Abstract
This study investigated the metabolic changes with age in the Fischer 344 × Brown Norway rat and its suitability as an animal model of postmaturational insulin resistance. Specifically, we determined whether an age-associated decrease in glucose disposal is associated with diminished whole body insulin responsiveness and/or a decrease in glucose transporter (GLUT-4) protein and mRNA content in medial gastrocnemius muscle of male Fischer 344 × Brown Norway rats of ages 8, 18, and 28 months. Fasting plasma glucose was unchanged with age. There was a significant age effect on visceral adiposity, fasting plasma insulin levels, insulin responsiveness, and GLUT-4 protein content. Insulin responsiveness and GLUT-4 protein were lower in the 18-month-old rats than in the 8-month-old rats. The findings of age-associated increases in visceral adiposity and insulin resistance, and decreases in GLUT-4 in the Fisher 344 × Brown Norway rat, suggest that this rat strain may be an appropriate model for studying the effects of aging on glucose homeostasis.
Aging is associated with a high rate of impaired glucose tolerance and diabetes (1). A decrease in insulin-mediated glucose disposal could contribute to the observed age-associated impairment in glucose tolerance and diabetes. Data from Houmard and colleagues (2) suggest that there is a negative correlation between advancing age and the concentration of insulin-stimulated glucose transporter (GLUT-4) in skeletal muscle that may partially contribute to the observed insulin resistance in humans. Rodent models previously used to study age-associated changes in glucose metabolism demonstrate declines in insulin action with rapid weight gain and the onset of puberty between 1 and 4 months of age (3, 4). This rapid decline in glucose metabolism appears to be inversely related to the maturational increase in lean body mass. Following stabilization in muscle mass with maturation, these strains do not demonstrate declines in insulin sensitivity (5–7). Thus, these rodent models are not applicable for studying mechanisms of the age-associated impairment of glucose tolerance that occur after stabilization of maturational increases in muscle mass.
The Fischer 344 (F344) rat strain has been used as a model for studying metabolic changes with aging because it develops less dramatic early-life adiposity compared with that of other rat strains. However, the F344 rat manifests age-associated complications such as renal failure, which could affect the interpretation of metabolic studies (8). More recently, the Fischer 344 × Brown Norway (F344 × BN) rat has been proposed as a better model for the study of aging in rodents (9). To date, two studies have investigated the effect of age on isolated muscle glucose uptake in the F344 × BN rat (10, 11). These in vitro studies suggest that, in the epitrochlearis muscle on the F344 × BN rat, there is no effect of age on basal glucose transport activity or GLUT-4 protein content (10,11) and a decrease in glucose transport with 100 µU/ml insulin concentration between 25 and 3.5 months of age (10). However, the epitrochlearis muscle, unlike the majority of skeletal muscles in the rat hind limb, does not show age-related atrophy and may not be representative of age-associated changes in muscle metabolism.
Therefore, the purpose of this study was to characterize a potentially useful new animal model for age-related changes in glucose metabolism. Specifically, we investigated age-associated changes in circulating insulin and glucose levels and whole body insulin responsiveness in the male F344 × BN rat. In addition, because skeletal muscle is the major site of glucose disposal (12) and glucose transport is the major rate-limiting step in skeletal muscle glucose disposal (3,13), we studied the relationship between insulin responsiveness in this strain and alterations in GLUT-4 protein and mRNA content in skeletal muscle.
In order to study the effect of age-associated changes on metabolic and contractile function in skeletal muscle, we needed to find a muscle that was large enough to allow for the collection of adequate samples for determining several assays of metabolic function and that was an accessible muscle with a mixed fiber-type composition. The mass of the medial gastrocnemius (GTN) muscle in a male F344 × BN rat is adequate to supply enough tissue to perform several metabolic measures. The medial GTN muscle is innervated by only one nerve (a branch of the tibial nerve), which makes monitoring of contractile properties feasible. In addition, by 8 months of age, the male F344 × BN GTN muscle has reached maximum muscle mass, suggesting that maximal muscle maturation has occurred by 8 months of age.
METHODS
Animals and Animal Care
To characterize the postpubescent age spectrum of this rat strain, male F344 × BN F1 hybrid rats of ages 8, 18, and 28 months were obtained from the National Institute on Aging’s animal colony maintained by the Harlan Sprague-Dawley Laboratory (Indianapolis, IN). The ages of these rats correspond to approximately 100% (8 months), 98% (18 months), and 80% (28 months) survival in this strain of rat (14). The median life span and the age of the tenth percentile survivors in this rat strain occur at 33 and 38 months, respectively (14). Rats were acclimated to our colony conditions, light cycle (12:12 light:dark), and temperature (20°C–22°C), for 2 weeks prior to experimentation. Rats were sheltered in a specific pathogen-free facility, housed individually in hanging plastic cages (28 cm × 56 cm), and fed Purina Rodent Chow 5001 laboratory chow and water ad libitum.
Although necropsy and microbiological examinations were not performed routinely on the 8-month-old and 18-month-old rats, all animals were inspected daily for external signs of disease. Only rats with no external signs of disease were used in this study. In this study, no animals with external signs of disease were eliminated. In addition, all 28-month-old animals were inspected upon dissection for gross pathologies and for the presence of radiculoneuropathy by the pathology division of the University of Michigan Medical School Unit for Laboratory Animal Medicine. In this study, no 28-month-old animals were eliminated because of the presence of radiculoneuropathy. The differences in the number of animals within each study were a result of loss of samples (Study 1) or nonpatent cannulae (Study 2).
