Abstract
Prenatal viral infection has been associated with neurodevelopmental disorders such as schizophrenia and autism. It has previously been demonstrated that viral infection causes deleterious effects on brain structure and function in mouse offspring following late first trimester (E9) and middle-late second trimester (E18) administration of influenza virus. Neurochemical analysis following infection on E18 using this model has revealed significantly altered levels of serotonin, 5-hydroxyindoleacetic acid, and taurine, but not dopamine. In order to monitor these different patterns of monoamine expression in exposed offspring in more detail and to see if there are changes in the dopamine system at another time point, pregnant C57BL6J mice were infected with a sublethal dose of human influenza virus or sham-infected using vehicle solution on E16. Male offspring of the infected mice were collected at P0, P14, and P56, their brains removed and cerebellum dissected and flash frozen. Dopamine and serotonin levels were then measured using HPLC-ED technique. When compared to controls, there was a significant decrease in serotonin levels in the cerebella of offspring of virally exposed mice at P14. No differences in levels of dopamine were observed in exposed and control mice, although there was a significant decrease in dopamine at P14 and P56 when compared to P0. The present study shows that the serotonergic system is disrupted following prenatal viral infection, potentially modelling disruptions that occur in patients with schizophrenia and autism.
Keywords: Viral infection, Schizophrenia, Autism, Dopamine, Serotonin
1. Introduction
According to the neurodevelopmental hypothesis of schizophrenia, pathophysiologically relevant brain developmental genes are perturbed in the second trimester of pregnancy, leading to subsequent disturbances in brain maturation, limbic disorganization, neurochemical alterations, and dysfunction of the monaminergic system (Weinberger, 1995). The efficacy of dopamine D2 receptor blocking drugs in the treatment of schizophrenia, as well as SPECT studies on neuroleptic naïve patients, suggest that dopamine hyperfunction in the ventral striatum and dopamine hypofunction in the prefontal cortex may be responsible for the positive symptomology of schizophrenia (Abi-Dargham et al., 2000, 2002). Additionally, electrophysiological studies have suggested increased serotonergic function in schizophrenia (Juckel et al., 2003, 2008).
Recent serologic evidence points to prenatal exposures to a number of viruses as causative factors in the rise of births leading to schizophrenia (Mednick et al., 1988; Brown et al., 2004; Dalman et al., 2008) and autism (Barak et al., 1995; Singh et al., 1997; Connolly et al., 1999).
Furthermore, recent findings from in vitro and in vivo studies emphasize the influence of enhanced anti-inflammatory cytokine signaling on early brain development (Meyer et al., 2008). Several groups have shown evidence for viral infections and/or immune challenges being responsible for production of abnormal brain structure and function in rodents whose mothers were exposed to viral insults throughout pregnancy (Fatemi et al., 2005; Meyer et al., 2006a,b). Furthermore, the immune systems of patients with schizophrenic disorders show clear signs of over-activation, and anti-inflammatory treatment leads to improvement of schizophrenic symptomatology (Rothermundt et al., 2001). A promising animal model of the inflammatory genesis of schizophrenia is that of prenatal viral infection of mice (Fatemi et al., 1999, 2002; Shi et al., 2003). Offspring of female mice that were exposed to a mouse-adapted influenza virus in the middle of pregnancy display several changes in brain morphology, physiology, and behavior that are comparable to those of patients with schizophrenia (Fatemi et al., 2002, 2005, 2008).
In the current study, we examined post-mortem levels of dopamine and serotonin in the cerebella of exposed mouse progeny following maternal infection at E16. The cerebellum is one of the key structures in the early course of schizophrenia, since dysdiadochokinesia, a neurological sign specific to cerebellar dysfunction, often presents at the beginning of this disease (Boks et al., 2000). Furthermore, there is growing evidence that the cerebellum plays a role in higher cortical functions in schizophrenia (Andreasen and Pierson, 2008). While the neurochemistry is cerebella in unknown in patients with schizophrenia, previous experiments using this animal model have revealed altered levels of serotonin (5-HT), its metabolite 5-hydroxy indole acetic acid (5-HIAA), and taurine, but not dopamine in the progeny of mice exposed to virus on E18 (Fatemi et al., 2008). In order to monitor these different patterns of monoamine expression in exposed offspring in more detail and to see if there are changes in the dopamine and serotonin systems at E16, levels of monoamines were measured at three different postnatal days (P0, P14 and P56) using high performance liquid chromatography with electrochemical detection (HPLC-ED). Our results again suggest that the serotonergic, but not the dopaminergic, system is impacted by maternal prenatal infection.
