Abstract
The protein kinase B (PKB)/mammalian target of rapamycin (mTOR) signaling pathway is thought to play a critical role in the regulation of protein synthesis and skeletal muscle mass. The purpose of the present study was to determine the effects of voluntary wheel running on the PKB/mTOR signaling pathway in muscles from aged mice (20–22 months). The total levels of mTOR were 65% higher in gastrocnemius muscles from aged mice subjected to wheel running compared to aged sedentary mice (p = 0.02). PKB phosphorlation on Ser473 was 45% higher in gastrocnemius muscles from aged mice subjected to wheel running compared to aged sedentary mice (p = 0.01). The total abundance of PKB was 50% higher in gastrocnemius muscles from wheel running mice compared to aged controls (p = 0.05). Three months of wheel running did not alter the total amount of p70 S6K in gastrocnemius muscle. Protein synthesis, as assessed by [14C]phenylalanine incorporation into protein was significantly higher in soleus muscles from aged mice subjected to wheel running compared to aged sedentary mice (p = 0.01). These findings indicate the aerobic exercise training may attenuate the age-related decline in protein synthesis, a process that appears to be due, in part, to increases in mTOR and PKB.
Keywords: Protein synthesis, Voluntary wheel running, mRNA translation, Contractile activity
1. Introduction
Aging is associated with a progressive decline in skeletal muscle mass in both humans and rodents (Lexall et al., 1988; Holloszy et al., 1991). The precise mechanism responsible for the age-associated deterioration of muscle mass is yet to be firmly established, however, several studies demonstrate that skeletal muscle protein synthesis decreases with advancing aging (Yarasheski et al., 1993; Rooyackers et al., 1996; Balagopal et al., 1997, 2001). Declines in the rates of myosin heavy chain and mitochondrial protein synthesis were associated with impaired muscle function, as evidenced by decreased muscular strength and oxidative capacity (Rooyackers et al., 1996; Balagopal et al., 1997). However, a recent study concluded that changes in skeletal muscle protein synthesis rates with advancing age did not explain the age-related loss of muscle mass (Volpi et al., 2001).
Although resistance training has emerged as an effective intervention for treating and preventing age-related muscle atrophy, the effect of aerobic exercise training on muscle mass in older individuals has not been studied. Studies have demonstrated that resistance training increases muscle strength (Fiatarone et al., 1990) as well as skeletal muscle protein synthesis rates in aged individuals (Yarasheski et al., 1999; Hasten et al., 2000; Balagopal et al., 2001). It appears that resistance training may prevent age-related muscle atrophy by increasing the rate of protein synthesis in skeletal muscle. Unlike resistance training, there is little information regarding the effects of aerobic exercise training on skeletal muscle protein synthesis rates. In rats, chronic voluntary wheel running not only increases skeletal muscle oxidative capacity (Rodnick et al., 1989; Lambert and Noakes, 1990), but also increases skeletal muscle protein synthesis rates (Munoz et al., 1994; Willis et al., 1998) and prevents age-related muscle atrophy (Brown et al., 1992). Taken together these findings suggest that in addition to improving VO2 max, aerobic exercise training may reduce the loss of muscle mass with advancing age by increasing protein synthesis rates. Therefore, aerobic exercise training may provide elderly individuals with a more feasible intervention for stimulating skeletal muscle protein synthesis and muscle hypertrophy than resistance training.
The cellular mechanisms responsible for the changes in skeletal muscle protein synthesis due to aging or resistance training remain obscure. Since insulin and amino acids enhance protein synthesis, perhaps the age-associated muscle atrophy is due, in part, to a decrease in the ability of insulin and amino acids to stimulate protein synthesis in skeletal muscle. The mammalian target of rapamycin (mTOR) is an insulin responsive and amino acid sensitive Ser/Thr kinase whose activity is suggested to be a marker for protein synthesis in skeletal muscle (Reynolds et al., 2002). Protein kinase B (PKB) is a signaling molecule in the phosphatidylinositol 3-kinase pathway that is thought to activate mTOR by promoting the phosphorylation of Ser2448 on mTOR’s carboxy terminus regulatory domain (Scott et al., 1998; Nave et al., 1999; Sekulic et al., 2000). The activation of mTOR leads to the phosphorylation of the mRNA translational regulators PHAS-1 and p70 S6 kinase (S6K), which enhances mRNA translation initiation and increases protein synthesis (Shah et al., 2000).
