Abstract
Objectives
To determine the relationship between depressed mood and the development of Alzheimer's Disease in cognitively normal individuals.
Design
Longitudinal. Observational.
Setting
Community-based cohort study.
Participants
288 participants in the Cardiovascular Health Study-Cognition Study (mean age=77.52, SD=3.65, range=70-89). All of the participants were adjudicated as cognitively normal in 1998/99, and all had at least three visits prior to 1998/99 with measures of cognition and mood state. The mean length of follow up from 1998-1999 to 2007 was 7.1 years (range 1-9 yrs, median=9 yrs).
Measurements
The Center for Epidemiological Studies-Depression Scale was used to index mood state, and the Modified Mini-Mental State Examination (3MSE) was the index of cognitive function among participants prior to 1998/99. These measures were considered in two ways: participants were classified according to: 1) whether or not they showed a high negative correlation between their CESD and 3MSE scores (i.e., indicating that greater depression was linked to poorer cognition), and 2) whether or not they showed persistently elevated CESD scores. The study outcome, development of dementia (n=48), was based on consensus classifications based on detailed neuropsychological and neurological exams.
Results
We could find no consistent relationship between mood state, either alone or in relation to cognitive status and the subsequent development of dementia. Those individuals whose cognitive functions were highly correlated with their mood state were no more likely to develop dementia than other participants. Those who had persistently depressed mood were also no more likely to develop dementia than those without persistently depressed mood.
Conclusion
Within the confines of this prospective, community-based study of elderly adults, we could not find strong evidence to support the hypothesis that mood disturbance was linked with the development of dementia.
Introduction
Studies from both epidemiological cohorts [1-3], and specialized clinic samples [4-7] have found that symptoms of depression are risk factors for dementia, especially Alzheimer's disease (AD) (See [8] for review). However, this is not a universal finding; other epidemiological studies have shown that cognitive decline over time in the elderly cannot be explained by symptoms of depression. While depressive symptoms may show cross-sectional associations with cognitive functions, they do not predict subsequent decline in function [9-11]. Although the analyses of dementia from the Cardiovascular Health Study have not previously focused on the relationship between depression and incident dementia [12, 13], there was an association between having a Center for Epidemiological Studies - Depression Scale (CESD) score greater than 8 in 1992-94 and incident dementia in 1998-99 (RR: 1.27 (95% CI: 1.01 – 1.59), p= .03). However, the association was attenuated when we controlled for age, education level, MRI-identified infarcts, gender, race, diabetes mellitus, hypertension, and heart disease (RR: 1.07 (95% CI: .83 – 1.39), p= .56). Thus, low mood state was occurring in the context of important medical comorbidities, and this may result in a spurious correlation between depression and dementia.
One way to view this paradoxical state of affairs is to consider that depression creates a vulnerability state for dementia. For example, depression in the elderly is associated with decreased hippocampal volume [14], and this association is stronger for those patients with longer histories of Major Depressive Disorder [15]. There is an association between small hippocampal volumes and incident dementia both in non-depressed individuals who have Mild Cognitive Impairment [16, 17] and in elderly depressed subjects [18]. However, not all subjects with depression have cognitive deficits or progress to AD. In addition, the progression to AD is not linear, and individuals may have episodes of depression with cognitive deficits that improve over time, either spontaneously or after treatment for their depression [19].
Thus, there is both physiological and behavioral (e.g., [20, 21]) support for suggesting that there is an increased vulnerability to dementia among individuals with mood disturbance. Indeed, this could be viewed in the context of a model of cognitive reserve [22, 23] such that the functional and structural CNS alterations associated with depression would reduce reserve capacity in a dose:response manner. With increasing severity of depressive symptomatology, cognitive test performance would decline. With decreasing severity of depressive symptomatology, cognitive test performance would improve.
In order to evaluate this model, we used data from a subset of the 927 participants who participated in the Cardiovascular Health Study – Cognition Study (CHS-CS) in Pittsburgh. We hypothesized that there is a vulnerability state related to symptoms of depression that lowers the threshold for the expression cognitive deficits related to an underlying degenerative condition, and that this increases the risk that these individuals will go on to develop dementia. We tested this hypothesis in all of the CHS-CS who were cognitively normal in 1998/99 and who were followed through 2007. Because we were not sure a priori how the vulnerability state would express itself, we defined it in two ways. First, we reasoned that those individuals in whom the link between symptoms of mood disturbance and cognition was strong would be especially vulnerable to subsequent dementia. Therefore, if we were to evaluate a patient in a clinical setting, and their cognitive functions became better or worse as their mood state improved or deteriorated, we might infer that their cognitive state was directly affected by their mood (a not uncommon situation in geriatric depression). Thus, we decided to identify those CHS-CS participants whose mood state was linked to their cognitive state over several study visits (i.e., years of observation) prior to the critical observation period. We measured the correlation between the CESD and 3MSE variables within-subject because this would give us a measure of the extent to which each participant's mood state and cognitive status covaried over time. We focused our analyses on those participants whose CESD:3MSE correlation was strongly negative (i.e., more mood disturbance, lower cognitive function, and vice-versa) relative to the remainder of the study volunteers. These were the study participants in whom there appeared to be a strong link between change in mood and abnormal cognition, and we reasoned that these were individuals in whom a lower mood state would “uncover” the underlying neurodegenerative process.
