Summary
During development, the interaction between tenocytes and myotubes leads to the formation of highly specialized muscle-tendon structural interfaces: myotendinous junctions (MTJs). Structural integrity of MTJs is critical for force transmission from contracting muscle through tendon to bone. We recently developed an in vitro model of three-dimensional (3-D) skeletal muscle-tendon constructs to address mechanisms of the MTJs development. We hypothesized that engineered in vitro 3-D skeletal muscle-tendon constructs would develop MTJs ultrastructurally resembling those found during fetal development in vivo. To test this hypothesis we compared MTJs structures in vivo to those developed in 3-D skeletal muscle constructs co-cultured with engineered self-organized tendon constructs (SOT), or segments of adult (ART) or fetal rat tail (FRT) by means of electron microscopy. Our study showed that at sites of termination some of the myofibers of the engineered 3-D skeletal muscle-FRT and -SOT constructs displayed emerging finger-like sarcolemmal projections surrounded by collagen fibers. These structures resemble fetal MTJs in vivo. Muscle-ART constructs did not develop MTJs. Muscle-FRT constructs in addition to muscle and tendon also demonstrated well developed cartilage, possessing high potential for development into bone. The muscle-FRT construct model could be used for studies of developmental mechanisms involved in the establishment of interfaces among all four muscular-skeletal tissues: muscle, tendon and cartilage/bone.
Keywords: Cell culture, Myoblasts, Tenocytes, Electron microscopy
Introduction
Myotendinous junctions (MTJs) are highly specialized and architecturally complex structures which form at the interface between muscle and tendon (reviewed in Kjaer, 2004). MTJs are important for force transmission from contracting muscle to tendon. Development of MTJs is influenced by the development of both muscle and tendon tissues and their interaction (reviewed in Tozer and Duprez, 2005) as well as cell-cell interactions within muscle and tendon (Benjamin and Ralphs, 2000). Recent data suggest that MTJ development is regulated by the retinoids (Rodriguez-Guzman et al., 2007). The ability to generate force, acquired by muscle during the last weeks of fetal and the first weeks of neonatal development, is of critical importance to the formation of functional MTJs structures. Structural and functional interaction between muscle and tendon cells at the MTJ, especially mechanical force transduction, results in the development of the adult phenotype. During embryonic/neonatal period tenoblasts in the developing tendon become embedded in a three-dimensional (3-D) matrix consisting of several types of collagens (type I, type III and type V), proteoglycans, elastin and fibronectin and are transformed into flat cells (tenocytes) with a large number of cellular protrusions (reviewed in Tozer and Duprez, 2005). Extracellular matrix (ECM) proteins in the tendon, especially collagen type I, as well as tenocytes and their cellular protrusions, are predominantly oriented along the direction of applied force. Neighboring tenocytes are connected with each other via gap junctions (McNeilly et al., 1996) forming an interconnected 3-D network. Type I collagen is the predominant ECM component comprising more than 60% of the tendon’s dry weight (reviewed in Kjaer, 2004). Adult tendon is a collagen-rich, poorly vascularized tissue populated with a small number of flat, mitotically inactive tenocytes (Ahmed et al., 1998). The composition and spatial orientation of ECM proteins and cells in the tendons explains the non-linear stress-strain response and large tangent modulus which characterize this tissue. The structure and composition of ECM in tendons can adapt to changes in mechanical loading applied by muscle contraction (reviewed in Kjaer, 2004). In long-term monolayer cultures, tenocytes lose their differentiation and diminish their ability to deposit type I collagen (Schwarz et al., 1976; Bernard-Beaubois et al., 1997). Muscle development involves fusion of mononucleated myoblasts and formation of elongated multinucleated muscle cells, also called myofibers. Later in development, the contractile proteins of the myofibers, mostly myosin and actin, orient longitudinally, form sarcomeres and acquire the ability to vigorously contract. Myofibers are surrounded by a collagen-rich ECM which is involved in transmission of lateral force between adjacent myofibers. The tapered ends of myofibers are embedded into the ECM of the tendon, forming the MTJ. Myofibers develop extensive folding of sarcolemma at this interface, resulting in a more than ten-fold increase in the surface area between myofibers and tendon. This increase in surface area enables the MTJ to withstand the high mechanical stresses (~1.8−3.5×104 N/m2) generated during muscle contraction (Tidball and Daniel, 1986).
Despite the importance of MTJs in force transmission, the mechanisms involved in the development of MTJs are not yet completely understood. To address this issue, we developed 3-D skeletal muscletendon constructs as an in vitro model to study MTJ development (Larkin et al., 2006). Previously we evaluated 3-D skeletal muscle constructs co-cultured with engineered self-organized tendon constructs (SOT), or segments of adult (ART) or fetal rat tail (FRT; E15) by means of light and fluorescent microscopy and tested tensile strength of the tendon-muscle constructs (Larkin et al., 2006). All three types of 3-D skeletal muscletendon constructs were very similar in structure based on our of light microscopy studies and were able to withstand tensile forces (Larkin et al., 2006). We hypothesized that engineered in vitro 3-D skeletal muscle-tendon constructs develop structurally organized interfaces resembling those found at the MTJ during fetal development in vivo. In the current study we use electron microscopy to further evaluate the ultrastructural characteristics of the constructs and determine which of the three 3-D tendon-skeletal muscle constructs used in the current study develops more advanced MTJs in vitro. We also compare in vitro developed structures with corresponding structures developed in vivo. The results of our study demonstrate that 3-D skeletal muscle-SOT and muscle-FRT constructs are better models for developing muscle-tendon interfaces than muscle-ART constructs. Our data establish 3-D skeletal muscle-tendon construct models as an advantageous tool for elucidating basic biological mechanisms underlying development of MTJs. Different biologically active compounds, for example inhibitors/stimulators of the signal transduction pathways, could be easily used with our model in order to elucidate their effect on the MTJs development.
