Abstract
We studied the effects of serum growth factors and of TNF-family proteins on osteoblast gap-junction connectivity. Serum starvation of human MG63 osteosarcoma cells or nontransformed osteoblasts decreased connexin43 protein. TNFα or TRAIL reduced connexin43 further. Serum starvation redistributed gap junctions but did not reduce intercellular diffusion. In contrast, TNFα or TRAIL reduced gap junctions on cell processes and decreased intercellular diffusion. Effects of TNFs on connexin43 were mediated by lysosomal proteolysis. Activating analogs of cAMP increased connexin43 protein, but did not block effects of serum starvation, TNFα, or TRAIL on connexin43 protein. Connexin43 and connectivity recovered overnight if stimuli were withdrawn. Surprisingly, connexin43 mRNA increased in serum starvation and with TNFα or TRAIL. Since β- catenin is a binding partner of connexin43, when connexin43 is degraded, β- catenin activation may contribute to a reflexive increase in connexin43 transcription. We conclude that osteoblast connectivity is regulated by a multifactorial system that maintains intercellular connections. Serum starvation, TNFα and TRAIL augmented connexin43 degradation and connexin43 transcription. Cell-cell communication was maintained in serum starvation, which may model response to acute injury, but was sensitive to TNFs. These inflammatory agents mediated selective, reversible removal of connexin43 from cell processes.
Keywords: DR4, CX43, osteoblast, osteocyte, gap junction
Introduction
Bone consists of functional multicellular units connected by gap junctions that secrete and maintain the bone matrix. Successive layers of osteoblasts are buried in the impervious bone matrix. These buried cells, osteocytes, communicate with each other and surface osteoblasts via cell processes within canaliculi [1, 2]. On these cell processes, hexamers of connexin43 protein dock with hexamers in neighboring cells forming gap junctions [3]. These junctions allow passive diffusion of molecules smaller than approximately 1000 daltons, such as nutrients, ions and signalling molecules. Connexin43 is an abundant protein in osteoblasts, and gap junctions are very prominently seen.
Regulation of osteoblast connections is incompletely understood. This is an important gap in understanding because cell-cell connections are regulated during normal bone function. For long periods, the bone forming unit is stable, with surface cells passing nutrients and signals to buried cells in the connected unit. However, during bone turnover, parts of the bone-forming unit are resorbed by osteoclasts, while other cells in the unit remain unaffected and alive. In some circumstances, such as in high concentrations of glucocorticoids, connected bone cells undergo widespread apoptosis [4] leading to important pathology. Neither the mechanisms that allow connected bone cells to be resorbed without damage to the unit, nor the reasons that connected cells die together in pathological conditions, are clear.
Connexin43 gap junctions are essential to normal bone function. Mineralization in connexin43 null osteoblasts is defective [5], and osteoblast response to anabolic signals is flawed [6]. Other connexins expressed by osteoblasts include connexins 31.9 and 45, although normally at protein levels a few percent that of connexin43. Their contributions to osteoblast communication are poorly understood, although their presence may be reflected in the fact that osteoblasts with connexin43 deletion can form bone, albeit with defects. Osteoblast synthesis of connexin43 is, in part, regulated by cAMP and cell stretch [7]. Connexin43 also may form hemi-gap junctions in osteoblasts, believed to mediate apoptosis [8].
Our previous work showed that short-term exposure to TRAIL, the TNFα- related apoptosis inducing ligand, reduced cell-cell processes in osteoblast cell cultures [9]. In fibroblasts, TNFα was recently shown to downregulate connexin43 and gap junctions [10]. These findings suggest that TRAIL or TNFα may modify osteoblast gap junctions via regulation of expression, distribution or function of connexin43. Here we studied the effects of TNFs on osteoblasts on connectivity in nontransformed human osteoblasts and in MG63 human osteosarcoma cells. This included connexin43 degradation and selective loss of connexons on connecting cell processes with a reduction of cell-cell communication after exposure to TRAIL and TNFα. We further examined intracellular mechanisms involved in regulation of connexon degradation and homeostasis in response to TNFs. This is potentially important because TNFα is a prominent product of macrophage-family cells during osteoclast differentiation [11], as well as being an important product of immune cells. TRAIL is a prominent product of osteoblasts, and may under some conditions thus affect neighboring cells, since it is almost entirely membrane bound when active, but is held in a precursor form in osteoblasts [9]. TRAIL is also strongly regulated in bone by soluble TNF (decoy) receptors including osteoprotegerin and DcR2 [9].
Materials and methods
Cell cultures
MG63 cells [9] were used at passages 83-95, and grown in RPMI 1640 medium with 10% fetal bovine serum (Gibco, Carlsbad, CA). Committed single donor pre-osteoblast (division competent) cells (Cambrex/Bio-Whittaker, East Rutherford, NJ) were grown to 10-20,000 per cm2. Additives to nontransformed cells for growth were pretested aliquots from Cambrex/Bio-Whittaker. Differentiation was induced with 100 μM ascorbate and 10 mM β-glycerol phosphate [9]. Media included streptomycin and penicillin.
Cytokines, antibodies, immune labeling, and Western blots
Human TNFα, RANKL, IL-1 and TRAIL were from RDI (Flanders, NJ) and were recombinant proteins produced in bacteria, expressing the ligand domains only. Monoclonal IgM anti-Fas was from MBL (Watertown, MA). Rabbit anti-connexin43 was an affinity isolated polyclonal antibody (Sigma, St Louis, MO), used at 1:200 for tissue labeling and 1:1000 for Western analysis. Alexafluor-488 goat anti-rabbit was from Molecular Probes (Eugene, OR), used at 1:500. The mouse antibody to β- catenin was from Santa Cruz (Santa Cruz, CA), while antibody recognizing active β- catenin, Ser37/Thr41 hypo-phosphorylated-β- catenin, was from Upstate (Charlottesville, VA). Cells for Western blots were lysed in 0.1% polyglycol ether (NP-40, Sigma) with 50 mM HEPES, 150 mM NaCl, 10 mM EGTA, 10 mM EDTA, 1 mM Na orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 1 mM ammonium molybdate, 50 μM NaF, 0.5 μM okadaic acid, 5 mM benzamidine, 200 μg/ml aprotinin, 50 μg/ml pepstatin at pH 7.0. Proteins were separated on 9 or 12% polyacrylamide in Laemmli buffers, and transferred to polyvinylidine fluoride membranes for immune labeling. Horseradish peroxidase anti-rabbit was from Sigma, and was detected by chemiluminescence using luminol (ECL Plus, Amesham Pharmacia Biotech). Apoptosis was determined by phosphatidyl-serine exposure using Alexafluor 488-labeled annexin V (Molecular Probes).