Because individuals with low levels of depressive symptoms (i.e., CESD score of 0-9) and small changes in cognition could easily be captured in that analysis, we tested the relationship between elevated symptom scores (by validated standards) and risk for AD. We hypothesized that those participants who had persistently elevated symptoms of mood disturbance (such as might be found in a specialty clinic for late-life mood disorders) would also be in a vulnerability state relative to individuals with no symptoms of mood disturbance, and those with only transiently depressed mood. Thus, we also created groups based on whether or not participants showed consistently elevated CESD scores (i.e., ≥ 10) in the five years prior to the observation period.
In summary, we classified each subject with regard to each of these two schemas. The critical issues for the analysis (and interpretation) of the data are the facts that (a) the classification was based on mood state prior to the observation period, and (b) all of the participants were known to be cognitively normal at the beginning of the observation period based on neuropsychological testing outcome. Using these two classification schemes, we then evaluated our hypothesis - that there was an increased vulnerability to AD among individuals with mood disturbance - in two separate sets of analyses.
Material and Methods
CHS Cognition Study
In 1988-89, 5201 noninstitutionalized individuals over the age of 65 were recruited in four communities from the Part A Medicare list (Pittsburgh, PA, Sacramento, CA, Winston-Salem, NC, Hagerstown, MD). The mean age of the cohort was 72 in 1989-90. In 1992-93, the fifth year of the study, 687 African-Americans were added to the study in the same manner, in three of the four centers; their mean age in 1992 was 71 years. The demographic characteristics of the total Cardiovascular Health Study cohort of 5888 participants has been described previously [24]. Extensive clinical, radiological, and laboratory data were obtained from all its participants and included the Modified Mini-Mental State Examination (3MSE) [25] and the Digit Symbol Substitution Test [26] annually. Information on cognition was obtained from proxies (e.g., spouses, children) using the Informant Questionnaire for Cognitive Decline in the Elderly [27], and the dementia questionnaire [28]. The CHS staff also obtained information from participants and next-of-kin regarding vision and hearing, the circumstances of the illness, functional status, and information about medication use and alcohol consumption. Data on instrumental activities of daily living were also collected at every clinic visit [29]. Therefore, we have a complete 12-13 year clinical history of each of the participants entered in this study.
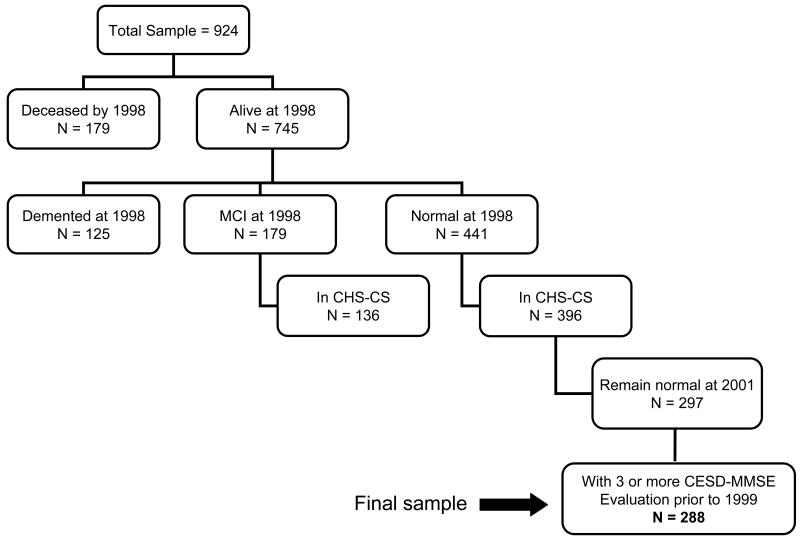
In 1998/99, 3608 CHS participants underwent a structural MRI scan of the brain, and 924 of these were scanned in Pittsburgh. In 2002, the CHS-CS study began at the Pittsburgh site only[30]; this study is designed to determine the incidence of MCI and AD among those participants who had been classified as either cognitively normal, or having MCI in 1998-99. From the 924 participants in Pittsburgh who were alive and were scanned in 1998/99; 297 were cognitively normal and were available for study in 2002-03 (i.e., could complete all study evaluations) (See Figure 1). The characteristics of the CHS cohort, and the Pittsburgh CHS-CS have been described previously [29-31].
Figure 1.
1) Flow chart of study enrollment. See text for details.
Study Sample
For the present analysis we selected those individuals who were classified as cognitively normal in 1998/99 (n=441)(see below), and who had at least three evaluations that included both the CESD and 3MSE (n=288) prior to and including the 1998/99 clinic visit (i.e., prior to the beginning of the observation period) (See Figure 1). The mean age of the group was 77.52 years (SD=3.65, range=70-89). We then evaluated the risk of developing AD during follow-up through 2007 among the 48 participants who converted to dementia. We compared the characteristics of the participants included in the analysis to those who were excluded, and found that the groups were compatible except that 5 of the 9 excluded cases were African-American.
