Abstract
Objective
To identify factors associated with peripartum hysterectomy performed within 30 days postpartum.
Methods
This was a population-based case-control study using Washington State birth certificate registry (1987-2006) linked to the Comprehensive Hospital Abstract Reporting System (CHARS). Cases underwent hysterectomy within 30 days postpartum. Controls were frequency matched 4:1. Exposures included factors related to hemorrhage, delivery method, multiple gestations, and infection. Incidence rates of peripartum hysterectomy and maternal and neonatal morbidity/mortality were assessed. Adjusted odds ratios (aOR) by maternal age, parity, gestational age, year of birth, and mode of delivery and 95% confidence intervals (CI) were computed.
Results
There were 896 hysterectomies. Incidence rates ranged from 0.25 in 1987to 0.82 per 1,000 deliveries in 2006 (χ2 for trend, p<0.001). Factors related to hemorrhage were strongly related to peripartum hysterectomy. Placenta previa (192 cases vs. 23 controls; aOR=7.9, 95% CI: 4.1– 15.0), abruptio placenta (71 vs. 55; aOR=3.2, 95% CI: 1.8–5.8), and retained placenta (214 vs. 28; aOR=43.0, 95% CI: 19.0–97.7) increased the risk of hysterectomy, as did uterine atony, uterine rupture, and thrombocytopenia. Having multiple gestations did not. As compared with vaginal delivery, vaginal delivery after cesarean (27 cases vs. 105 controls; aOR=1.9, 95% CI: 1.2–3.0), primary cesarean (270 vs. 504; aOR=4.6, 95% CI: 3.5–6.0), and repeat cesarean (296 vs. 231; aOR=7.9, 95% CI: 5.8-10.7) increased the risk of peripartum hysterectomy. Among the 111 women who had hysterectomy on readmission (12.8% of cases), hemorrhage- and infection-related factors were still strongly associated with peripartum hysterectomy.
Conclusion
Incidence rates of peripartum hysterectomy are increasing over time. The most important risk factor for peripartum hysterectomy is hemorrhage, most notably caused by uterine rupture, retained placenta, and atony of uterus.
INTRODUCTION
Peripartum hysterectomy, a surgical procedure performed at the time of delivery or in the immediate postpartum period, although a rare event, is associated with increased morbidity and mortality. Moreover, it is considered one of the most devastating complications in obstetrics resulting in high costs to the health care system1,2 and adverse outcomes for women desiring to maintain their fertility. The main complications related to emergency peripartum hysterectomy include transfusions1,3,4, need for re-exploration because of persistent bleeding and febrile morbidity5-7, major surgical complications or maternal death8,9.
Many studies have estimated an incidence rate in the US between 0.8 and 1.5 per 1,000 deliveries5,10,11 although, the incidence has been reported to be as high as 2.28 per 1,000 deliveries12. This variation is due in part to the different definitions regarding the time period for peripartum hysterectomy used in different studies, either within 24 hours of a delivery12 or during the same hospitalization period5,10.
Previous reports have found that peripartum hysterectomy is associated with cesarean delivery15. A prior cesarean delivery is associated with an increased rate of abnormal placentation, including placenta previa, and placenta accreta in subsequent pregnancies15. In addition, it is hypothesized that uterine scarring, especially with increasing number of previous cesarean deliveries, also increases the risk of peripartum hysterectomy, even in the absence of placenta previa13,14.
Although some risk factors for peripartum hysterectomy have been established, including mode of delivery10,15 or multiple births10,15, it is important to note that many reports were limited by lack of adequate control for potential confounders15,16. Moreover, most of the studies were not able to measure the magnitude of the associations due to the small sample sizes6,14. In addition, these studies were conducted in single tertiary care institutions, diminishing their generalizability4,5,17,18, and most of these studies did not have a comparison group.
The purpose of this project was to identify obstetric related factors associated with peripartum hysterectomy in the State of Washington and to provide estimates of the magnitude of the risk for each of those factors. We focused on hemorrhage, mode of delivery, multiple gestations and infection related factors, while adjusting for confounders such as maternal age, race, number of previous births, gestational age, and delivery method, when appropriate. We also evaluated potential maternal factors (gestational diabetes and preeclampsia) and infant factors (birth weight). Furthermore, we explored whether risk factors for hysterectomy performed during the delivery admission differed from risk factors for hysterectomy performed in a subsequent admission before 30 days postpartum. We hypothesized that hemorrhage related factors would be associated with hysterectomy in the delivery admission while infection related factors would be associated with the readmitted group.
METHODS
We performed a population-based case-control study using data from the Washington State birth certificates from 1987 to 2006. Birth certificates record demographic characteristics as well as certain medical and clinical information from mothers and newborns at all non-federal hospitals and birthing centers in Washington State. Birth certificate information was linked to the Washington State hospital inpatient discharge data from the Comprehensive Hospital Abstract Reporting System (CHARS) through unique identifiers. Since 1992, over 90% of hospitals have linked to birth certificates and CHARS information for birth hospitalization is available both for the baby and the mother since 1987. Cases were women who underwent a peripartum hysterectomy within 30 days after delivery. The identification of cases was based on the ICD-9 procedure codes (68.3-68.7, and 68.9) using the CHARS from 1987 to 2006. Controls were women randomly selected from the Washington State birth certificate records with linkage to CHARS that did not have a peripartum hysterectomy, frequency matched to cases by year of delivery in a 4:1 ratio. Singletons as well as multiple gestations were included for cases and controls. Sample size was determined by including the 896 women with the procedure codes for peripartum hysterectomy during this period, with an estimated power of over 90% to detect an odds ratio of 2.00 or more at the 5% level of statistical significance, assuming exposures among controls of 2.5% or greater.
