Abstract
Objective
To assess the effect of weight loss by bariatric surgery on metabolic syndrome (MetS) prevalence and to examine predictors of MetS resolution.
Patients and Methods
We performed a population-based, retrospective study between January 1st,1990 and December 31st,2003 of patients evaluated for bariatric surgery with AHA/NHLBI-defined MetS (increased triglycerides, low high density lipoprotein, increased blood pressure, increased fasting glucose, and a measure of obesity). There were 180 Roux-en-Y gastric bypass patients and 157 non-operative patients assessed in a weight-reduction program. We determined the change in MetS prevalence and used logistic regression models to determine predictors of MetS resolution.
Results
Mean follow-up was 3.4 years. All MetS components improved in the surgical group and medication use decreased. Non-operative patients had improvements in high density lipoprotein. Of the 180 surgical patients, MetS prevalence decreased from 156 patients (87%) to 53 (29%), and from 133 patients (85%) to 117 (75%) in the non-operative group. There was a relative risk reduction of 0.59 (95% CI 0.48-0.67; p<0.001)] with bariatric surgery patients having MetS at follow-up. The number needed to treat with surgery to resolve one case of MetS was 2.1. Results were similar after excluding patients with diabetes or cardiovascular disease or after using non-BMI diagnostic criteria for MetS. Significant predictors of MetS resolution included a 5% loss in excess weight (OR 1.26; 95%CI 1.19-1.34;p<0.001) and diabetes (OR 0.32; 95%CI 0.15-0.68;p=0.003).
Conclusion
Roux-en-Y gastric bypass induces considerable and persistent improvement in MetS prevalence. Our results suggest that reversibility of MetS depends more on the amount of excess weight lost than on other parameters.
Keywords: Bariatric Surgery, Metabolic Syndrome, Weight Loss, Obesity
Introduction
The components of the metabolic syndrome (MetS) account for a substantial portion of the attributable risk for atherosclerotic cardiovascular (CV) diseases. All five components of the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) definition of MetS have been linked independently to CV diseases, including increased serum triglycerides (TG), low serum high density lipoprotein cholesterol (HDL-C), elevated blood pressure, increased fasting plasma glucose, and an increased waist circumference 1. With the increasing prevalence of MetS and its strong association with the development of diabetes and CV disease, this syndrome is a significant public health concern2-4.
Substantial evidence suggests that insulin resistance is the underlying abnormality in the pathophysiology of MetS5 and that lifestyle modifications represent the cornerstone of management6,7. Increased physical activity and a healthy diet in people with impaired fasting blood glucose reduces the incidence of type 2 diabetes mellitus8, even when participants experience only modest weight loss of <10% 9. Because most dietary interventions fail to achieve more than a 10% weight loss and most lost weight is regained, the net effect of significant and long-lasting weight loss on MetS is unknown.
Bariatric surgery, an approved treatment for obesity when other measures have failed10, induces longstanding, profound weight loss11. Most patients eligible for weight reduction by bariatric procedures have a substantial number of components of MetS with most of the weight loss attributed to reduced caloric intake and, to some extent, partial malabsorption of nutrients or bypass of the duodenum by Roux-en-Y gastric bypass (RYGB). This patient population presents a unique opportunity to determine the effect of major weight loss on MetS prevalence with little confounding by changes in moderate-intense physical activity. We evaluated the effect of bariatric surgery on MetS in a population-based cohort of patients with morbid class II-III obesity, with a body mass index (BMI) ≥35kg/m2, undergoing RYGB and in a control group of patients who were non-operative.
Patients and Methods
Study Setting
We performed a population-based, retrospective cohort study of all Olmsted County patients referred for bariatric surgery at Mayo Clinic between January 1st, 1990 and December 31st, 2003. Patients were identified using a centralized diagnostic index and the Rochester Epidemiology Project. All bariatric interventions in the county are performed at our institution. The Rochester Epidemiology Project is a comprehensive, record-linkage system funded continually by the Federal government since 1966 in its use in disease-related epidemiology12. Olmsted County patients have all of their medical care indexed, allowing complete ascertainment of patients' medical histories. The county is a relatively isolated and self-contained area. Medical care is provided predominantly by the Mayo Clinic, its hospitals, and Olmsted Medical Center and its hospital. Healthcare delivery by a limited number of individual private practitioners is also captured. This study was approved by both the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Study Cohort
The patient's primary place of residence was determined using baseline demographic information, and county residence was verified using United States Postal Services zip codes. The surgical cohort was identified using the Mayo Surgical Index whose primary indication was weight reduction using RYGB. We identified 231 Olmsted county residents having undergone RYGB, and excluded 16 patients with a BMI <35kg/m2, and 35 patients with either incomplete data or whose follow-up was less than three months, yielding a surgical cohort of 180 patients.
Our comparison group included non-operative patients evaluated for bariatric surgery at the multidisciplinary Mayo Clinic Nutrition Center who did not undergo surgery. Reasons for not undergoing bariatric surgery were: voluntary decline, ineligibility due to denial by insurance providers, or those who did not maintain lifestyle interventions during their evaluation. A minority were excluded due to psychiatric reasons. As is often the case in clinical practice, patient exclusion from bariatric interventions was multifactorial. We identified 252 Olmsted county patients and excluded 19 with a BMI <35kg/m2, the majority of whom underwent revisional bariatric operative procedures for complications, and 76 who did not have any further medical follow-up or whose follow-up was less than three months. Our final comparison cohort consisted of 157 patients. All operative and non-operative patients were managed medically with a multidisciplinary program consisting of medical care, dietetic care, and extensive counseling about the importance of physical activity.
Time of bariatric surgery was considered the baseline time for the surgical group and time of nutrition consultation was considered the baseline time for the non-operative group. We defined our baseline variables based on information present in the medical record from the baseline time or earlier, while the follow-up variables were based on information at time of last follow-up evaluation. As major weight loss or significant metabolic changes do not normally occur prior to three months, all patients in our study had a minimum follow-up of at least three months. Height and weight were measured in a standardized manner by a trained nurse, and BMI was calculated by dividing weight (kg) by height (in meters) squared. We used the method of Robinson et al13 to calculate ideal body weight.