Clinical examination
Neuropsychological examination
The neuropsychological battery included tests of premorbid intelligence, memory, language, visuoperceptual/visuoconstructional, attention/executive, and fine motor control functions. Details of the neuropsychological battery, and normative data have been previously published [32].
Neurological exam
The neurological exam included a brief mental status examination, as well as cranial nerves testing, motor tone, abnormal movements, strength, deep tendon reflexes, release signs, plantar response and clonus, cerebellar testing, primary sensory testing, gait, and postural stability. The examiner also completed the Unified Parkinson's Disease Rating Scale [33] and the Hachinski Ischemic Scale [34].
Psychiatric examination
Symptoms of depression were measured with the modified 10-item version of the Center for Epidemiology Studies Depression Scale (CESD) [35]. A score of 10 or greater on the CESD indicates significant depressive symptoms comparable to a score of 16 or greater on the full-length scale, which has good sensitivity and specificity for a major depressive disorder diagnosis [36]. Additional psychiatric information was obtained using the Neuropsychiatric Inventory [37].
Diagnostic criteria
The diagnosis of dementia was made by the Adjudication Committee using all available data from each participant [31]. The presence of dementia was established based on impairments in performance in two or more cognitive domains that were of sufficient severity to affect the participants' activities of daily living, and a history of normal intellectual function before the onset of cognitive abnormalities. An abnormal domain was present when at least two tests in the same domain were abnormal. The Adjudication Committee assigned specific diagnoses (e.g., AD, Vascular Dementia, etc.) using the accepted research standard classifications, including DSM-IV, NINCDS/ADRDA, NINDS-AIREN, and ADDTC. However, all of the cases in this study sample met criteria for Probable or Possible AD [38], and these criteria have shown a sensitivity of 98% and a specificity of 88% for AD relative to neuropathological diagnosis [39].
Statistical Analysis
Analysis 1
For each individual participant, a correlation coefficient was computed over that person's serial assessments collected between 1992-1999 (i.e., a within-subject correlation) to reflect the strength of the association between the CESD and 3MSE for that individual. This new variable, reflecting the temporal covariation between depression and cognition was examined as a predictor of the development of dementia between 2002-2007. Specifically, it was hypothesized that a strong negative within-subject correlation between CESD and 3MSE scores over time would be associated with the greatest risk of developing AD. Therefore, we identified those participants whose CESD:3MSE correlation was in the lower 25% of the distribution (i.e., strongest negative correlation) and compared them to the remaining participants in terms of the incidence of AD. The linkage between the covariation variable and dementia incidence was examined at the simple bivariate level and then with multivariate logistic regression to adjust for other known risk factors for dementia (e.g., age, hypertension, heart disease, diabetes mellitus, MRI-infarcts, white matter lesions).
Analysis 2
For the second analysis we used the 1994-1998 CESD data (i.e. 5 years of data) to create three sub-groups modeled after those used by Lenze and colleagues in a prior analysis of the CHS [40]: 1) non-depressed (n=205), 2) transiently depressed (n=56), and 3) persistently depressed (n=27). If all the CESD scores in those 5 years were less than 10, the participant was defined as ‘non-depressed’. If at least 75% of the CESD scores in those 5 years were greater than 9 or the CESD scores in the last 2 years (1997, 1998) were greater than 9, then the person was considered ‘persistently depressed’; otherwise, the participant was considered ‘transiently depressed’. We predicted that those participants who were persistently depressed in the period prior to 1998/99 would have an increased risk for AD after 1998/99.
Cox proportional hazard models of the factors associated with the development of dementia were completed; both models (i.e., both Analysis 1 and 2) satisfy the proportional hazard assumptions. The study participants who died before the end of the observation period (i.e., 2007) were censored at the year of death. Those participants who were still alive and cognitively normal at the end of the observation period, were censored at 2007. The mean length of follow up from 1998-1999 to 2007 was 7.1 years (range 1-9 yrs, median=9 yrs). The results described below are for the analyses that account for critical covariates.
Results
Analysis 1
A total of 288 cognitively normal CHS-CS participants had at least 3 pairs of 3MSE and CESD scores prior to 1997/98; data from these participants were used for this analysis. Descriptive characteristics of these participants are shown in Table 1. In the sample used in this analysis, there was no significant association between critical medical comorbidities such as hypertension and MRI infarcts and the mood-state grouping variables (See Table 2A).