Since aging appears to decrease skeletal muscle protein synthesis, the present study investigated whether or not aerobic exercise training could enhance the PKB/mTOR signaling pathway as well as the rate of protein synthesis in muscles from aged mice. Specifically, we examined the effects of 3 months of voluntary wheel running on PKB expression and PKB phosphorylation on Ser473 in gastrocnemius (GAS) muscles from aged mice. PKB Phosphorylation on Ser473 is necessary for full kinase activity (Alessi et al., 1996). We also assessed the total abundance of mTOR and S6K in GAS muscles, as well as the rate of protein synthesis in soleus muscle from aged mice subjected to 3 months of wheel running.
2. Methods and materials
2.1. Animals and housing
Specific pathogen-free male B6C3F1 mice were obtained from the National Institute on Aging breeding colony maintained by Harlan Sprague-Dawley (Indianapolis, IN) at the age of 16–19 months. Upon arrival, mice were housed in a temperature-controlled specific pathogen-free animal room maintained on a 12:12-h light–dark cycle at the Unit for Laboratory Animal Medicine at the University of Michigan. The animals were ad libitum fed Lab Diet 5001 rodent chow (Richmond, IN) and water. Following a 1-month acclimatization period, mice were randomly assigned to either an aged control group (AG, n = 5) or aerobic exercise training group that consisted of voluntary wheel running (AG-WR, n = 5). The AG-WR group was given free access to a running wheel for 3 months. Wheel revolutions were recorded daily by a digital counter. At 8 h following the removal of running wheels, mice were anesthetized and GAS muscle were isolated and rapidly frozen with metal tongs cooled to the temperature of liquid N2. Soleus muscles were isolated intact for the assessment of protein synthesis. At the time of the sacrifice the AG and AG-WR mice were 20–22 months old. All animal care and surgery were in accordance with the National Institute of Health guide for Care and Use of Laboratory Animals (DHEW Publication No. 85–23). All experimental protocols were approved by the University Committee for the Use and Care of Animals.
2.2. Preparation of GAS muscle extracts
The frozen GAS muscles from AG and AG-WR mice were manually ground with a porcelain mortar and pestle chilled in liquid N2. Powdered tissue was homogenized on ice using a motor-driven tissue grinder (Teflon-glass) in 3 ml of Homogenization Buffer, which was composed of Buffer A (50 mM NaCl, 10 mM NaF, 0.25% Tween-20, 10% glycerol, 0.1 mM dithiothreitol, 500 nM microcystin-LR, 50 mM Tris/HCl, pH 7.4) supplemented with 0.1 mM phenylmethylsulfonyl fluoride, and 10 µg/ml each of aprotinin, leupeptin, and pepstatin-A. The homogenates were rotated at 4 °C for 1 h and then centrifuged at 8900g for 30 min at 4 °C. The protein concentrations of the supernatants were determined by the BCA method (Pierce, Inc). The remaining skeletal muscle extract was utilized for electrophoretic analysis and immuno-blotting experiments.
2.3. Affinity purification of mTOR
To purify mTOR, 100 µg of a recombinant glutathione S-transferase-FKBP12 fusion protein (GST-FKBP12), prepared as previously described (Sabers et al., 1995; Brunn et al., 1997; Reynolds et al., 2002), were incubated with 25 µl of glutathione-Sepharose beads in Buffer B (145 mM NaCl and 10 mM sodium phosphate, pH 7.4) containing 10% bovine serum albumin (BSA) for 1 h at 21 °C. The beads were then washed 3 times with Homogenization Buffer (1 ml/wash), and incubated with 1 ml GAS muscle extract (1 mg protein) plus 10 µM rapamycin (Calbiochem). After 90 min at 4 °C, the beads were washed twice with Buffer A, twice with Buffer A plus 500 mM NaCl, and twice in Buffer A.
2.4. Electrophoretic analyses and immuno-blotting of mTOR, PKB, and S6K
Samples of affinity-purified mTOR and skeletal muscle extracts were subjected to SDS-PAGE (7.5% polyacrylamide gel). The proteins were then electrophoretically transferred to Immobilon membranes, and immuno-blotted with mTOR, PKB, or S6K antibodies as described previously (Reynolds et al., 2002). After washing the membranes, the light generated by the alkaline-phosphatase conjugated secondary antibody and Tropix reagent was detected using X-ray film (Kodak XAR-5). Relative signal intensities of the PKB, mTOR, and S6K bands were determined by using a scanning laser densitometer (Molecular Dynamics).