Experimental Procedures
One set of animals was used for determining fasting plasma glucose and insulin levels, body fat, and medial GTN muscle GLUT-4 transporter protein and mRNA levels. These animals were anesthetized with halothane, and a cannula was implanted in the left carotid artery. The animals were allowed to recover from surgery for 24 hours without food (to obtain fasting blood values). Beginning at 7:30 am, a 2-ml arterial blood sample was obtained via the carotid cannula and placed into pre-chilled tubes, which contained EGTA (ethylene glycol-bis [(β-aminoethyl ether]-N,N,N′,N′-tetraacetic acid). The sample was immediately centrifuged at 4°C and plasma was stored at −70°C for a subsequent analysis of glucose and insulin. The animals were then euthanized with sodium pentobarbital, delivered through the carotid cannula; both GTN muscles (lateral and medial) were quickly dissected in their entirety, freeze clamped, weighed, and stored at −70°C for a subsequent analysis of citrate synthase activity and GLUT-4 glucose transporter protein and mRNA content. Epididymal, retroperitoneal, and mesenteric adipose tissue depots were dissected and weighed for use as an index of visceral fat (expressed as a percentage of total body weight). The bodies were then eviscerated and the resulting carcass weights determined.
Hyperinsulinemic-Euglycemic Clamp
In a separate group of animals, insulin responsiveness was studied by using a hyperinsulinemic-euglycemic clamp technique. Briefly, animals were anesthetized with halothane and one cannula was implanted in the left carotid artery for blood collection. Two additional cannulae were placed in the jugular vein for infusion of insulin and glucose. Animals were allowed to recover for 5 days prior to the clamp procedure. Following the 5-day recovery from surgery, there was a small decline in body weight ranging from 3% to 18%, with an average weight loss of 7.5%. There was no significant difference in weight loss among the three age groups.
Animals were fasted overnight and basal glucose was obtained before insulin infusion. A continuous infusion of regular porcine insulin (20 mU/kg per min) was administered at a rate of 10 µl/min along with a variable infusion of 50% glucose solution for a total of 120 minutes. The dose of insulin used for infusion was selected because it raises plasma insulin to approximately 4000 pM, a concentration that has been shown to fully suppress hepatic glucose output and maximally stimulate skeletal muscle glucose uptake in male Sprague-Dawley rats (15) and in male Wistar rats ages 1–20 months (4). Every 5 minutes, blood was sampled from the carotid cannula and blood glucose was determined with a Beckman One Touch Glucometer (Beckman Instruments, Inc., Fullerton, CA); the rate of glucose infusion was then varied to maintain blood glucose at a euglycemic level (5 mM). Plasma samples were obtained at baseline and every 30 minutes during the clamp for determination of plasma insulin. Insulin responsiveness was determined by measuring the glucose infusion rate normalized for body mass [(mg/kg/min)] during the last 30 minutes of the 2-hour clamp.
Analytical Procedures
Plasma glucose was analyzed by the glucose oxidase method (Beckman Glucose Analyzer 2, Beckman Instruments; plasma insulin was analyzed by radioimmunoassay in the Core Laboratories of the Michigan Diabetes Research and Training Center at the University of Michigan. Muscle homogenates (700 × g supernatant) were used to determine citrate synthase (CS) activity (assay run at 25°C), using the method of Serre (16). Protein concentrations on the 700 × g supernatant (CS assay) and on total membranes (GLUT-4 assay) were measured by using a Bradford protein assay kit from Bio-Rad (Richmond, CA).
GLUT-4 Western Blotting
The protocol used for the isolation of total membranes was previously described by Klip and colleagues (17). Briefly, frozen tissues (200 mg) were powdered in a mortar and pestle that was cooled in liquid N2. Powdered tissues were suspended in 5.0 ml of ice-cold homogenization buffer (10mM NaHCO3, 0.25M sucrose, and 5.0mM NaN3) and homogenized twice for 10 seconds by using a Brinkman Polytron (setting 7). Homogenates were centrifuged at 1200 × g for 10 minutes, at 4°C; pellets were resuspended in 5 ml of ice-cold homogenization buffer and centrifuged at 1200 × g for 10 minutes, at 4°C. Supernatants were combined and centrifuged at 190,000 × g for 66 minutes at 4°C. Pellets were then resuspended in 500 µl of ice-cold homogenization buffer and stored at −70°C.
The procedure used for Western analysis of GLUT-4 glucose transporters was a modified version of a method previously described by Kahn and Pedersen (18). Briefly, vertical slab polyacrylamide gel electrophoresis was performed in the presence of sodium dodecyl sulfate on molecular-weight markers (Amersham, Arlington Heights, IL) and aliquots of muscle membranes each containing 30 µg of protein. An internal control sample was run on all gels, which consisted of an aliquot (30 µg of protein) of a muscle membrane pool obtained from combined muscle homogenates. Samples were separated on a 10% polyacrylamide resolving gel and then electrophoretically transferred onto Immobilon PVDF (polyvinylidene difluoride) membrane (Millipore, Milford, MA). Immunoblotting was performed by using a rabbit polyclonal antibody against glucose transporter (GLUT-4) protein (East Acres Biologicals, Southbridge, MA), followed by 125I-labeled antirabbit immunoglobulin (Amersham) and exposure of the transfer membrane to Kodak X-OMAT film at −80°C for 24 hours. There was a single band for GLUT-4 protein at a molecular weight of 45 kDa quantitated by densitometry.