2. Experimental procedures
2.1. Viral infection and brain collection and dissection
All experimental protocols used in this study were approved by the Institute for Animal Care and Use and Institutional Biosafety Committees at the University of Minnesota. Influenza A/NWS/33 (H1N1) virus was obtained from R.W. Cochran, University of Michigan (Ann Arbor). A virus pool was prepared in Maden Darby canine kidney (MDCK) cells; the virus was ampuled and frozen at − 80 °C until used. Data were expressed as log10 cell culture infectious doses (CCID50)/ml by the method of Reed and Muench (1938). By this titration, it was determined that at a dilution of 10− 4.5, none of the mice died of the infection but displayed a mean lung consolidation scores and mean lung weights similar to those obtained by Fatemi et al. (2002) and had a mean virus titer of 105.25 CCID50/ml, indicating that a moderate but sublethal infection had been induced. This was the virus dose selected for use in the pregnant mouse study. On day 16 of pregnancy, C57BL6J mice (Charles River, Wilmington, MA) were anesthetized using 200 µl isoflurane, and intranasally (i.n.) administered a dilution of 10− 4.5 of 6.5 log 10 (CCID50) per 0.1 ml human influenza virus A/NWS/33 in 90 µl of minimum essential medium (MEM). Sham infected mothers were treated identically but administered i.n. 90 µl MEM. After being infected, their drinking water contained 0.006% oxytetracycline (Pfizer, New York, NY) to control possible bacterial infections. Pregnant mice were allowed to deliver pups. The day of delivery was considered day 0. Groups of infected and sham-infected neonates were deeply anesthetized and killed on postnatal day (P) P0, P14, and P56. Offspring were weaned from mothers at P21, and males and females were caged separately in groups of 2–4 littermates. Groups of infected (N = 3) and sham-infected male neonates (N = 3) were deeply anesthetized. Brains were snap-frozen by immersion in liquid nitrogen and stored at − 85 °C for all HPLC studies.
Cerebellum was identified using coronal images derived from the stereotaxic mouse brain atlas (Franklin and Paxinos, 1997) and using the following coordinates: starting at − 1.40 mm to − 3.68 mm from interaural line and from − 5.20 mm to − 7.48 mm from bregma. Additionally, sagitally, cerebellum is easily recognized using the boundary between the brainstem and the lateral recess of the 4th ventricle to the anterolateral edge of cerebellum.
2.2. Neurochemical analyses
For neurochemical analyses, frozen samples were weighed and homogenized at 4 °C by ultrasonication in 20 volumes of 0.1 N perchloric acid. Subsequently, 100 µl of the homogenate was added to an equal volume of 1 N sodium hydroxide for the measurement of protein content. The homogenates were centrifuged at 17,000 g and 4 °C for 10 min. The levels of monoamines (dopamine and serotonin) were measured by high performance liquid chromatography (HPLC) with electrochemical detection as previously described (Felice et al., 1978; Sperk et al., 1981; Sperk, 1982). Levels of monoamine concentrations are given as µmol/g protein.
2.3. Statistical analysis
Data were statistically analyzed using SigmaStat version 3.0. A one or two-way analysis of variance (ANOVA) was used for all comparisons (treatment, postnatal day) and the Holm–Sidak method was used for post hoc analysis when indicated. A probability level (p) of less than 0.05 was considered statistically significant.
3. Results
Levels of dopamine (DA) and serotonin (5-HT) were measured in the cerebella of offspring of exposed and control mice. Two-way ANOVA revealed a significant interaction of treatment condition (viral exposure vs. control) and levels of 5-HT (F(1,17) = 6935; p < 0.018), but not levels of DA (F(1,17) = 0,01; p < 0.99) (Table 1). Post hoc testing revealed a significant reduction in levels of 5-HT at postnatal day P14 (Table 1, Fig. 1). One-way ANOVA further revealed significant decreases in levels of DA between P0 and later dates (P14 and P56) in both the control (F(3,8) = 24.831, p < 0.001) and exposed mice (F(3,8) = 5.770, p < 0.021).