Table 1. Characteristics of Study Sample in 1998/99 (see text for details).
| Analysis 1 | Analysis 2 | |||||
|---|---|---|---|---|---|---|
| CESD: 3MSE Corr lower 25th | CESD: 3MSE Corr middle 50th | CESD: 3MSE Corr upper 25th | Non-Depressed | Transiently Depressed | Persistently Depressed | |
| N= | 73 | 143 | 72 | 205 | 56 | 27 |
| Race, European American: n (%) | 56 (76.71) | 123 (86.01) | 59 (81.94) | 167 (81.46) | 47 (83.93) | 24 (88.89) |
| Sex, male: n (%) | 27 (36.99) | 58 (40.56) | 21 (29.17) | 78 (38.05) | 20 (35.71) | 8 (29.63) |
| Education (% >HS) | 42 (57.53) | 95 (66.43) | 40 (55.56) | 131 (63.90) | 31 (55.36) | 15 (55.56) |
| 3MSE <90, n (%) | 4 (5.80) | 16 (11.43) | 5 (7.25) | 15 (7.54) | 7 (12.96) | 3 (12.00) |
| 90-97 | 26 (37.68) | 49 (35.00) | 23 (33.33) | 70 (35.18) | 23 (42.59) | 5 (20.00) |
| >97 | 39 (56.52) | 75 (53.57) | 41 (59.42) | 114 (57.29) | 24 (44.44) | 17 (68.00) |
| APOE*4 (% Present) | 12 (18.72) | 24 (18.32) | 15 (22.06) | 37 (19.17) | 9 (19.15) | 5 (21.74) |
| Diabetes (%Yes) | 7 (10.29) | 9 (6.43) | 10 (14.49) | 19 (9.60) | 4 (7.41) | 3 (12.00) |
| Hypertension (% Yes) | 30 (43.48) | 63 (44.37) | 36 (51.43) | 92 (45.45) | 26 (48.15) | 11 (44.00) |
| Smoking (%Yes) | 41 (59.42) | 78 (56.11) | 31 (44.28) | 107 (53.50) | 31 (57.41) | 12 (50.00) |
| Cholesterol (% >218) | 21 (32.31) | 37 (27.01) | 20 (29.85) | 63 (32.64) | 13 (25.00) | 2 (8.33) |
| MRI Infarct 1998 (% Yes) | 13 (17.81) | 28 (19.58) | 14 (19.44) | 39 (19.02) | 12 (21.43) | 4 (14.81) |
Table 2.
Associations with Incident AD (through 2007) among Cognitively Normal Participants (1998)
| A: CESD: 3MSE Correlation (2) | |||
|---|---|---|---|
| Variable | Wald χ2 | Hazard Ratio (95% CI) | p |
| Age | 10.54 | 1.15 (1.06, 1.25) | <.001 |
| Sex (Male) | 0.40 | 0.81 (0.42, 1.57) | .53 |
| Education (≤HS) | 0.04 | 0.94 (0.49, 1.81) | .85 |
| Race (White) | 0.24 | 0.81 (0.35, 1.89) | .62 |
| Large infarct 1 | 0.96 | 0.65 (0.27, 1.55) | .33 |
| Ventricular grade ≥5 1 | 6.69 | 2.60 (1.26, 5.36) | .01 |
| White matter grade ≥3 1 | 0.04 | 1.07 (0.53, 2.17) | .84 |
| Diabetes | 0.34 | 1.35 (0.49, 3.71) | .56 |
| Hypertension | 0.33 | 0.83 (0.44, 1.56) | .56 |
| Heart disease | 0.06 | 0.86 (0.26, 2.91) | .81 |
| Correlation Group <25th | 0.91 | 0.83 (0.34, 2.01) | .63 |
| middle 50th | 0.91 | 0.69 (0.32, 1.48) | .63 |
| B: Mood-State Grouping (2) | |||
| Variable | Wald χ2 | Hazard Ratio (95% CI) | p |
| Age | 9.93 | 1.15 (1.05, 1.25) | <.001 |
| Sex (Male) | 0.44 | 0.80 (0.41, 1.55) | .51 |
| Education (≤HS) | 0.15 | 0.88 (0.45, 1.70) | .70 |
| Race (White) | 0.34 | 0.78 (0.34, 1.79) | .56 |
| Large infarct 1 | 0.76 | 0.68 (0.29, 1.61) | .38 |
| Ventricular grade ≥5 1 | 6.71 | 2.62 (1.26, 5.42) | .01 |
| White matter grade ≥3 1 | 0.01 | 1.03 (0.51, 2.09) | .94 |
| Diabetes | 0.48 | 1.42 (0.53, 3.85) | .49 |
| Hypertension | 0.12 | 0.90 (0.48, 1.67) | .73 |
| Heart disease | 0.01 | 0.95 (0.28, 3.25) | .94 |
| Depression - persistent | 1.78 | 1.33 (0.49, 3.65) | .41 |
| transient | 1.78 | 1.62 (0.78, 3.35) | .41 |
1) Using CHS Criteria for grading MRI scans [53]
2) All df=1.
The multivariate model revealed that in terms of the development of AD, only age (Wald χ2=10.54, df=1, p<.001, HR=1.15 (1.06, 1.25)) and ventricular grade (χ2=6.69, df=1p=.01, HR=2.60 (1.26-5.36)) were significantly associated with conversion. There was no significant increase in HR among those participants with the highest negative correlation between the CESD and 3MSE scores (See Table 2A).