GLUT-4 Northern Blotting
RNA was isolated from 100 mg of frozen GTN muscle by using TRI REAGENT (Molecular Research Center, Inc., Cincinnati, OH; 19). Ten micrograms per lane of RNA was electrophoresed on 1% formaldehyde-agarose gels and was transferred onto nylon Biotrans membranes (Boehringer Mannheim, Indianapolis, IN). GLUT-4 cDNA (obtained from Dr. Jessica Schwartz, at the University of Michigan, Ann Arbor, MI) was used to synthesize a cRNA probe by using T7 polymerase and the Genius 4 RNA labeling kit as described by the manufacturer (Boehringer Mannheim). Detection of digoxigenin-labeled GLUT-4 mRNA was performed by using the chemiluminescent substrate Lumi-Phos 530, Genius System (Boehringer Mannheim) and by exposing the membrane to Kodak X-OMAT film at 25°C for 1.5 hours. The abundance of GLUT-4 mRNA signal was determined by using a densitometer.
Statistics
Values are presented as means ± standard error. Statistical analysis was performed by using Statview 4.01 (Abacus Concepts, Berkeley, CA). An analysis of variance (ANOVA) was used to test for overall age effects. Because it is well documented that insulin action declines with age, we used a one-tailed ANOVA to determine the effect of age on glucose infusion rate during the 2-hour hyperinsulinemic-euglycemic clamp. When a significant main effect was found, the Fischer least significant difference post hoc test was used to determine differences between the age groups. Differences were considered significant at p ≤ .05.
RESULTS
Body, Carcass, GTN, and Visceral Fat Weight
Body, carcass, GTN, and visceral fat weight for the three age groups of animals are shown in Table 1. There was a significant age effect for all of these measures. The weights of epididymal, retroperitoneal, and mesenteric adipose tissue depots and the combined weight of all three fat pads (visceral fat) were greater in the 18-month-old and 28-month-old rats compared with the 8-month-old rats, as was the percentage of visceral fat. Except for GTN muscle weight, there were no significant differences in any of these measures between 18- and 28-month-old animals. GTN muscle weight was significantly less in the 28-month-old rats compared with the 8- and 18-month-old rats, with no significant differences in muscle weight between 8- and 18-month-old rats.
Table 1.
Characteristics of Male F344 × BN Rats
| Age (mo)† | ||||
|---|---|---|---|---|
| Parameter | 8 | 18 | 28 | Effect of Age‡ |
| Body weight (g) | 440 ± 6 | 534 ± 13* | 544 ± 7* | p < .0001 |
| (n = 10) | (n = 10) | (n = 12) | ||
| Carcass weight (g) | 347 ± 5 | 406 ± 10* | 413 ± 6* | p < .0001 |
| (n = 10) | (n = 10) | (n = 12) | ||
| Gastrocnemius weight (g) | 2.78 ± 0.04 | 2.74 ± 0.07 | 2.41 + 0.06* | P < .0001 |
| (n = 10) | (n = 10) | (n = 12) | ||
| Fat pad content | (n = 10) | (n = 10) | (n = 12) | |
| epididymal fat (g) | 7.6 ± 0.3 | 14.3 ± 0.7* | 13.7 ± 0.5* | p < .0001 |
| retroperitoneal fat (g) | 5.4 ± 0.2 | 12.2 ± 0.8* | 11.8 ± 0.6* | p < .0001 |
| mesenteric fat (g) | 3.8 ± 0.2 | 12.8 ± 1.0* | 13.3 ± 0.7* | p < .0001 |
| Visceral fat = EPI + RP + MES | 16.9 ± 0.6 | 39.3 ± 2.4* | 38.9 ± 1.7* | p < .0001 |
| % Fat = visceral fat/body wt. | 3.8 ± 0.1 | 7.3 ± 0.3* | 7.2 ± 0.3* | p < .0001 |
| Glucose (mmol/L) | 6.2 ± 0.1 | 6.1 ± 0.2 | 5.9 ± 0.3 | NSD |
| (n = 10) | (n = 10) | (n = 12) | ||
| Insulin (pmol/l) | 181 ± 11 | 269 ± 19* | 244 ± 33* | p = .036 |
| (n = 10) | (n = 10) | (n = 10) | ||
| Citrate synthase activity (µmol/min/µg protein) | 99.1 ± 2.6 | 104 ± 2.8 | 111.1 ± 6.3 | NSD |
| (n = 9) | (n = 9) | (n = 12) | ||
Values are means ± standard error.
Determined by an analysis of variance.
Significant difference p < .05 vs 8-mo-old rats.
NSD = no significant difference.
Fasting Plasma Glucose and Insulin Concentrations
The fasting plasma glucose concentration did not significantly differ between the 8-, 18-, and 28-month-old groups (Table 1). In contrast, fasting plasma insulin concentrations were significantly greater in the 18- and 28-month-old rats compared with the 8-month-old rats (Table 1).