Kinase and proteinase inhibitors, protein and activity assays
The Src inhibitor PP2, 4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine and inactive congener PP3, 4-Amino-7-phenylpyrazol[3,4-d]pyrimidine were from Calbiochem (San Diego, CA). The hydrolysis-resistant cGMP analog 8-pCPT-cGMP (8-(4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphate), and its adenosine homolog, 8-(4CPT)-cAMP were from Biolog (Bremen, Germany). A hydrolysis-resistant cGMP antagonist Rp-cGMPS (8-(Rp-4-chlorophenylthio)-guanosine-3′,5′-cyclic monophosphorothioate) was also from Biolog. Lactacystin (clasto-lactacystin β- lactone), an irreversible proteasomal inhibitor [12], was from AG Scientific (San Diego, CA). The cysteine proteinase inhibitor leupeptin and the protein kinase C activator phorbol myristate acetate (PMA) were from Sigma (St Louis, MO). The cAMP-dependent protein kinase Inhibitor Peptide PKI(6-22), TYADFIASGRTGRRNAI was from Upstate (Lake Placid, NY). Protein was measured by alkaline reduction of copper with bicinchoninic acid relative to albumin standards measured as absorbance at 662 nm (BCA, Pierce, Rockford, IL).
Real-time PCR
Messenger RNA was isolated on oligo-dT columns (RNeasy then Oligotex, QIAGEN, Santa Clarita, CA) and quantified spectrophotometrically (A260/A280 ≥1.8). Three μg of each RNA was reverse transcribed using Superscript (Invitrogen, Carlsbad, CA). The resulting cDNA was diluted four-fold and stored at -20°C. Human GAPDH primers: forward 5′-CCCATGTTCGTCATGGGGT-3′ at positon 437-453 and reverse 5′-TGGTCATGAGTCCTTCCACGATA-3′ at position 559-581 (accession number BC083511). Human connexin43 primers were forward 5′-AGGTTGCCCAAACTGATGGTG-3′ and reverse 5′-AACCCCCCTCGCATTTTC-3′. Human α- actin primers were forward 5′-AGGCATCCTCACCCTGAAGTA-3′ and reverse 5′-CACACGCAGCTCATTGTAGA-3′. Primers were tested for single amplification products at calculated sizes and amplification efficiency at a common annealing temperature (58 °C) using conventional PCR (Mastercycler Gradient, Epindorf-Brinkman, Westbury NY). Real time PCR used an ABI Prism 7000 instrument (Applied Biosystems, Foster City, CA) with 5 μl of cDNA, 200 pM of forward and reverse primers and 12.5 μl SYBR Green PCR Master Mix (Applied Biosystems) in a total volume of 25 μl. PCR conditions were 50°C for 2 min, 95°C for 10 min, followed by 50 cycles with 15 sec at 95°C and 1 min at 58°C. The relative cDNA content was determined from standard curves constructed from serial diluted cDNA and normalized to α- actin or GAPDH in each sample.
Imaging and Lucifer yellow dye diffusion
To determine extent of gap junction-based intercellular communication, live osteoblasts or MG63 cells were micro-injected into the cytoplasm using a manually-controlled but computer-injection Eppendorf Transjector (model name and number in 723a; Hamburg, Germany). Lucifer yellow potassium salt (Sigma/Aldrich, St Louis) was used as a 5% solution in 100mM LiCl, 5mM Tris pH 7, using back filled Femtotips (Eppendorf) injected at 86 hPa for 500 msec. Images were obtained with a 12 bit 1600 × 1200 pixel monochrome CCD (Spot, Diagnostic Instruments, Sterling Heights, MI) using a Nikon TE2000 inverted microscope. Color, where shown, is false color at approximate emission color. Fluorescent images were acquired as follows: green fluorescence with an excitation of 450-490 nm, a 510 nm dichroic mirror and a 520 nm barrier filter; red fluorescence with an excitation of 536-556 nm, a 580 nm dichroic mirror and a 590 nm barrier filter; lucifer yellow with an excitation of 450-490 nm, a 510 nm dichroic mirror and a 540 nm barrier filter. Phase-fluorescence photographs used a NA 0.95 long working distance 40× objective. Other fluorescence was photographed using 1.3 NA 40× or 100× oil objectives.
Results
Osteoblasts resist apoptosis in TRAIL or TNFα, but cell connectivity changes
Osteoblasts express the TNF-receptor family proteins TNFR-1 (p55), VEGI/TL1a (Apo3, DR3), TRAIL-R1 (DR4) and FAS (Apo1) [9, 13]. Activation of transmembrane receptors for TNFs should affect cell survival or differentiation. In MG63 cells, Fas crosslinking caused apoptosis and crosslinking of DR3 affects the balance of fibroblast-like growth with bone matrix production, and causes apoptosis under some conditions [13]. However, in several studies, including 12 hour treatment of serum starved cells with 100 ng/ml of TRAIL or TNFα, apoptosis was at background despite strong apoptosis in Fas-crosslinked controls (Figure 1A). Nontransformed osteoblasts after serum starvation gave the same result, with neither TRAIL nor TNFα inducing apoptosis. Resistance of osteoblasts to apoptosis in TRAIL or TNFα under most conditions was consistent with previous reports, and this was not pursued further. However, TRAIL or TNFα at 10 ng/ml altered cell shape and connectivity. Specifically, serum starvation separated cell bodies in MG63 cultures, which then resembled nontransformed osteoblasts (Figure 1B). After serum starvation, connections between MG63 cells were reduced by TRAIL or TNFα. We also observed that, in nontransformed osteoblasts, TNFα and TRAIL increased numbers of rounded cells, but the effects, by phase microscopy, were subtle and are not illustrated. Measurements of changes of MG63 shape with TRAIL in similar cell cultures were published previously [9]. However, the effects of TRAIL or TNFα on cellular connectivity had not been studied systematically. Thus, we analyzed the effects of serum starvation and TNFs on osteoblast connexons and on intercellular diffusion.
Figure 1. Effect of TNFα and TRAIL on osteoblast survival and cell shape.

A. Fas causes apoptosis in starved MG63 cells, but TRAIL and TNFα do not. Cells are shown after being serum starved 5 hours, treated with anti-Fas, TRAIL or TNFα and labeled with annexin V (green label) two hours later. Sporadic dead cells occurred with starvation (top left), while crosslinking with anti-human Fas (0.1 μg/ml, IgM) caused nearly quantitative apoptosis (top right). Apoptosis in 100 ng/ml TRAIL and TNFα (bottom left and right) was not significantly above background. Results at times up to 24 hours also showed no apoptosis above background with TRAIL and TNFα. Propidium iodide nuclear labeling occurred after annexin V labeling of Fas-crosslinked cells indicating apoptosis. Most annexin V labeled cells in other groups also labeled with propidium iodide. Photograph fields are 220 μm square. Apoptosis in Fas antibody was essentially quantitative, while cell death under other conditions was typically less than 5% and was not statistically different between serum starvation, TRAIL, and TNFα treatment.
B. Effect of serum starvation and TRAIL on MG63 cell connectivity and comparison with nontransformed osteoblasts. The top left panel shows MG63 cells with epithelioid appearance when growing in RPMI 1640 with 10% fetal bovine serum. After five hours without serum (top right), the cell bodies retract leaving connecting processes between the cells. Addition of 10 ng/ml TRAIL reduces the connections (bottom left). Some cells have no connections and smaller cell diameter (arrows). Similar effects were seen in cells after addition of 10 ng/ml of TNFα (bottom right). Photograph fields are 100 μm square.