Several maternal characteristics, such as age, race, educational level, number of previous births, smoking status and marital status were obtained from the Washington state birth certificate. Gestational age was also obtained from the birth certificate. The median income level of residence census track was obtained from the US Census data from 1987 to 2006. Classification of urban or rural residency was determined according to the 2000 Census data.
First, factors related to maternal hemorrhage were considered in the analysis. Abnormal placentation included placenta previa, abruptio placenta, and retained placenta. They were identified from check boxes on the birth certificates and the following ICD-9 codes in the CHARS: for placenta previa 641.0, 641.1, 762.0; for abruptio placenta, 641.2. 762.1; and for retained placenta 666.0 and 666.2 (includes placenta accreta, percreta and increta). Atony of uterus was identified by the ICD-9 code 666.1, uterine rupture by the birth certificates and ICD-9 codes 665.0 and 665.1, and thrombocytopenia by the ICD-9 codes 287.3, 387.4, and 287.5. Other hemorrhagic factors included vasa previa (663.6) and coagulation defects (666.3 and 286.6). Delivery method (vaginal birth, primary cesarean, repeat cesarean, or vaginal birth after cesarean section, and spontaneous, forceps, vacuum) was then identified from the birth certificates. Multiple gestations (yes/no) were also identified by check boxes on the birth certificates. Other potential risk factors were evaluated including birth weight (from birth certificates), gestational diabetes (648.8 and birth certificates), preeclampsia (642.3, 642.4, 642.5, 642.7, and birth certificates), chorioamnionitis (658.4 and birth certificates), and other infection related factors, which included inflammatory diseases of the uterus, including endometritis (ICD-9 codes 615.x), major puerperal infection (670.x), infection of genitourinary tract in pregnancy (646.6), puerperal fever (672.x), and sepsis (038.x, 995.91, 995.92, 790.7, and 785.52).
Adjusted odds ratios (aOR) were calculated to measure the association between peripartum hysterectomy and the different risk factors using stratified analysis and the Mantel-Haenszel estimates. Mantel-Haenszel is a computational feasible method used for computing adjusted estimates of association in case-control studies without making model assumptions19. Variables considered as potential confounders and/or effect modifiers included maternal age (<25, 25-29, 30-34, 35-39, 40+ years), race (white, black, Asian, Hispanic, and others), number of previous births (0, 1, 2, 3+), educational level (<12, 12, 12+ years), income (<20K, 20K-29999, 30K-39999, 40K-49999, 50K-59999, 60K+), gestational age in weeks (<29, 29-32, 33-36, 37-41, 41+), and smoking during pregnancy (yes/no). Only those factors that altered the risk estimates appreciably (> 10%) were retained in the analysis. Hemorrhage related factors, multiple gestations, maternal infection, birth weight, gestational diabetes, and preeclampsia were also adjusted for delivery method.
Using the same methodology, we performed sub-analyses calculating the association between peripartum hysterectomies performed at birth admission and peripartum hysterectomies performed on readmission evaluating the same risk factors.
Incidence rates for peripartum hysterectomy were calculated based on the total number of hysterectomies and the number of births per year from 1987 to 2006 using Washington State birth certificate data. A χ2 test for trend in 2xK tables was used20, 21. Confidence intervals for the incidence rates were based on the Poisson distribution for counts of 100 or less19. Maternal and infant deaths rates were calculated using the Washington State death certificate data and birth certificates. Lastly, maternal and infant morbidity were assessed as determined by maternal transfusion, admission to the intensive care unit (ICU), Apgar scores and admission to the neonatal intensive care unit (NICU), all factors identified from the birth certificates, available from 2003-2006 only. Statistical tests and confidence intervals for proportions were based on the binomial distribution.
Statistical analysis was performed using STATA 10 for Macintosh and Windows (STATA Corp LP, College Station, TX, USA). The protocol for this study was approved by the Institutional Review Boards for Protection of Human Subjects at the Washington State Department of Health and the University of Washington prior to conducting of the study.
RESULTS
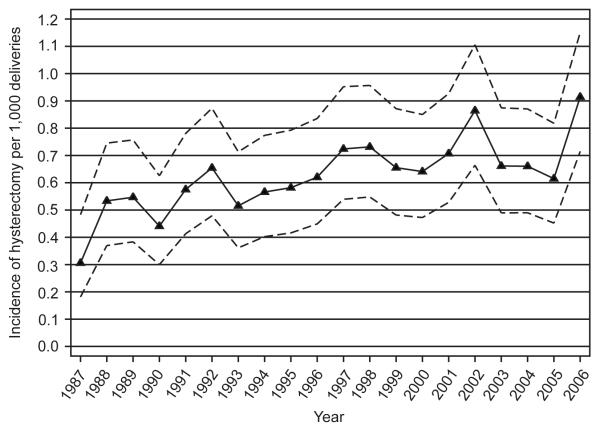
From 1987 to 2006, 896 women had a peripartum hysterectomy within the first 30 days after delivery in Washington State. The incidence rate averaged 0.56 (95% CI: 0.5 – 0.62) per 1,000 deliveries; however, rates varied from 0.25 (95% CI: 0.14 – 0.40) in 1987 to 0.82 (95% CI: 0.64 – 1.03) in 2006 (χ2 for trend, p<0.001) (Figure 1). Twenty-nine women who underwent a peripartum hysterectomy had cervical cancer, cervical carcinoma in situ, or ovarian cancer and were removed in the subsequent analyses. No controls were diagnosed with these illnesses. We excluded these women as we were interested in factors related to complications of pregnancy. Among the remaining 867 cases, 756 (87.2%) had a hysterectomy during the same hospitalization as the delivery; while 111 (12.8%) had the procedure on readmission to a hospital to perform the procedure, 85 (76.6%) of whom were emergent or urgent re-admissions. The median number of days between delivery and hospitalization for hysterectomy was 12 days (inter-quartile range: 9-21 days).