Citrate Synthase Activity
There was no effect of age on CS activity [expressed as (µmol/min)/µg protein] in the GTN muscle (Table 1).
Hyperinsulinemic-Euglycemic Clamp
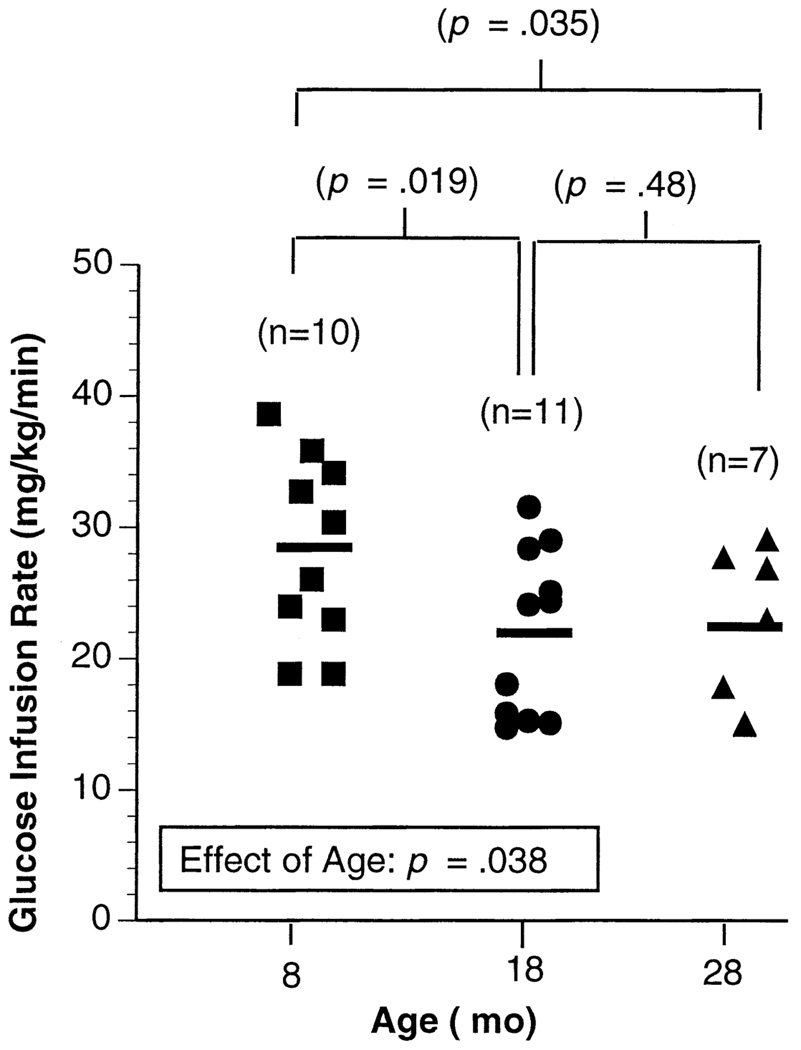
Insulin responsiveness was assessed by determining glucose infusion rate [mg/kg/min] during the last 30 minutes of the 2-hour clamp procedure. The rate of glucose infusion did reach steady state during the last 30 minutes of the clamp. As with the first group of animals, body weight significantly increased from 8 to 18 months of age, with no further increase between 18 and 28 months of age (Table 2). Basal plasma glucose and plasma glucose and insulin during the last 30 minutes of the 2-hour clamp were not significantly different between the age groups (Table 2). Overall there was a significant age effect on insulin responsiveness, as shown in Figure 1. Insulin responsiveness was lower in the 18- and 28-month-old rats compared with that of the 8-month animals (Figure 1). There was no significant difference between the values of the 18- and 28-month-old animals.
Table 2.
Characteristics of Male F344 × BN Rats During the Hyperinsulinemic-Euglycemic Clamp
| Age (mo)† | Effect of Age‡ |
|||
|---|---|---|---|---|
| Parameter | 8 | 18 | 28 | |
| Body weight (g) | 389 ± 14 | 472 ±11* | 505 ± 17* | p < .0001 |
| (n = 9) | (n = 11) | (n = 7) | ||
| Basal glucose (mmol/l) | 4.7 ± 0.2 | 4.8 ± 0.2 | 4.5 ± 0.2 | p = .54 |
| (n = 9) | (n = 11) | (n = 7) | ||
| Plasma glucose (mmol/l) | 5.0 ± 0.1 | 4.9 ± 0.1 | 5.0 ± 0.1 | p = .82 |
| mean last 30 min | (n = 9) | (n = 11) | (n = 7) | |
| Plasma insulin (pmol/l) | 4411 ± 74 | 4130 ± 135 | 4261 ± 226 | p = 0.37 |
| at 120 min | (n = 9) | (n = 11) | (n = 7) | |
Values are means ± standard error.
Determined by an analysis of variance.
Significant difference p < .05 vs 8-mo-old rats.
Figure 1.
Glucose infusion rate during the last 30 minutes of the 2-hour hyperinsulinemic-euglycemic clamp procedure in 8-, 18-, and 28-month-old male F344 × BN rats. The solid horizontal line represents the mean value for each age group. There was a significant age effect by analysis of variance.
