletion. Loss of estrogen appears to diminish serotonin concentrations, activity and metabolism17–21 Thus, we may not have accomplished further lowering of central serotonin despite the observed changes in peripheral tryptophan and TRP/LNAA ratios. This would need to be tested in a subsequent study using cerebral spinal fluid sampling.
If central serotonin levels are already low in these women, an alternative approach would be to provide additional tryptophan by dietary supplementation. However, this assumes a substrate limitation rather than an enzyme limitation. If menopause alters the enzymes involved in converting tryptophan to serotonin, adding more substrate would not produce a response. However, there is evidence suggesting that tryptophan supplementation may have merit. For example, tryptophan supplements have been suggested as a potential hot flash treatment62 and appear to impact other symptoms. For example, in women, tryptophan significantly reduced premenstrual dysphoric symptoms compared to placebo.63, 64 In humans, primates, and rats, tryptophan decreases mild depressive symptoms and aggressive behavior.65, 66 Chronic tryptophan supplementation raises plasma tryptophan and central serotonin and potentiates SSRI effectiveness in rats.67, 68 Although contaminated tryptophan supplements were thought to cause eospinophilia myalgia syndrome,69 today, dietary manipulation of tryptophan or L-tryptophan/5-hydroxytryptophan supplementation is considered to be a safe intervention.65, 66, 68, 70
A second explanation for negative study findings may be that acute tryptophan depletion alone, without an additional stressor such as a thermal challenge, may not have been adequate to invoke hot flashes. A similar paradigm has been seen in panic and obsessive compulsive disorders. Tryptophan depletion alone does not provoke panic, however, it increases susceptibility to panic so that if a stressor is applied during tryptophan depletion (i.e., flumazenil), panic symptoms are exacerbated.28 Similarly, symptoms of obsessive compulsive disorder emerge after acute tryptophan depletion only when an additional stressor is present.71 In this study, tryptophan depletion may have increased susceptibility to hot flashes but normal environmental stressors were controlled due to the laboratory setting (e.g., absence of temperature and humidity fluctuations, absence of emotional stressors). This hypothesis could be tested in a follow-up study by comparing time to hot flash onset or hot flash frequency, intensity or bother following a stressor such as application of heating pads during acute tryptophan depletion and control conditions.
Study findings should be considered in light of study limitations. Although the sample size was comparable or larger than that seen in over 70 similar studies included in a recent review,27 the sample was limited in terms of ethnic and racial diversity, yet heterogeneous in relation to breast cancer disease and treatment characteristics. In addition, this sample of breast cancer survivors does not provide any indication of how healthy menopausal women who have not undergone breast cancer treatment would respond. Finally, the design was limited in that it did not include a study arm to evaluate the effects of tryptophan or 5-hydroxytryptophan supplementation. Some previous studies have used tryptophan supplementation for comparison.28 However, because of reported variable increases in tryptophan and serotonin with supplementation32 and anticipated difficulties in recruiting women to take part in an additional study visit, we chose to use a one-quarter strength drink as the control condition. This control condition reduced plasma tryptophan levels more than the 25% that was expected and the reasons for this are unknown. However, the control drink did differ from the full strength depletion in that depletion occurred to a lesser degree and with faster recovery.32
In summary, to our knowledge, this was the first study to examine hot flash physiology using acute tryptophan depletion. Further testing of our study hypothesis may be warranted through tryptophan supplementation or a tryptophan depletion with stressor paradigm. Findings indicate that additional research on mechanistic pathways is needed to guide appropriate treatment in clinical practice.
ACKNOWLEDGEMENTS
This research was supported with an Idea Award from the Department of Defense Breast Cancer Program (BC043199, DAMD W81XWH-05-1-0326) and by the Indiana University General Clinical Research Center (NIH M01 RR00750).