Figure 1.
Trends of peripartum hysterectomy in Washington State from 1987 to 2006. The dashed lines represent the 95% confidence intervals.
Cases and controls were similar in median family income, type of residency (urban/rural), and smoking status (Table 1). Cases and controls were generally similar in race distributions, although a higher proportion of cases were Asians (14.7%) as compared to controls (7.1%). Cases tended to be older, have a greater number of prior pregnancies, and to deliver at earlier gestational ages compared to controls.
Table 1.
Demographic characteristics of women with and without peripartum hysterectomy, Washington State, 1987-2006
| Maternal characteristics | Cases (N = 867) | Controls (N = 3584) |
|---|---|---|
| n (%) | n (%) | |
| Age§ | ||
| < 25 years | 93 (10.7) | 1237 (34.5) |
| 25 – 29 years | 184 (21.3) | 1000 (27.9) |
| 30 – 34 years | 261 (30.1) | 864 (24.1) |
| 35 – 39 years | 237 (27.4) | 399 (11.1) |
| = 40 years | 91 (10.5) | 82 (2.3) |
| Race§ | ||
| White | 578 (68.6) | 2636 (75.3) |
| African American | 45 (5.3) | 144 (4.1) |
| Asian | 124 (14.7) | 249 (7.1) |
| Hispanic | 74 (8.8) | 386 (11.0) |
| Others | 22 (2.6) | 87 (2.5) |
| Educational level§ | ||
| < 12 years | 90 (13.8) | 516 (18.9) |
| 12 years | 182 (28.0) | 830 (30.3) |
| > 12 years | 379 (58.2) | 1388 (50.8) |
| Number of previous births§ | ||
| 0 | 149 (17.8) | 1477 (42.0) |
| 1 | 262 (31.3) | 1137 (32.4) |
| 2 | 207 (24.8) | 515 (14.7) |
| 3+ | 218 (26.1) | 384 (10.9) |
| Family income (dollars) | ||
| < 20000 | 33 (4.1) | 127 (3.8) |
| 20000 – 29999 | 139 (17.3) | 615 (18.2) |
| 30000 – 39999 | 232 (28.8) | 942 (27.9) |
| 40000 – 49999 | 164 (20.4) | 792 (23.5) |
| 50000 – 59999 | 122 (15.1) | 482 (14.3) |
| = 60000 | 116 (14.4) | 417 (12.3) |
| Gestational age§ | ||
| < 29 weeks | 22 (2.7) | 19 (0.6) |
| 29 – 32 weeks | 60 (7.5) | 33 (1.0) |
| 33 – 36 weeks | 156 (19.5) | 219 (6.6) |
| 37 – 41 weeks | 549 (68.5) | 2963 (89.5) |
| 42+ weeks | 15 (1.9) | 77 (2.3) |
| Unmarried§ | 191 (22.2) | 989 (27.7) |
| Ruralresidence | 203 (25.8) | 807 (24.5) |
| Smoking during pregnancy | 126 (15.4) | 460 (13.3) |
Numbers might no add to totals due to missing values
P < 0.05
Hemorrhage-related factors were strongly related to peripartum hysterectomy. As compared to women with normal placentation, greater risk for peripartum hysterectomy was observed among women who had placenta previa (aOR=7.9, 95% CI: 4.1–15.0) or abruptio placenta (aOR=3.2, 95% CI: 1.8–5.8). Retained placenta (including placenta accreta, percreta and increta) had the greatest risk associated with peripartum hysterectomy with a 43-fold increased risk (aOR=43.0, 95% CI: 19.0–97.7). Atony of the uterus, uterine rupture as well as other factors related to hemorrhage (vasa previa and coagulation defects) were also strongly associated with peripartum hysterectomy.
The risk of peripartum hysterectomy varied with delivery method, with almost twice the risk for vaginal delivery after a prior cesarean as compared to vaginal without prior cesarean delivery (aOR=1.9, 95% CI: 1.2–3.0) (Table 2). For cesarean deliveries, the risk increased as the number of cesarean births increased, with the risk of primary cesarean delivery being more than 4 times the risk of vaginal delivery (aOR=4.6, 95% CI: 3.5–6.0), and approximately 8 times for repeat cesarean (aOR=7.9, 95% CI: 5.8–10.7). Women who had instrumented vaginal deliveries had an almost 2-fold increased risk of peripartum hysterectomy as compared with women who had vaginal births, but only vacuum deliveries were statistically significant (aOR=1.7, 95% CI 1.1-2.6).
Table 2.