GLUT-4 Protein and mRNA Content
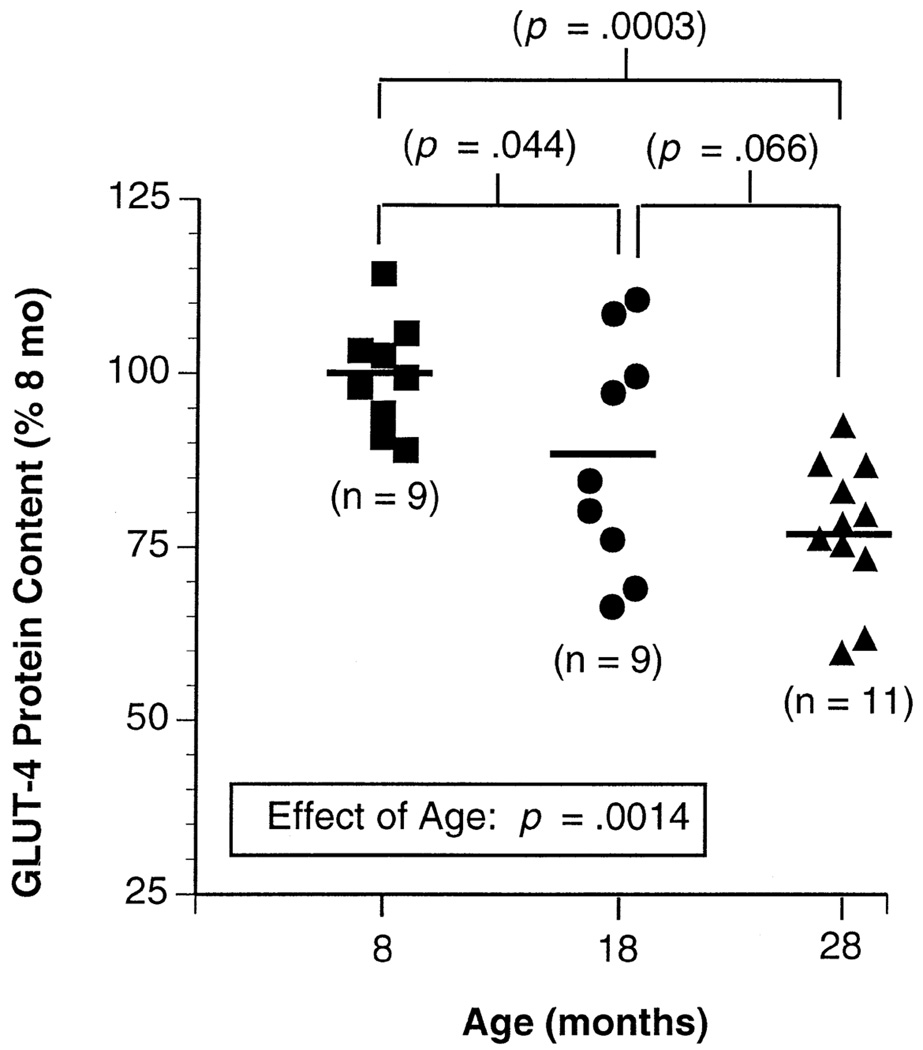
Overall there was a highly significant effect of age on GLUT-4 protein content in muscle (Figure 2). GLUT-4 protein content was 12% lower in the 18-month-old group and 25% lower in the 28-month-old group compared with that of the 8-month-old group (p < .05 and p < .001, respectively). Muscle GLUT-4 content was 10% lower on average in the 28-month-old animals compared with the 18-month-old animals, but this was of borderline significance (p = .066). The correlation between plasma insulin level and GLUT-4 protein content (r = .23) was not significant. As shown in Figure 3, there was a trend toward decreased GLUT-4 mRNA in the 28-month-old animals compared with that of the two younger groups, but this value did not reach statistical significance.
Figure 2.
GLUT-4 (glucose transporter) protein content in gastrocnemius muscle membranes from 8-, 18-, and 28-month-old male F344 × BN rats. Values are expressed as a percentage of the mean value for the 8-mo-old animals. The solid horizontal line represents the mean value for each age group. There was a significant age effect by an analysis of variance.
Figure 3.
GLUT-4 (glucose transporter) mRNA content in gastrocnemius muscle membranes from 8-, 18-, and 28-month-old male F344 × BN rats. Values are expressed as a percentage of the mean value for the 8-mo-old animals. The solid horizontal line represents the mean value for each age group. The effect of age was of borderline significance by an analysis of variance.
DISCUSSION
The present study demonstrates that, unlike previous rodent studies investigating age-associated alterations in glucose homeostasis, male F344 × BN rats have a significant age-associated increase in plasma insulin, a decline in whole body insulin responsiveness, a decline in skeletal muscle GLUT-4 protein content, and a tendency for decline in GLUT-4 mRNA content. Thus, the male F344 × BN rat may be a suitable animal model for the age-associated changes insulin resistance occurring after stabilization of maturational increases in muscle mass.