REFERENCES
- 1.Canney PA, Hatton MQ. The prevalence of menopausal symptoms in patients treated for breast cancer. Clin Oncol (R Coll Radiol) 1994;6:297–299. doi: 10.1016/s0936-6555(05)80270-5. [DOI] [PubMed] [Google Scholar]
- 2.Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage. 2002;23:501–509. doi: 10.1016/s0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JS. State of the science: Hot flashes and cancer. Part 1: Definition, scope, impact, physiology, and measurement. Oncol Nurs Forum. 2005;32:959–968. doi: 10.1188/05.ONF.959-968. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter JS. State of the science: Hot flashes and cancer. Part 2: Management and future directions. Oncol Nurs Forum. 2005;32:969–978. doi: 10.1188/05.ONF.969-978. [DOI] [PubMed] [Google Scholar]
- 5.Gupta P, Sturdee DW, Palin SL, et al. Menopausal symptoms in women treated for breast cancer: The prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9:49–58. doi: 10.1080/13697130500487224. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16–E25. doi: 10.1188/02.ONF.E16-E25. [DOI] [PubMed] [Google Scholar]
- 7.Love RR. Tamoxifen therapy in primary breast cancer: Biology, efficacy, and side effects. J Clin Oncol. 1989;7:803–815. doi: 10.1200/JCO.1989.7.6.803. [DOI] [PubMed] [Google Scholar]
- 8.Love RR, Cameron L, Connell BL, Leventhal H. Symptoms associated with tamoxifen treatment in postmenopausal women. Arch Intern Med. 1991;151:1842–1847. [PubMed] [Google Scholar]
- 9.Pasacreta JV, McCorkle R. Providing accurate information to women about tamoxifen therapy for breast cancer: Current indications, effects, and controversies. Oncol Nurs Forum. 1998;25:1577–1583. [PubMed] [Google Scholar]
- 10.Carpenter JS, Andrykowski MA, Cordova M, et al. Hot flashes in postmenopausal women treated for breast carcinoma: Prevalence, severity, correlates, management, and relation to quality of life. Cancer. 1998;82:1682–1691. [PubMed] [Google Scholar]
- 11.Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: Results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 12.Reichman BS, Green KB. Breast cancer in young women: Effect of chemotherapy on ovarian function, fertility, and birth defects. J Natl Cancer Inst Monogr. 1994:125–129. [PubMed] [Google Scholar]
- 13.Kendall A, Dowsett M, Folkerd E, Smith I. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17:584–587. doi: 10.1093/annonc/mdj127. [DOI] [PubMed] [Google Scholar]
- 14.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer--is it safe?), a randomised comparison: Trial stopped. Lancet. 2004;363:453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 15.Berendsen HH. The role of serotonin in hot flushes. Maturitas. 2000;36:155–164. doi: 10.1016/s0378-5122(00)00151-1. [DOI] [PubMed] [Google Scholar]
- 16.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes D. Hot flushes. Lancet. 2002;360:1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 17.Gonzales GF, Carrillo C. Blood serotonin levels in postmenopausal women: Effects of age and serum oestradiol levels. Maturitas. 1993;17:23–29. doi: 10.1016/0378-5122(93)90120-7. [DOI] [PubMed] [Google Scholar]
- 18.Blum I, Vered Y, Lifshitz A, et al. The effect of estrogen replacement therapy on plasma serotonin and catecholamines of postmenopausal women. Isr J Med Sci. 1996;32:1158–1162. [PubMed] [Google Scholar]
- 19.Halbreich U, Rojansky N, Palter S, Tworek H, Hissin P, Wang K. Estrogen augments serotonergic activity in postmenopausal women. Biol Psychiatry. 1995;37:434–441. doi: 10.1016/0006-3223(94)00181-2. [DOI] [PubMed] [Google Scholar]
- 20.Bethea CL, Mirkes SJ, Shively CA, Adams MR. Steroid regulation of tryptophan hydroxylase protein in the dorsal raphe of macaques. Biol Psychiatry. 2000;47:562–576. doi: 10.1016/s0006-3223(99)00156-0. [DOI] [PubMed] [Google Scholar]
- 21.Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 22.Mohyi D, Tabassi K, Simon J. Differential diagnosis of hot flashes. Maturitas. 1997;27:203–214. doi: 10.1016/s0378-5122(97)83974-6. [DOI] [PubMed] [Google Scholar]
- 23.Schnirer II, Yao JC, Ajani JA. Carcinoid--a comprehensive review. Acta Oncol. 2003;42:672–692. doi: 10.1080/02841860310010547. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Lu X, Huang Y, Xin X, Ye X. [Changes of plasma serotonin precursor metabolite concentrations in postmenopausal women with hot flushes] Zhonghua Fu Chan Ke Za Zhi. 2002;37:726–728. [PubMed] [Google Scholar]