Risk factors associated with peripartum hysterectomy, Washington State, 1987-2006
| Risk factor | Cases (N = 867) |
Controls (N = 3584) |
Adjusted OR (95% CI)§ |
|
|---|---|---|---|---|
| n (%) | n (%) | |||
| Hemorrhage related factors¥ | ||||
| Placenta abnormalities | ||||
| Placenta previa | 192 (22.2) | 23 (0.6) | 7.9 | (4.1 – 15.0) |
| Abruptio placenta | 71 (8.2) | 55 (1.5) | 3.2 | (1.8 – 5.8) |
| Retained placenta | 214 (24.7) | 28 (0.8) | 43.0 | (19.0 – 97.7) |
| Atony of uterus | 253 (29.2) | 111 (3.1) | 21.4 | (14.1 – 32.5) |
| Uterine rupture | 77 (8.9) | 1 (0.0) | 165.4 | (12.4 – 2208) |
| Thrombocytopenia | 13 (1.5) | 18 (0.5) | 3.7 | (1.3 – 10.5) |
| Other hemorrhage related factors‡ |
186 (21.5) | 8 (0.2) | 266.9 | (56.7 – 1256) |
| Delivery method | ||||
| Vaginal | 270 (31.3) | 2739 (76.5) | 1 | |
| Vaginal birth after cesarean | 27 (3.1) | 105 (2.9) | 1.9 | (1.2 – 3.0) |
| Primary cesarean | 270 (31.3) | 504 (14.1) | 4.6 | (3.5 – 6.0) |
| Repeat cesarean | 296 (34.3) | 231 (6.5) | 7.9 | (5.8 – 10.7) |
| Instrumentation (only vaginal) | ||||
| Spontaneous | 204 (77.6) | 2249 (83.5) | 1 | |
| Vacuum | 39 (14.8) | 311 (11.6) | 1.7 | (1.1 – 2.6) |
| Forceps | 20 (7.6) | 132 (4.9) | 1.7 | (0.8 – 3.6) |
| Multiple gestations¥ | 63 (7.3) | 99 (2.8) | 0.8 | (0.5 – 1.4) |
| Infection related factors¥ | ||||
| Chorioamnionitis | 39 (4.5) | 66 (1.8) | 3.6 | (1.7 – 7.4) |
| Other infection factors† |
109 (12.2) | 106 (3.0) | 4.0 | (2.7 – 6.1) |
| Other¥ | ||||
| Birth weight | ||||
| 2500 – 3999 grams | 553 (65.7) | 2868 (80.2) | 1 | |
| < 2500 grams | 180 (21.1) | 208 (5.8) | 1.1 | (0.7 – 1.9) |
| ≥ 4000 gram s | 122 (14.3) | 499 (14.0) | 1.5 | (1.1 – 1.9) |
| Gestational diabetes | 70 (8.1) | 158 (4.4) | 1.3 | (0.9 – 2.0) |
| Preeclampsia | 87 (10.0) | 269 (7.5) | 1.4 | (1.0 – 2.0) |
Adjusted for maternal age, number of previous births, gestational age, and birth year.
Adjusted for the previous variables and delivery method.
Includes vasa previa and coagulation defects.
Includes endometritis, major puerperal infection, infection of genitourinary tract in pregnancy, puerperal fever, and sepsis.
After adjusting for delivery method, there was no evidence that women who had multiple gestations were at increased risk of peripartum hysterectomy compared with women with a singleton delivery (aOR=0.8, 95% CI: 0.5–1.4). Maternal preeclampsia slightly increased the risk of peripartum hysterectomy (aOR=1.4, 95% CI: 1.0–1.4), as did macrosomia (birth weight ≥ 4000 grams) and infection related factors, including chorioamnionitis.
When the cases were stratified among those who had a hysterectomy during the same hospitalization as the delivery and those who had peripartum hysterectomy on readmission within 30 days of delivery, hemorrhage following delivery was still the strongest risk factor for hysterectomy among the readmitted women (Table 3). Retained placenta had almost a 19-fold increased risk for those readmitted (aOR=18.9; 95% CI: 4.2–85.2). Abruptio placenta was no longer significantly associated with hysterectomy when it was performed on readmission. Peripartum hysterectomy was associated with uterine atony whether the procedure was performed at delivery admission or on readmission, although the risk was higher on the birth admission. Thrombocytopenia was related to peripartum hysterectomy at the birth delivery admission (aOR=6.0, 95% CI 1.8–20.0) but other hemorrhagic related factors (vasa previa and other coagulation defects) were more strongly associated with peripartum hysterectomy for the readmitted group. Women with repeat cesarean delivery had an increased risk of hysterectomy on readmission as compared to women who had a vaginal delivery (aOR=1.9; 95% CI: 1.0–3.6); women with primary cesarean and vaginal birth after cesarean delivery did not have an increased risk at readmission. Postpartum infection related factors had an over 2-fold increased risk of peripartum hysterectomy at birth admission but over a 20-fold if performed on readmission within 30 days of delivery. In summary, factors associated with hysterectomy on birth admission only were placenta previa, abruption, uterine rupture, thrombocytopenia, primary cesarean delivery and chorioamnionitis, although differences should be interpreted with caution due to small numbers of women readmitted. There were no factors related to only the readmission hysterectomy, but the magnitude of the associations for infections other than chorioamnionitis differed dramatically (aOR=2.5, 95% CI: 1.5-4.1 at birth hospitalization and aOR=20.8, 95% CI: 8.6-50.2 at readmission). Factors related to either birth or readmission hysterectomy were hemorrhagic related factors, repeat cesarean delivery, and postpartum-infection related factors.
Table 3.