Materials and methods
Animals
Tissue engineering studies were carried out using female Fischer 344 pregnant rats obtained from the Charles River Laboratories, Inc. (Wilmington, MA). At day E15 of gestation, soleus muscles, Achilles and tail tendons were dissected from the pregnant rats. E15 fetuses were cesarean derived and fetal tails were obtained. Cells isolated from soleus muscles and Achilles tendon were used to form in vitro engineered muscle and tendon constructs, respectively. Adult and fetal tails were used as anchors for muscle-tendon co-cultures. All surgical procedures were performed in an aseptic environment with animals in a deep plane of anesthesia induced by intra-peritoneal injections of sodium pentobarbital. Supplemental doses of pentobarbital were administered as required to maintain an adequate depth of anesthesia. All animal care and animal surgery were in accordance with The Guide for Care and Use of Laboratory Animals (Public Health Service, 1996, NIH Publication No. 85-23); the experimental protocol was approved by the University Committee for the Use and Care of Animals.
Preparation of the solutions, media, culture dishes and anchors
They were the same as described in details previously (Larkin et al., 2006).
Preparation of self organized tendon constructs (SOT)
SOT constructs were engineered as described previously (Larkin et al., 2006). In short: Achilles tendon cells were isolated by dissociating the tendons in type II collagenase (Worthington Biochemical, Lakewood, NJ) solution. After the tissue dissociation cells were pelleted by centrifugation, resuspended in growth medium, expanded in 100 mm diameter tissue culture dishes and then passaged at ~60% confluence. Cells were trypsinized between the 1st and 4th passages and 2×105 cells suspended in 2 mL growth medium were seeded onto each plate and supplemented with Lascorbic acid 2-phosphate (100 µg/mL; Sigma-Aldrich, St. Louis, MO), a stable derivative of ascorbic acid. Fresh ascorbic acid was added each time the growth medium was changed, every 4 days. After approximately 10–14 days, growth medium was substituted for differentiation medium to induce construct formation. The differentiation medium was changed every 3–4 days and fresh ascorbic acid, TGF1 and insulin were added each time. Cell layers delaminated after approximately one month, forming a 15 mm long, 0.5 mm in diameter, construct constrained by the minutien pins. Constructs were cut in half and pinned onto monolayers of muscle cells.
Preparation of muscle and isolation of satellite cells
Both soleus muscles were surgically removed under aseptic conditions, weighed, sterilized in 70% ETOH, and incubated for 5 min in transport Medium. A single-edged razor blade and a pair of forceps were then used to slice each soleus muscle longitudinally into 3 strips. Sylgard treated 35 mm plates were sterilized in 70% ETOH, and muscle slices were pinned to length, two muscle strips per plate. Then plating medium was added to each of the plate, and plates were UV treated for 90 min. The plates were then placed in a 37°C 5% CO2 incubator for 50 h. The remaining muscle strips were then removed from the plates and incubated in Dispase and Collagenase solution. Once the muscle was fully digested, approximately 4 hours, the dissociated cells were filtered through a 100 micron filter and centrifuged at 700 g for 10 min at 25°C. Finally, the supernatant was aspirated from the vials, and the pellet was re-suspended in growth media to obtain a concentration of 10 mg of dissociated muscle per 2 ml media.
Cell culture and muscle-tendon construct formation
Two mL of the cell suspension were plated onto each of the previously prepared laminin-coated culture plates and placed in a 37°C 5% CO2 incubator. Growth media was changed every 48 h until the cells became confluent. At confluence, cells were fed with differentiation media every 48 h until myocytes fused to form spontaneously contracting multinucleated myotubes. Two weeks after the plating of the muscle stem cells, two anchors consisting of either engineered SOT or segments of ART or FRT were pinned onto the muscle cell monolayer ~15 mm apart. About one week later, monolayers rolled up around the anchors and formed cylindrical constructs. Seven to ten days post formation, ~14–17 days of muscle-tendon co-culture, constructs were fixed and prepared for light and transmission electron microscopy as described below.
Light and transmission electron microscopy
For in vivo studies muscle-tendon interfaces were harvested from adult rats and 3–4 day old Fisher rat pups. Specimens of in vivo and in vitro developed muscle-tendon interfaces were fixed in a 2.5% formaldehyde–glutaraldehyde in 0.1 M sodium cacdylate buffer solution, pH 7.4 (Electron Microscopy Sciences, Fort Washington, PA) for 5–12 hours at 4°C. Samples were washed in 0.25 M sucrose and then post-fixed in 1% OsO4 for 1 hour at 4°C. The construct pieces were dehydrated in a graded series of ethanol and absolute acetone at room temperature and then embedded in a mixture of Epon/Araldite using an Eponate 12-Araldite 502 Kit (Ted Pella, Inc., Redding, CA).
For light microscopy, semi-thin sections, 0.5 µm, were cut with an ultramicrotome (Sorvall, Newtown, CT), mounted on glass microscope slides and stained with 1% (w/v) toluidine blue solution. Ultra-thin sections (75–85 nm) were mounted on uncoated copper grids and stained with aqueous uranyl acetate and lead citrate. The ultrastructure of the samples was investigated with a Philips CM100 transmission electron microscope (Philips Medical Systems, Bothell, WA).