2.5. In vitro muscle preparation and incubation
Intact soleus muscles were blotted on gauze, weighed, and transferred to 25 ml Erlenmeyer flasks (1 muscle/flask) containing 3 ml of Krebs Henseleit buffer containing 0.1% BSA, 1000 µU/ml insulin, 10 mM glucose, and 200 µM valine, 170 µM leucine, 100 µM isoleucine. Soleus were incubated for 30 min at 37 °C in a shaking water bath and then transferred to a second set of 25 ml Erlenmeyer flasks (1 muscle/flask) containing fresh media and were incubated for a 3 h experimental period at 37 °C with shaking. In order to measure protein synthesis rates, [14C]phenylalanine (0.05 µCi/ml) was included in the media. All flasks were pre-gassed with 95% O2/5% CO2 for 3 min immediately prior to both incubations as well as every 20 min (15 s) during the experimental incubation period. The muscles were frozen with metal tongs cooled to the temperature of liquid N2 and stored at −80 °C.
2.6. Assessment of protein synthesis rates
Protein synthesis was measured by assessing the incorporation of [14C]phenylalanine into protein as described previously (Tischler, 1992). Briefly, muscles were homogenized in Kontes Tubes (Kontes, Inc., Vineland, NJ) containing 1.5 ml of 10% TCA. The homogenates were centrifuged at 15,000g and the resulting pellets were washed 5 times with 1.5 ml of 10% TCA. Following the final wash the pellets were solubilized in 0.5 ml 1N NaOH for 1 h at 55 °C and then neutralized with 0.5 ml 1N HCl. Following neutralization, 5 ml of Ready Safe (Beckman, Fullerton, CA) scintillation fluid was added to a 200 µl of sample and the amount of [14C]phenylalanine incorporation in skeletal muscle protein was assessed by liquid scintillation (Packard 2500 TR, Meriden, CT). Protein synthesis values for each soleus muscle from a mouse were pooled and expressed as nmol phenylalnine/mg/3 h.
2.7. Assessment of citrate synthase activity
Freeze-clamped GAS muscles were weighed, powdered with at mortar and pestle in liquid N2, and homogenized on ice with a Brinkman polytron homogenizer at 1:40 (w/v) in a buffer containing 100 mM sodium phosphate and 2.0 mM EDTA supplemented with 0.1 mM phenylmethylsulfonyl fluoride, and 10 µg/ml each of aprotinin, leupeptin, and pepstatin-A. Muscle homogenates were centrifuged at 700g. The supernatant was saved and the pellet was resuspended and centrifuged at 700g. The supernatants were combined and used for the determination of citrate synthase activity as described by Srere (1969).
2.8. Statistical analysis
The effects of aerobic exercise training on the PKB/mTOR signaling pathway, the rate of protein synthesis, citrate synthase activity, body mass, and muscle mass were analyzed using a one-way analysis of variance. Data are expressed as means ± SEM, and the level of statistical significance was set at p < 0.05.
3. Results
3.1. Body mass, muscle mass, protein content, and citrate synthase activity
Body mass declined significantly following 3 months of voluntary wheel running (p = 0.001). GAS muscle mass and soleus muscle mass were not significantly different between AG and AG-WR mice (GAS, p = 0.45; Soleus, p = 0.20). Citrate synthase activity increased by 45% in AG-WR compared to AG mice, but this effect was not statistically significant (p = 0.066; Table 1).
Table 1.