Risk factors associated with peripartum hysterectomy performed on birth hospitalization versus readmission, Washington State, 1987-2006
| Risk factors | Controls (N = 3584) |
Birth Hospitalization Cases (N = 756) |
Readmission Cases (N = 111) |
||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) § | n (%) | OR (95% CI) § | |||
| Hemorrhage related factors¥ | |||||||
| Placenta abnormalities | |||||||
| Placenta previa | 23 (0.6) | 191 (25.3) | 9.2 | (4.7 – 17.9) | 1 (0.9) | -- | -- |
| Abruptio placenta | 55 (1.5) | 67 (8.9) | 3.5 | (1.9 – 6.4) | 4 (3.6) | 1.1 | (0.2 – 5.8) |
| Retained placenta | 28 (0.8) | 206 (27.3) | 49.0 | (20.7 – 116.2) | 8 (7.2) | 18.9 | (4.2 – 85.2) |
| Atony of uterus | 111 (3.1) | 242 (32.0) | 33.1 | (20.0 – 54.9) | 11 (9.9) | 2.8 | (1.3 – 5.9) |
| Uterine rupture | 1 (0.0) | 75 (9.9) | 193.8 | (11.9 – 3149) | 2 (1.8) | 40.5 | (0.6 – 2867) |
| Thrombocytopenia | 18 (0.5) | 13 (1.7) | 6.0 | (1.8 – 20.0) | 0 (0.0) | -- | -- |
| Other hemorrhage related factors‡ | 8 (0.2) | 114 (15.1) | 182.2 | (35.5 – 934.4) | 72 (64.9) | 1041 | (34.8 – 3115) |
| Delivery method | |||||||
| Vaginal | 2739 (76.5) | 199 (26.5) | 1 | 71 (64.0) | 1 | ||
| Vaginal birth after cesarean | 105 (2.9) | 22 (2.9) | 1.9 | (1.2 – 3.1) | 5 (4.5) | 1.7 | (0.6 – 4.9) |
| Primary cesarean | 504 (14.1) | 253 (33.6) | 5.8 | (4.4 – 7.7) | 17 (15.3) | 1.2 | (0.6 – 2.4) |
| Repeat cesarean | 231 (6.5) | 278 (37.0) | 10.4 | (7.4 – 14.7) | 18 (16.2) | 1.9 | (1.0 – 3.6) |
| Instrumentation (only vaginal) | |||||||
| Spontaneous | 2249 (83.5) | 149 (77.6) | 1 | 55 (77.5) | 1 | ||
| Vacuum | 311 (11.6) | 30 (15.6) | 1.7 | (1.0 – 2.7) | 9 (12.7) | 1.9 | (0.8 – 4.7) |
| Forceps | 132 (4.9) | 13 (6.8) | 1.4 | (0.6 – 3.3) | 7 (9.9) | 2.8 | (0.8 – 10.0) |
| Multiple gestations¥ | 99 (2.8) | 59 (7.8) | 0.8 | (0.4 – 1.4) | 4 (3.6) | 1.2 | (0.4 – 4.5) |
| Infection related factors¥ | |||||||
| Chorioamnionitis | 66 (1.8) | 37 (4.9) | 4.7 | (2.2 – 10.0) | 2 (1.8) | 0.7 | (0.1 – 8.5) |
| Other infection related factors† | 106 (3.0) | 76 (9.7) | 2.5 | (1.5 – 4.1) | 33 (29.7) | 20.8 | (8.6 – 50.2) |
| Other¥ | |||||||
| Birth weight | |||||||
| 2500 – 3999 grams | 2868 (80.2) | 459 (61.7) | 1 | 94 (84.7) | 1 | ||
| < 2500 grams | 208 (5.8) | 172 (23.1) | 1.1 | (0.7 – 2.0) | 8 (7.2) | 0.9 | (0.3 – 3.0) |
| ≥ 4000 grams | 499 (14.0) | 113 (15.2) | 1.7 | (1.3 – 2.3) | 9 (8.1) | 0.5 | (0.3- 1.2) |
| Gestational diabetes | 158 (4.4) | 66 (8.7) | 1.5 | (1.0 – 2.4) | 4 (3.6) | 0.4 | (0.1 – 1.7) |
| Preeclampsia | 269 (7.5) | 81 (10.7) | 1.4 | (0.9 – 2.0) | 6 (5.4) | 1.6 | (0.6 – 4.1) |
Adjusted for maternal age, number of previous births, gestational age, and birth year.
Adjusted for the previous variables and delivery method.
Includes vasa previa and coagulation defects.
Includes endometritis, major puerperal infection, infection of genitourinary tract in pregnancy, puerperal fever, and sepsis.
Overall, there were 15 (1.7%, 95% CI: 1.0%–2.8%) maternal deaths among the cases and 16 (0.4%, 95% CI: 0.3%–0.7%) maternal deaths among controls from 1987 to 2006. Eight of these deaths among cases occurred within 30 days of delivery compared to none among controls (p<0.001). Six of the 8 women had a hysterectomy during the delivery admission while 2 had hysterectomy on readmission. Among those who had hysterectomy, 33 (3.8%, 95% CI: 2.6%– 5.3%) had infants who died, whereas 32 (0.9%, 95% CI: 0.6%–1.3%) infants died among controls (p<0.001).
Measures of morbidity such as maternal transfusion, admission to ICU or NICU were available in the State of Washington during the period 2003-3006. When the population study was restricted to this period, there were 213 cases (24.6%) and 872 controls (24.3%). Of the 213 cases, 186 (87.3%) had the hysterectomy during the same hospitalization as the delivery and 27 (12.7%) had the procedure on readmission to a hospital. Overall, women with hysterectomy were more likely to be admitted to the ICU (16.9% of cases vs. no controls, p<0.001) and to be transfused (28.0% of cases vs. 0.7% of controls, p<0.001). Likewise, hysterectomy was associated with Apgar score less than 7 at 5 minutes (14.2% of newborns from cases vs. 3.7% of newborns from controls, p<0.001) and admission to NICU (22.3% of newborns from cases vs. 6.5% of newborns from controls, p<0.001).