MetS was diagnosed according to the criteria of the AHA/NHLBI described in 20051. The five components include: increased serum triglycerides (TG), low serum high density lipoprotein cholesterol (HDL-C), increased blood pressure, increased fasting plasma glucose, and increased waist circumference. As waist circumference was not documented routinely in the medical record, we used BMI as a surrogate for central obesity because data have shown that most patients with a BMI≥30kg/m2 have a large waist circumference14. Patients on fibrates or nicotinic acid or whose serum TG≥150mg/dL were classified as having hypertriglyceridemia. A low serum HDL-C was considered to be <40mg/dL in males, <50mg/dL in females, or on treatment with nicotinic acid or fibrates specifically for this disorder. We classified patients as having hypertension if their blood pressure was >135/85mmHg, or if they were on any medications specifically for hypertension, including β-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazides, or loop or potassium-sparing diuretics. These medications were included only if the patient had a coexisting documented diagnosis of hypertension. Increased fasting plasma glucose concentration was defined as greater than 100mg/dL without the diagnosis of diabetes. Patients were considered as having diabetes mellitus if the fasting glucose was >126mg/dL or if they were on insulin or oral hypoglycemic agents. A diagnosis of MetS required ≥3 criterion. Finally, we also determined the impact on MetS prevalence if it was defined as ≥2 components, without obesity as a diagnostic criterion. In a subanalysis of patients with greater than one year follow-up, of the surgical patients with a baseline diagnosis of MetS, we defined patients as MetS responders if they no longer fulfilled criteria for the MetS at most recent evaluation. MetS non-responders consisted of surgical patients who still had ≥3 criteria of MetS at follow-up.
Statistical Analysis
Continuous data are presented as means±standard deviations, and categorical data are presented as numbers and percent. Within-group comparisons between baseline and follow-up were compared using a two-sided, paired t-test and Wilcoxon Signed Rank for continuous variables and McNemar test for categorical variables. Inter-group comparisons between the baseline non-operative and surgical patients, and changes between groups were compared using a two-sample t-test of unequal variances, the Wilcoxon Rank Sum test, Chi-square orFisher exact tests depending on the type of data. For changes in categorical variables between groups, we compared the three point scale distributions (improved, no change, worsened) and applied the Cochran-Armitage trend test. Due to the skewness of triglycerides, logarithmic transformation was applied.
We sought to determine if the amount of weight loss, defined as the percent of excess weight lost, was the main predictor for MetS resolution. We constructed multiple logistic regression models using backward selection to identify predictors of MetS resolution at follow-up in patients with MetS at baseline. Our inclusion threshold was p<0.25, and the exclusion threshold for variables was a p-value of 0.10. Initially, we excluded patients with a follow-up less than one year, as previous studies have suggested that weight loss in this population does not occur linearly in this time period 11, and normally plateaus following the one year period. The overall cohort of patients with MetS at baseline were stratified by quartiles according to their follow-up time. Because of the limited number of events in each group, we adjusted only for age and sex in these multivariate analyses. Models were created to test whether our primary predictor, % excess weight loss, was significantly different in these groups. We additionally determined whether the within-quartile beta coefficients differed within each cohort to further elicit the impact of follow-up time. As no differences were observed, we re-ran the analysis on the entire cohort of MetS patients at baseline with a follow-up greater than one year. Univariate predictors (p<0.10) were entered subsequently into a multivariate model, with the exception of any measures related to weight or glucose, because of collinearity with % excess weight loss and diabetes, both predictors of interest. The first model (Model 1) defined MetS as ≥3 components. Because patients undergoing bariatric surgery have previously been shown to lose weight, they may be cured potentially of their obesity criterion, increasing the likelihood for MetS response. Therefore, we also performed a second model (Model 2) defining MetS as ≥2 components, without considering obesity as a component, to have a MetS definition independent of BMI. This allowed us to determine the potential role of percent excess weight loss on the resolution of other components of MetS. We examined all patients, regardless of whether they underwent surgery, who had MetS at baseline, and included covariates in both models such as age, sex, baseline TG, the presence of diabetes at baseline, use of an angiotensin converting enzyme inhibitor or blockers, and % excess weight lost. Additionally, we separately adjusted for follow-up time as an additional co-variate in a multiple logistic regression analysis for MetS≥3 components (Model 1A), and MetS≥2 component (Model 1B), to ascertain whether this variable altered our results. Finally, we tested Model 1 solely on bariatric surgery patients with baseline MetS (≥3 components) whose follow-up was greater than one year, to determine possible relationships with MetS resolution. We included the above predictors in addition to initial HDL levels. In the non-operative group, we independently assessed % excess weight loss on its own as a predictor in Model 1 and 2, in addition to incorporating follow-up time in Model 1A and 2A, because we had little power in detecting the few MetS resolution outcomes for this particular analysis. The strength of association between follow-up time and % excess weight loss using a correlation coefficient was also determined.
Sensitivity Analysis
Sensitivity analyses were performed on our data using a carry forward method of imputation for patients lost to follow-up. Patients excluded from the analysis due to missing data (n=35 in the bariatric group, and n=76 in the non-operative group), were assumed to have MetS both at baseline and at follow-up, to determine what the within-group change in MetS prevalence would have been using this approach. We also determined the impact of excluding patients who had a baseline diagnosis of both diabetes and CV disease (74 patients (41%) in the bariatric group and 56 patients (36%) in the non-operative group), and of patients with only a diagnosis of diabetes (58 patients (32%) in the bariatric group and 40 patients (25%) in the non-operative group). A p-value <0.05 was considered statistically significant. All analyses were performed using JMP for SAS (Windows version 7.0.0, SAS Institute Inc, Cary, NC).