Results
Neonatal and adult MTJs in vivo
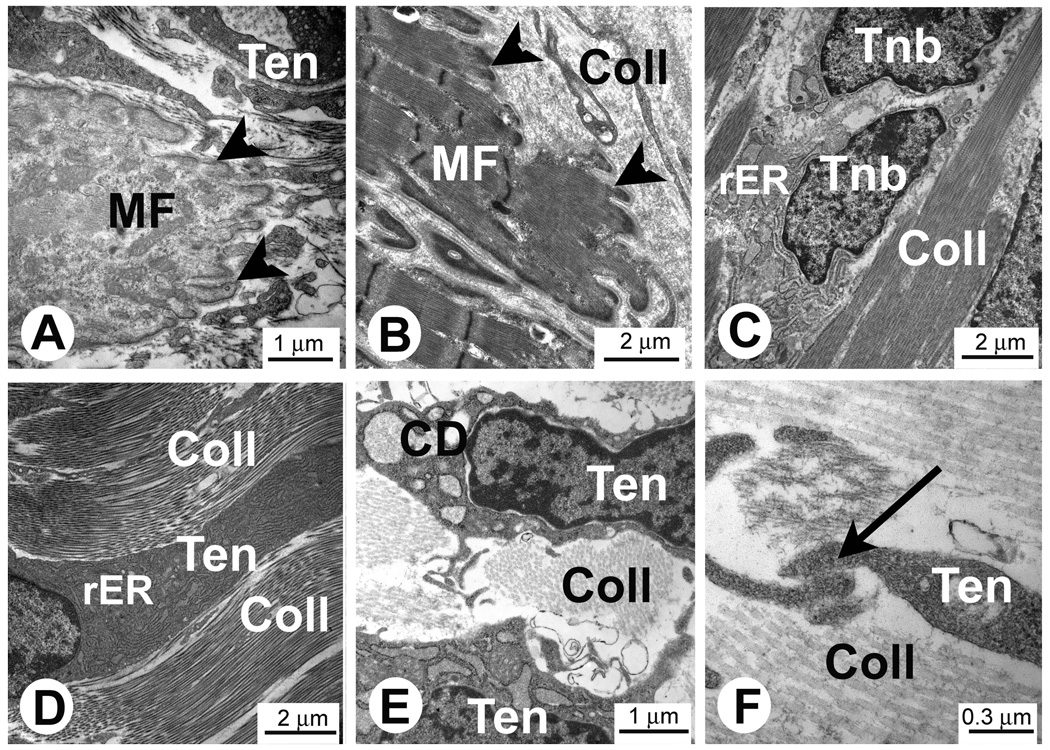
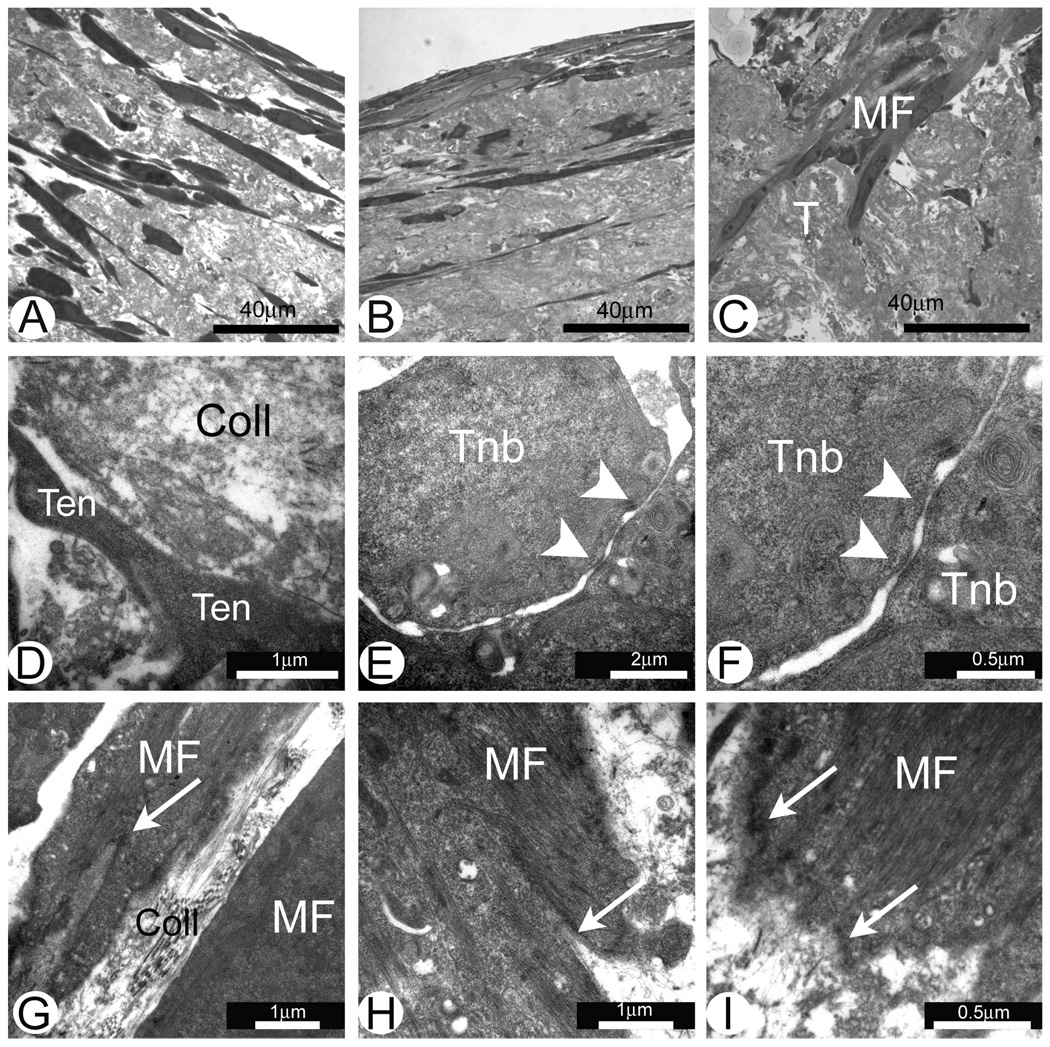
Structural analysis of gastrocnemius muscle MTJs from 3 day old rats (Fig. 1A) showed that at this stage of development MTJs do not have the complex architectural structure found in MTJs of the adult rats (Fig. 1B). Neonatal tendons have high numbers of cells surrounded by a collagen-rich ECM (Fig. 1C–F). Two phenotypically different types of cells can be found in neonatal tendons. The first type of cell resembles tenocytes found in adult tendons. These are flat cells with long cellular protrusions located between bundles of collagen fibers (Fig. 1D). These cells have well developed rough endoplasmic reticulum (rER) (Fig. 1D) and are likely actively synthesizing, storing (Fig. 1E) and depositing matrix proteins. Long cellular protrusions of neighboring tenocytes form well developed cell-to-cell contacts (Fig. 1F). The second type of cell in neonatal tendons has a more rounded shape and these cells could be considered tenoblasts (Fig 1C). Two closely located tenoblasts in Fig. 1C suggest that these cells were produced by a recent cell division.