Characteristics of AG and AG-WR male B6C3F1 mice
| AG | AG-WR | p-value | |
|---|---|---|---|
| Body mass (kg) | 37.0 ± 0.6 | 33.2 ± 0.4 | 0.001 |
| GAS muscle mass (mg) | 155.9 ± 5.0 | 165.6 ± 16.7 | 0.45 |
| GAS muscle protein (mg/g tissue) |
73.5 ± 7.1 | 84.7 ± 9.1 | 0.40 |
| Soleus muscle mass (mg) | 10.6 ± 0.7 | 12.0 ± 0.8 | 0.20 |
| Citrate synthase activity (umol/g tissue/min) |
92.3 ± 11.8 | 132.0 ± 13.0 | 0.066 |
3.2. mTOR expression levels
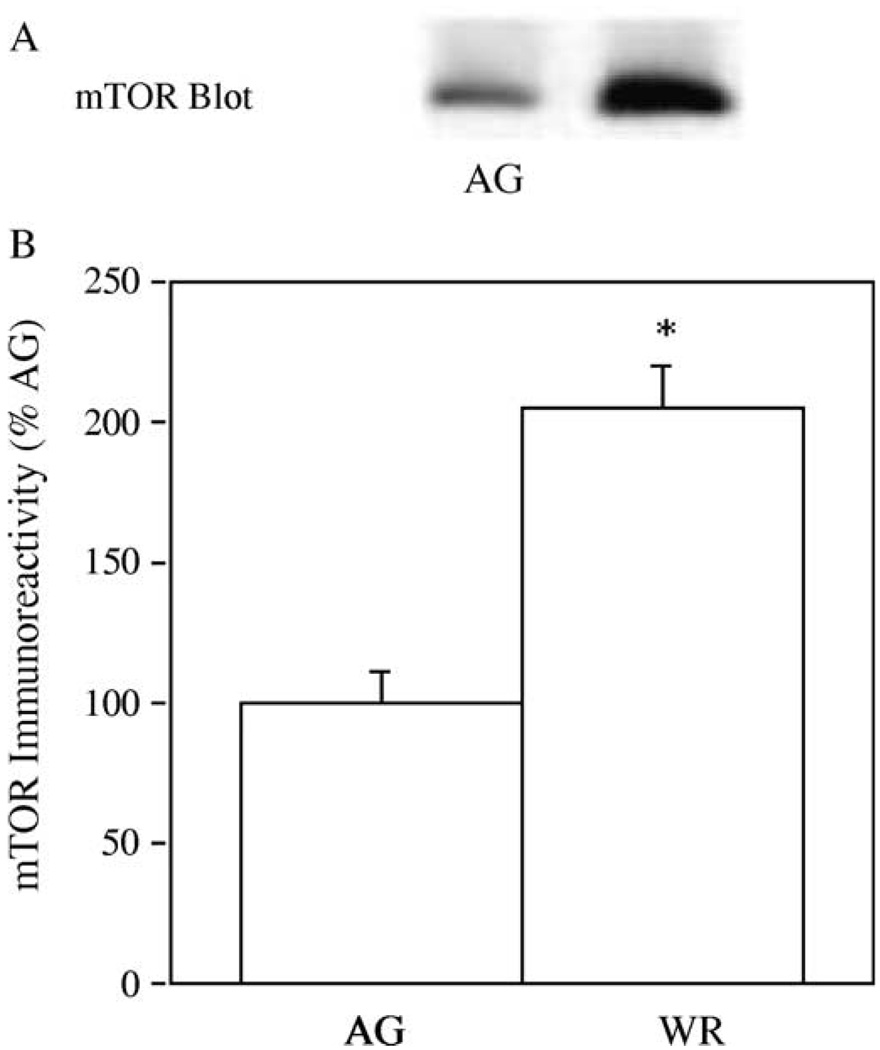
Since mTOR has recently been shown to be a crucial regulatory of skeletal muscle mass (Bodine et al., 2001; Reynolds et al., 2002), we conducted immuno-blotting experiments to determine if aerobic exercise training altered the total amounts of mTOR in GAS muscle. In Fig. 1(A), samples of GAS muscles from AG and AG-WR mice were immuno-blotted with the mTOR antibody, mTAb2. Immuno-reactivity of mTOR increased 65% in GAS muscles from AG-WR mice compared to AG mice (Fig. 1(B); p = 0.01).
Fig. 1.
The effect of 3 months of voluntary wheel running on mTOR levels in GAS muscles from AG and AG-WR B6C3F1 mice. A representative immuno-blot prepared with the mTAb2 antibody is shown (A) and quantification of mTOR immuno-reactivity is expressed as percentages of AG (B). *Significantly different from AG.