Results
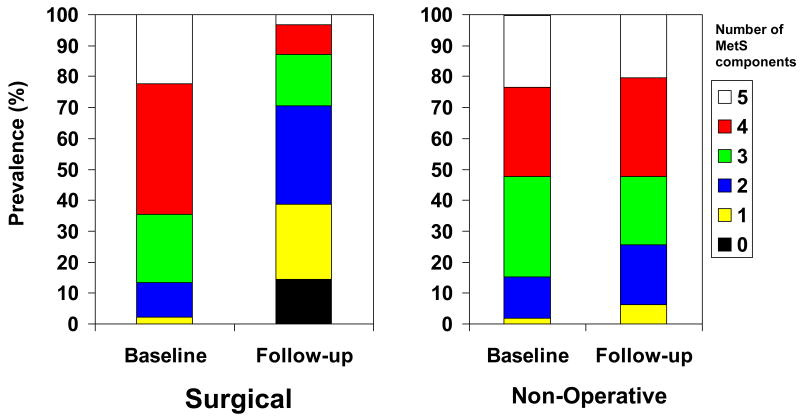
The baseline characteristics of the cohorts are shown in Table 1. Bariatric surgery patients had a higher BMI than the non-operative patient group, and were more likely to be taking insulin. Prevalence of MetS at baseline (156 patients (87%) vs. 133 patients (85%), p=0.61) and duration of follow-up (3.4±2.5 vs. 3.5±2.6 years; p=0.79) were similar between groups. Follow-up ranged between 0.4-12.8 years (IQR: 0.4-12.8) and 0.4-13.8 years (IQR:1.4-4.4), in the surgical and non-operative groups, respectively. Of those with a follow-up of less than one year, there were 14 subjects (7.8%) and 15 subjects (9.6%) in these groups, respectively. The correlation between duration of follow-up and % excess weight loss was 0.14. A greater number of patients in the surgical group had four MetS components when compared to the non-operative group (76 patients (42%) vs. 45 patients (29%); p=0.001) (Figure 1).
Table 1.
Baseline Characteristics of Olmsted County Residents Evaluated for Roux-en-Y Gastric Bypass a
| Surgical N=180 |
Non-Operative N=157 |
P-valueb | |
|---|---|---|---|
| Age, years | 45±10 | 44±11 | 0.39 |
| Female sex (%) | 143 (79) | 113 (72) | 0.11 |
| Duration of follow-up | 3.4±2.5 | 3.5±2.6 | 0.79 |
| Body Mass Index, kg/m2 | 49±9 | 44±6 | <0.001 |
| Excess Weight, kg | 78±24 | 63±17.3 | <0.001 |
| Systolic Blood Pressure, mmHg | 134±16 | 134±18 | 0.79 |
| Diastolic Blood Pressure, mmHg | 80±10 | 77±10 | 0.03 |
| Serum Biochemical Parameters: | |||
| Total Cholesterol, mg/dL | 200±39 | 208±45 | 0.08 |
| LDL-C, mg/dL | 118±33 | 122±36 | 0.26 |
| HDL-C, mg/dL | 45±11 | 45±14 | 0.65 |
| Triglycerides, mg/dL | 190±119 | 219±155 | d0.10 |
| Glucose, mg/dL | 118±38 | 121±51 | 0.66 |
| Creatinine, mg/dL | 1.0±0.2 | 1.0±0.2 | 0.32 |
| Diabetes (%) | 58 (32) | 40 (26) | 0.17 |
| Ever Smoker (%) | 25 (14) | 29 (19) | 0.25 |
| Cardiovascular Disease (%) | 29 (16) | 23 (15) | 0.71 |
|
| |||
| Medications | |||
| Statins (%) | 26 (14) | 19 (12) | 0.53 |
| β -Blockers (%) | 36 (20) | 22 (14) | 0.15 |
| Calcium Channel Blockers (%) | 17 (9) | 8 (5) | 0.13 |
| ACE-I/ARB (%) | 39 (22) | 36 (23) | 0.78 |
| Diuretics (%) | 46 (26) | 36 (23) | 0.58 |
| Insulin (%) | 30 (17) | 7 (5) | <0.001 |
| Oral Diabetes Medications (%) | 26 (14) | 24 (15) | 0.83 |
|
| |||
| Metabolic Syndrome (%) | 156 (87) | 133 (85) | 0.61 |
| Obesity Component (%) | 180 (100) | 157 (100) | 1.00 |
| Hypertriglyceridemia (%) | 110 (61) | 99 (63) | 0.71 |
| Low HDL (%) | 123 (68) | 111 (71) | 0.64 |
| Elevated Blood Pressure (%) | 155 (86) | 120 (76) | 0.02 |
| Impaired Fasting Glucose (%) | 112 (62) | 88 (56) | 0.25 |
All values are represented as mean ± standard deviation or count (percent) for categorical variables and are rounded to the nearest integer unless otherwise indicated. ACE – angiotensin converting enzyme inhibitors; ARB – angiotensin receptor blockers; HDL-C – High density lipoprotein cholesterol; LDL-C – Low density lipoprotein cholesterol
Unpaired t-test with unequal variances and Wilcoxon Rank Sum for continuous data, and Chi-Square test and Fisher exact test for non-parametric data.
SI conversion factors: To convert total cholesterol to mmol/L, multiply by 0.0259;, to convert LDL-C to mmol/L, multiply by 0.0259; to convert HDL-C values to mmol/L,multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113; to convert glucose to mmol/L, multiply by 0.0555; to convert creatinine to μmol/L, multiply by 88.4.
p-value based on logarithmic transformation of Triglycerides
Figure 1. Change in Metabolic Syndrome Components.
aBaseline and follow-up prevalence of Metabolic Syndrome components in bariatric surgery and non-operative patient groups.