Fig. 1.
Electron micrographs of the tendon and MTJs in rat muscle in vivo. A and C–F. MTJs of the gastrocnemius muscle from 3 day old rats. B. Adult rat MTJs. Note close interaction of the developing muscle fibers (MF) and tenocytes (Ten) in the area of MTJs. Finger-like protrusions are located at the ends of the muscle fibers in the area of MTJs (arrowheads in Figures A and B). Note longitudinally aligned collagen type I deposits (Coll) separating tenoblasts (Tnb) and tenocytes (Ten) in C–F. C. Two recently divided tenoblasts (Tnb) with large euchromatic nuclei. Note that at this stage of the development tenocytes have large intracellular deposits of collagen (CD in Figure E). Long cytoplasmic processes of tenocytes (Ten) form cell-to-cell contacts (arrow in Figure F).
Fetal and adult tail tendons in vivo
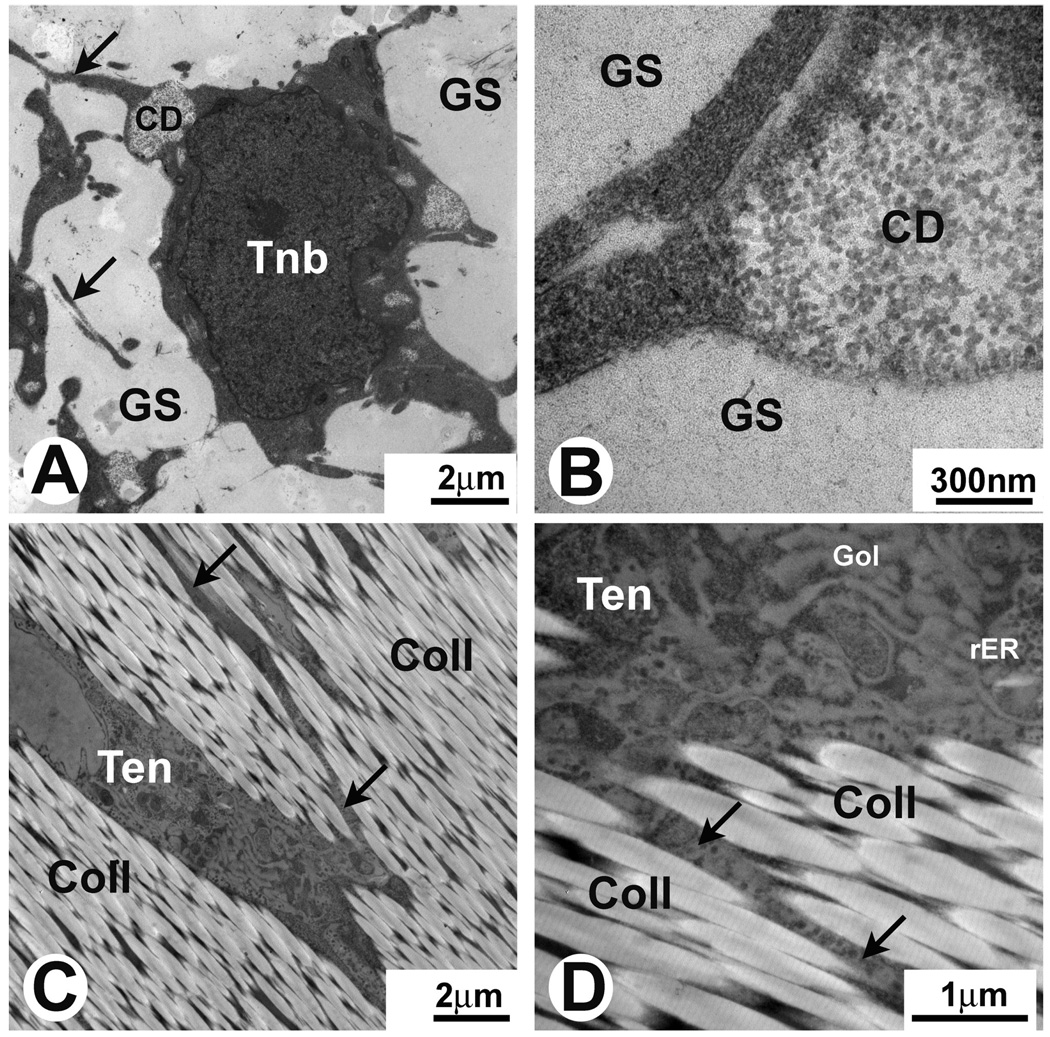
The structure of FRT and ART before placement in culture is shown in Fig. 2. We consider study of these structures in their in vivo state very important since it allows evaluation of changes in these structures that occur when they are placed in culture conditions during muscle-FRT or ART construct formation. On electron micrographs, the developing tenoblasts in FRT had a round shape with multiple small cellular processes (Fig. 2A,B). Cells were embedded in a gel-like ground substance (Fig. 2A,B). The tenoblasts in FRT did not display any preferential 3-D orientation. No extracellular collagen fibrils were detected at this stage of development (Fig. 2A,B). In contrast, tenocytes had multiple areas of developing intracellular collagen fibrils located in the cytoplasm close to the periphery of the cells (Fig. 2A,B). Tenocytes in ART on electron micrographs appeared as elongated cells with definitive longitudinal orientation (Fig. 2C,D) and were embedded in tightly packed collagen type I fibrils displaying a characteristic striation pattern (Fig. 2D). Tenocytes possessed multiple processes located between tightly packed collagen fibrils. The processes extended to great distances from the cell bodies (Fig. 2C,D). The presence of a high number of ribosomes, including those in cellular processes, and extensive Golgi apparatus (Fig. 2D) suggest that tenocytes in ART are actively synthesizing proteins and are participating in the maintenance and repair of the surrounding extracellular matrix.