- 25.Weitzner MA, Moncello J, Jacobsen PB, Minton S. A pilot trial of paroxetine for the treatment of hot flashes and associated symptoms in women with breast cancer. J Pain Symptom Manage. 2002;23:337–345. doi: 10.1016/s0885-3924(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 26.Kockler DR, McCarthy MW. Antidepressants as a treatment for hot flashes in women. Am J Health Syst Pharm. 2004;61:287–292. doi: 10.1093/ajhp/61.3.287. [DOI] [PubMed] [Google Scholar]
- 27.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 28.Bell C, Forshall S, Adrover M, et al. Does 5-HT restrain panic? A tryptophan depletion study in panic disorder patients recovered on paroxetine. J Psychopharmacol. 2002;16:5–14. doi: 10.1177/026988110201600116. [DOI] [PubMed] [Google Scholar]
- 29.Menkes DB, Coates DC, Fawcett JP. Acute tryptophan depletion aggravates premenstrual syndrome. J Affect Disord. 1994;32:37–44. doi: 10.1016/0165-0327(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 30.Reilly JG, McTavish SF, Young AH. Rapid depletion of plasma tryptophan: A review of studies and experimental methodology. J Psychopharmacol. 1997;11:381–392. doi: 10.1177/026988119701100416. [DOI] [PubMed] [Google Scholar]
- 31.Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. Br J Psychiatry. 2001;178:399–405. doi: 10.1192/bjp.178.5.399. [DOI] [PubMed] [Google Scholar]
- 32.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: Review. Mol Psychiatry. 2003;8:951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 33.Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61 Suppl 1:5–12. [PubMed] [Google Scholar]
- 34.Salomon RM, Kennedy JS, Johnson BW, et al. Association of a critical CSF tryptophan threshold level with depressive relapse. Neuropsychopharmacology. 2003;28:956–960. doi: 10.1038/sj.npp.1300098. [DOI] [PubMed] [Google Scholar]
- 35.Kang RH, Choi MJ, Paik JW, Hahn SW, Lee MS. Effect of serotonin receptor 2A gene polymorphism on mirtazapine response in major depression. Int J Psychiatry Med. 2007;37:315–329. doi: 10.2190/PM.37.3.h. [DOI] [PubMed] [Google Scholar]
- 36.Maron E, Toru I, Must A, et al. Association study of tryptophan hydroxylase 2 gene polymorphisms in panic disorder. Neurosci Lett. 2007;411:180–184. doi: 10.1016/j.neulet.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 37.Lane HY, Liu YC, Huang CL, et al. Prefrontal executive function and D1, D3, 5-HT2A and 5-HT6 receptor gene variations in healthy adults. J Psychiatry Neurosci. 2008;33:47–53. [PMC free article] [PubMed] [Google Scholar]
- 38.Smith AK, Dimulescu I, Falkenberg VR, et al. Genetic evaluation of the serotonergic system in chronic fatigue syndrome. Psychoneuroendocrinology. 2008;33:188–197. doi: 10.1016/j.psyneuen.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Avis NE, McKinlay SM. The Massachusetts Women's Health Study: An epidemiologic investigation of the menopause. J Am Med Womens Assoc. 1995;50:45–49. 63. [PubMed] [Google Scholar]
- 40.Brambilla DJ, McKinlay SM, Johannes CB. Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol. 1994;140:1091–1095. doi: 10.1093/oxfordjournals.aje.a117209. [DOI] [PubMed] [Google Scholar]
- 41.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 42.Salomon RM, Miller HL, Krystal JH, Heninger GR, Charney DS. Lack of behavioral effects of monoamine depletion in healthy subjects. Biol Psychiatry. 1997;41:58–64. doi: 10.1016/0006-3223(95)00670-2. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann RC, McDougle CJ, Schumacher M, Olcese J, Heninger GR, Price LH. Urinary 6-hydroxymelatonin sulfate as a measure of melatonin secretion during acute tryptophan depletion. Psychoneuroendocrinology. 1993;18:567–578. doi: 10.1016/0306-4530(93)90034-i. [DOI] [PubMed] [Google Scholar]
- 44.Krahn LE, Lu PY, Klee G, Delgado PR, Lin SC, Zimmermann RC. Examining serotonin function: A modified technique for rapid tryptophan depletion. Neuropsychopharmacology. 1996;15:325–328. doi: 10.1016/0893-133X(95)00273-G. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter JS, Gautam S, Freedman RR, Andrykowski M. Circadian rhythm of objectively recorded hot flashes in postmenopausal breast cancer survivors. Menopause. 2001;8:181–188. doi: 10.1097/00042192-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Carpenter JS, Andrykowski MA, Freedman RR, Munn R. Feasibility and psychometrics of an ambulatory hot flash monitoring device. Menopause. 1999;6:209–215. doi: 10.1097/00042192-199906030-00006. [DOI] [PubMed] [Google Scholar]