Analysis 2
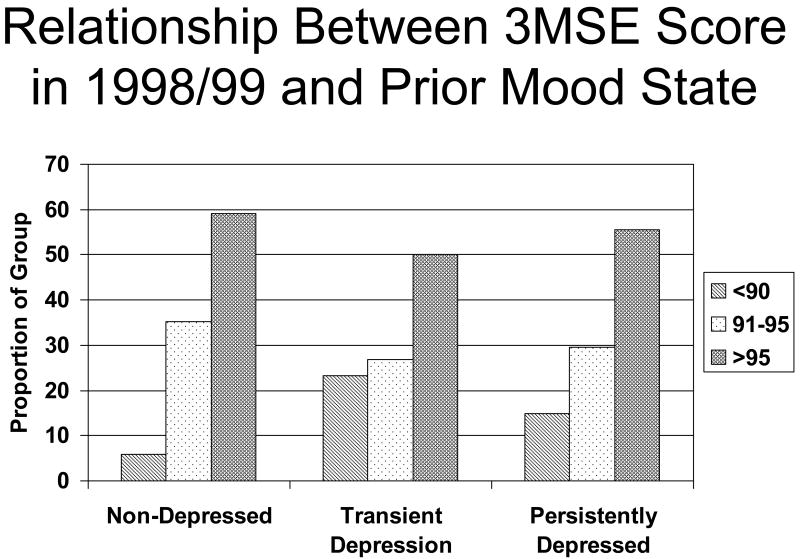
Table 1 shows the descriptive characteristics of the participants, grouped according to mood state during the five years prior to the 1998/99 evaluation (right hand panels). There was a significant difference in the scores on the 3MSE in 1998/99 between the three groups (See Figure 2). Specifically, the likelihood that a participant would have a score less than 90 (i.e., poorer performance on the 100 point scale) differed significantly between the groups (χ2 =15.4, df=2, p<.001), with the non-depressed participants being the least likely to have scores below 90.
Figure 2.
2) Relationship Between 3MSE Score in 1998/99 and Prior Mood State. Proportion of participants with 3MSE scores in the range of <90, 91-95 and >95 (vertical axis) as a function of the persistence of depressed mood (horizontal axis).
In terms of the development of AD, only age (Wald χ2=9.93, df=1, p<.001, HR=1.15 (1.05, 1.25)) and ventricular grade (χ2=6.71, df=1, p=.01, HR=2.62 (1.26-5.42)) were significantly associated with incident AD. There was no significant increase in HR among those participants with either transient or persistent depression relative to the non-depressed participants (See Table 2B).
Discussion
Within the confines of this longitudinal, community-based sample of elderly adults, we did not find strong evidence to support the hypothesis that mood disturbance was linked with the development of dementia. In the first analysis we identified CHS-CS participants in whom the scores on the 3MSE and the CESD were strongly negatively correlated. That is, among these participants when their CESD scores increased (indicating greater mood symptoms), their scores on the 3MSE at the same study visit decreased (indicating poorer cognitive functions). Our assumption was that in these participants the link between mood state and cognitive state was stronger than it was in other participants, and that this might indicate a greater vulnerability to dementia due to a decrease in cognitive reserve. However, there was no association between this correlation and subsequent cognitive decline. In the second analysis we defined mood disturbance based on the CESD score, and the persistence of any elevation during the five years prior to our observation period. Again, we did not find a significant link between persistently low mood state and the development of dementia. We thus conclude, as have others [9], that while depression may be transiently association with poor cognition, it does not increase the rate at which people develop AD.
Our findings generally argue against the vulnerability hypothesis of depression in the elderly. Because there was a 3 year period between the last clinic contact in 1999 and the evaluation in 2002-03, we were expecting that the persistence of depression (from 1992 to1999) or a strong covariation between depression and cognitive functioning would have resulted in an increased rate of dementia; however, we did not find such an association. We did find, as we have in the past, that there is a group of cognitively normal individuals whose global cognitive functions (i.e., 3MSE scores) declined when depressive symptoms increased (or improved with decreased symptoms of depression), but this relationship did not convey any additional risk to develop dementia over time. To the extent that the such changes in global cognition may be severe enough to warrant a classification of Mild Cognitive Impairment (especially in the context of depression), this may explain why we find a link between depressive symptoms and Mild Cognitive Impairment in cross-sectional studies [41, 42], or in studies with short-term follow-up[43].
Establishing that a personal characteristic such as depressed mood serves as a risk factor, rather than a correlate or outcome of cognitive impairment, requires that participants be free of cognitive impairment at the point(s) of risk factor assessment. When the normal/abnormal classification of dementia is made based on a cut-off of a global cognitive measure (e.g., [2, 3]), there is a risk of including in the “normal group”, individuals with early dementia. Even if the “miss rate” of a given two-stage screening protocol is known (e.g., [13]), the fact remains that the group classified as cognitively normal likely includes individuals in the earliest stages of dementia. Thus, mood symptoms that accompany the development of memory loss and other symptoms of the impending dementia may be found in participants who, although classified as cognitively normal based on the screening tests, are actually in the earliest stages of clinical dementia. In that case, we would predict that the risk of dementia in such cases would actually decline over time - once the early AD cases (who were considered normal) had developed dementia, there would be no additional increased rate of conversion. This is exactly what Andersen and colleagues found; the association between history of depression and incident AD after two years of follow-up (OR: 1.9, 95% CI: 1.0-3.3) was attenuated after 5 years follow up (OR: 1.6, 95%CI: 0.9-2.7) [44].