Fig. 2.
Electron micrographs of FRT and ART in vivo. A and B. Different magnifications of the same area of FRT. Tenoblasts (Tnb) have a round shape and are surrounded by a small amount of ground substance (GS). No collagen fibers are observed in the ECM at this stage of development. Note intracellular deposits of collagen (CD in A and B) in the developing tenoblasts (Tnb) and multiple tenoblast protrusions (arrows in A). C and D. Different magnifications of the same area of ART. Note that elongated tenocytes (Ten) have multiple cellular protrusions (arrows in C and D) surrounded by type I collagen (Coll) with well distinguished striation pattern. Cellular protrusions extend to large distances from cell bodies. Tenocytes have abundant rough endoplasmic reticulum (rER) and extensive Golgi apparatus (Gol).
3-D skeletal muscle-tendon constructs engineered with fetal and adult tail tendons in vitro
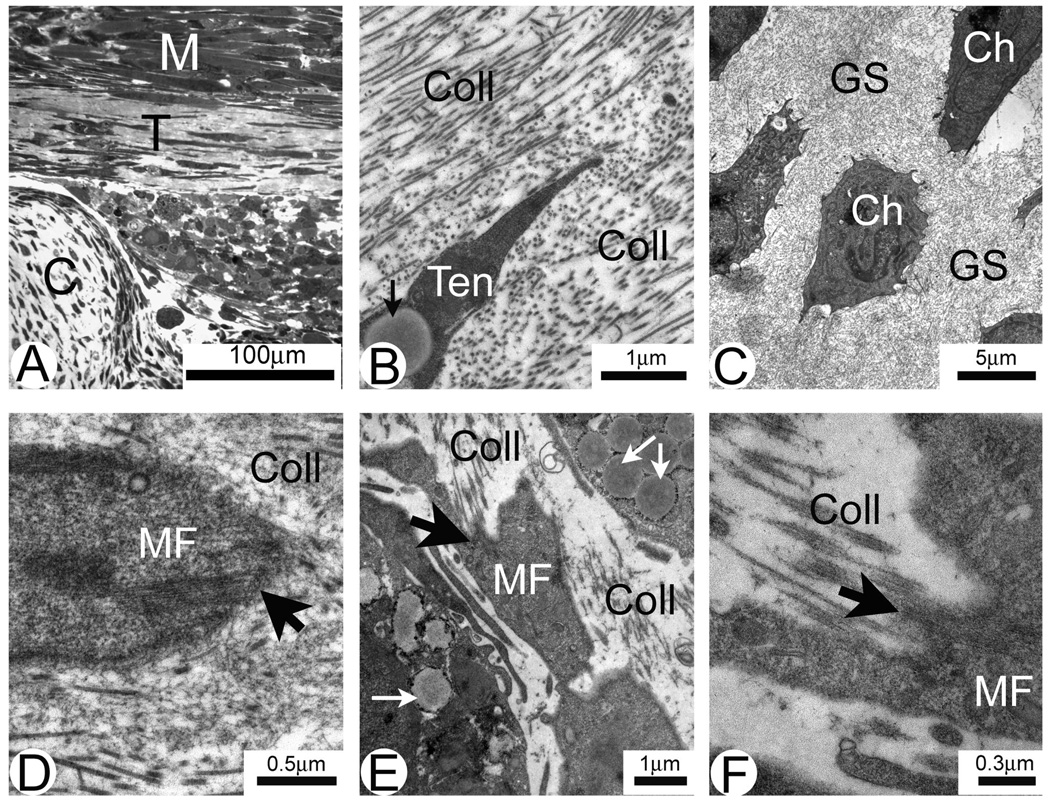
The structure of muscle-FRT constructs was evaluated with light and electron microscopy (Fig. 3). The constructs displayed several well defined tissues: multinucleated myotubes, cartilage and connective tissue/tendon with elongated tenocytes (Fig. 3A). Cartilage of muscle-FRT constructs had a large number of well formed chondrocytes with multiple short cellular processes located in ground substance (Fig. 3C) and was much more developed than the chondrocyte precursors found in FRT in vivo (not shown). On electron micrographs, extracellular type I collagen deposits surrounding tenocytes were well identified (Fig. 3B,E) which represents significant advancement compared with FRT in vivo (Fig. 1A,B). Tenocytes often had clusters of intracellular lipid droplets (Fig. 3B,E). Several MTJ-like structures in different stages of development were identified at the end of the developing myofibers in places of close contact with collagen deposits (Fig. 3D–F). At early stages of development MTJ-like structures were represented by the sub-sarcolemmal densities (Fig. 3D). At later stages developing MTJs had well defined folding of plasma membrane and were surrounded by extracellular type I collagen with the characteristic striation pattern (Fig. 3E,F).