TRAIL or TNFα reduce connexin43 protein in osteoblasts
Connexin43 was studied in Western blots of lysates of sub-confluent cell cultures of nontransformed osteoblasts or MG63 cells. Figure 2A shows connexin43 in cells serum starved six hours and treated with 10 ng/ml of TNFα or TRAIL for 4 hours. Time-courses showed that the reduction in connexin43 was in some cases maintained to 18 hours in the TNFs, but results were most consistent when assays were done at 2-4 hours. If TNFs were withdrawn, cells recovered to control connexin43 levels overnight. An example of recovery is shown in Figure 2A, left panel. Both nontransformed and MG63 cells responded more consistently to TNFα than to TRAIL, although in some cases TRAIL was as effective. In some cases connexin43 decreased by 90% or more. The variability of the effect is presumably related to effects of other cytokines; we were unable to determine a specific cause of this variability beyond that high density cell cultures typically had reduced response to TNFα or TRAIL (see cytokine regulation, below). Additional controls included the TNF family protein RANKL and IL-1. RANKL is a TNF-family protein for which osteoblasts do not have the receptor [9]. IL-1 is an important cytokine stimulating receptors in the toll-like superfamily with signalling divergent from TNF receptors. No meaningful differences in connexin43 occurred after 4 hour exposure to RANKL or IL-1 in serum starved cells. Effects of serum starvation and of TNFs were most consistent when cells were studied at 30-70% confluence. The lack of response in very sparse cultures could reflect cells being too distant to form gap junctions. Dense cultures, on the other hand, could produce large quantities of TNF-binding scavenger proteins, and may produce cytokines that modify cellular response. Since the response of transformed and nontransformed cells were similar, additional experiments used MG63 cells with only selected comparisons to nontransformed cells. This reduced difficulties with variability of cell differentiation which occur in nontransformed osteoblasts.
Figure 2. Connexin43 in osteoblasts is reduced by TRAIL or TNFα.
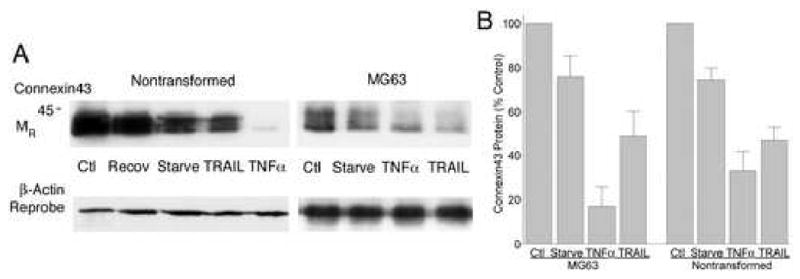
A. Nontransformed osteoblasts (left) and MG63 cells (right) had reduced connexin43 protein after serum starvation and exposure to 10 ng/ml TRAIL or TNFα. The data are Western blots of cell lysates made after six hours of serum starvation followed by four hours of treatment, except for control cells which were not serum starved, serum starved controls (which had 10 hours without serum) and a culture which was serum starved and treated with TNFα but then allowed to recover in serum without added TNFα overnight (Recov, second lane of left panel). In some cases, the decrement in connexin43 was more than 90%, although not all experiments showed strong responses, and especially in TRAIL in long time-course experiments. This may reflect in part that inhibitors of TRAIL are secreted by osteoblasts (see Discussion).
B. Response of connexin43 to serum starvation and TRAIL or TNFα. Five MG63 and five nontransformed osteoblast preparations were studied by Western blot as in (A). Optical density of connexin43 was measured and values normalized to control to allow comparisons to be displayed as mean ± SEM. Nontransformed and transformed cells were sensitive to TNFα and TRAIL, but effects of TRAIL were less consistent. Both MG63 and nontransformed cells make large quantities of TRAIL-binding soluble proteins [9]. The TNFα effect in both cells types relative to the starved cells is different at p < 0.01, while the TRAIL effect is variable and in this data set is different from serum starved in the MG63 cells at p < 0.05 and the difference in nontransformed cells has p ∼ 0.10. Data are from sub-confluent cells at 50-75% density.
Decreased connexin43 in TNFα and TRAIL reflect lysosomal degradation
We investigated the connexin43 degradation mechanism; which had proteasomal and lysosomal precedents [14]. Inhibiting proteinase with 1 mg/ml leupeptin or eliminating the lysosomal pH gradient with 1mM NH4+ blocked the effect of TNFα or TRAIL on connexin43 protein quantity. Under these conditions no significant reduction in connexin43 protein occurred after TNFα addition. The proteasomal inhibitor lactacystin, 5 μM, did not effect on reduction of Cx43 quantity by TNFα in any of four experiments, one of which is illustrated in Figure 3A. Lactacystin may have effects on the cells that cause changes in connexin43 phosphorylation, but these are beyond the scope of this study (compare Figures 3A and 2). Thus, increased connexin43 degradation in the presence of TNFα or TRAIL reflects increased lysosomal cycling. No effects on connexin43 degradation were observed when calpeptin, a calpain inhibitor, was added.
Figure 3. Effect of TNFs on degradation of connexin43 and on connexin43 mRNA.

A. Effect of proteasomal and lysosomal inhibitors. Western blots for Connexin43 in MG63 cells serum starved two hours and treated with 10 ng/ml of TRAIL or TNFα for four hours after the addition of 5 μM lactacystin, 1 mg/ml leupeptin, or 1 mM ammonium chloride. In lactacystin, which inhibits proteasomal degradation of ubiquitinated proteins, TRAIL and TNFα increased degradation of connexin43 essentially as in untreated cells (See Figure 2). Leupeptin and ammonia, however, eliminated almost all of the TNF-dependent connexin43 loss. Blots from three experiments with leupeptin or ammonium chloride gave similar results. Blots were reprobed for actin to show similar protein loads, and meaningful differences were not seen. Densitometry showed that decrements in connexin43 in lactacystin were not statistically different from levels shown in Fig 1B, while in in leupeptin or ammonium chloride, there was no statistically significant decrement in connexin43 with TRAIL or TNFα treatment.
B. Effect of starvation and TNF-family proteins on connexin43, actin, and glycerol-3-phosphate dehydrogenase (GAPDH) mRNAs. Three independent real-time PCR reactions were run, each in duplicate or triplicate, measuring connexin43, two measuring actin and one measuring GAPDH. All gave similar results indicating that serum starvation increased connexin43 mRNA relative to α-actin and GAPDH. Within starved groups, the TNFs consistently further increased connexin43 mRNA over controls, but only 20-40% relative to serum starved individually with p values of ∼0.1 to ∼0.35. On the other hand, differences between controls and serum starvation relative to actin or GAPDH were both significant, p < 0.05. The bars show mean ± SEM, N=3, with controls normalized to 1.0.