Age-associated insulin resistance is primarily due to decreased glucose disposal in insulin-sensitive peripheral tissues such as adipose and skeletal muscle (20–22). In this study of male F344 × BN rats, diminished glucose disposal and insulin resistance was demonstrated by a 22% decrease in the amount of infused glucose needed to maintain euglycemic conditions during a 2-hour hyperinsulinemic clamp in 18- and 28-month-old animals compared with 8-month-old animals, with no significant difference between the 18- and 28-month-old animals. Therefore, in this rat strain the age-associated changed in insulin resistance coincided with the increase in adiposity and was not further exacerbated by advancing age alone. Because we measured whole body insulin responsiveness, one cannot discount the contribution of glucose uptake into adipose tissue as a contributing factor to the observed decrease in glucose disposal. Further studies have to be done to specifically investigate age-associated changes in glucose uptake into adipose and skeletal muscle tissues in this rat strain.
The concentration of GLUT-4 protein has been shown to be a predominant determinant of the capacity for glucose disposal in skeletal muscle (23). Therefore, an age-related decline in the concentration or functional state of the GLUT-4 glucose transporter in skeletal muscle could explain an age-associated impairment in glucose disposal. Previous studies in rodents demonstrated a rapid decline in GLUT-4 protein content during pubescence but no change in GLUT-4 protein content following maturational increases in muscle mass (5–7). In the present study, skeletal muscle GLUT-4 content was lower in 18- and 28-month-old male F344 × BN animals than in 8-month-old animals with a tendency (p = .066) for further age-associated decline between 18- and 28-month-old animals. Thus, this decrease in skeletal muscle GLUT-4 protein content could contribute, in part, to the age-associated decline in insulin responsiveness observed in these animals.
A previous study done by Spangler and colleagues (24) indicates that there is a decrease in 24-hour activity levels with age in both the F344 and the F344 × BN rat. In addition, Spangler observed that there is a decrease in 24-hour activity in the F344 × BN compared with the F344 rat, which may, in part, explain the differences in GLUT-4 content and glucose disposal observed between the two strains of rat. Furthermore, Spangler found an increase in radiculo-neuropathy in 31-month-old rats that resulted in foot dragging or hind-limb paresis and may explain the diminished activity observed in his study. Knowing the increase in the incidence of radiculoneuropathy in the F344 × BN strain, we specifically tested all of our 28-month-old animals used in this study for this pathology. Necropsy results indicated no incidence of radiculoneuropathy in the 28-month-old animals used in the present study. In this study, when CS activity, an indicator of skeletal muscle oxidative capacity, is expressed as micromoles of activity per minute per microgram of protein, no age-associated alteration was observed in the 18- and 28-month-old rats compared with the 8-month-old animals. Oxidative capacity of the muscle is positively correlated with the level of physical activity experienced by the muscle (25). Even when the age-associated decrease in GTN muscle weight is taken into account, total CS activity is not significantly different in the 28-month-old than in the 8- and 18-month-old rats. The absence of radiculoneuropathy in this cohort of 28-month-old animals may explain the maintenance of oxidative capacity in the hind-limb muscles of these animals. Because we observed no significant age-related difference in CS activity of the muscle, it is unlikely that the age-associated decrease in GLUT-4 content is related to a decrease in physical activity in the oldest animals.
Increased adiposity appears to be a major factor contributing to an age-associated decrease in insulin action, both in humans and animals (26, 27). A previous study done by Berthelier and colleagues (28) in female Wistar rats shows a significant decrease in whole body glucose utilization rates (GUR) between 2 months and 18 months. However, the greatest decline (47%) in GUR was observed between 12 and 18 months of age. Body composition data were not presented in this paper; however, food intake and weight gain data suggest that these animals increased their body fat content between 12 and 18 months of age. The Fischer 344 rat, which does not demonstrate an age-associated increase in adiposity (29), does not demonstrate alterations in insulin responsiveness following the stabilization of maturational increases in muscle mass (5). The male F344 × BN rats in our study demonstrate an age-associated increase in visceral adiposity and a decrease in insulin responsiveness, supporting a link between increased adiposity and decreased peripheral insulin action. In fact, the percent visceral adiposity did not increase further between the 18- and 28-month-old animals, and insulin responsiveness remained unchanged as well. These data suggest that insulin responsiveness may be influenced more by the level of adiposity than by the age of the animal. Recent studies by Barzilai and coworkers (30–32), have suggested that it is the alterations in visceral and not whole body fat content that account for alterations in insulin action. More specifically, Barzilai and coworkers have demonstrated that a decrease in visceral adiposity by surgical removal of visceral fat pads, leptin treatment, beta-3-adre-noreceptor agonist, or severe calorie restriction improve hepatic insulin action (31,32). However, improved peripheral insulin action was only accomplished in the leptin-treated animals. Studies investigating the effect of leptin treatment on visceral adiposity and peripheral insulin action in the aged F344 × BN rat may help to examine influences of age versus adiposity on peripheral insulin action.