Fig. 3.
Semi-thin section (A) and electron micrographs (B–F) of FRT/muscle construct in vitro. Three distinct areas are identified in the construct shown in A: muscle (M), tendon (T) and cartilage (C). B. Tendon area showing a tenocyte with intracellular lipid droplet (arrow). Collagen fibrils (Coll) surrounding tenocyte are sectioned both in cross and longitudinal directions. C. Developing cartilage. Note large individually positioned chondrocytes (Ch) surrounded by the ground substance (GS). D–F. Different stages of the development of MTJ (large arrows) located at the tapered ends of muscle fibers (MF). Collagen deposits (Coll) in the ECM and intracellular lipid inclusions (small arrows in E) are well identified.
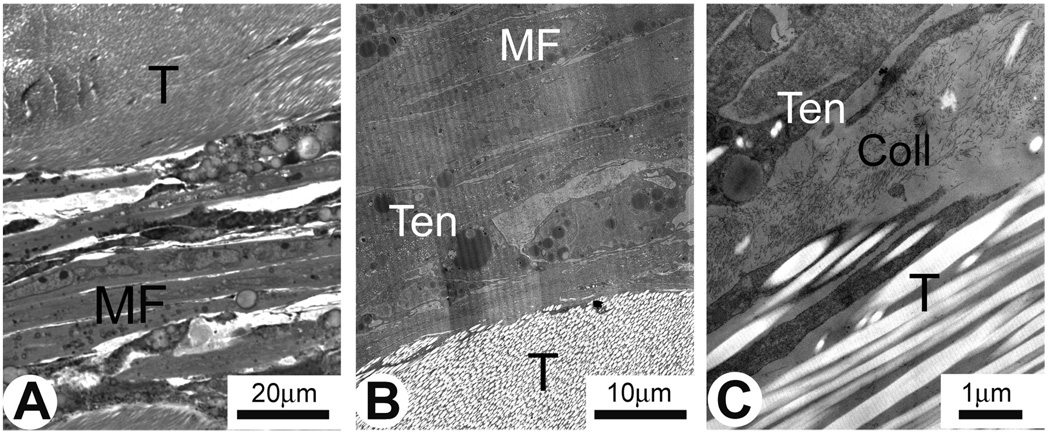
The structure of muscle-ART constructs is shown in Fig 4. In these constructs, myofibers did not form close contact with ART and were separated from it by a layer of collagen deposits and fibroblasts (Fig. 4A–C). ART collagen fibrils were well preserved and displayed a characteristic type I collagen striation pattern (Fig. 4C). Interestingly, the peripheral collagen fibrils in ART were tightly incorporated into the newly deposited collagen-rich matrix (Fig. 4C). There was no direct structural connection between myofibers and ART and here were no MTJ-like structures found in muscle-ART constructs. Therefore muscle-ART constructs are not good models for studies of developing muscle-tendon interfaces.
Fig. 4.
Semi-thin section (A) and electron micrographs (B–C) of ART/muscle construct in vitro. Developing muscle fiber bundles (MF) are separated from ART (T) by newly deposited collagen (A). B. Low magnification electron micrograph of the construct showing three distinct areas: muscle (MF), tenocutes (Ten) and ART (T). C. Higher magnifications of the same construct. Muscle fibers (MF) are separated from ART (T) by newly deposited collagen (Coll) and tenocytes (Ten) with long cellular protrusions.
Engineered self-organized tendons and 3-D skeletal muscle-engineered tendon constructs in vitro
The structure of SOT and muscle-SOT constructs is shown in Fig. 5. SOT consists of collagen-rich deposits and flattened, longitudinally-oriented tenocytes (Fig. 5A). Note the presence of areas of close cell-to-cell contact (Fig. 5D) resembling those found in the developing tendon in vivo (Fig. 1F).
Fig. 5.
Semi-thin section (A) and electron micrograph (D) of SOT only construct and semi-thin sections (B and C) and electron micrographs (E–I) of SOT/muscle construct in vitro. Note that most of the flat tenocytes are oriented longitudinally in parallel with the SOT length (A and B). Tendon only area of SOT/muscle construct is almost identical to the same of SOT only construct (B). Development of the MTJ could be observed at the muscle (MF)/tendon (T) interface (C). Cell-to-cell contacts are formed by the cellular protrusions of two neighboring tenocytes (Ten) (D). Note desmosomal connections (arrowheads in E and F) between two round shape neighboring tenoblasts (Tnb). MTJs (arrows in G–I) at different stages of development could be observed at tapered ends of the muscle fibers (MF). Note collagen type I deposits (Coll) surrounding relatively well developed MTJ (G).