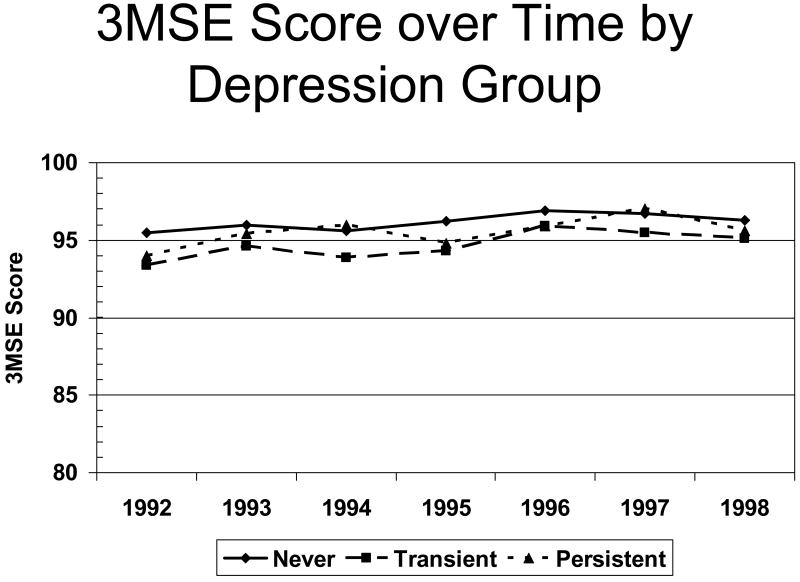
In the CHS the mean 3MSE score of the AD cases was 88.1 [45], which is well above the standard cut-off of normal cognition (i.e., greater than 80/100 [46]). Had we used the standard criterion for determining normal (or “not impaired”) cognitive state, we would have under-estimated the true prevalence of impairment, but more important our “normal” group would have contained mild AD cases thus biasing studies of incident AD such as this one. Because we have historical information on the cognitive functions of the study participants, we can determine whether or not there is evidence of decline in cognitive functions - even if the summary measures remain within normal limits. Figure 3 shows that there was no decline in 3MSE scores across the years prior to the observation period as a function of whether the participants reported persistent symptoms of mood disturbance. Thus, we feel confident that our group of cognitively normal participants was as free from early clinical dementia as we could reasonably expect. We not only have a less biased group of cognitively normal participants at the beginning of the observation period, but we have good ascertainment of the dementia outcome at its earliest onset.
Figure 3.
3) 3MSE Score over Time by Depression Group. Scores on the 3MSE (vertical axis) in the five years prior to the observation period (horizontal axis) as a function of the persistence of depressed mood.
There are several strengths of to this study. First, the original CHS participants were selected based on their inclusion on the Medicare lists, and not because of any mood or cognitive complaints or symptoms [24]. Second, there was a wealth of data for the 10 years prior to the beginning of the observation period that were used to control for possible co-morbid factors that could affect the risk of dementia. Third, the ascertainment of AD was done using a neuropsychological assessment and neurological exam, coupled with interviews with informants. Fourth, the participants in the study had documented normal cognition at the beginning of the observation period, and for a period of time prior. (See Figure 3).
However, there are also limitations to these data that need to be kept in mind. First, we did not ascertain syndromal Mood Disorder using standardized interviews (e.g., SCID [47], or MINI [48]). Thus, while we have excellent visit-to-visit measurement of mood state, we do not have research-level diagnoses of any mood disorders. Second, the sample size is small by the standards of epidemiological studies (n ∼ 300); however, with our sample size we were able to detect a HR of 3.2 with 80% power and a 2-sided alpha of 0.05. Said another way, assuming that HRs that we measured are valid indicators of the true HR, then we would have needed a sample of in excess of 4100 participants to detect them as statistically significant. Thus, we feel that we have adequate power to detect relevant effects, and that what we report is, in fact, a “null” finding. Third, the average age of the participants was 77 years, and our findings may be affected by a survivor bias. In order to be included in this study, the participants had to be cognitively normal in 1998/99. Thus, individuals who had developed dementia prior to 1998/99, or who had Mild Cognitive Impairment were excluded from this study. If depression were to exert effects to alter the risk of dementia, it would do so at an earlier age; those individuals who survived until their late 70's or early 80's without a cognitive impairment would be viewed as relatively immune to the additive effects of depression. This hypothesis is consistent with recent findings from the Rotterdam study [49], although the nature of that two-stage screening protocol makes it difficult to draw firm conclusions.
One finding that bears discussion was our failure to find an association between incident dementia and level of education. Within the CHS study [50] differences in incidence rates as a function of education level were significant only among white participants. Whites with less than high school education had an age-adjusted dementia incidence of 41.9 per 1,000 person-years, compared with 36.6 for high school graduates and 30.6 for individuals with at least some college education (p<.0001). Age-adjusted incidence of dementia for African Americans was 67.4 for those with less than high school, 55.8 for high school graduates, and 42.1 for college attendees (p=.79). Further, the age of this sample again raises the possibility that survivor bias may be affecting the results.