Table 1.
Concentrations of monoamines in infected vs. control mice.
| Neurotransmitter (µmol/g protein) |
controls | VEA |
|---|---|---|
| Postnatal day O | ||
| DA | 13.4+/−0.2## | 14.1+/−4.1# |
| 5-HT | 32.7+/−3.1 | 28.9+/−4.6 |
| Postantal day 14 | ||
| DA | 6.0+/−1.1 | 4.8+/−0.3 |
| 5-HT | 37.2+/−2.0 | 23.8+/−2.5* |
| Postnatal day 56 | ||
| DA | 2.9+/−0.5 | 3.1+/−0.8 |
| 5-HT | 34.8+/−4.7 | 29.4+/−4.6 |
DA, dopamine; 5-HT, serotonin
p < 0.05 (t-test)
p < 0.05
p < 0.01 (one way ANOVA)
VEA, virally exposed animals
Figure 1.
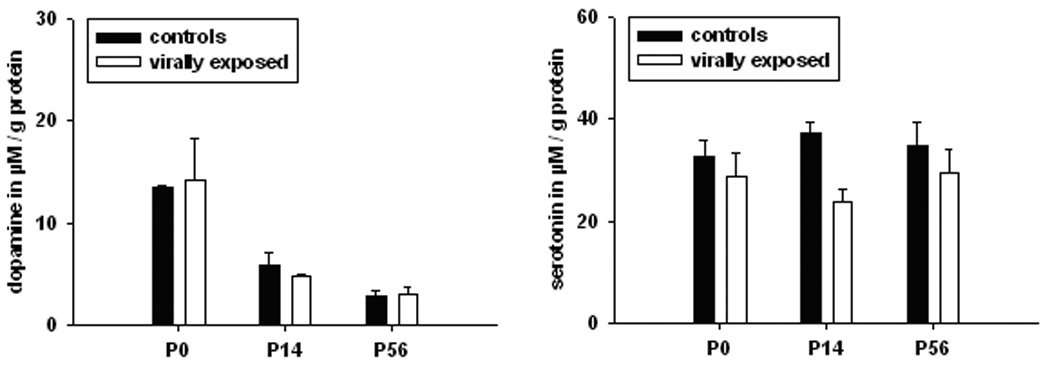
Effect of maternal influenza infection at embryonic day E16 on monoamines in the cerebellum of the offspring at postnatal day P0, P14, and P56, respectively. Shown are mean values ± the standard error of the mean of the monoamines (A) dopamine (DA) and (B) serotonin (5-HT). Data are separately indicated for postnatal day 0, 14, and 56. For comparisons between groups (controls versus virally exposed animals), significance levels (based on T-tests) are denoted by: * p < 0.05, and for comparisons within groups (between postnatal groups), significance levels (based on one way ANOVA) are denoted by: # p < 0.05.
4. Discussion
The present study revealed decreased levels of serotonin in the cerebella of offspring of virally exposed mice at P14, while no differences in levels of dopamine were observed. Interestingly, there were higher levels of dopamine in both groups at P0 as compared to P14 and P56, possibly related to an early pruning process.
Our findings are in contrast to the hypothesis that schizophrenia is associated with an up-regulation of both dopaminergic and serotonergic systems (Abi-Dargham et al., 2000, 2002; Juckel et al., 2003, 2008). However, several points should to be taken into account: 1) the current study focused on the cerebellum, not the ventral striatum where such increases have been previously observed in patients with schizophrenia (Abi-Dargham et al., 2002); 2) high levels of dopamine have been demonstrated to be phasic events in patients with schizophrenia (Abi-Dargham et al., 2000); 3) the animal model investigated here shows changes only at P14, i.e., long before adulthood. Therefore, our data are not comparable to neurochemical levels in adult patients with schizophrenia.