Bariatric Surgery Group
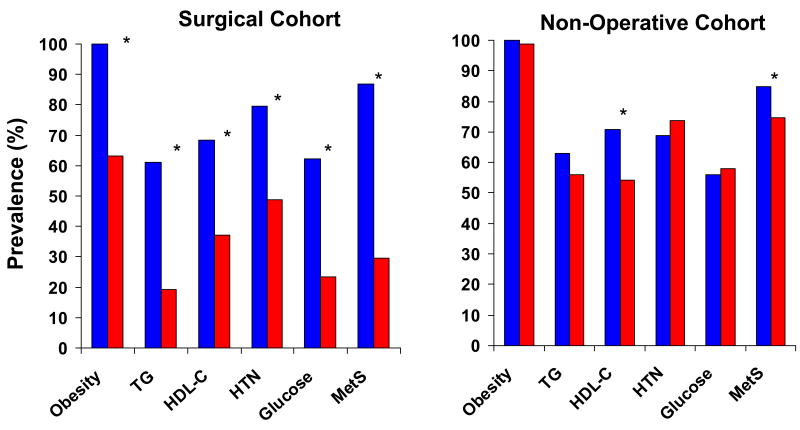
All MetS components improved at follow-up when measured as continuous or as categorical variables (Figure 2). The components that showed the most improvement when measuring the change in prevalence before and after bariatric surgery were hypertriglyceridemia, glucose intolerance/diabetes mellitus and obesity, with a 42%, 39% and 37% decrease in prevalence, respectively. Mean lipid and blood pressure values decreased despite a decrease in the use of statins, diuretics, beta-blockers, and angiotensin converting enzyme inhibitors (Table 2).
Figure 2. Change in Metabolic Syndrome Parameters.
aChange in each of the five components of the Metabolic Syndrome and in the prevalence of the Metabolic Syndrome as defined by the American Heart Association / National Heart, Lung and Blood Institute. Significant changes (P<0.05) are represented by an asterix (*).
bAbbreviations: TG – Triglycerides; HDL – High Density Lipoprotein Cholesterol; HTN – Hypertension; MetS – Metabolic Syndrome
Table 2.
Change in Parameters from Baseline to Follow-up in Olmsted County Residents Referred for Roux-en-Y Gastric Bypass a
| Surgical N=180 |
Non-Operative N=157 |
P-value b | |
|---|---|---|---|
| Age, years | 3.4±2.5 | 3.5±2.6 | 0.74 |
| Body Mass Index, kg/m2 | -16±6 | -0.1±6.0 | <0.001 |
| Excess Weight, kg | -44±17 | -0.3±16 | <0.001 |
| % Excess Weight Lost | 59±20 | -0.2±29 | <0.001 |
| Systolic Blood Pressure, mmHg | -13±18 | -6±21 | <0.001 |
| Diastolic Blood Pressure, mmHg | -8±13 | -2±13 | <0.001 |
| Serum Biochemical Parameters: | |||
| Total Cholesterol, mg/dL | -46±39 | -15±43 | <0.001 |
| LDL-C, mg/dL | -41±34 | -13±34 | <0.001 |
| HDL-C, mg/dL | 9±11 | 4±11 | <0.001 |
| Triglycerides, mg/dL | -80±103 | -44±112 | 0.001 |
| Glucose, mg/dL | -24±34 | -4±47 | <0.001 |
| Creatinine, mg/dL | -0.05±0.15 | 0±0.15 | 0.02 |
| Diabetes (%) | -37/180 (-21) | 14/157 (9) | <0.001 |
| Cardiovascular Disease (%) | 4/180 (2) | 6/157 (4) | 0.39 |
|
| |||
| Medications | |||
| Statins (%) | -14/180 (-7) | 31/157 (20) | <0.001 |
| β -Blockers (%) | -15/180 (-8) | 11/157 (7) | <0.001 |
| Calcium Channel Blockers (%) | -1/180 (1) | 1/157 (1) | 0.75 |
| ACE-I/ARB (%) | -9/180 (5) | 21/157 (13) | <0.001 |
| Diuretics (%) | -17/180 (9) | 17/157 (11) | <0.001 |
| Insulin (%) | -24/180 (13) | 12/157 (8) | <0.001 |
| Oral Diabetes Medications (%) | -24/180 (-13) | -14/157 (-9) | 0.29 |
Values are represented as mean difference ± standard deviation for continuous variables. Differences in each cohort represent ValueFOLLOW-UP - ValueBASELINE. For categorical values, the change in the number of patients (Follow-up – Baseline) re-normalized to the baseline counts are represented (percent) and are rounded to the nearest integer, unless otherwise indicated. A negative value represents an improvement in the variable for continuous data, and a reduction in the net number (% reduction) of patients for categorical data. ACE – angiotensin converting enzyme inhibitors; ARB – angiotensin receptor blockers; HDL-C – High density lipoprotein cholesterol; LDL-C – Low density lipoprotein cholesterol
unpaired t-test for unequal variances and Wilcoxon Rank Sum used to test between the differences between Surgical and Non-Operative data for continuous data and Cochran-Armitage trend test for categorical variables.
SI conversion factors: To convert total cholesterol to mmol/L, multiply by 0.0259; to convert LDL-C to mmol/L, multiply by 0.0259;, to convert HDL-C to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113; to convert glucose to mmol/L, multiply by 0.0555; to convert creatinine to μmol/L, multiply by 88.4.
Non-Operative Group
Patients had improvements in their systolic blood pressure and overall lipid values, but the magnitudes of these improvements were much less than those of the bariatric surgery group and were associated, moreover, with a significant increase in the use of statins and antihypertensive agents at follow-up. The prevalence of MetS criteria other than low HDL did not change. There was also a greater number of patients with type 2 diabetes at follow-up and a greater proportion of patients taking insulin.