Geriatric depression is associated with an increased rate of medical comorbidities, and an increase in premature death [51]. This explains why, in the earlier study from the CHS [12] depression-dementia links were fully mediated by measurers of cardio-and cerebrovascular health (cf., [52]). In the present study, there were no differences in the rates of medical problems between the study groups, which suggests the possibility that the sicker, depressed participants had either already developed dementia (as above) or had died. Thus, our failure to find a link between depression and incident dementia could be viewed as evidence that depression exerts its “effect” on cognition by having the unwanted fellow travelers of medical disease. The clinical ramification of this conclusion is that physicians caring for elderly patients with depression need to pay particular attention to caring for the conditions/diseases that affect the cardio- and cerebrovasculature. By doing so, they may reduce the medical burden of their patients and reduce the likelihood of their developing cognitive dysfunction.
One other important characteristic of the sample that bears emphasizing is that the rate of use of anti-depressant medications was low. Among the individuals classified as persistently depressed, only 2/27 (7.4%) were being treated. While this may mean that these individuals did not, in fact, meet criteria for Major Depressive Disorder, it is more likely the case that their mood state was not appreciated by the treating physician [51].
These data emphasize the importance of attending to critical differences between studies conducted in referral research clinics and those conducted using community-based samples. In addition to questions related to the extent to which the samples accurately reflect the characteristics of the population of interest, the “portal of entry” into the study may be critical for the results of any studies of associations. For example, while participants in referral memory disorder clinics (e.g., Alzheimer's Disease Research Centers) by definition have complaints of cognitive impairment, fewer than 10% of the CHS-CS participants classified as demented had been previously diagnosed [13]. Thus, the CHS-CS is identifying AD in its mildest (or earliest) manifestations. The same could hold true for studies of mood disturbance. Participants in typical research clinics are recruited based on the presence of diagnosed mood disorder, which means that either the patient or the family felt that the dysphoria was severe enough to warrant care at a specialty clinic (as opposed, for example, to treatment by the Primary Care Physician). By the same token, individuals with severe depressed mood may be the ones who are less likely to agree to enroll in a study such as the CHS-CS, or who will have the lowest rate of follow-up.
Taken in context, these data suggest that the link between mood disturbance and the development of dementia is not as strong as might be expected. In our view, the data that are needed to fully address this issue simply do not exist. In studies such as the CHS-CS that focused on cognitive outcomes, the assessment of diagnosable mood disorders is sub-optimal. Among those studies using research-level psychiatric diagnoses, the ascertainment of cognitive impairment is not as strong as it should be. Thus, there is a critical need for a single study that does a good job of assessing both types of outcomes. When coupled with a good evaluation of cardiovascular and cerebrovascular factors (including MRI scans of the brain), and when begun prior to the development of even the earliest symptoms of age-associated cognitive dysfunction (e.g., 45-55 years old at time of enrollment), that type of large scale study could provide a wealth of critical information regarding the earlier life events that affect the risk of developing clinical dementia.
Acknowledgments
The authors have no competing financial or other interests that might have affected this research. The study reported in this article was supported, in part, by funds from the National Institute of Aging to O.L.L. (AG 20098) and L.K. (AG15928), and by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. In addition, partial support was provided by P30-MH71944, the UPMC Endowment in Geriatric Psychiatry, and the John A. Hartford Foundation Center of Excellence in Geriatric Psychiatry at the University of Pittsburgh.
References
- 1.Devanand DP, et al. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53(2):175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 2.Geerlings MI, et al. Depressive symptoms and risk of Alzheimer's disease in more highly educated older people. J Am Geriatr Soc. 2000;48(9):1092–1097. doi: 10.1111/j.1532-5415.2000.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 3.Palmer K, et al. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;88:1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 4.Modrego PJ, Ferrandez J. Depression in patients with mild cognitive impaiment increases the risk of developing dementia of Alzheimer type. Arch Neurol. 2004;61:1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 5.Green RC, et al. Depression as a risk factor for Alzheimer's disease. The MIRAGE Study. Arch Neurol. 2003;60:753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 6.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer's disease. Dement Geriatr Cogn Disorder. 2007;24(4):253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- 7.Steffens DC, et al. Methodology and preliminary results from the neurocognitive outcomes of depression in the elderly study. J Geriatr Psychiatry Neurol. 2004;17(4):202–11. doi: 10.1177/0891988704269819. [DOI] [PubMed] [Google Scholar]
- 8.Ownby RL, et al. Depression and risk for Alzheimer's disease. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganguli M, et al. Depressive symptoms and cognitive decline in late lfie. Arch Gen Psychiatry. 2006;63:153–160. doi: 10.1001/archpsyc.63.2.153. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay J, et al. Risk factors for Alzheimer's disease: a prosepctive analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 11.Panza F, et al. Impact of depressive symptoms on the rate of progression to dementia in patients affected by mild cognitive impairment. The Italian Longitudinal Study on Aging. Int J Geriatr Psychiatry. 2008 doi: 10.1002/gps.1967. [DOI] [PubMed] [Google Scholar]