- 47.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 50.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 51.McNair DM, Lorr M, Droppelman LF. POMS manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 52.Curran SL, Andrykowski M, Studts JL. Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychol Assess. 1995;7:80–83. [Google Scholar]
- 53.Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26:1311–1317. [PubMed] [Google Scholar]
- 54.Carpenter JS, Andrykowski MA, Cordova MJ, Cunningham LL, Studts JL. Do participants' reports of symptom prevalence or severity vary by interviewer gender? Nurs Res. 1999;48:276–279. doi: 10.1097/00006199-199909000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Carpenter JS. Physiological monitor for assessing hot flashes. Clin Nurse Spec. 2005;19:8–10. doi: 10.1097/00002800-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR. Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors. Menopause. 2004;11:375–381. doi: 10.1097/01.gme.0000113848.74835.1a. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter JS, Monahan PO, Azzouz F. Accuracy of subjective hot flush reports compared with continuous sternal skin conductance monitoring. Obstet Gynecol. 2004;104:1322–1326. doi: 10.1097/01.AOG.0000143891.79482.ee. [DOI] [PubMed] [Google Scholar]
- 58.Freedman RR. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26:573–579. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 59.Xiao R, Beck O, Hjemdahl P. On the accurate measurement of serotonin in whole blood. Scand J Clin Lab Invest. 1998;58:505–510. doi: 10.1080/00365519850186319. [DOI] [PubMed] [Google Scholar]
- 60.Carpenter JS, Rand KL. Modeling the hot flash experience in breast cancer survivors. Menopause. 2008;15:469–475. doi: 10.1097/gme.0b013e3181591db7. [DOI] [PubMed] [Google Scholar]
- 61.Sambeth A, Blokland A, Harmer CJ, et al. Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: A pooled analysis of nine studies. Neurosci Biobehav Rev. 2007;31:516–529. doi: 10.1016/j.neubiorev.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Curcio JJ, Kim LS, Wollner D, Pockaj BA. The potential of 5-hydryoxytryptophan for hot flash reduction: A hypothesis. Altern Med Rev. 2005;10:216–221. [PubMed] [Google Scholar]
- 63.Steinberg S, Annable L, Young SN, Liyanage N. A placebo-controlled study of the effects of L-tryptophan in patients with premenstrual dysphoria. Adv Exp Med Biol. 1999;467:85–88. doi: 10.1007/978-1-4615-4709-9_11. [DOI] [PubMed] [Google Scholar]
- 64.Steinberg S, Annable L, Young SN, Liyanage N. A placebo-controlled clinical trial of L-tryptophan in premenstrual dysphoria. Biol Psychiatry. 1999;45:313–320. doi: 10.1016/s0006-3223(98)00005-5. [DOI] [PubMed] [Google Scholar]
- 65.Young SN, Leyton M. The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels. Pharmacol Biochem Behav. 2002;71:857–865. doi: 10.1016/s0091-3057(01)00670-0. [DOI] [PubMed] [Google Scholar]
- 66.Orosco M, Rouch C, Beslot F, Feurte S, Regnault A, Dauge V. Alpha-lactalbumin-enriched diets enhance serotonin release and induce anxiolytic and rewarding effects in the rat. Behav Brain Res. 2004;148:1–10. doi: 10.1016/s0166-4328(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 67.Feurte S, Gerozissis K, Regnault A, Paul FM. Plasma Trp/LNAA ratio increases during chronic ingestion of an alpha-lactalbumin diet in rats. Nutr Neurosci. 2001;4:413–418. doi: 10.1080/1028415x.2001.11747377. [DOI] [PubMed] [Google Scholar]
- 68.van der Stelt HM, Broersen LM, Olivier B, Westenberg HG. Effects of dietary tryptophan variations on extracellular serotonin in the dorsal hippocampus of rats. Psychopharmacology (Berl) 2004;172:137–144. doi: 10.1007/s00213-003-1632-6. [DOI] [PubMed] [Google Scholar]
- 69.Williamson BL, Benson LM, Tomlinson AJ, Mayeno AN, Gleich GJ, Naylor S. On-line HPLC-tandem mass spectrometry analysis of contaminants of L-tryptophan associated with the onset of the eosinophilia-myalgia syndrome. Toxicol Lett. 1997;92:139–148. doi: 10.1016/s0378-4274(97)00048-9. [DOI] [PubMed] [Google Scholar]
- 70.Das YT, Bagchi M, Bagchi D, Preuss HG. Safety of 5-hydroxy-L-tryptophan. Toxicol Lett. 2004;150:111–122. doi: 10.1016/j.toxlet.2003.12.070. [DOI] [PubMed] [Google Scholar]
- 71.Berney A, Sookman D, Leyton M, Young SN, Benkelfat C. Lack of effects on core obsessive-compulsive symptoms of tryptophan depletion during symptom provocation in remitted obsessive-compulsive disorder patients. Biol Psychiatry. 2006;59:853–857. doi: 10.1016/j.biopsych.2005.08.023. [DOI] [PubMed] [Google Scholar]