Comparison of Bariatric Surgery and Non-Operative Groups
Both bariatric surgery and non-operative patients had significant improvements in the overall prevalence of MetS (Figure 2), decreasing by 58% in the surgical group from 87% to 29% (P<0.001), and by 10% in the non-operative group, from 85% to 75% (P<0.001). The absolute difference in prevalence reduction between groups was 48% (95% CI 38-58%; p<0.001), yielding a relative risk reduction with bariatric surgery of 0.59 (95% CI 0.48-0.67; p<0.001). The number of patients needed to treat in order to cure one patient with MetS with bariatric surgery was 2.1. This was based on all patients with MetS at baseline, calculating the difference between the proportion of non-operative patients with MetS at follow-up (82%) minus the proportion with MetS at follow-up among bariatric surgery patients (34), and divided by the control proportion (82%). The mean number of components decreased from 3.7 to 1.9 (p<0.001) in the bariatric surgery group and changed slightly from 3.6 to 3.4 (p=0.04) in the non-operative group. From the 157 non-operative patients at baseline, all met criteria for obesity, and 155 patients (99%) remained obese at follow-up. In the operative group, all 180 patients (100%) met criteria for obesity at baseline, whereas only 114 (63%) were obese at follow-up. After omitting BMI as a categorical variable for MetS and defining MetS as ≥2 components, the prevalence in the surgical group decreased from 158 patients (88%) to 67 patients (37%) (p<0.001), while in the non-operative group it decreased much less, from 138 patients (88%) to 117 patients (75%) (p<0.001). Examination of our cohort, stratified by quartiles of follow-up time, demonstrated that the % excess weight lost, as the main predictor of MetS resolution, was highly significant and did not change. Subsequently, performing multiple logistic regression on the entire cohort of patients with MetS at baseline, demonstrated that % excess weight loss was the main predictor of MetS resolution (Table 4). Again, results were no different after incorporating follow-up time as a co-variate in the overall cohort after adjusting for follow-up time (Model 1A and 2A). By separately examining the odds ratios of MetS resolution in the operative group (Table 4) and non-operative group (data not shown) for % excess weight loss as our primary predictor, there were no differences between these values after adjustment for follow-up time. All the above analyses suggest that follow-up time was not a significant contributor to MetS resolution
Table 4.
Multivariate Logistic Regression Models Identifying Predictors of Metabolic Syndrome Resolution in Patients with a Follow-up Greater than One Year a
| Overall Cohort with Metabolic Syndrome at Baseline | Surgical Responders vs. Non-Responders with Metabolic Syndrome at Baseline | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 1Ac | Model 2 | Model 2Ac | Model 1 | Model 1Ac | |||||||
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | OR | P-value | |
| Age | 0.94 | 0.001 | 0.94 | 0.001 | 0.95 | 0.003 | 0.95 | 0.003 | 0.93 | 0.009 | 0.93 | 0.009 |
| Male Sex | 1.07 | 0.86 | 1.09 | 0.83 | 0.87 | 0.69 | 0.85 | 0.66 | 1.24 | 0.71 | 1.23 | 0.73 |
| Triglycerides | 0.28 | 0.001 | 0.28 | 0.001 | 0.35 | 0.002 | 0.35 | 0.002 | 0.33 | 0.03 | 0.33 | 0.03 |
| Diabetes(Yes/No)b | 0.32 | 0.003 | 0.32 | 0.003 | 0.26 | <0.001 | 0.26 | <0.001 | 0.41 | 0.06 | 0.41 | 0.06 |
| ACE/ARB | 0.40 | 0.02 | 0.40 | 0.02 | 0.54 | 0.11 | 0.55 | 0.11 | 0.34 | 0.03 | 0.34 | 0.03 |
| HDL | --- | --- | --- | --- | --- | --- | --- | --- | 1.02 | 0.37 | 1.02 | 0.38 |
| % EWLb | 1.05 | <0.001 | 1.05 | <0.001 | 1.03 | <0.001 | 1.03 | <0.001 | 1.05 | <0.001 | 1.05 | <0.001 |
| 5% change in %EWL | 1.26 | <0.001 | 1.26 | <0.001 | 1.17 | <0.001 | 1.18 | <0.001 | 1.28 | <0.001 | 1.28 | <0.001 |
| Follow-up Time | --- | --- | 0.98 | 0.75 | --- | --- | 1.03 | 0.65 | ---- | ---- | 1.01 | 0.92 |
Separate models were constructed for the overall cohort consisting of both operative and non-operative patients with MetS at baseline, and for bariatric surgery patients with MetS at baseline. All patients had a follow-up of greater than one year. MetS was defined as ≥ 3 of 5 components of AHA/NHLBI-defined MetS in Model 1. Model 2 consists of ≥ 2 of 4 components (excluding the obesity component). Co-variates in the overall cohort for Models 1 and 2 included: age, sex, baseline serum triglycerides, the presence of diabetes at baseline (yes/no), the use of angiotensin converting enzyme inhibitors or blockers at baseline (yes/no), and % excess weight loss. For the surgical cohort alone (MetS responders and MetS non-responders), HDL was added as an additional co-variate in the multivariate analysis for Model 1. Follow-up time was included as a covariate in the multiple logistic regression models in models labeled with the suffix “A”. BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; HDL-C – high density lipoprotein cholesterol; LDL-C – low density lipoprotein cholesterol; CCB – Calcium Channel Blocker; CV – cardiovascular; IBW – ideal body weight; ACE/ARB – angiotensin converting enzyme inhibitor; EWL – excess weight loss; MetS – metabolic syndrome; OR – Odds Ratio; Total-C – Total cholesterol
per 1 % unit change in excess weight lost
models including time as a co-variate
Surgical Metabolic Syndrome Responders vs. Metabolic Syndrome Non-Responders
Of the 156 bariatric surgery patients with MetS at baseline, 13 (8%) with a follow-up of less than one year were excluded for this particular analysis. Of the remaining 143 patients (79%) with a baseline diagnosis of MetS, forty-five (32%) did not have resolution of their MetS during follow-up and were considered MetS non-responders. The median number of components decreased from 4 to 2 in the MetS responders and from 4 to 3 in the MetS non-responders. There was a decrease from 98 patients (100%) to 51 patients (52%) that fulfilled the obesity criterion in the MetS responders, while in the non-responders the number fulfilling the obesity criterion decreased from 45 patients (100%) to 40 patients (89%). These MetS non-responders were older, had a higher baseline BMI, had a lower percent of excess weight lost, higher serum levels of TGs, higher fasting blood glucose, a greater prevalence of baseline diabetes, and increased usage of angiotensin converting enzyme inhibitors or blockers (Table 3). Percent excess weight loss was a highly significant predictor of MetS resolution, even after adjusting for follow-up time (Table 4).
Table 3.