3.3. PKB phosphorylation on Ser473 and PKB expression levels
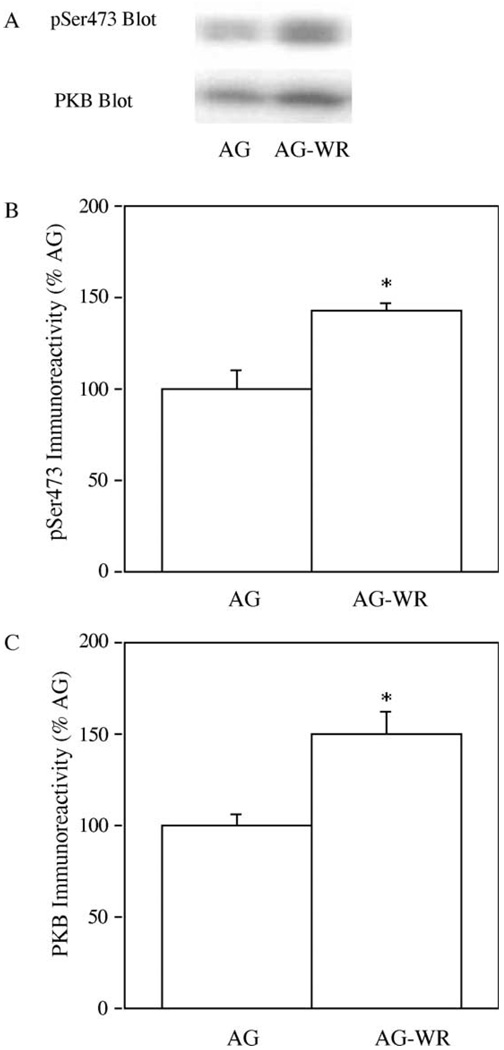
Because PKB is thought to activate mTOR, we examined the effect of aerobic exercise training on PKB phosphorylation on Ser473, a site whose phosphorylation is necessary for full kinase activity (Alessi et al., 1996) in GAS muscle. We also assessed the total amounts of PKB in GAS muscles. In order to measure changes in PKB phosphorylation on Ser473 and total PKB levels with aerobic exercise training, samples of GAS muscles from AG and AG-WR mice were immuno-blotted with either a phospho-specific Ser473 PKB antibody (pSer 473) that recognizes only PKB phosphorlation on Ser473 or a PKB antibody that recognizes all PKB regardless of its phosphorylation state (Fig. 2(A)). The amount of PKB phosphorylated on Ser473 increased significantly (p = 0.01); as evidenced by a 45% increase in pSer473 immuno-reactivity, in GAS muscle from AG-WR mice compared to AG mice (Fig. 2(B)). The total abundance of PKB increased 50% in GAS muscles from AG-WR compared to muscles from AG mice (p = 0.05; Fig. 2(C)).
Fig. 2.
The effect of 3 months of voluntary wheel running on PKB levels in GAS muscles from AG and AG-WR B6C3F1 mice. Representative immuno-blots prepared with phospho-specific Ser473 (pSer473) antibody and PKB antibody is shown (A). Quantification of pSer473 immunoreactivity (B) and PKB-β immuno-reactivity (C) are expressed as percentages of AG. *Significantly different from AG.
3.4. S6K expression levels
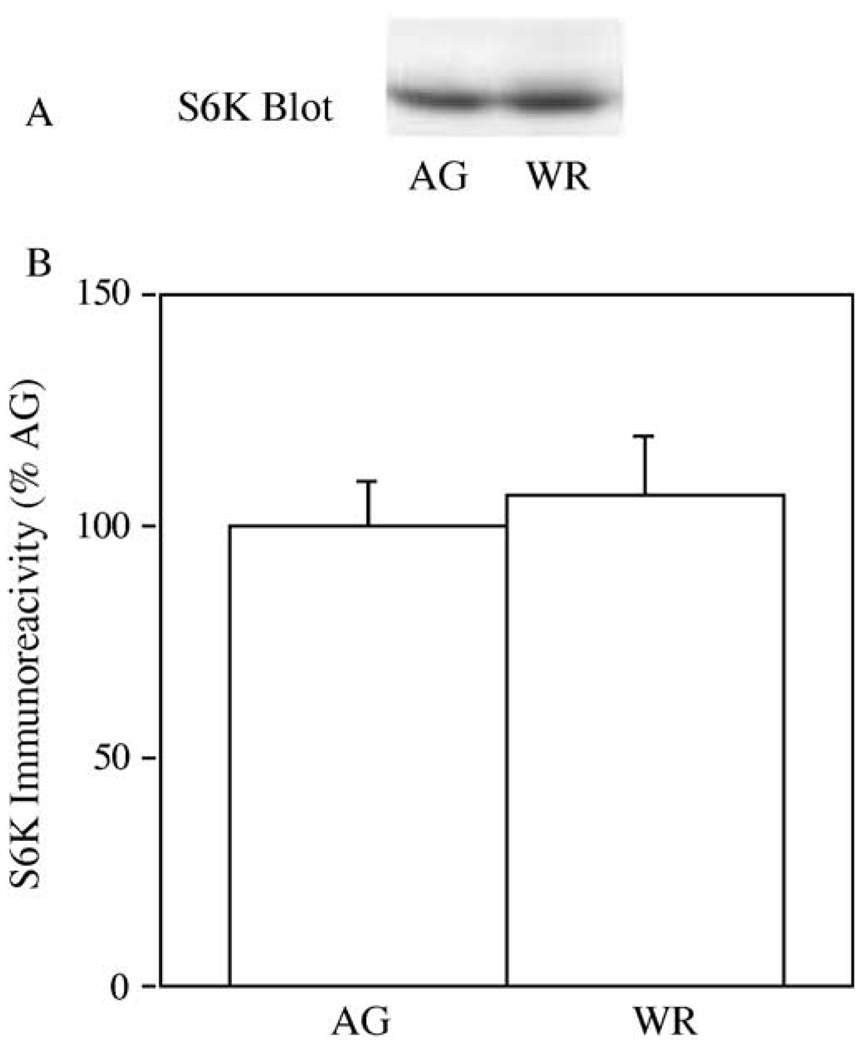
Since S6K is a downstream target of mTOR, we also performed immuno-blotting experiments investigating the effects of aerobic exercise training on total levels of this kinase in skeletal muscle. In Fig. 3(A), samples of GAS muscle from AG and AG-WR mice were immuno-blotted with an S6K antibody. S6K immuno-reactivity was similar in GAS muscle from AG and AG-WR mice (p = 0.69).
Fig. 3.
The effect of 3 months of voluntary wheel running on S6K levels in GAS muscles from AG and AG-WR B6C3F1 mice. A representative immuno-blot prepared with a S6K antibody is shown (A) and quantification of S6K immuno-reactivity is expressed as percentages of AG (B).
3.5. The rate of protein synthesis