However, a lack of change in the dopaminergic system, coupled with diminished levels of cerebellar serotonin has been demonstrated previously in a study of mice virally exposed at E18 (Fatemi et al., 2008). Thus, it may be the case that the cerebellum in patients with schizophrenia is characterized by low serotonergic activity and stable dopaminergic activity. Cerebellar serotonin and its alterations are closely related to motor functions, especially to those with highly repetitive character such as diadochokinetic movements (Jacobs and Fornal, 1999). Therefore, it is possible that altered levels of serotonin may represent the neurobiological underpinning of neurological soft signs displayed in the early course of psychosis or in children who develop schizophrenia later in life (Walker et al., 1994). Furthermore, one can speculate whether early disturbances in the one neurotransmitter system, i.e. serotonin, induce changes in the other system (i.e. dopamine) later, since there is a broad literature of serotonergic modulation of dopaminergic neurotransmission (Esposito, 2006).
In recent years, altered cerebellar serotonin levels during early postnatal brain development have been discussed as an important etiopathological mechanism contributing to autism (Chugani, 2002). On the other hand, cerebellar and serotonergic pathologies have been commonly reported in models of schizophrenia as well as of autism (Fatemi et al., 2008; Mittleman et al., 2008; Meyer et al., 2008). Up to the present, no neurochemical or neuroanatomical marker has been established to unambiguously differentiate between patients suffering from schizophrenia and autism, respectively.
A recent study from our laboratory demonstrated reduced serotonin in the hippocampus, nucleus accumbens and lateral globus pallidus of offspring exposed to PolyI:C, a potential pro-inflammatory substance, at E9 (Winter et al., unpublished observation). In contrast, offspring exposed to PolyI:C showed increased dopamine in prefrontal cortex and lateral globus pallidus. The lack of an increase in dopamine following prenatal viral infection could represent an important difference between the two animal models. Further studies of additional brain regions using our model are necessary for confirmation. The findings of low serotonergic levels across different brain regions could imply that the two inflammatory animal models reported here – from a neurochemical point of view – may reflect features of the negative symptomatology of schizophrenia which is known to be accompanied by reduced activity in this neurotransmitter system (e.g. Silver, 2004). Further hints for this perspective could be the data from Shi et al. (2003) showing that locomotion is highly restricted to one corner during the open field test, and that social interactions are extremely reduced in the offspring of mice exposed to virus on E9.
Another reasonable explanation for unchanged dopamine levels observed in our infection model would be that the precise timing of prenatal infection and/or immune activation may critically influence the extent to which postnatal dopamine levels are affected by the prenatal manipulation. This is supported by recent experimental findings highlighting the critical influence of the prenatal timing on dopamine-associated structural and functional brain changes later in life (Meyer et al., 2007, 2008).
Some limitations and open questions of this study are as follow: 1) there was a small sample size with N = 3 per group; 2) dopamine and serotonin were only analyzed in the cerebellum but not in other brain regions; 3) changes in serotonin levels were observed only at P14 but not at P56, pointing to important limitations of the influenza animal model to mimic developmental cerebellar changes in adolescence and adulthood; and 4) in the present study there was no use of in vivo microdialysis, which is essential to assess real neurochemical alterations in distinct brain regions. Therefore, our next study using this model will monitor various neurotransmitters by microdialysis at several time points across the life span.
Acknowledgments
Role of the funding source
We are thankful to the Faculty program of Humboldt-University (Berlin) for giving us a grant for the project ″Neuroinflammation in Schizophrenia” (GJ). Grant support by National Institute of Child Health and Human Development (#1R01 HD046589-01A2) to SHF is gratefully acknowledged. The funding sources had no influence on the study and its publication.