- 12.Kuller LH, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology. 2005;64:1548–1552. doi: 10.1212/01.WNL.0000160115.55756.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuller LH, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 14.Steffens DC, et al. Hippocampal volume in geriatric depression. Biological Psychiatry. 2000;48(4):301–9. doi: 10.1016/s0006-3223(00)00829-5. [DOI] [PubMed] [Google Scholar]
- 15.Bell-McGinty S, et al. Brain morphometric abnormalities in geriatric depression : long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 16.Bell-McGinty S, et al. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 17.Jack CR, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffens DC, et al. Hippocampal volume and incident dementia in geriatric depression. American Journal of Geriatric Psychiatry. 2002;10(1):62–71. [PubMed] [Google Scholar]
- 19.Butters MA, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 20.Butters MA, Whyte E, Nebes R. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 21.Bhalla RK, et al. Persistence of neuropsychological deficits in the remitted state of late-life depression. Am J Gen Psychiatry. 2006;14(5):419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 22.Satz P. Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence of threshold theory. Neuropsychology. 1991;7(3):273–295. [Google Scholar]
- 23.Stern Y, et al. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol. 1992;32(3):371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, et al. The Cardiovascular Health Study: Design and Rationale. Ann Epidemiol. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Memory Scale- Revised Manual. New York: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- 27.Jorm AF, Jacomb PA. The informant questionnaire on cognitive decline in the elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 28.Kawas C, et al. A validation study of the dementia questionnaire. Arch Neurol. 1994;51:901–906. doi: 10.1001/archneur.1994.00540210073015. [DOI] [PubMed] [Google Scholar]
- 29.Lopez OL, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognitive Study Part 1. Arch Neurology. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 30.Lopez OL, et al. Incidence of dementia in Mild Cognitive Impairment in the Cardiovascular Health Study. Arch Neurol. 2007;64:416–420. doi: 10.1001/archneur.64.3.416. [DOI] [PubMed] [Google Scholar]
- 31.Lopez OL, et al. Evaluations of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22(1):1–12. doi: 10.1159/000067110. [DOI] [PubMed] [Google Scholar]
- 32.Lopez OL, et al. Neuropsychological characteristics of mild cognitive impairment subgroups. J Neurol Neurosurg Psychiatry. 2006;77:159–165. doi: 10.1136/jnnp.2004.045567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fahn S, Elton RI. UPDRS Development Committee: Unified Parkinsons Rating Scale. In: Fahn S, et al., editors. Recent developments in Parkinson's disease. MacMillan Healthcare Information; Florham Park: 1987. pp. 153–163. [Google Scholar]
- 34.Hachinski VC, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, et al. Risk factors for 5-year mortality in older adults: The cardiovascular health study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 36.Andresen EM, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 37.Cummings JL, et al. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 39.Lopez OL, et al. Research evaluation and diagnosis of possible Alzheimer's disease over the last two decades: I. Neurology. 2000;55:1854–1862. doi: 10.1212/wnl.55.12.1854. [DOI] [PubMed] [Google Scholar]
- 40.Lenze EJ, et al. The course of functional decline in older people with persistently elevated depressive symptoms: longitudinal findings from the Cardiovascular Health Study. J Am Geriatr Soc. 2005;53(4):569–75. doi: 10.1111/j.1532-5415.2005.53202.x. [DOI] [PubMed] [Google Scholar]
- 41.Lopez OL, et al. Risk factors for mild cognitive imapirment in the Cardiovascular Health Study Cognition Study: Part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 42.Solfrizzi V, et al. Incident occurrence of depressive symptoms among patients with mild cognitive impairment - the Italian longitudinal study on aging. Dement Geriatr Cogn Disord. 2007;24(1):55–64. doi: 10.1159/000103632. [DOI] [PubMed] [Google Scholar]
- 43.Barnes D, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: Results from the Cardiovascular Health Study. Neurology. 2005;64(Suppl 1):A127. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 44.Andersen K, et al. Depression and the risk of Alzheimer disease. Epidemiology. 2005;16(2):233–8. doi: 10.1097/01.ede.0000152116.32580.24. [DOI] [PubMed] [Google Scholar]
- 45.Lopez OL, et al. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology. 2005;64:1538–1547. doi: 10.1212/01.WNL.0000159860.19413.C4. [DOI] [PubMed] [Google Scholar]
- 46.Graham JE, Rockwood K, Beattie EL. Prevalence and severity of cognitive impairment with and without dementia in an elderly opoulation. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 47.Spitzer RL, et al. Structured Clinical Interview for DSM-III-R. New York: Biometrics Research Department, NY State Psychiatric Institute; 1990. [Google Scholar]
- 48.Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 20:22–33. quiz 34-57. [PubMed] [Google Scholar]
- 49.Geerlings MI, et al. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258–64. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick AL, et al. Incidence and prevalence of dementia in the cardiovascular health study. J Am Geriatr Soc. 2004;52(2):195–204. doi: 10.1111/j.1532-5415.2004.52058.x. [DOI] [PubMed] [Google Scholar]
- 51.Lebowitz BD, et al. Diagnosis sand treatment of depression in late life. Cognitive statement update. JAMA. 1997;278(14):1186–1190. [PubMed] [Google Scholar]
- 52.Butters MA, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–57. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bryan RN, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]