The rate of protein synthesis was assessed by measuring [14C]phenylalanine incorporation into protein during in vitro incubation of intact soleus muscles from AG and AG-WR mice. Similar to the GAS muscle, the soleus muscle is a hindlimb muscle that is recruited during wheel running exercise. The rate of protein synthesis in soleus muscles isolated from AG-WR mice was significantly higher (30%) than muscles from AG mice (0.054 ± 0.005 vs 0.071 ± 0.006 nmol/mg/3 h, p = 0.01).
4. Discussion
Three months of voluntary wheel running increased the PKB phosphorylation on Ser473, and total levels of PKB and mTOR in GAS muscles from aged mice. In addition, wheel running produced an increase in the rate of protein synthesis in soleus muscles isolated from aged mice. These findings are the first evidence that aerobic exercise training enhances the PKB/mTOR signaling pathway and may have important implications regarding the regulation of skeletal protein synthesis with advancing age.
Recently, the PKB/mTOR signaling pathway has been shown to play a critical role in the regulation of muscle size (Bodine et al., 2001; Reynolds et al., 2002). Bodine et al. (2001) demonstrated that muscle hypertrophy induced by synergist muscle ablation was associated with a 4-fold increase in PKB levels and a 9-fold increase in the phosphorylation of PKB on Ser473, a site whose phosphorylation is required to fully activate the kinase (Alessi et al., 1996). Alternatively, muscle atrophy induced by hindlimb suspension was associated with marked decreases in both PKB expression and phosphorylation (Bodine et al., 2001). We demonstrated that chronic voluntary wheel running resulted in a 45% increase in PKB phsophorylation on Ser473 and a 50% increase in PKB expression in GAS muscles for AG-WR compared to AG sedentary mice. In contrast to our results, studies have reported no change in either PKB phosphorylation (Tanner et al., 2002; Jessen et al., 2003) or PKB expression (Chibalin et al., 2000; Jessen et al., 2003) following aerobic exercise training. However, insulin-stimulated PKB phosphorylation appears to be increased following aerobic exercise training (Chibalin et al., 2000; Luciano et al., 2002).