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The Role of the Cerebellum in Schizophrenia. Biol. Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Ring A, Sulkes J, Gabbay U, Elizur A. Season of birth and autistic disorder in Israel. Am. J. Psychiatry. 1995;152:798–800. doi: 10.1176/ajp.152.5.798. [DOI] [PubMed] [Google Scholar]
- Boks MP, Russo S, Knegtering R, van den Bosch RJ. The specificity of neurological signs in schizophrenia: a review. Schizophr. Res. 2000;43:109–116. doi: 10.1016/s0920-9964(99)00145-0. [DOI] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Chugani DC. Role of altered brain serotonin mechanisms in autism. Mol. Psychiatry. 2002;7(Suppl 2):S16–S17. doi: 10.1038/sj.mp.4001167. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK. Serum autoantibodies to brain in Landau–Kleffner variant, autism, and other neurologic disorders 6. J. Pediatr. 1999;134:607–613. doi: 10.1016/s0022-3476(99)70248-9. [DOI] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Gunnell D, Harrison G, Kristensson K, Lewis G, Lofving S, Rasmussen F, Wicks S, Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am. J. Psychiatry. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- Esposito E. Serotonin–dopamine interaction as a focus of novel antidepressant drugs. Current Drug Targets. 2006;7:177–185. doi: 10.2174/138945006775515455. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol. Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell. Mol. Neurobiol. 2002;22:25–33. doi: 10.1023/a:1015337611258. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, Smee DF, Pearce DA, Winter C, Sohr R, Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felice LJ, Felice JD, Kissinger PT. Determination of catecholamines in rat brain parts by reverse-phase ion-pair liquid chromatography. J. Neurochem. 1978;31:1461–1465. doi: 10.1111/j.1471-4159.1978.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Franklin BJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Jacobs BL, Fornal CA. Activity of serotonergic neurons in behaving animals. Neuropsychopharmacology. 1999;21(2 Suppl):9S–15S. doi: 10.1016/S0893-133X(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Juckel G, Gallinat J, Riedel M, Sokullu S, Schulz C, Moller HJ, Muller N, Hegerl U. Serotonergic dysfunction in schizophrenia assessed by the loudness dependence measure of primary auditory cortex evoked activity. Schizophr. Res. 2003;64:115–124. doi: 10.1016/s0920-9964(03)00016-1. [DOI] [PubMed] [Google Scholar]
- Juckel G, Gudlowski Y, Müller D, Özgürdal S, Brüne M, Gallinat J, Frodl T, Witthaus H, Uhl I, Wutzler A, Pogarell O, Mulert C, Hegerl U, Meisenzahl EM. Loudness dependence of the auditory evoked N1/P2-component as an indicator of serotonergic dysfunction in patients with schizophrenia—a replication study. Psychiatry Res. 2008;158:79–82. doi: 10.1016/j.psychres.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006a;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Immunological stress at the maternal–foetal interface: a link between neurodevelopment and adult psychopathology. Brain Behav. Immun. 2006b;20:378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62:544–550. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Rothermundt M, Arolt V, Bayer TA. Review of immunological and immunopathological findings in schizophrenia. Brain Behav. Immun. 2001;15:319–339. doi: 10.1006/brbi.2001.0648. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver H. Selective serotonin re-uptake inhibitor augmentation in the treatment of negative symptoms of schizophrenia. Expert Opin. Pharmacother. 2004;5:2053–2058. doi: 10.1517/14656566.5.10.2053. [DOI] [PubMed] [Google Scholar]
- Singh VK, Singh EA, Warren RP. Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biol. Psychiatry. 1997;41:753–755. doi: 10.1016/S0006-3223(96)00522-7. [DOI] [PubMed] [Google Scholar]
- Sperk G. Simultaneous determination of serotonin, 5-hydroxindoleacetic acid, 3,4-dihydroxyphenylacetic acid and homovanillic acid by high performance liquid chromatography with electrochemical detection. J. Neurochem. 1982;38:840–843. doi: 10.1111/j.1471-4159.1982.tb08708.x. [DOI] [PubMed] [Google Scholar]
- Sperk G, Berger M, Hortnagl H, Hornykiewicz O. Kainic acid-induced changes of serotonin and dopamine metabolism in the striatum and substantia nigra of the rat. Eur. J. Pharmacol. 1981;74:279–286. doi: 10.1016/0014-2999(81)90046-7. [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr. Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. In: Schizophrenia as neurodevelopmental disorder. Hirsch SR, Weinberger DR, editors. Oxford: Schizophrenia, Blackwell; 1995. pp. 293–323. [Google Scholar]