Concurrent with TRAIL and TNFα reducing connexin43 protein, mRNA levels increase
We examined the effect of TNFα and TRAIL on mRNA levels by real-time PCR. This showed that connexin43 mRNA synthesis increased under conditions promoting connexin43 degradation, with the largest and most significant effect due to serum starvation. The highest mRNA levels occurred with TNF· or TRAIL also present, but the significance of the TNFs in further augmenting connexin43 mRNA was unclear (Figure 3B).
Effects of TRAIL or TNFα on intercellular transport
Reduced connexin43 was expected to alter intercellular gap junction communication. To test this, we studied diffusion of the low molecular-weight fluorescent dye lucifer yellow (Figure 4). Dye diffusion was maximal at 5-10 min and at longer periods faded, but the patterns did not change to ∼40 min. In MG63 cells, serum starvation separated cell bodies, changed the pattern of cell processes, and increased the number of connected cells. Dye diffusion in serum-starved MG63 cells labeled ∼20 cells; this pattern was not affected by RANKL or by IL-1. Both TRAIL and TNFα reduced dye diffusion. The consistency of TRAIL effects may reflect that these assays used short exposures, when synthesis of TRAIL-binding scavenger proteins may be unimportant (see Discussion). Assays were repeated with similar results; quantification of a second experiment is shown (Figure 4A, bar graph). Nontransformed cells grow less uniformly than MG63 cells, and serum starvation in these cells had only minor effects on the cell shape, but response of lucifer yellow diffusion to TRAIL or TNFα was otherwise similar to that in MG63 cells (Figure 4B). Groups of ∼10 cells labeled after a single dye injection in either serum-replete or serum-starved conditions.
Figure 4. Serum starvation, TNFα and TRAIL alter osteoblast connectivity.
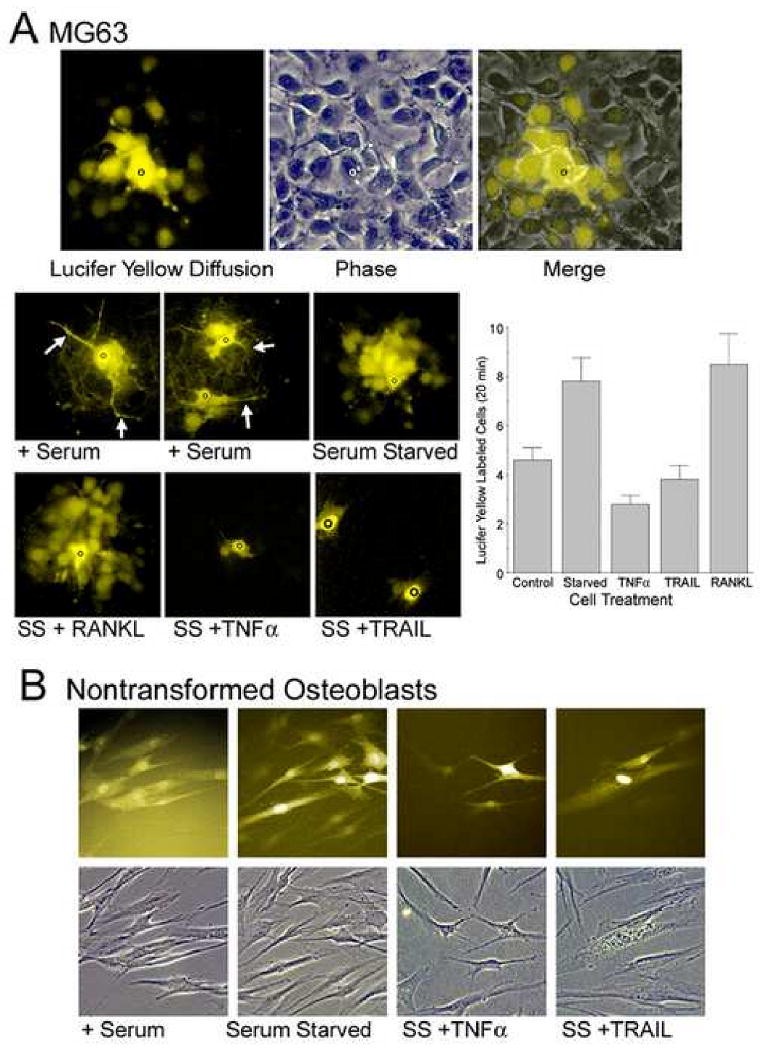
A. Dye diffusion in serum starved MG63 cells. Randomly selected individual cells in MG63 cells serum starved 4 hours were injected with 100 mM lucifer yellow (black circle). After 10 minutes, fluorescent images demonstrated intercellular diffusion. A typical result is shown in the top images with phase comparison. Approximately 20 cells labeled, most of them were adjacent but a few productive connections skip intermediate cells. Fields are 150 μm across. The lower panels show the effect of serum starvation and TNFs on dye diffusion. MG63 cells in growth media with serum (+ serum; three injections shown in two fields) gave diffusion involving ∼5 cells and showed prominent cell processes (arrows). In serum starved cultures intercellular processes were greatly narrowed, but several times as many cell bodies labeled. Both TRAIL or TNFα reduced dye diffusion but the TNF family protein RANKL, for which receptors are absent in osteoblasts, had no effect. 220 μm square fields. The graph shows results from a second experiment with numbers of cells labeled at 20 minutes after lucifer yellow injection in sequential cultures. The number of labeled cells was significantly less in serum starved cells exposed 2 hours to TNFα or TRAIL (p<0.05) than in serum starved or RANKL treated osteoblasts (n=5 except for RANKL, n=4).
B. Dye diffusion in nontransformed osteoblasts. Nontransformed osteoblasts are less uniform than MG63 cells in shape. These cells had strong diffusion involving neighboring cells (left), even at low density. In these cells, serum starvation had smaller effects on cell shape. However, cell processes and connectivity were reduced by TNFα (third pair) and TRAIL (fourth pair). Each panel is 150 μm square.
Effect of TNFα or TRAIL on gap junctions and intracellular connexin43
Sub-confluent MG63 cultures were labeled for filamentous actin with phalloidin rhodamine and with Alexafluor-488 anti-connexin43 (Figure 5). Cultures had abundant connexin43 in gap junctions as well as perinuclear and cytoplasmic labeling. Gap junctions were almost exclusively on cell-process after serum starvation. The connexons on cell processes were seen as linear groupings between cell bodies with a long overlap of processes of connected cells, in keeping with the rapid dye diffusion (Figure 4). There was occasional membrane labeling of connexin43 not clearly at cell junctions, possibly representing hemi-gap junctions. An example is seen, brightly labeled, at the superior margin of a cell in the lower left panel of Figure 5. After TRAIL or TNFα treatment, gap junctions were seen only between a few adjacent cells. While many cells still had some processes, connexin43 labeled gap junctions were reduced. Additional controls included RANKL and IL-1, which had no significant effect on connexin43 distribution. There were changes in the cell processes of serum starved cells that were consistent with changes seen in dye diffusion studies. In nontransformed cells, the difference in cell shape and actin organization with serum starvation was indistinct, but there was a redistribution of connexin43 from cell bodies to cell processes. TNFα or TRAIL reduced connexin43 labeling on cell processes. However, the morphology was more complex, and did not add to the results with MG63 cells.