Since PKB is thought to activate mTOR by phosphorylating Ser 2448 of mTOR’s carboxy terminal regulatory domain (Scott et al., 1998; Nave et al., 1999; Sekulic et al., 2000), an increased expression and/or phosphorylation of PKB might result in a greater ability to activate mTOR. Reynolds et al. (2002) observed an increase in Ser 2448 phosphorylation of mTOR in muscles undergoing hypertrophy by synergist muscle ablation without an increase in mTOR expression. The level of mTOR expression increased by 65% in GAS muscles from aged mice that underwent 3 months of voluntary wheel running. Although we did not assess Ser 2448 phosphorylation, increasing mTOR expression by 65% could possibly allow for greater Ser 2448 phosphorylation, particularly since PKB phosphorylation and expression were increased markedly in muscles from AG-WR mice. To the best of our knowledge, no other studies have examined the effect of aerobic or resistance exercise training on mTOR phosphorylation and/or expression.
S6K is a downstream target of mTOR and its phosphorylation is associated with increased S6K activity (Shah et al., 2000). Therefore, investigators have examined S6K phosphorylation in muscle undergoing experimentally induced hypertrophy and atrophy. Muscle hypertrophy following resistance training was associated with increased S6K phosphorylation (Baar and Esser, 1999). Muscle hypertrophy induced by synergist muscle ablation was associated with an increase in S6K activity (Bodine et al., 2001). The present study observed no effect of chronic voluntary wheel running on total S6K levels or phosphorylation status in GAS muscles from mice. In the face of the substantial increases in PKB and mTOR expression observed in the present study, it would be expected that S6K phosphorylation be enhanced. However, our mice were studied in the fasted state when plasma levels of insulin and amino acids are low, therefore, we may have missed an upward shift in the electrophoretic mobility of S6K indicative of phosphorylation. Perhaps if we studied mice in the post-prandial state an S6K gel shift might have been observed and differences between AG and AG-WR mice revealed.
Despite the present increases in PKB and mTOR in GAS muscle and the increase in the rate of protein synthesis in the soleus muscle, muscle mass of either GAS or soleus muscles was not altered by 3 months of wheel running in aged mice. In contrast to this study, Munoz et al. (1994) observed an increase in soleus muscle mass and protein content in mice following 1 week of voluntary wheel running in young rats. Willis et al. (1998) also detected a significant increase in soleus muscle mass from aged mice following 3–9 months of voluntary wheel running. Brown et al. (1992) demonstrated that 27 months of voluntary wheel running prevented age-related muscle atrophy in the soleus muscles. In rodents, age-related muscle atrophy may only be consistently detected in the oldest of old animals (>25 months) (Brooks and Faulkner, 1998; Brooks et al., 2001). Perhaps if we initiated the wheel running intervention in older mice or increased the training duration we might have observed an increased muscle mass in AG-WR compared to sedentary AG mice. However, conducting such studies is problematic due to the rapid increase in the mortality of 24–25-monthold mice (NIA: Biology of Aging Program, PCR Survival Curves). Nonetheless, the absence of an increase in muscle mass following the wheel running intervention is different from the increase in muscle mass that typical occurs following resistance training or muscle loading.
In summary, the present study demonstrates that voluntary wheel running increases PKB phosphorylation on Ser473, PKB expression, and mTOR expression in skeletal muscle from aged mice. These findings indicate that aerobic exercise training may attenuate or prevent age-related declines in skeletal muscle protein synthesis by increasing the capacity of the PKB/mTOR signaling pathway. This idea is supported by the present increase in the rate of protein synthesis in soleus muscles from aged mice subjected to 3 months of wheel running.
Acknowledgements
THR was supported by an Institutional National Research Service Award (T32-AG00114, ‘Multidisciplinary Research Training in Aging’) and a Time Release Grant from Ithaca College. DRD was supported by a Department of Veteran Affairs VA Merit Award and LML was supported by KO1-AG00710 from the National Institutes of Health. This work was also supported by the University of Michigan Geriatrics Center and University of Michigan Claude D. Pepper Older American Independence Center. The authors are indebted to the support of Dr John C. Lawrence Jr., Departments of Pharmacology and Medicine, University of Virginia, Charlottesville, VA.