DISCUSSION
Our results confirm that the most important risk factor for peripartum hysterectomy is hemorrhage most notably due to uterine rupture, retained placenta, and atony of uterus. Despite medical advances, hemorrhage continues to be an important contributor to maternal morbidity and mortality. In our study, we were most interested to explore those factors that might be known antepartum and could be potentially modified with preventative measures. We found that uterine rupture, placenta previa, abruptio placenta, retained placenta, and atony of the uterus were associated with peripartum hysterectomy, findings consistent with previous reports6,8,16,17. Some of these factors are potentially recognizable antepartum with the exception of some forms of retained placenta, uterine rupture and uterine atony. Abnormal placentation has been shown to be associated with a previous uterine scar and subsequent bleeding complications, hysterectomy, and longer maternal hospital stays8,16. These life-threatening abnormal placental complications require aggressive blood transfusion therapy and the decision of invasive treatment must be considered within no more than 30 minutes if previous measures have failed22. With today’s imaging capabilities, knowledge of abnormal placentation can lead clinicians to prepare for delivery with interventional radiologists, uterotonics, uterine balloon compression devices, transfusion services, and the optimal surgical team available23, 24.
Primary and repeat cesarean sections, as well as vaginal birth after cesarean were associated with peripartum hysterectomy, as has been previously described10, 15, 25. Repeat cesarean deliveries were associated with the highest risk for peripartum hysterectomy. One hypothesis is that uterine scarring, especially with increasing number of previous cesarean deliveries, increases the risk of peripartum hysterectomy, even in the absence of placenta previa13,14. Interestingly, assisted vaginal delivery was also related to risk of peripartum hysterectomy. A previous report found the same association15 which could indicate the possibility of damage to cervical or vaginal tissues, resulting in hemorrhage. Previous studies have related the use of vacuum and forceps to perineal tears26, and birth canal lacerations27, as well as unnoticed cervical and uterine damage during the delivery or the formation of unrecognized hematomas28.
We did not find that multiple gestation deliveries were significantly associated with peripartum hysterectomy, as has been described previously10,15. This null result could be explained by several reasons. The null result could potentially be due to the lack of power in our study. However, there are also differences in our analyses as compared with other studies. In Knight et al.15, it is unclear which factors were adjusted for as they used a step-wise model, an exploratory analysis with the potential of false-positive results due to the number of statistical tests used in the modeling. Whiteman et al.10 adjusted for factors that do not seem to be related to either multiple births or hysterectomy, such as hospital region, insurance type or hospital type, and that could have introduced bias. Francois et al. previously reported that multiple gestations had significantly greater risk of hysterectomy, hypothesizing that preterm labor requiring tocolysis and uterine distension from more than one fetus contribute to uterine atony and hemorrhage12. However, that study failed to adjust for potential covariates such as delivery method and gestational age, which could have confounded the results.
We found that women with gestational diabetes did not have an increased risk for peripartum hysterectomy as compared with women without gestational diabetes. However, birth infant weight ≥4000 grams was found to be related to our outcome due perhaps to its association with risk of hemorrhage secondary to uterine atony22.
This study also reports risk factors associated with peripartum hysterectomy for women who were readmitted within the first 30 days after delivery. Among this group of women who were readmitted, hemorrhage- and infection-related factors were strongly associated with peripartum hysterectomy. Retained placenta is associated with hemorrhage and infection as well as possible complications related to instrumentation with its removal29. Atony of the uterus was also an important risk factor for peripartum hysterectomy on readmission.
We found an increasing trend in the incidence rates of peripartum hysterectomy from 1987 to 2006 (Figure 1). Three recent studies have reported trends of peripartum hysterectomy. Whiteman et al.10 found an increase of incidence rates from 1998 to 2003 nationwide, although the increase was not statistically significant in this 5-year period. Those rates were only slightly higher than what we found in the State of Washington. Another study from Denmark observed a statistically increased risk of peripartum hysterectomy in later years when comparing rates in 1978-1984 to those in 1995-200413. A third study in the Calgary Health region in Canada found no difference of incidence rates from 1999-2006. However, the number of hysterectomies was so small that it was difficult to interpret those findings6. However, all these rates are crude estimates and comparisons are only valid as long as the populations are similar in factors such as age and time periods being evaluated are similar.
The main strengths of this study are a relatively large and population-based sample in the main analysis, facilitating estimates of odds ratios adjusted for possible confounding factors. Only two previous studies have had large enough sample sizes to be able to adjust for confounding factors10,15. However, the only obstetrics factors for which adjusted estimates of odds ratios were provided in one of the studies were delivery type, and multiple gestations10. In the UK study, with about half the sample size of our study, only crude estimates for many obstetric factors were provided15. In addition, we evaluated risk factors associated with peripartum hysterectomy performed in the birth hospitalization and on a subsequent readmission. Nevertheless, our study also has several limitations. The most important one is that our results depend on the accuracy of the diagnoses listed on birth certificates and hospital discharge summaries. We combined check box diagnoses in birth certificates and ICD-9 diagnosis and procedures codes to reduce misclassification of potential risk factors and confounders, as suggested by previous studies30,31. The coding for retained placenta limited our ability to distinguish between placenta accreta, percreta and increta from other forms of retained placenta. Without doing a chart review, we were unable to determine the indication for the peripartum hysterectomy nor the time of hemorrhage (antepartum, intrapartum, postpartum). Another limitation is the use of readmission criterion as a surrogate of the interval between delivery and hysterectomy as well as the small number of readmitted cases. Although we could not obtain the exact time interval between the two events, our results for the women who had a hysterectomy during their delivery hospitalization are in agreement with previous reports evaluating emergency peripartum hysterectomy5,32,33, indicating that probably most of these women had emergency hysterectomies, while the magnitude of the associations for the readmitted women seemed to be different. Lastly, the number of cases and/or controls for some of the factors that were examined were relatively small and, as a consequence, the confidence intervals for the aOR are wide. This is especially the case for some of the factors in the readmitted group. However, the findings on women with readmission peripartum hysterectomy are novel and merit presentation.