Figure 5.
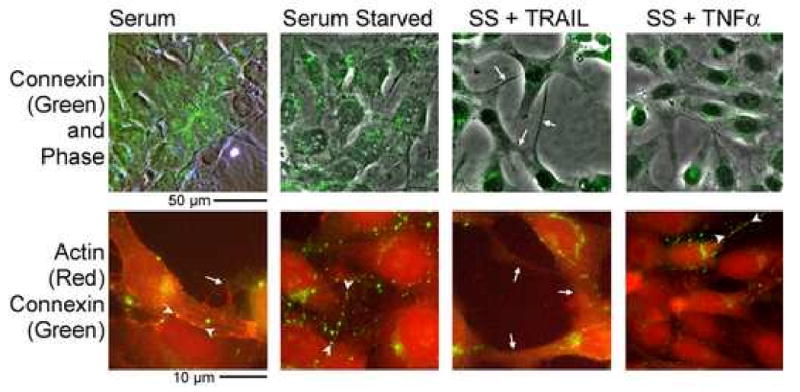
Effect of TRAIL and TNFα on gap junctions and the actin cytoskeleton. MG63 cells were grown and labeled with phalloidin rhodamine for filamentous actin (red) and Alexafluor-488 anti-connexin43 (green) after three hour treatment with 10 ng/ml TNFα or TRAIL. Phase with connexin43 is shown at low power in the top frames and the bottom frames show actin and connexin43 at high power. Serum starvation reduced junctions between juxtaposed cell bodies and instead lines of connexons occurred on cell processes (arrowheads in the bottom frames of the serum and serum starved photographs). Connexin43 on cell processes was reduced in TRAIL or TNFα, with a large portion of the connexin43 labeling in a vacuolar distribution (masses of green material within cells). There was variation with occasional cells retaining processes with connexons, as shown by the arrowheads, in the bottom right frame, after TNFα treatment. The top panels are 150 μm across. The bottom panels are 40 μm across.
Kinase and cytokine regulation of connexin43
The effect of serum starvation on connexin43 suggested that deprivation of growth factors reduces connexin43 and sensitizes the cells to the TNFs. A number of kinases and hormones associated with connexin43 and osteoblast maturation may participate in these effects. We examined this using kinase activators, inhibitors and selected hormones. The assays shown in Figure 6 used MG63 cells and were performed by adding test kinase activators and inhibitors and TNFs for two hours after six hours of serum starvation, except that the hormone PTH was added at the time of serum starvation. Activating protein kinase A increased connexin43 relative to serum starvation independently of TNF effects, while a cAMP-dependent protein kinase inhibitor peptide reduced connexin43 (Figure 6A). PTH and PTHrP are osteoblastic differentiation agents that activate cAMP-dependent protein kinase. High concentrations of PTH 1-34 added with serum starvation increased connexin43 and blunted response to TNFα and TRAIL (Figure 6B). Connexin43 blots showed connexin43 phosphorylation to varying extents. Although study of connexin43 phosphorylation is beyond the scope of our work, the bands with higher apparent relative molecular size in Figure 6B suggest possible increased phosphorylation of connexin43 in PTH; phosphorylation is well known to regulate gap junctional communication. Activating PKG did not affect the results with TNFα or TRAIL (Figure 6C). We also studied effects of the PKC activator PMA and the Src inhibitor PP2, relative to controls without PMA or with the inactive PP2 analog PP3. No consistent effects on connexin43 were seen. Other negative findings included no consistent effect of the glucocorticoid analog dexamethasone, 100 nM, on connexin43 protein or on TNF response.
Figure 6.
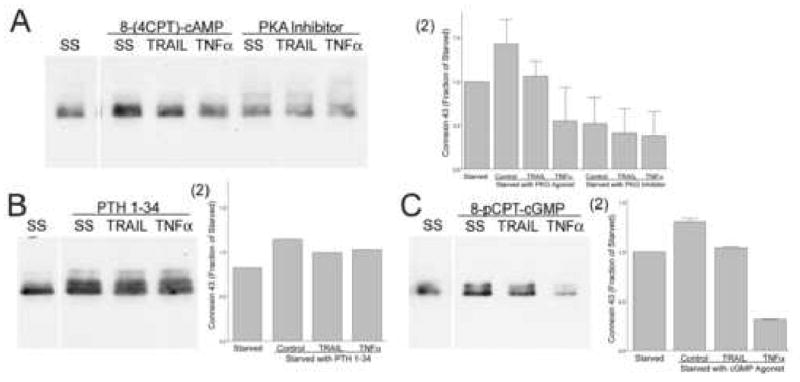
Effects of major kinase pathways and cytokines on osteoblast connexin43. All cultures were serum starved six hours prior to addition of stimuli, except that PTH was added at the time of serum starvation, with lysates collected after an additional two hours of incubation. TRAIL or TNFα were used at 10 ng/ml. Lysate proteins were measured and 25 μg loaded in each lane.
A. Protein kinase A. Activating protein kinase A with the non-hydrolyzable cAMP analog 8-(4-CPT)-cAMP, 100 μM increased connexin43 relative to serum starvation, while PKA inhibitor peptide at 50 μM reduced connexin43 (left blots). The cAMP analogs did not abolish TRAIL or TNFα response. Similar PKA effects were seen in replicates. (2) Densitometry of the blot and a replicate, normalized to the density of serum starved cultures; Mean ± Range.
B. PTH. PTH 1-34, 10 nM, increased connexin43 relative to serum starved cells, and blunted response of the TNFs (right lanes). (2) Densitometry of the blot normalized to the serum starved control.
C. Protein kinase G. The nonhydrolyzable cGMP analog 8-pCPT-cGMP, 100 μM reduced connexin43. However, response to TNFα was not affected. Replicates gave the same result. (2) Densitometry of the blot and a replicate, normalized to the density of serum starved cultures; Mean ± Range.
Connexon degradation and β- Catenin
Serum starvation with or without TRAIL or TNFα caused reversible lysosomal degradation of connexons, but increased connexin43 mRNA. Since β- catenin is a binding partner of connexins, connexin degradation may cause β- catenin activation. Activation of β- catenin is known to increase transcription of connexin43 and other proteins [15]. Therefore, we studied β- catenin dephosphorylation after serum starvation with TRAIL treatment in MG63 cells. Figure 7A shows Western blots of cultures of MG63 cells after 2 hour serum starvation followed by treatment with 10 ng/ml of TNFα or TRAIL. Under these circumstances, total β- catenin on average remained near control levels. This is not surprising because only a minor part of β- catenin is associated with connexons, even in connexin43 rich cells. However, there was generally an increase in serine37/threonine41-hypo-phosphorylated active β- catenin at 1-2 hours. Active β- catenin stimulates connexin43 transcription so, if confirmed, this mechanism may mediate rapid recovery of gap junctions from TNF-mediated degradation (Figure 2). However, because connexin-related β- catenin is a small component of beta-catenin in the cells, there is considerable difficulty of observation and the mechanism will need further study by more sensitive methods. Immune localization of β- catenin showed that the effects of TRAIL and TNFα on distribution of β- catenin in the peripheral cell were consistent with the effects of the TNFs on degradation of connexons (Fig 7B). These subconfluent osteoblast cultures are proliferating, for which reason β- catenin also occurs in the nucleus by participation in independent cyclin-related pathways. Thus, nuclear β- catenin is seen in all instances with significant variation related to cell cycling (note mitotic figure, lower right panel). Nonetheless, attachment-related β- catenin was seen at cell junctions and, after serum starvation, on intercellular processes. This was reduced by 2 hour treatment with 10 ng/ml of TRAIL or TNFα in keeping with possible peripheral β- catenin activation (Fig 7A) and supporting a probable role for canonical Wnt signaling in rescuing the connexin43 expression during serum starvation.