Baseline Characteristics of 143 Roux-en-Y Gastric Bypass Patients with MetS at Baseline Comparing MetS Responders vs. MetS Non-Responders with a Follow-up of Greater than One Yeara,b
| MetS Responders N=98 |
MetS Non-Responders N=45 |
Inter group P-value c |
|
|---|---|---|---|
| Age, years | 43±10 | 48±10 | 0.009 |
| Female sex (%) | 77 (79) | 35 (78) | 0.91 |
| Body Mass Index, kg/m2 | 49±8 | 52±11 | 0.05 |
| Excess Weight, kg | 76±24 | 82±25 | 0.20 |
| % Excess Weight Lost | 63±20 | 49±18 | <0.001 |
| Systolic blood pressure, mmHg | 134±16 | 135±16 | 0.82 |
| Diastolic blood pressure, mmHg | 80±10 | 79±11 | 0.74 |
| Serum Biochemical Parameters: | |||
| Total Cholesterol, mg/dL | 199±41 | 199±37 | 0.62 |
| LDL-C, mg/dL | 119±33 | 110±28 | 0.12 |
| HDL-C, mg/dL | 44±9 | 41±11 | 0.07 |
| Triglycerides, mg/dL | 177±88 | 254±169 | <0.001 |
| Glucose, mg/dL | 118±38 | 133±40 | 0.03 |
| Creatinine, mg/dL | 1± 0.2 | 1±0.2 | 0.71 |
| Diabetes (%) | 28 (29) | 28 (62) | <0.001 |
| Ever Smoker (%) | 18 (18) | 5 (11) | 0.27 |
| Cardiovascular Disease (%) | 15 (15) | 9 (20) | 0.49 |
|
| |||
| Medications | |||
| Statins (%) | 16 (16) | 7 (16) | 0.91 |
| β-Blockers (%) | 19 (19) | 12 (27) | 0.33 |
| Calcium Channel Blockers (%) | 10 (10) | 6 (13) | 0.58 |
| ACE-I/ARB (%) | 15 (15) | 16 (36) | 0.006 |
| Diuretics (%) | 26 (27) | 14 (31) | 0.57 |
| Insulin (%) | 16 (16) | 10 (22) | 0.40 |
| Oral Diabetes Medications (%) | 13 (13) | 7 (16) | 0.71 |
| eMetabolic Syndrome Components | |||
| Obesity Component (%) | 51 (52) | 40 (89) | <0.001 |
| Hypertriglyceridemia (%) | 6 (6) | 23 (51) | <0.001 |
| Low HDL (%) | 28 (29) | 27 (60) | <0.001 |
| Elevated Blood Pressure (%) | 38 (39) | 37 (82) | <0.001 |
| Impaired Fasting Glucose (%) | 9 (9) | 30 (67) | <0.001 |
All variables are mean ± standard deviation for continuous variables, or count (percent) for categorical variables and are rounded to the nearest integer, unless otherwise indicated. ACE – angiotensin converting enzyme inhibitors; ARB – angiotensin receptor blockers; HDL-C – High density lipoprotein cholesterol; LDL-C – Low density lipoprotein cholesterol
Responders are defined as Roux-en-Y gastric bypass patients with Metabolic Syndrome (MetS) at baseline that are cured of MetS at follow-up
Unpaired t-tests with unequal variances and Wilcoxon Rank Sum for continuous data and Chi-square or Fisher exact testing for categorical data. P-values are testing the differences in baseline parameters between MetS responders and MetS non-responders.
SI conversion factors: To convert total cholesterol to mmol/L, multiply by 0.0259;, to convert LDL-C to mmol/L, multiply by 0.0259; to convert HDL-C to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, multiply by 0.0113; to convert glucose to mmol/L, multiply by 0.0555; to convert creatinine to μmol/L, multiply by 88.4.
Metabolic Syndrome Components at follow-up
Sensitivity Analysis
Our sensitivity analysis assuming that patients with missing data would not have MetS resolution at follow-up demonstrated a 49% reduction in MetS prevalence in the 215 patients in the bariatric cohort, from 194 patients (90%) to 88 patients (41%), and a 7% reduction, from 209 patients (90%) to 193 patients (83%), in the 233 non-operative patients. In another sensitivity analysis, after excluding patients with a baseline diagnosis of both diabetes and CV disease, there were no differences in baseline prevalence of MetS (p=1.00). Of the 101 non-operative patients, 79 (78%) had MetS at baseline, while 66 (65%) had it at follow-up (p=0.02). In the operative cohort, of the 106 patients without baseline diabetes or CV disease, 83 (78%) had MetS at baseline, compared to 19 (18%) at follow-up (p<0.001). Results were essentially similar after excluding patients with baseline diabetes.
Discussion
Our population-based study demonstrates that MetS is a largely reversible phenomenon in patients with class II-III obesity and that reversibility of MetS depends more on the % of excess weight lost than on other clinical or demographic characteristics.
Although the reversibility is possibly influenced by or associated with other factors, including age, baseline serum TG, and baseline diabetes status, these factors, although significant in our univariate analysis at follow-up, were not as strongly predictive for MetS resolution as % excess weight loss. This relationship persisted even in the BMI-independent definition of MetS suggesting its importance on the other MetS components. Patients in the structured, multidisciplinary, non-surgical program demonstrated minimal weight changes associated with improvements in systolic blood pressure and lipid parameters; however, much of these improvements may have been related to more aggressive pharmacotherapy, as a significantly greater number of non-operative patients at follow-up were on statins or antihypertensive agents.
Relatively few studies have used established MetS criteria in evaluating outcomes after bariatric surgery15,16. All have demonstrated marked improvements in the prevalence and in the number of components as outlined by the definition of MetS and confirm our results. Several studies have examined non-American populations16-19, in whom RYGB is often not used. Of studies performed in the United States with RYGB, Madan and colleagues20 demonstrated a decrease in MetS as defined by Adult Treatment Panel (ATP) III from 78% to 2%; however, this study was relatively small, did not have a non-operative group, and follow-up was limited to one year. Another study by Mattar et al21 found a decrease from 70 to 14% in a series of 70 patients.