In conclusion, peripartum hysterectomy rates are increasing over time, possibly related to increasing cesarean deliveries, and other factors, such as abnormal placentation, that are known to be associated with increasing maternal age and delayed childbearing in today’s society. Our results suggest that all factors that have the potential to lead to hemorrhage and infection increase the risk for peripartum hysterectomy and particular attention should be paid to these factors known antepartum by the clinician. Although it is not possible to prevent all cases of hysterectomy, women at particularly high risk should be counseled and preventative steps comprising early assessment and recognition of a woman’s potential risks34 should be employed. Techniques like arterial embolization might be useful in treating obstetric hemorrhage especially in those women with greater risk23. Placement of catheters prior to delivery among high risk women could also decrease the risk of peripartum hysterectomy. In addition, delivery in, or in close proximity to, the angiographic suite with interventional radiologists on site should be considered, particularly for women who wish to retain childbearing. Also, as previously described, use of intrauterine balloon compressive devices have been shown to successfully tamponade uterine bleeding to prevent hysterectomy24. Having all potentially life-saving devices ready and assembling the appropriate team prior to delivery in at risk situations could potentially decrease the maternal and neonatal morbidity and mortality associated with peripartum hysterectomy that we observed in the last two decades in Washington State.
ACKNOWLEDGMENTS
The authors thank the Washington State Department of Health for data access, Mr. William O’Brien for help with the data.
DISCLOSURE OF FUNDING Dr. Bodelon was funded by the Cancer Epidemiology Biostatistics Training Grant T32 CA009168.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
SOURCE OF DATA From the Washington State Department of Health.
PRECIS Women who experience significant antepartum hemorrhage or infection are at increased risk for peripartum hysterectomy.
REFERENCES
- 1.Briery CM, Rose CH, Hudson WT, et al. Planned vs emergent cesarean hysterectomy. Am J Obstet Gynecol. 2007;197:154.e1–154.e5. doi: 10.1016/j.ajog.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Knight M. Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG. 2007;114:1380–1387. doi: 10.1111/j.1471-0528.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 3.Engelsen IB, Albrechtsen S, Iversen OE. Peripartum hysterectomy-incidence and maternal morbidity. Acta Obstet Gynecol Scand. 2001;80:409–412. [PubMed] [Google Scholar]
- 4.Selo-Ojeme DO, Bhattacharjee P, Izuwa-Njoku NF, Kadir RA. Emergency peripartum hysterectomy in a tertiary London hospital. Arch Gynecol Obstet. 2005;271:154–159. doi: 10.1007/s00404-004-0715-x. [DOI] [PubMed] [Google Scholar]
- 5.Forna F, Miles AM, Jamieson DJ. Emergency peripartum hysterectomy: a comparison of cesarean and postpartum hysterectomy. Am J Obstet Gynecol. 2004;190:1440–1444. doi: 10.1016/j.ajog.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Glaze S, Ekwalanga P, Roberts G, et al. Peripartum hysterectomy: 1999 to 2006. Obstet Gynecol. 2008;111:732–738. doi: 10.1097/AOG.0b013e31816569f2. [DOI] [PubMed] [Google Scholar]
- 7.Kastner ES, Figueroa R, Garry D, Maulik D. Emergency peripartum hysterectomy: experience at a community teaching hospital. Obstet Gynecol. 2002;99:971–975. doi: 10.1016/s0029-7844(02)01999-3. [DOI] [PubMed] [Google Scholar]
- 8.Gould DA, Butler-Manuel SA, Turner MJ, Carter PG. Emergency obstetric hysterectomy - an increasing incidence. J Obstet Gynaecol. 1999;19:580–583. doi: 10.1080/01443619963761. [DOI] [PubMed] [Google Scholar]
- 9.Kwee A, Bots ML, Visser GH, Bruinse HW. Emergency peripartum hysterectomy: A prospective study in The Netherlands. Eur J Obstet Gynecol Reprod Biol. 2006;124:187–192. doi: 10.1016/j.ejogrb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Whiteman MK, Kuklina E, Hillis SD, et al. Incidence and determinants of peripartum hysterectomy. Obstet Gynecol. 2006;108:1486–1492. doi: 10.1097/01.AOG.0000245445.36116.c6. [DOI] [PubMed] [Google Scholar]
- 11.Zelop CM, Harlow BL, Frigoletto FDJ, Safon LE, Saltzman DH. Emergency peripartum hysterectomy. Am J Obstet Gynecol. 1993;168:1443–1448. doi: 10.1016/s0002-9378(11)90779-0. [DOI] [PubMed] [Google Scholar]
- 12.Francois K, Ortiz J, Harris C, Foley MR, Elliott JP. Is peripartum hysterectomy more common in multiple gestations? Obstet Gynecol. 2005;105:1369–1372. doi: 10.1097/01.AOG.0000161311.31894.31. [DOI] [PubMed] [Google Scholar]