Figure 7. Effect of TRAIL and TNFα on β- catenin activation in MG63 cells.
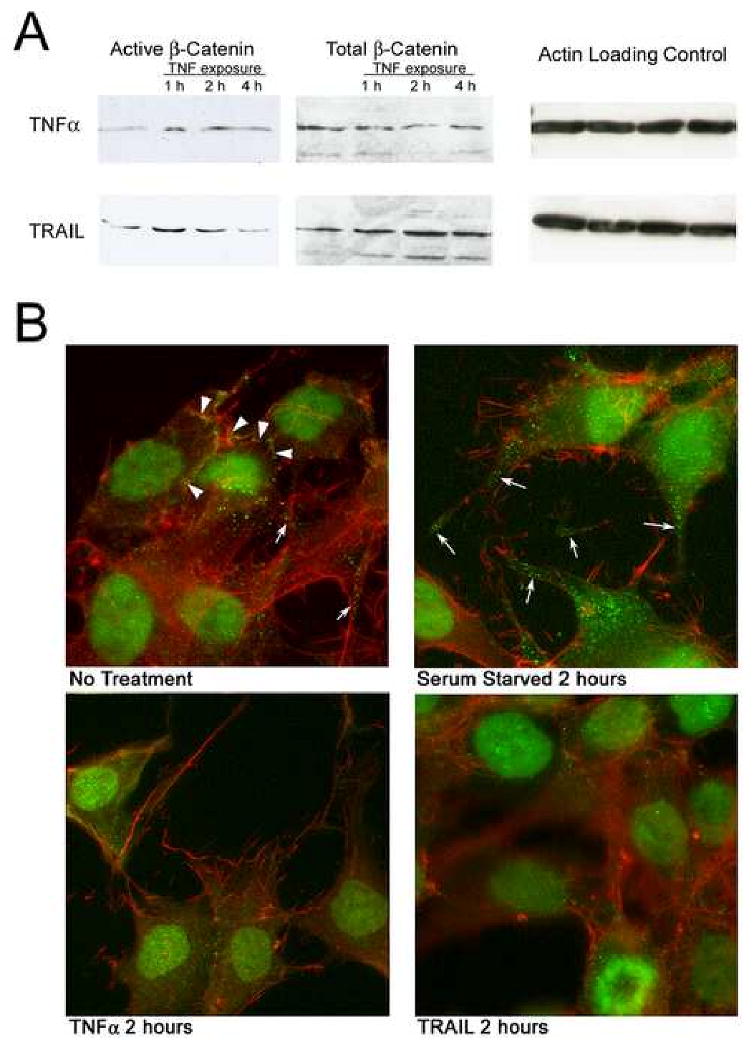
A. Western blots of MG63 cultures after 2 hour serum starvation and subsequent treatment with 10 ng/ml of TNFα or TRAIL. An increase in active, serine37/threonine41 hypo-phosphorylated β-catenin was generally observed at 1-2 hours. β- catenin stimulates connexin43 transcription, in keeping with rapid recovery from TNF effects (Figure 2) and with increased connexin43 mRNA during TNF treatment. Also shown are total β- catenin and actin labeling controls. In some cases total β- catenin appeared to be decreased with treatment, but the connexin-associated β- catenin is a relatively small fraction of the total and this observation was not consistent. In some cases β-catenin activation appeared to be prolonged beyond 2 hours but this was also inconsistent.
B. Effect of TRAIL and TNFα on peripheral distribution of β- catenin. In subconfluent MG63 cells, some β- catenin (green) is concentrated densely at cell borders (arrowheads), while occasional peripheral punctate concentrations are also seen. Under all conditions, there is also significant nuclear β- catenin. The cytoskeleton is also visualized using rhodamine phalloidin (red). After serum starvation, peripheral β- catenin is prominent on cell processes in patterns similar to those of connexin43, in keeping with the distribution of connexons in this manner and with β- catenin being a component of the connexon. Consistent with the degradation of most connexin43 in, the peripheral distribution of β- catenin was greatly reduced by10 ng/ml TRAIL or TNFα at 2 hours. Effects of the TNFs on β- catenin distribution in nontransformed cells are not illustrated but were were similar to those observed in MG63 cells. In these proliferating cell cultures, β- catenin is also seen in the nucleus due to unrelated activity in cell-cycle regulation; note the mitotic figure at the bottom of the TRAIL-treated culture, lower right panel (see text). Each frame is 70 μm square.
Discussion
Despite a clear involvement of TNFα in bone damage in disease, including in rheumatoid arthritis, no cellular function clearly attributable to TNFα and TRAIL was known in osteoblasts. TNFα or TRAIL do not cause osteoblast apoptosis acutely under most conditions, although some osteoblast-like cells can be sensitized for TNF apoptosis by inhibitors of protein synthesis or by special growth conditions [9, 16-19]. We studied the effects of serum starvation and TNFs on osteoblast connectivity under conditions where apoptosis does not occur. We show that osteoblast intercellular transport can be regulated by TNFα or TRAIL. This finding followed our observations that TRAIL affects MG63 cell shape and cell processes [9]. In addition, Hao et al. [10] reported that fibroblast gap junctions are reduced in TNFα. The requirement for removing fetal bovine serum for TNF effects to be apparent indicates that this response is regulated by hormonal factors, possibly PTH or PTHrP in osteoblasts.
The effect of TNFα and TRAIL on osteoblast connectivity is likely to have significant consequences in bone physiology. Both TRAIL and TNFα are prominent products of bone cells, whose functions in osteoblasts have not been clear. It was recently discovered that TNFα is produced during osteoclast differentiation [11]; TNFα is also well known to regulate bone as a product of lymphocytes. TRAIL is produced by osteoblasts [9], but no functions of TRAIL in osteoblasts were known. TRAIL is mainly present in osteoblasts in a precursor form, and when TRAIL is active it is almost entirely membrane bound. TRAIL is regulated by soluble receptors including osteoprotegerin and DcR2 [9], which may under most circumstances limit TRAIL activity to low levels in bone. That TRAIL is synthesized in quantity, however, suggests strongly that it is under some circumstances an important signal. Our work suggests that TNFα and TRAIL activity may terminate osteoblast gap-junction connectivity, such as during bone resorption. Further work will be required to test this hypothesis fully, however. Particularly, there was significant variability in the TNF effect on connexons, with reduced effects in high density cells possibly reflecting secondary effects of density on cell maturation.