In muscle-SOT constructs three distinct areas were identified: SOT (Fig. 5B), SOT/muscle interfaces (Fig. 5C) and the muscle construct area (not shown). SOT areas of SOT/muscle constructs structurally resembled those in SOT-only constructs (Fig. 5A,B, respectively). The tenoblasts found in the constructs were still mitotically active as shown by two cells that probably have recently divided and were still connected by desmosomes (Figs. 5E.F). Myofibers were tightly integrated into the collagen-rich matrix at the SOT/muscle interface (Fig. 5C, G–I). Developing MTJs were well identified at the tapered ends of myofibers (Figs. 5G–I) and resembled those found in neonatal MTJs (Fig. 1A). Some of the MTJ-like structures had advanced structure with well developed folding of plasma membrane and were surrounded by extracellular type I collagen with the characteristic striation pattern (Fig. 5G).
Discussion
We previously described 3-D skeletal muscle-tendon model engineered in vitro (Larkin et al., 2006). Light microscopy was used to evaluate the structure of the 3-D skeletal muscle-tendon constructs and we determined that all three 3-D tendon-skeletal muscle constructs (muscle-FRT, -ART and -SOT) have sound structural interfaces capable to withstand tensile forces (Larkin et al., 2006). Nevertheless on the light microscopy level it was difficult to evaluate which of the 3-D skeletal muscle-tendon constructs develop interfaces that ultrastructurally resemble those found in the fetal/neonatal MTJs in vivo. Therefore in the current study we used electron microscopy to study MTJs developed in the 3-D skeletal muscle-tendon constructs in vitro and compare them to the fetal/neonatal MTJs developed in vivo.
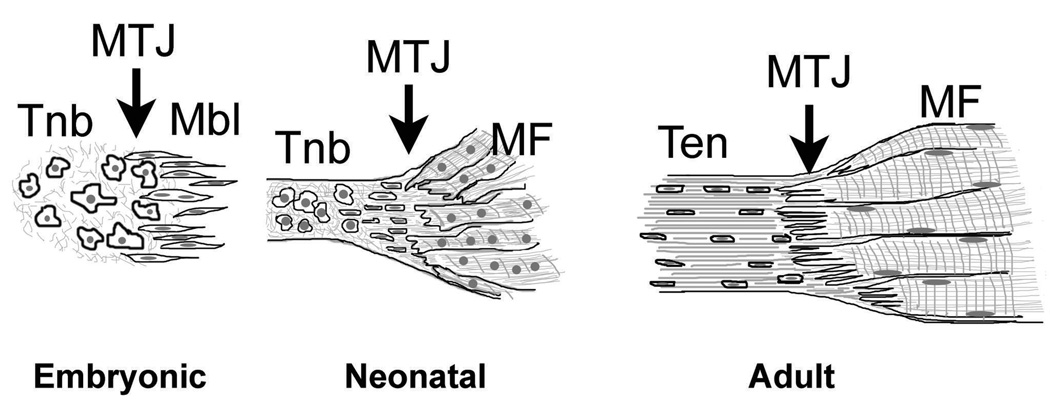
Our studies of MTJs in vivo confirmed previous observations that tendon-muscle development and MTJ formation is a gradual process that starts during embryogenesis and continues into neonatal period (Benjamin and Ralphs, 2000). A characteristic feature of developing tendon in vivo is a high density of large round cells (tenoblasts). Previous studies have shown that rER and Golgi complexes are prominent features of tenocytes during early development when they require the synthesis and deposition of large quantities of ECM, particularly type I collagen (Benjamin and Ralphs, 2000). In agreement with previous observations, our study showed that fetal and neonatal tenoblasts and tenocytes in vivo possess well developed protein synthesis machinery, and actively synthesize and deposit type I collagen into ECM. Tendons can develop autonomously in the absence of muscles however molecules expressed by the muscle may be essential for tendon maintenance (Edom-Vovard et al., 2002). For example, during avian limb development transient tendons are formed in the absence of muscle bellies, but undergo degeneration shortly after the formation by cellular dislocation and cell death (Kieny and Chevallier, 1979; Kardon, 1998). Although the exact mechanisms of tendon development are not well understood, we hypothesize that the tendon’s interactions with muscle, especially the increase in force applied to tendon by muscle contraction during both fetal and neonatal development, helps to determine the tendon’s morphology. A schematic picture of the tendon, muscle and MTJ development is presented in Fig. 6. During embryonic development, non-differentiated tendon and muscle precursor cells cohabitate at the site of future MTJ development. In response to the contractile force of the developing muscle during the late fetal and early neonatal period, tenocytes and extracellular matrix components start to align themselves in the direction of the applied force. As observed during tendon development in vivo as well as in 3-D skeletal muscle-tendon constructs in vitro, neighboring tenocytes which were created through division of the same initial tenoblast, retained close contact through long cytoplasmic processes. Tenocytes begin to form longitudinal rows of cells in the proximity of the MTJ. MTJs are not very developed and tendon-muscle interdigitations are not as extensive and long as in adults. In adult tendon, neighboring rows of cells are separated by large deposits of type I collagen and other extracellular matrix components. Tenocytes have long cell processes and gap junction-based cell-to-cell contacts that allow contact of tenocytes located in different rows. Therefore in the adult state tendon is not simply composed of a number of individual cells dispersed in the ECM but becomes a cohesive structure in which all of the cells are interconnected via long cytoplasmic processes and form an extensive network for cell-to-cell communication (McNeilly et al., 1996). In long-term monolayer cultures, tenocytes loose their differentiation and diminish their ability to deposit type I collagen (Schwarz et al., 1976; Bernard-Beaubois et al., 1997). Recently Schulze-Tanzil and colleagues (2004) used a three-dimensional high-density culture system for long-term cultivation of phenotypically and functionally differentiated human tenocytes. In these cultures tenocytes displayed typical spindle-like shape morphology and sheet-like cytoplasmic processes, expressed tenocyte-specific transcriptional factor-scleraxis and were able to deposit type I collagen, fibronectin and proteoglycans. Neighboring tenocytes formed contacts with each other via gap junctions. Tenocytes in 3-D tendon and skeletal muscle-tendon constructs described in our study were morphologically similar to those reported by Schulze-Tanzil and colleagues as the tenocytes in our study deposited extracellular matrix fibrils some of which had the typical type I collagen cross-striations (Schulze-Tanzil et al., 2004). In addition, in both 3-D tendon and in skeletal muscle-tendon constructs tenocytes developed long sheet-like cytoplasmic processes and formed cell-to-cell contacts via gap junctions.