- 13.Sakse A, Weber T, Nickelsen C, Secher NJ. Peripartum hysterectomy in Denmark 1995-2004. Acta Obstet Gynecol Scand. 2007;86:1472–1475. doi: 10.1080/00016340701692651. [DOI] [PubMed] [Google Scholar]
- 14.Yucel O, Ozdemir I, Yucel N, Somunkiran A. Emergency peripartum hysterectomy: a 9-year review. Arch Gynecol Obstet. 2006;274:84–87. doi: 10.1007/s00404-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 15.Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Cesarean delivery and peripartum hysterectomy. Obstet Gynecol. 2008;111:97–105. doi: 10.1097/01.AOG.0000296658.83240.6d. [DOI] [PubMed] [Google Scholar]
- 16.Usta IM, Hobeika EM, Musa AA, Gabriel GE, Nassar AH. Placenta previa-accreta: risk factors and complications. Am J Obstet Gynecol. 2005;193:1045–1049. doi: 10.1016/j.ajog.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Habek D, Becarevic R. Emergency peripartum hysterectomy in a tertiary obstetric center: 8-year evaluation. Fetal Diagn Ther. 2007;22:139–142. doi: 10.1159/000097114. [DOI] [PubMed] [Google Scholar]
- 18.Kayabasoglu F, Guzin K, Aydogdu S, Sezginsoy S, Turkgeldi L, Gunduz G. Emergency peripartum hysterectomy in a tertiary Istanbul hospital. Arch Gynecol Obstet. 2008 doi: 10.1007/s00404-007-0551-x. [DOI] [PubMed] [Google Scholar]
- 19.Koepsell TD, Weiss NS. Epidemiologic Methods. Studying the Occurrence of Illness. Oxford University Press; New York: 2003. [Google Scholar]
- 20.Daya S. Chi-square test for trend (2xc contingency table) Evidence-based Obstetrics and Gynaecology. 2001;3:116–117. [Google Scholar]
- 21.Van Belle G, Fisher LD, Heagerty PJ, Lumley T. Biostatistics. A Methodology for the Health Sciences. Wiley; New York: 2004. [Google Scholar]
- 22.Mercier FJ, Van de Velde M. Major obstetric hemorrhage. Anesthesiol Clin. 2008;26:53–66. vi. doi: 10.1016/j.anclin.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Ojala K, Perala J, Kariniemi J, Ranta P, Raudaskoski T, Tekay A. Arterial embolization and prophylactic catheterization for the treatment for severe obstetric hemorrhage. Acta Obstet Gynecol Scand. 2005;84:1075–1080. doi: 10.1111/j.0001-6349.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 24.Dabelea V, Schultze PM, McDuffie RSJ. Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol. 2007;24:359–364. doi: 10.1055/s-2007-984402. [DOI] [PubMed] [Google Scholar]
- 25.Kacmar J, Bhimani L, Boyd M, Shah-Hosseini R, Peipert J. Route of delivery as a risk factor for emergent peripartum hysterectomy: a case-control study. Obstet Gynecol. 2003;102:141–145. doi: 10.1016/s0029-7844(03)00404-6. [DOI] [PubMed] [Google Scholar]
- 26.Caughey AB, Sandberg PL, Zlatnik MG, Thiet MP, Parer JT, Laros RKJ. Forceps compared with vacuum: rates of neonatal and maternal morbidity. Obstet Gynecol. 2005;106:908–912. doi: 10.1097/01.AOG.0000182616.39503.b2. [DOI] [PubMed] [Google Scholar]
- 27.Shihadeh A, Al-Najdawi W. Forceps or vacuum extraction: a comparison of maternal and neonatal morbidity. East Mediterr Health J. 2001;7:106–114. [PubMed] [Google Scholar]
- 28.Johnson JH, Figueroa R, Garry D, Elimian A, Maulik D. Immediate maternal and neonatal effects of forceps and vacuum-assisted deliveries. Obstet Gynecol. 2004;103:513–518. doi: 10.1097/01.AOG.0000114985.22844.6d. [DOI] [PubMed] [Google Scholar]
- 29.Chhabra S, Dhorey M. Retained placenta continues to be fatal but frequency can be reduced. J Obstet Gynaecol. 2002;22:630–633. doi: 10.1080/0144361021000020402. [DOI] [PubMed] [Google Scholar]
- 30.Lydon-Rochelle MT, Holt VL, Cardenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193:125–134. doi: 10.1016/j.ajog.2005.02.096. [DOI] [PubMed] [Google Scholar]
- 31.Lydon-Rochelle MT, Holt VL, Nelson JC, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19:460–471. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 32.Akar M Erman, Yilmaz E Saygili, Yuksel B, Yilmaz Z. Emergency peripartum hysterectomy. Eur J Obstet Gynecol Reprod Biol. 2004;113:178–181. doi: 10.1016/j.ejogrb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Sheiner E, Levy A, Katz M, Mazor M. Identifying risk factors for peripartum cesarean hysterectomy. A population-based study. J Reprod Med. 2003;48:622–626. [PubMed] [Google Scholar]
- 34.El-Jallad MF, Zayed F, Al-Rimawi HS. Emergency peripartum hysterectomy in Northern Jordan: indications and obstetric outcome (an 8-year review) Arch Gynecol Obstet. 2004;270:271–273. doi: 10.1007/s00404-003-0563-0. [DOI] [PubMed] [Google Scholar]