TNF-family receptors and gap junctional communication occur in many mesenchymal cell types aside from osteoblasts and fibroblasts [10], including the stromal cell networks within bone that regulate hematopoiesis [20]. Therefore, the mechanisms studied here may be important in other contexts. Osteoblasts express several TRAIL-binding proteins, so the importance of TRAIL in regulating osteoblast connectivity may be high, even though the effects on connexin43 here were clearer with TNFα stimulus. TNFα is an inflammatory cytokine that has local and systemic effects. In contrast, TRAIL functions, as do most TNF-family proteins, mainly in cell-cell signalling via membrane-bound protein. Although we know no precedents for TNF stimulating degradation of osteoblast connexons, this appears to occur by a lysosomal mechanism, which is one of two mechanisms established to regulate connexin43 degradation [21-23].
We found no clear cooperativity with kinases that regulate connexin43 in osteoblasts. These included cAMP upregulation and cGMP downregulation, in keeping with reports that connexin43 is a cAMP- or cGMP-dependent kinase target [3] and a precedent for PKA increasing connexin43 in osteoblasts [7]. However, PTH, a cAMP stimulus, blunted response to TNFs. It is not clear why this occurred, but PTH has complex effects on osteoblast function including being a strong cAMP stimulus. This may reflect, in part, differences between nonspecific global cAMP activation and site-directed cAMP-dependent protein kinase A activity. PTH is a recognized stimulus for connexin43 expression in bone [21]. Additional kinases associated with connexin degradation include PKC and Src, which regulate proteasomal degradation of connexin43 in other cell systems. We did not see clear effects of PKC activators or Src inhibitors in osteoblast response to TNFs. This is not surprising in that the precedents point to proteasomal mechanisms [24, 25], but our results suggest a lysosomal degradation in this system. Because of the finding of apoptosis of connected groups of osteoblasts and osteocytes in high-dose glucocorticoids [4], we also examined the effect of dexamethasone, but found no consistent response in connexin43 expression. This suggests that effects of glucocorticoids on osteoblast connectivity, if they occur, are likely to be indirect.
The established role of connexin43 in osteoblast communication [26], and the TNF-dependent reduction in intercellular dye diffusion suggest that this effect may be physiologically important. It may contribute to the uncoupling of osteoblasts, such as during bone resorption. The importance of the TNF mechanism may also be cell-context dependent. Elevated intracellular calcium inhibits lucifer yellow diffusion in osteoblasts [27], and gap junctional activity is also regulated by many kinases independently of connexin expression. Demonstrating TNF effects on connexin43 in dense osteoblast cultures, particularly in mineralizing osteoblasts, was difficult. This may in part reflect that connexin43 expression is enhanced by the alkaline pH [28] that is produced during mineral deposition in bone. This may be a mechanism protecting new bone formation from TNF effects, although this possibility is purely hypothetical at this point.
Connexin43 expression, and increases in expression in dense osteoblastic cell cultures have been appreciated for a long time [26, 29]. Indeed, it is surprising that connexin43 knockout animals can make bone at all, albeit abnormal bone [5, 6]. This lack of lethal effect may reflect alternative genes with overlapping function. In rodents, some types of osteoblast-like cells also express connexin45, although this appears to be less important for cell communication [30]. Another alternative connexin, connexin46, may also function under some conditions in the osteoblast [31] Only the predominant protein, connexin43 was studied in this work. Our cRNA screening of human osteoblasts for connexins showed very strong connexin45 expression, and definite but much lower expression of connexin31.9 and connexin45. However, other connexins may be expressed in the absence of connexin43; we have not studied this possibility.
Osteoblasts produce several nonsignalling TNF-receptors that limit the activity of TNFs. These include osteoprotegerin, which binds RANKL and TRAIL, DcR1 and DCR2, which bind TRAIL and probably other TNFs, a DR3 isoform that binds TWEAK and Apo3L, and probably a soluble isoform of Fas. Expression of TRAIL binding soluble receptors is probably an important reason that TRAIL effects were more difficult to observe than those of TNFα. TNFα, largely a product of inflammatory cells, may act by similar mechanisms to TRAIL but with the osteoblast not having a strong natural regulatory system for it. Osteoblasts also synthesize significant quantities of TRAIL, but its secretion appears to be highly regulated [9]. Hence, TRAIL is probably a heavily regulated endogenous effector, while TNFα is an inflammatory and exogenous signal.
Connexin43 in connexons is part of a large protein complex that include β- catenin [3]. Our findings suggest that β- catenin may be activated when connexons are trafficked to lysosomal degradation. The major effect on connexin43 mRNA occurred after serum starvation, with minor increments when TRAIL or TNFα were added. This may reflect that, despite the clear effect of TNFs on cell process connexons and intercellular communication (Figures 4-5), connexin43 degradation and downstream β- catenin effects may be activated to near-maximal levels by serum starvation. In addition to connexin43 [32], β- catenin changes expression of many proteins including cell cycle regulating kinases [33]. However, additional studies such as siRNA-mediated β- catenin knockdown along with serum starvation will be essential to confirm the role of canonical Wnt signaling in connexin43 re-expression. In osteoblast-like cells, transfection with connexin43 increases alkaline phosphatase expression and cell proliferation [34]. In preliminary studies of effects of TNFs on osteoblast proliferation and alkaline phosphatase, we did not see clear and consistent differences. This may reflect the transient and reversible nature of TNF effects on osteoblast intercellular connections; further studies will also be required to resolve the point. Intermediate steps connecting TNF activity to increased non proteasomal degradation are as yet unknown, and further analysis of effects by trafs and kinase activity known to be linked to TNF receptor activation would be useful.
We conclude that TRAIL and TNFα cause rapid disassembly of connexin43 gap junctions in human osteoblasts by promoting lysosomal degradation. Connexin43 degradation was also stimulated by serum starvation, which was coupled, with or without further stimulation by TNFs, to increased connexin43 mRNA synthesis. This may be, at least in part, linked to β- catenin dephosphorylation coupled to redistribution of β- catenin that occurs during connexon degradation. Changes in connexin43 degradation in response to TNFs appeared to be independent of kinase activation pathways that regulate lysosomal or proteasomal degradation of connexin43 in other circumstances. Strong stimulation by PTH, however, or antagonists of lysosomal function, eliminated the effects of TRAIL or TNFα on osteoblast connexin43.