References
- Alessi DR, Andjelkovic M, Caudwell FB, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance training. Am. J. Physiol. 1999;45:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997;273(36):E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades P, Adey D, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2001;280(2):E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Opiteck JA, Faulkner JA. Conditioning of skeletal muscles in adult and old mice for protection from contraction-induced injury. J. Gerontol. 2001;56(4):B163–B171. doi: 10.1093/gerona/56.4.b163. [DOI] [PubMed] [Google Scholar]
- Brown M, Ross TP, Holloszy JO. Effects of aging and exercise on soleus and extensor digitorum longus muscles of female rates. Mech. Ageing Dev. 1992;63:69–77. doi: 10.1016/0047-6374(92)90017-8. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Fadden P, Haystead TAJ, Lawrence JC. The mammalian target of rapamycin phosphorylates sites having a (Ser/Thr)-Pro motif and is activated by antibodies to a region near its COOH terminus. J. Biol. Chem. 1997;272:32547–32550. doi: 10.1074/jbc.272.51.32547. [DOI] [PubMed] [Google Scholar]
- Chibalin AV, Yu M, Ryder JW, Song XM, Galuska D, Krook A, Wallberg-Henriksson H, Zierath JR. Exercise-induced changes in expression and activity of proteins involved in insulin signal transduction in skeletal muscle: differential effects on insulin-receptor substrates 1 and 2. Proc. Natl Acad. Sci. USA. 2000;97(1):38–43. doi: 10.1073/pnas.97.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263(22):3029–3034. [PubMed] [Google Scholar]
- Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am. J. Physiol. Endocrinol. Metab. 2000;278(4):E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Chen M, Cartee GD, Young C. Skeletal muscle atrophy in old rats: differential changes in three fiber types. Mech. Ageing Dev. 1991;60:199–213. doi: 10.1016/0047-6374(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Jessen N, Pold R, Buhl ES, Jensen LS, Schmitz O, Lund S. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles. J. Appl. Physiol. 2003;94(4):1373–1379. doi: 10.1152/japplphysiol.00250.2002. [DOI] [PubMed] [Google Scholar]
- Lambert MI, Noakes TD. Spontaneous running increases VO2 max and running performance in rats. J. Appl. Physiol. 1990;68:400–403. doi: 10.1152/jappl.1990.68.1.400. [DOI] [PubMed] [Google Scholar]
- Lexall J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size, and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Luciano E, Carneiro EM, Carvalho CR, Carvalheira JB, Peres SB, Reis MA, Saad MJ, Boschero AC, Velloso LA. Endurance training improves responsiveness to insulin and modulates insulin signal transduction through the phosphatidylinositol 3-kinase/Akt-1 pathway. Eur. J. Endocrinol. 2002;147(1):149–157. doi: 10.1530/eje.0.1470149. [DOI] [PubMed] [Google Scholar]
- Munoz KA, Aannestad A, Tischler ME, Henriksen EJ. Skeletal muscle protein content and synthesis after voluntary running and subsequent unweighting. Metabolism. 1994;43(8):994–999. doi: 10.1016/0026-0495(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Nave BT, Ouwens DM, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem. J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- Reynolds TH, Bodine SC, Lawrence JC. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J. Biol. Chem. 2002;277(20):17657–17662. doi: 10.1074/jbc.M201142200. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J. Appl. Physiol. 1989;66(3):1250–1257. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl Acad. Sci. USA. 1996;93(26):15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J. Biol. Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Scott PH, Brunn GJ, Kohn AD, Roth RR, Lawrence JC. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc. Natl Acad. Sci. USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60(13):3504–3513. [PubMed] [Google Scholar]
- Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E- BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am. J. Physiol. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Meth. Enzymol. 1969;13:3–5. [Google Scholar]
- Tanner CJ, Koves TR, Cortright RL, Pories WJ, Kim YB, Kahn BB, Dohm GL, Houmard JA. Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in middle-aged men. Am. J. Physiol. Endocrinol. Metab. 2002;282(1):E147–E153. doi: 10.1152/ajpendo.2002.282.1.E147. [DOI] [PubMed] [Google Scholar]
- Tischler ME. Estimation of protein synthesis and proteolysis in vitro. In: Nissen S, editor. Modern methods in protein nutrition and Metabolism. New York: Academic Press; 1992. pp. 225–250. [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis PE, Chadan S, Baracos V, Parkhouse WS. Restoration of insulin-like growth factor I action in skeletal muscle of old mice. Am. J. Physiol. 1998;275:E525–E530. doi: 10.1152/ajpendo.1998.275.3.E525. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Zachwieja JJ, Bier DM. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. 1993;265(2):E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >76 yr old. Am. J. Physiol. 1999;277(1):E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]