The relationship between increased visceral adiposity with age in these animals and the observed decrease in GLUT-4 content is uncertain. Obesity in rodents, whether genetically or environmentally induced, appears to have little consistent effect on the expression of GLUT-4 transporter content. In genetically induced obesity, increased adiposity has been associated with either a decrease or no change in GLUT-4 content in skeletal muscle (33–35). The large deficit observed in insulin-mediated glucose uptake in genetically induced obese animals cannot be fully explained by the slight decreases observed in skeletal muscle GLUT-4 content. A study investigating the effect of diet-induced obesity on GLUT-4 content has shown that 7 weeks of a high-calorie diet resulted in a 30% increase in epididymal fat pads, with no change in GLUT-4 content compared with ad-libitum chow-fed controls (18). In our study, 18-month-old male F344 × BN rats on an ad-libitum chow diet had a 90% greater epididymal fat pad weight than 8-month-old animals, with significantly lower skeletal muscle GLUT-4 content. Thus, it is possible that the length of time exposed to the obese state and the magnitude of visceral adiposity may be factors that affect the expression of GLUT-4 protein in skeletal muscle. However, the trend toward a further age-associated (28 vs 18 months) decrease in GLUT-4 content in male F344 × BN rats was not accompanied by a further increase in visceral adiposity from 18 to 28 months, suggesting a possible age effect that is independent of visceral adiposity. Similar findings were observed in the vastus lateralis but not the GTN muscle in humans (2). Data from the Houmard and colleagues study suggest that, in humans, muscle groups that are relatively inactive show a more pronounced decrease in GLUT-4 content and that because the decrease in skeletal muscle GLUT-4 content was positively correlated with whole body insulin sensitivity, the diminished GLUT-4 content may in part contribute to the age-associated increase in insulin resistance observed in humans.
In conclusion, we observed an age-associated decline in insulin responsiveness in 18- and 28-month-old rats compared with 8-month-old male F344 × BN rats. This decreased responsiveness with advanced age may be contributed to by a small, but significant, decrease in GLUT-4 glucose transporter protein content in skeletal muscle. The initial decline in GLUT-4 content from 8 to 18 months occurred in association with an increase in visceral adiposity, whereas the continued apparent decline in GLUT-4 content in the 28-month-old group may be an effect that is independent of visceral adiposity and insulin responsiveness. Further studies have to be done to determine the mechanisms involved in the development of obesity-induced insulin resistance as distinct from age-associated insulin resistance. The age-associated increase in visceral adiposity and insulin resistance, and the decrease in skeletal muscle GLUT-4 content, in the male F344 × BN rat suggests that this rat strain may be an appropriate rat model for studying the effects of aging on metabolic parameters such as glucose homeostasis occurring after stabilization of maturational increases in muscle mass.
ACKNOWLEDGMENTS
This research was supported in part by a National Institute on Aging training grant (Grant T32-AG00114) and by the Core Facility for Aged Rodents, sponsored by the Claude Pepper Older Americans Independence Center (Grant AG00880) and Institute of Gerontology, University of Michigan; by the Geriatric Research, Education, and Clinical Center, Department of Veterans Affairs Medical Center, Ann Arbor, Michigan; and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health (Grant DK43051) and from the U.S. Department of Agriculture.
The authors thank Andrezej Galecki, Jodi L. Kreuger, Eric Leiendecker, and Marla Smith for their technical assistance.
REFERENCES
- 1.Halter JB. Carbohydrate Metabolism. In: Masoro EJ, editor. Handbook of Physiology: Aging. New York: Oxford University Press; 1995. pp. 119–145. [Google Scholar]
- 2.Houmard JA, Weidner MD, Dolan PL, et al. Skeletal muscle GLUT-4 protein concentration and aging in humans. Diabetes. 1995;44:555–560. doi: 10.2337/diab.44.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Goodman MN, Dluz SM, McElaney MA, Belur E, Rudderman NP. Glucose uptake and insulin sensitivity in rat muscle: changes during 3–96 weeks of age. Am J Physiol. 1983;244:E93–E100. doi: 10.1152/ajpendo.1983.244.1.E93. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Kuzuya H, Okamoto M, et al. Change of insulin action with aging in conscious rats determined by euglycemic clamp. Am J Physiol. 1988;254:E92–E98. doi: 10.1152/ajpendo.1988.254.1.E92. [DOI] [PubMed] [Google Scholar]
- 5.Barnard R, Lawani L, Martin D, Youngren J, Singh R, Scheck S. Effects of maturation and aging on the skeletal muscle glucose system. Am J Physiol. 1992;262:E619–E626. doi: 10.1152/ajpendo.1992.262.5.E619. [DOI] [PubMed] [Google Scholar]
- 6.Gulve EA, Henriksen EJ, Rodnick KJ, Youn JH, Holloszy HO. Glucose transporters and glucose transport in skeletal muscles of 1- to 25-month-old rats. Am J Physiol. 1993;264:E319–E327. doi: 10.1152/ajpendo.1993.264.3.E319. [DOI] [PubMed] [Google Scholar]