Acknowledgments
Supported in part by the US National Institutes of Health awards AR053976 and GM069668, and by the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. doi: 10.1359/jbmr.2000.15.2.209. [DOI] [PubMed] [Google Scholar]
- 2.Cooper DM, Turinsky AL, Sensen CW, Hallgrimsson B. Quantitative 3D analysis of the canal network in cortical bone by micro-computed tomography. Anat Rec B New Anat. 2003;274:169–79. doi: 10.1002/ar.b.10024. [DOI] [PubMed] [Google Scholar]
- 3.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 4.Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid treated rabbits. Endocrinology. 2001;142:1333–1340. doi: 10.1210/endo.142.3.8048. [DOI] [PubMed] [Google Scholar]
- 5.Furlan F, Lecanda F, Screen J, Civitelli R. Proliferation, differentiation and apoptosis in connexin43-null osteoblasts. Cell Commun Adhes. 2001;8:367–371. doi: 10.3109/15419060109080755. [DOI] [PubMed] [Google Scholar]
- 6.Chung DJ, Castro CH, Watkins M, Stains JP, Chung MY, Szejnfeld VL, Willecke K, Theis M, Civitelli R. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci. 2006;119:4187–4198. doi: 10.1242/jcs.03162. [DOI] [PubMed] [Google Scholar]
- 7.Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX. Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the PG EP2 receptor. J Biol Chem. 2003;278:43146–43156. doi: 10.1074/jbc.M302993200. [DOI] [PubMed] [Google Scholar]
- 8.Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 9.Bu R, Borysenko CW, Li Y, Sabokbar A, Blair HC. Expression of TNF-family proteins and Receptors in Human Osteoblasts. Bone. 2003;33:760–770. doi: 10.1016/j.bone.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Hao JL, Suzuki K, Lu Y, Hirano S, Fukuda K, Kumagai N, Kimura K, Nishida T. Inhibition of gap junction-mediated intercellular communication by TNF-alpha in cultured human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46:1195–1200. doi: 10.1167/iovs.04-0840. [DOI] [PubMed] [Google Scholar]
- 11.Nakao A, Fukushima H, Kajiya H, Ozeki S, Okabe K. RANKL-stimulated TNFα production in osteoclast precursor cells promotes osteoclastogenesis by modulating RANK signaling pathways. Biochem Biophys Res Commun. 2007;357:945–950. doi: 10.1016/j.bbrc.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 12.Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 2007;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- 13.Borysenko CW, Garcia-Palacios V, Griswold RD, Li Y, Iyer AK, Yaroslavskiy BB, Sharrow AC, Blair HC. Death receptor-3 mediates apoptosis in human osteoblasts under narrowly regulated conditions. J Cell Physiol. 2006;209:1021–1028. doi: 10.1002/jcp.20812. [DOI] [PubMed] [Google Scholar]
- 14.Laing JG, Tadros PN, Westphale EM, Beyer EC. Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res. 2007;236:482–492. doi: 10.1006/excr.1997.3747. [DOI] [PubMed] [Google Scholar]
- 15.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 16.Hill PA, Tumber A, Meikle MC. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology. 1997;138:3849–58. doi: 10.1210/endo.138.9.5370. [DOI] [PubMed] [Google Scholar]
- 17.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 18.Atkins GJ, Bouralexis S, Evdokiou A, Hay S, Labrinidis A, Zannettino AC, Haynes DR, Findlay DM. Human osteoblasts are resistant to Apo2L/TRAIL-mediated apoptosis. Bone. 2002;31:448–456. doi: 10.1016/s8756-3282(02)00858-x. [DOI] [PubMed] [Google Scholar]
- 19.Evdokiou A, Bouralexis S, Atkins GJ, Chai F, Hay S, Clayer M, Findlay DM. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL induced apoptosis. Int J Cancer. 2002;99:491–504. doi: 10.1002/ijc.10376. [DOI] [PubMed] [Google Scholar]
- 20.Montecino-Rodriguez E, Dorshkind K. Regulation of hematopoiesis by gap junction-mediated intercellular communication. J Leukoc Biol. 2001;70:341–347. [PubMed] [Google Scholar]
- 21.Mitchell JA, Ou C, Chen Z, Nishimura T, Lye SJ. Parathyroid hormone-induced up-regulation of connexin-43 messenger ribonucleic acid (mRNA) is mediated by sequences within both the promoter and the 3′untranslated region of the mRNA. Endocrinology. 2001;142:907–915. doi: 10.1210/endo.142.2.7930. [DOI] [PubMed] [Google Scholar]
- 22.Lan Z, Kurata WE, Martyn KD, Jin C, Lau AF. Novel rab GAP-like protein, CIP85, interacts with connexin43 and induces its degradation. Biochemistry. 2005;44:2385–2396. doi: 10.1021/bi048306w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leithe E, Brech A, Rivedal E. Endocytic processing of connexin43 gap junctions: a morphological study. Biochem J. 2005;393:59–67. doi: 10.1042/BJ20050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leithe E, Brech A, Rivedal E. Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem. 2004;279:50089–50096. doi: 10.1074/jbc.M402006200. [DOI] [PubMed] [Google Scholar]
- 25.Leithe E, Rivedal A. Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci. 2004;117:1211–1220. doi: 10.1242/jcs.00951. [DOI] [PubMed] [Google Scholar]
- 26.Chiba H, Sawada N, Oyamada M, Kojima T, Iba K, Ishii S, Mori M. Hormonal regulation of connexin 43 expression and gap junctional communication in human osteoblastic cells. Cell Struct Funct. 1994;19:173–177. doi: 10.1247/csf.19.173. [DOI] [PubMed] [Google Scholar]
- 27.Schirrmacher K, Nonhoff D, Wiemann M, Peterson-Grine E, Brink PR, Bingmann D. Effects of calcium on gap junctions between osteoblast-like cells in culture. Calcif Tissue Int. 1996;59:259–264. doi: 10.1007/s002239900120. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi DT, Ma D. Mechanism of pH regulation of connexin 43 expression in MC3T3-E1 cells. Biochem Biophys Res Commun. 2003;304:736–739. doi: 10.1016/s0006-291x(03)00633-8. [DOI] [PubMed] [Google Scholar]
- 29.Civitelli R, Beyer EC, Warlow PM, Robertson AJ, Geist ST, Steinberg TH. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. J Clin Invest. 1993;91:1888–1896. doi: 10.1172/JCI116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg TH, Civitelli R, Geist ST, Robertson AJ, Hick E, Veenstra RD, Wang HZ, Warlow PM, Westphale EM, Laing LG. Connexin43 and connexin45 form gap junctions with different molecular permeabilities in osteoblastic cells. EMBO J. 1994;13:744–750. doi: 10.1002/j.1460-2075.1994.tb06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koval M, Harley JE, Hick E, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137:847–857. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin 43 in cardiac myocytes. J Clin Invest. 2000;105:161–171. doi: 10.1172/JCI7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamei J, Toyofuku T, Hori M. Negative regulation of p21 by beta-catenin/TCF signaling: a novel mechanism by which cell adhesion molecules regulate cell proliferation. Biochem Biophys Res Commun. 2003;312:380–387. doi: 10.1016/j.bbrc.2003.10.129. [DOI] [PubMed] [Google Scholar]
- 34.Gramsch B, Gabriel HD, Wiemann M, Grummer R, Winterhager E, Bingmann D, Schirrmacher K. Enhancement of connexin 43 expression increases proliferation and differentiation of an osteoblast like cell line. Exp Cell Res. 2001;264:397–407. doi: 10.1006/excr.2000.5145. [DOI] [PubMed] [Google Scholar]