Fig. 6.
Schematic picture of the MTJ structural development from early embryonic and neonatal to adult stages. MTJ: myotendinous junctions; Tnb: tenoblasts; Mbl: myoblasts; Ten: tenocytes; MF: muscle fibers.
Studies of developing chicken embryos have shown that a specific sequence of events is associated with the formation of MTJs in vivo (Tidball and Lin, 1989). At 9–10 days post fertilization (E9–E10) tenoblasts accumulate at the junctional regions of myogenic cells. At E13 sub-sarcolemmal densities appear and at E15 myofibrils are inserted into these sub-sarcolemmal densities and invaginations of sarcolemma are initiated. At E17, well distinguished MTJs are formed and development of MTJs continues during subsequent days of embryogenesis. Our studies of 3-D skeletal muscle-tendon constructs showed that structurally developed in vitro MTJs resembled those found in chicken embryos in vivo. The main difference was that often in the same construct different myofibers displayed MTJs corresponding to several different stages of development, from E13 to E17. Some myofibers clearly displayed the formation of sub-sarcolemmal densities, insertion of longitudinally oriented myofibrils into sub-sarcolemmal densities and early stages of sarcolemmal invagination corresponding to E13–E16 of development in vivo. Occasionally we observed well developed MTJs and deposits of large striated collagen type I fibrils structurally resembling MTJs found in E17–E18 chicken embryos in vivo (Tidball and Lin, 1989).
In conclusion, 3-D skeletal muscle-FRT and -SOT constructs engineered in vitro develop MTJs with ultrastructural features resembling those found in the fetal/neonatal MTJs in vivo and represent an excellent model for studies of mechanisms implicated in the MTJs development. Muscle- ART constructs do not develop MTJs and therefore have less potential for the future studies. Different biologically active compounds could be easily used with our 3-D skeletal muscle-FRT and -SOT constructs in order to elucidate their effect on the MTJs development. Moreover, 3-D skeletal muscle-tendon constructs could be useful in clinical applications for the repair of damaged muscle-tendon structures. In our current experiments we are using the in vitro developed muscle-SOT constructs to investigate the effect of implantation of these constructs into rat hind limb on further development of the MTJ structures.
Acknowledgements
We thank Ms. Dorothy Sorenson and Dr. Krystyna A. Pasyk from the University of Michigan Microscopy Imaging Laboratory for help with TEM and Michael Wellington for his technical support in fabrication and maintenance of the engineered constructs. This research was supported by NIH R21 award (5R21AR54359-2), by DARPA Biomolecular Motors Program, USAF, AFOSR Award Number FA9550-05-1-0015 and by the NSF, Civil and Mechanical Systems grant #CMS9988693. Sarah Calve was partially supported by a University of Michigan, GE-Rackham Merit Fellowship.
Abbreviations
- MTJs
myotendinous junctions
- ECM
extracellular matrix
- rER
rough endoplasmic reticulum
- SOT
self-organized tendon
- ART
adult rat tail
- FRT
fetal rat tail
References
- Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK. Blood supply of the Achilles tendon. J. Orthop. Res. 1998;16:591–596. doi: 10.1002/jor.1100160511. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int. Rev. Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- Bernard-Beaubois K, Hecquet C, Houcine O, Hayem G, Adolphe M. Culture and characterization of juvenile rabbit tenocytes. Cell. Biol. Toxicol. 1997;13:103–113. doi: 10.1023/b:cbto.0000010395.51944.2a. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev. Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J. Embryol. Exp. Morphol. 1979;49:153–165. [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004;89:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Larkin LM, Calve S, Kostrominova TY, Arruda EM. Structure and Functional Evaluation of Tendon-Skeletal Muscle Constructs Engineered in vitro. Tissue Eng. 2006;12:3149–3158. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J. Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Guzman M, Montero JA, Santesteban E, Gañan Y, Macias D, Hurle JM. Tendon-muscle crosstalk controls muscle bellies morphogenesis, which is mediated by cell death and retinoic acid signaling. Dev. Biol. 2007;302:67–80. doi: 10.1016/j.ydbio.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, Shakibaei M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004;122:219–228. doi: 10.1007/s00418-004-0694-9. [DOI] [PubMed] [Google Scholar]
- Schwarz R, Colarusso L, Doty P. Maintenance of differentiation in primary cultures of avian tendon cells. Exp. Cell. Res. 1976;102:63–71. doi: 10.1016/0014-4827(76)90299-8. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Daniel TL. Myotendinous junctions of tonic muscle cells: structure and loading. Cell Tissue Res. 1986;245:315–322. doi: 10.1007/BF00213937. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Lin C. Structural changes at the myogenic cell surface during the formation of myotendinous junctions. Cell Tissue Res. 1989;257:77–84. doi: 10.1007/BF00221636. [DOI] [PubMed] [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res. C. Embryo. Today. 2005;75:226–236. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]