- 7.Larkin L, Leiendecker ER, Supiano M, Halter JB. Glucose transporter content and enzymes of metabolism in nerve-repair grafted muscle of aging Fischer 344 rats. J Appl Physiol. 1997;83:1623–1629. doi: 10.1152/jappl.1997.83.5.1623. [DOI] [PubMed] [Google Scholar]
- 8.Lipman RD, Dallal GE, Bronson RT. Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F344, BN, and BNF3F1 rats. J Gerontol Biol Sci. 1999;54:B478–B491. doi: 10.1093/gerona/54.11.b478. [DOI] [PubMed] [Google Scholar]
- 9.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of Brown Norway, Brown Norway × Fischer 344, and Fischer 344 × Brown Norway rats with relation to age. J Gerontol Biol Sci. 1996;51A:B54–B59. doi: 10.1093/gerona/51A.1.B54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. J Appl Physiol. 1993;75:972–978. doi: 10.1152/jappl.1993.75.2.972. [DOI] [PubMed] [Google Scholar]
- 11.Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995;268:E902–E909. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo R, Jacot E, Jequier E, Wahren J, Felber J. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- 13.Richter E, Garetto L, Goodman M, Rudderman N. Muscle glucose metabolism following exercise in the rat. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol Biol Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 15.Smith D, Rossetti L, Ferrannini E, et al. In vivo glucose metabolism in the awake rat: tracer and insulin clamp studies. Metabolism. 1987;36:1167–1174. doi: 10.1016/0026-0495(87)90244-7. [DOI] [PubMed] [Google Scholar]
- 16.Serre PA. Citrate synthase. chap 13. In: Lowenstein JM, editor. Methods in Enzymology. New York: Academic Press; 1969. [Google Scholar]
- 17.Klip A, Ramal T, Young DA, Holloszy JO. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987;224:224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- 18.Kahn BB, Pedersen O. Suppression of GLUT-4 expression in skeletal muscle of rats that are obese from high fat feeding but not from high carbohydrate feeding or genetic obesity. Endocrinology. 1993;132:13–22. doi: 10.1210/endo.132.1.8419118. [DOI] [PubMed] [Google Scholar]
- 19.Chomczynski P. A reagent for the single-step simultaneous isolation of RNA, DNA, and proteins from cell and tissue samples. Bio Tech. 1993;15:532–537. [PubMed] [Google Scholar]
- 20.Defronzo RA. Glucose intolerance and aging. Evidence for tissue insensitivity to insulin. Diabetes. 1979;28:1095–1101. doi: 10.2337/diab.28.12.1095. [DOI] [PubMed] [Google Scholar]
- 21.Fink RI, Kolteman OG, Griffin J, Olefsky JM. Mechanisms of insulin resistance in aging. J Clin Invest. 1983;71:1523–1535. doi: 10.1172/JCI110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redhead S. Glucose transporter defects underlie insulin resistance. J NIH Res. 1990;2:58–62. [Google Scholar]
- 23.Kern M, Wells JA, Stephaens JM, et al. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J. 1990;270:397–400. doi: 10.1042/bj2700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 25.Tesch PA. Skeletal muscle adaptations consequent to long–term heavy resistance exercise. Med Sci Sports Exerc. 1988;20:S132–S134. doi: 10.1249/00005768-198810001-00008. [DOI] [PubMed] [Google Scholar]
- 26.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- 27.Lawrence JC, Jr, Colvin J, Cartee GD, Holloszy JO. Effects of aging and exercise on insulin action in rat adipocytes are correlated with changes in fat cell volume. J Gerontol Biol Sci. 1989;44:B88–B92. doi: 10.1093/geronj/44.4.b88. [DOI] [PubMed] [Google Scholar]
- 28.Berthelier C, Kergoat M, Portha B. Lack of deterioration of insulin action with aging in the GK rat: A contrasted adaptation as compared with nondiabetic rats. Metabolism. 1997;46:890–896. doi: 10.1016/s0026-0495(97)90075-5. [DOI] [PubMed] [Google Scholar]
- 29.McDonald RB, Horwitz BA, Hamilton JS, Stern JS. Cold- and norepi-nephrine-induced thermogenesis in younger and older Fischer 344 rats. Am J Physiol. 1988;254:R457–R462. doi: 10.1152/ajpregu.1988.254.3.R457. [DOI] [PubMed] [Google Scholar]
- 30.Barzilai N, Gupta G. Interaction between aging and syndrome X: new insights on the pathophysiology of fat distribution. Ann NY Acad Sci. 1999;892:58–72. doi: 10.1111/j.1749-6632.1999.tb07785.x. [DOI] [PubMed] [Google Scholar]
- 31.Barzilai N, She L, Liu L, et al. Decreased visceral adiposity accounts for leptin effect on hepatic but not peripheral insulin action. Am J Physiol. 1999;277:E291–E298. doi: 10.1152/ajpendo.1999.277.2.E291. [DOI] [PubMed] [Google Scholar]
- 32.Cases JA, Barzilai N. The regulation of body fat distribution and the modulation of insulin action. Int J Obes Relat Metab Disord. 2000;24:S63–S66. doi: 10.1038/sj.ijo.0801508. [DOI] [PubMed] [Google Scholar]
- 33.Friedman JE, Sherman WM, Reed MJ, Elton CW, Dohm GL. Exercise training increases glucose transporter protein GLUT-4 in skeletal muscle of obese Zucker (fa/fa) rats. FEBS Lett. 1990;268:13–16. doi: 10.1016/0014-5793(90)80960-q. [DOI] [PubMed] [Google Scholar]
- 34.Handberg A, Kayser L, Hoyer PE, Voldstedlund M, Hansen HP, Vinten J. Metformin ameliorates diabetes but does not normalize the decreased GLUT 4 content in skeletal muscle of obese (fa/fa) Zucker rats. Diabetologia. 1993;36:481–486. doi: 10.1007/BF02743261. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto T, Fukumoto H, Koh G, et al. Liver and muscle-fat type glucose transporter gene expression in obese and diabetic rats. Biochem Biophys Res Commun. 1991;175:995–1002. doi: 10.1016/0006-291x(91)91663-w. [DOI] [PubMed] [Google Scholar]