Although their follow-up was somewhat greater in duration, they had similar limitations to the Madan study. Mornigo's group22 examined RYGB using criteria from the ATP-III and demonstrated a decrease in MetS prevalence from 55% to 36% at six weeks, and to 11% at 52 weeks, but their study was limited to 36 patients without a control group. Our study confirms and expands these findings with the use of state-of-the-art epidemiologic methods supporting these conclusions. To the best of our knowledge, our study is the first to assess predictors of MetS resolution in patients with class II-III obesity, showing that % excess weight loss is the main contributor of whether a patient will be cured of MetS at follow-up. The use of an obesity-independent definition of MetS confirmed the importance of excess weight loss on resolution of MetS. Our study provides robust data to practicing clinicians regarding potential counseling regarding weight reduction in MetS patients.
The implications of our results are clinically important because our findings provide further understanding of the possible reversibility of MetS with weight loss. Does this reduction in MetS prevalence after bariatric surgery, and specifically after RYGB, reduce CV disease? There have been no studies using risk-prediction functions in MetS patients after bariatric surgery; however, in the past year, there have been studies projecting significant reductions in CV risk and death, including our own study that used risk modeling derived from the National Health and Nutrition Examination Surveys and applied it to this specific cohort23. This study estimated that 4 overall deaths and 16 CV events would be prevented by bariatric surgery per 100 patients at 10-years. Only a few studies have examined specifically the decrease of actual CV events and overall mortality, and limitations due to lack of follow-up information or use of hospital controls likely underestimate the value bariatric surgery may offer 24-26. Most recently, two studies have confirmed our previously published estimated risk reduction after bariatric surgery. One study examined 9,949 patients having undergone RYGB matched to obese controls demonstrated a 40% survival benefit27, with cause-specific mortality due to coronary artery disease reduced by 56%. Furthermore, the Swedish Obesity Study also demonstrated a risk reduction of 29% at 10 years 28. It is possible that mortality rates after bariatric surgery decrease in part because of the total or partial resolution of MetS.
Bariatric surgery decreases fat stores 29 and provides a better understanding of the reversibility of MetS with profound weight loss. Weight loss is known to reduce blood leptin and ghrelin levels, increase adiponectin levels, improve insulin sensitivity, and reduce fatty acid turnover, and is associated with a decrease in systemic inflammation and improved endothelial function30. Whether there are differential effects of a duodenal bypass procedure as compared to gastric banding on the effects of incretins, insulin sensitivity, and the presence of glucose intolerance requires further investigation.
The main strength of our study lies in the use of the Rochester Epidemiology Project to ascertain all patients and outcomes referred for RYGB in Olmsted County, Minnesota. By using a population-based cohort, we minimized selection and referral bias often observed at tertiary care institutions performing this surgery. Previous studies have demonstrated reasonable extrapolation of data to other parts of the country using this population 12. The ability to abstract a patient's entire medical record ensures that all information and outcomes relevant to this study were available. Although there have been a few studies examining the impact of bariatric surgery in patients with MetS, none have had a non-operative group whose characteristics were similar to the operative patients, and many have been performed in Europe where alternative techniques were used, including biliopancreatic bypass and gastroplasty. In addition, none of the recent studies have used the most updated MetS criteria published by the AHA/NHLBI. In studies examining MetS parameters after RYGB, the majority of studies examine outcomes up to one year, while our study provided follow-up of over three years. The use of an obesity-independent definition also provides credence to our results. We also performed a sensitivity analysis to determine the impact of missing patients on our intra-group results. Such an analysis ensures that in a worst case scenario, where these missing patients would not have metabolic improvements, the impact on our study results would be minimal, allowing adequate generalization of these results.
As with any retrospective study, recording and measurement bias are inherent issues in the study design. We had no control over when each patient had their laboratory or clinical assessment. Information regarding exercise, dietary habits, diagnosis or management of obstructive sleep apnea, leptin or insulin sensitivity, all of which are known confounders, was unavailable. These issues, however, would be present in both groups and at both points in time. The variability in the follow-up time created inherent challenges in analyzing our data. Normally, Cox-proportional hazard models should be utilized in inception cohorts where time to last follow up is variable, subjects are exposed to the predictor of interest since the beginning of the follow up and the risk is proportional to the time of follow-up and when the time when the outcome has occurred is well known. However, in our study group none of the latter three assumptions were met. Weight loss, particularly with bariatric surgery, normally occurs in a non-linear fashion within the first year 11, with subsequent minimal weight regain following this time. On the contrary, using logistic regression appeared to be more appropriate than Cox proportional hazard models after proving that length of follow-up did not influence the association between % of excess weight loss and the resolution of MetS. We acknowledged the limitations of both approaches, and attempted to prove, by eliminating patients with less than one year of follow-up and stratifying the remaining cohort by follow-up time, that % excess weight loss was still significant in each of these analyses. Furthermore, adjusting our models by follow-up time also confirmed these results. This approach suggests that one year after baseline time, % excess weight is still the main predictor of MetS resolution.
The decision to undergo bariatric surgery cannot be allocated randomly. Finally, we made no attempt to match our non-operative group to our bariatric surgery group. In a matched analysis, ideally we would have required a greater number of non-operative patients, of which we were inherently limited. In addition, our non-operative group should not be considered as a true “control” group, but rather as a population-based cohort of patients referred for surgical intervention that did not undergo operative treatment, the results which may not be generalizable to all patients attempting weight loss with non-surgical approaches. Furthermore, very few of the non-operative patients achieved a weight loss that exceeded the mean weight loss by the operative group, implying that no significant impact on our results would be observed, even if we eliminated specific groups of patients, such as the underinsured. Our findings can only be applied to patients with class II-III obesity, who have undergone evaluation for RYGB bariatric surgery, the patient population that is most eligible for bariatric procedures10, and hence cannot be extrapolated to non-obese patients with MetS or to patients treated with other types of bariatric procedures, including gastric banding.
Although MetS is thought to lead to the development of both type 2 diabetes mellitus and CV disease, we included patients with both of these diagnoses. One of the primary controversies in the MetS literature is the inclusion of diabetes into the ATP-definition criteria. Our study showed that the results were not altered after excluding patients with pre-existing diabetes or CV disease at baseline in our sensitivity analysis.
Conclusions
Bariatric surgery is an effective means of reversing or controlling MetS in patients eligible for surgically-induced weight loss. This current study provides strong evidence that % excess weight lost after RYGB is a primary contributor for reduced MetS prevalence independent of the obesity component. We suggest that RYGB should be considered as a treatment in patients with MetS who have failed conservative measures.
Acknowledgments
Financial Disclosure: Dr. Somers is supported by NIH grants HL-65176, HL-70302, HL-73211 and M01-RR00585. Dr. Lopez-Jimenez is a recipient of a Clinical Scientist Development Award from the American Heart Association. Dr. Romero-Corral is supported by a Postdoctoral Fellowship from the American Heart Association. This study was made possible by the Rochester Epidemiology Project (Grant #R01 - AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases).
Abbreviations
- ATP
Adult Treatment Panel
- AHA/NHLBI
American Heart Association / National Heart, Lung, Blood Institute
- BMI
Body Mass Index
- CV
Cardiovascular
- HDL-C
High density lipoprotein cholesterol
- MetS
Metabolic syndrome
- RYGB
Roux-en-Y gastric bypass
- TG
Triglycerides
Footnotes
Conflict of Interest: None
Presented in part at the 68th Annual Scientific Session of the American Diabetes Association, San Francisco, CA, June 6th - June 10th, 2008 (Abstract #1747-P).
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–2749. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 4.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Einhorn D, Reaven GM, Cobin RH, et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 6.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. Jama. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care. 2005;28:1195–1200. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 8.Florez H, Tremposa M, Orchard TJ, et al. Diabetes Prevention Program Research Group. Metabolic Syndrome increases diabetes risk in IGT subjects; Effects across Diabetes Prevention Program Interventions. American Diabetes Association 67th Scientific Session; Chicago, IL. 2007. [Google Scholar]
- 9.Burnet DL, Elliott LD, Quinn MT, Plaut AJ, Schwartz MA, Chin MH. Preventing diabetes in the clinical setting. J Gen Intern Med. 2006;21:84–93. doi: 10.1111/j.1525-1497.2005.0277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med; NIH conference; 1991. pp. 956–961. [PubMed] [Google Scholar]
- 11.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JD, Lupkiewicz SM, Palenik L, Lopez LM, Ariet M. Determination of ideal body weight for drug dosage calculations. Am J Hosp Pharm. 1983;40:1016–1019. [PubMed] [Google Scholar]
- 14.Okosun IS, Chandra KM, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960-2000. Prev Med. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne GH, Svahn J, Capella RF, et al. Predictors of prolonged hospital stay following open and laparoscopic gastric bypass for morbid obesity: body mass index, length of surgery, sleep apnea, asthma, and the metabolic syndrome. Obes Surg. 2004;14:1042–1050. doi: 10.1381/0960892041975460. [DOI] [PubMed] [Google Scholar]
- 16.Scopinaro N, Papadia F, Marinari G, Camerini G, Adami G. Long-term control of type 2 diabetes mellitus and the other major components of the metabolic syndrome after biliopancreatic diversion in patients with BMI < 35 kg/m2. Obes Surg. 2007;17:185–192. doi: 10.1007/s11695-007-9045-y. [DOI] [PubMed] [Google Scholar]
- 17.Gazzaruso C, Giordanetti S, La Manna A, et al. Weight loss after Swedish Adjustable Gastric Banding: relationships to insulin resistance and metabolic syndrome. Obes Surg. 2002;12:841–845. doi: 10.1381/096089202320995673. [DOI] [PubMed] [Google Scholar]
- 18.Coppini LZ, Bertevello PL, Gama-Rodrigues J, Waitzberg DL. Changes in insulin sensitivity in morbidly obese patients with or without metabolic syndrome after gastric bypass. Obes Surg. 2006;16:1520–1525. doi: 10.1381/096089206778870030. [DOI] [PubMed] [Google Scholar]
- 19.Alexandrides TK, Skroubis G, Kalfarentzos F. Resolution of diabetes mellitus and metabolic syndrome following Roux-en-Y gastric bypass and a variant of biliopancreatic diversion in patients with morbid obesity. Obes Surg. 2007;17:176–184. doi: 10.1007/s11695-007-9044-z. [DOI] [PubMed] [Google Scholar]
- 20.Madan AK, Orth W, Ternovits CA, Tichansky DS. Metabolic syndrome: yet another comorbidity gastric bypass helps cure. Surg Obes Relat Dis. 2006;2:48–51. doi: 10.1016/j.soard.2005.09.014. discussion 51. [DOI] [PubMed] [Google Scholar]
- 21.Mattar SG, Velcu LM, Rabinovitz M, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–617. doi: 10.1097/01.sla.0000179652.07502.3f. discussion 618-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morinigo R, Casamitjana R, Delgado S, et al. Insulin resistance, inflammation, and the metabolic syndrome following Roux-en-Y gastric bypass surgery in severely obese subjects. Diabetes Care. 2007;30:1906–1908. doi: 10.2337/dc07-0189. [DOI] [PubMed] [Google Scholar]
- 23.Batsis JA, Romero-Corral A, Collazo-Clavell ML, et al. Effect of weight loss on predicted cardiovascular risk: change in cardiac risk after bariatric surgery. Obesity (Silver Spring) 2007;15:772–784. doi: 10.1038/oby.2007.589. [DOI] [PubMed] [Google Scholar]
- 24.Omalu BI, Ives DG, Buhari AM, et al. Death rates and causes of death after bariatric surgery for Pennsylvania residents, 1995 to 2004. Arch Surg. 2007;142:923–928. doi: 10.1001/archsurg.142.10.923. discussion 929. [DOI] [PubMed] [Google Scholar]
- 25.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004;199:543–551. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004;240:416–423. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 28.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 29.Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16:1602–1608. doi: 10.1381/096089206779319347. [DOI] [PubMed] [Google Scholar]
- 30.Eckel RH, Barouch WW, Ershow AG. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on the pathophysiology of obesity-associated cardiovascular disease. Circulation. 2002;105:2923–2928. doi: 10.1161/01.cir.0000017823.53114.4c. [DOI] [PubMed] [Google Scholar]