Abstract
Bacterial group IIC introns are a subclass of group II intron ribozymes that are typically located downstream from transcriptional terminators. Class IIC-attC introns constitute a monophyletic subset of subgroup IIC, which preferentially insert into site-specific recombination sequences for integron integrases (attC). attCs are a diverse family of nucleotide sequences composed of conserved inverted repeats that flank a variable, but palindromic, central region. In this study, we used both PCR and colony patch hybridization methods to determine the basis for recognition of the attCaadA1 stem–loop motif by the Serratia marcescens intron (S.ma.I2) in vivo. The quantitative results showed that mobility into the wild-type site occurs at a frequency of 18%, and is strongly biased by the orientation of the homing site relative to the direction of DNA replication. S.ma.I2 mobility results into mutant attCaadA1 sites are consistent with recognition of stem–loop motifs in unwound DNA. The homing frequency results showed that, while the entire attC sequence is not necessary for recognition of the insertion site, short deletions of the attC stem–loop motif inhibited the intron mobility. Moreover, our data show that S.ma.I2 requires a bulged base in the folded attC stem for high homing frequency. We demonstrate that the IBS1/IBS3 motifs and two bulge bases conserved among attCs determine S.ma.I2 homing specificity for the attC bottom strand. These results suggest that class IIC-attC introns tolerate attC variation by recognition of a bulged hairpin DNA motif rather than a specific sequence.
Keywords: cassette, integron, resistance, retrohoming, ribozyme
INTRODUCTION
Group II introns are retroelements coding for a large structured catalytic RNA and a multidomain intron-encoded protein (IEP), which has maturase, reverse transcriptase (RT) and in some cases endonuclease activities (Lambowitz and Zimmerly 2004). They are found in organelles (from lower eukaryotes and higher plants), and in bacterial and archaeal genomes (Ferat and Michel 1993; Martinez-Abarca and Toro 2000; Bonen and Vogel 2001; Toro 2003; Pyle and Lambowitz 2006). Group II introns share very little sequence similarity but have a conserved intron RNA secondary structure, composed of six structural domains, which coevolved with the intron's RT (Toor et al. 2001). Phylogenetic analyses, based on IEP sequences, allow separation of group II introns into several lineages (chloroplast-like, mitochondrial-like, bacterial classes A–F) (Zimmerly et al. 2001; Simon et al. 2008). Together, the IEP and the spliced intron RNA form a ribonucleoprotein (RNP) complex, which mediates intron mobility and invasion of DNA targets (Zimmerly et al. 1995; Matsuura et al. 1997; Cousineau et al. 1998). The intron RNA catalyzes excision (splicing) from RNA transcripts and integration (reverse splicing) into one strand of the new allele target. The IEP facilitates these reactions by stabilizing the intron RNA structure (maturase activity) and by cleaving the strand opposite to the reverse spliced intron (endonuclease activity) to generate the primer for reverse transcription of the intron RNA (Lambowitz and Zimmerly 2004). However, over half of the IEPs in bacteria lack the endonuclease domain (Toro 2003). It has been shown that nascent strands at DNA replication forks can also serve as primers for reverse transcription of the intron RNA (by the IEP's RT activity) in the absence of second strand cleavage (Martinez-Abarca et al. 2000; Jimenez-Zurdo et al. 2003; Munoz-Adelantado et al. 2003; Zhong and Lambowitz 2003). This mechanism of mobility shows a pronounced strand bias and dependence on replication. Homing site recognition uses both the IEP and base pairing of the intron RNA with a specific DNA sequence (Guo et al. 1997). In a first step, the RNP binds DNA nonspecifically and then searches for a small number of specific bases in the distal 5′-exon and 3′-exon regions of the target site via major groove interactions. These base interactions enhance local DNA unwinding and a conformational change associated with identification of the specific binding site (Singh and Lambowitz 2001). In a second step, two exon binding sites (EBS1 and EBS2), located in the intron RNA, are involved in 5′ exon recognition through base pairing interactions with the target's intron binding sites (IBS1 and IBS2). Similarly, the 3′ exon is recognized by either the δ–δ′ or EBS3–IBS3 interactions (Jacquier and Michel 1987; Michel and Ferat 1995).
Bacterial class IIC introns are a phylogenetic subclass of group II introns that exhibit unique RNA structure (e.g., the EBS2 motif is missing; they have shorter EBS1–EBS3 motifs and a shorter catalytic domain 5) (Toor et al. 2001; Toor et al. 2008) and self-splicing properties (e.g., they generally self-splice in vitro through a hydrolytic pathway resulting in linear intron intermediates) (Podar et al. 1998). Furthermore, it was shown that a stem–loop motif located a few nucleotides upstream of the IBS1 was implicated in recognition of the 5′ exon during both splicing and reverse-splicing events (Toor et al. 2006; Robart et al. 2007). The targeted stem–loop structures are mostly transcriptional terminators (Granlund et al. 2001), which show low levels of sequence identity but have conserved stem–loop structures. Interestingly, a specialized lineage within class C introns, named class IIC-attC introns, preferentially inserts into the inverted repeats of integron attC sites (Centron and Roy 2002; Quiroga et al. 2008).
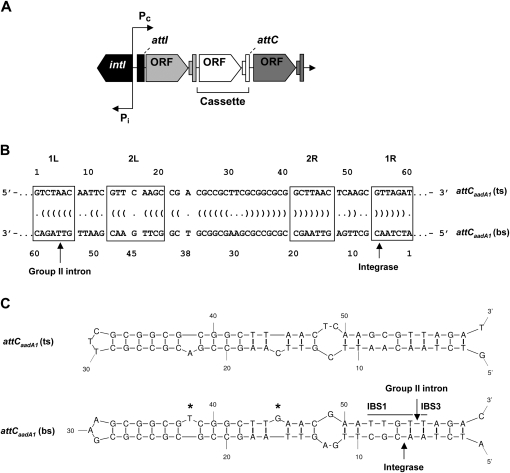
attC sites (or 59-base elements) are site-specific recombination sequences located at the 3′ end of integron gene cassettes (Hall et al. 1991). Integron integrases mediate site-specific recombination of DNA sequences flanking open reading frames or structural genes (e.g., drug resistance genes) using two distinct sites, a proximal attI and a cassette-associated attC site. The key elements of integrons are: an integrase gene (intI), a promoter for expression of cassettes (Pc), and a recombination site (attI) (Fig. 1A; Stokes and Hall 1989; Mazel 2006). Alignment of different attCs shows that they vary in length from 57 to 141 base pairs (bp), and that their sequence similarities are primarily restricted to the boundaries, which correspond to two inverted integrase-binding repeats, 1L-2L separated by a spacer of 5 bp and 2R-1R separated by 6 bp (Fig. 1B; Francia et al. 1997; Stokes et al. 1997). The central part between the 2L and 2R repeats varies both in length and sequence but is an imperfect inverted repeat. It was shown by electrophoretic mobility shift assays (EMSA) that integron integrase preferentially binds to single-strand DNA of the attCs (Francia et al. 1999). Interestingly, binding of integrase is strand specific for the bottom strand (bs) of the attC. It was suggested that formation of a cruciform (i.e., stem–loop structure on each strand) and imperfections in the inverted repeats (Fig. 1B) were of importance for attC site recognition by integrase and for further recombination reactions (Hall et al. 1991; Stokes et al. 1997; Francia et al. 1999). A recent study demonstrates that integrase specificity for the attC bottom strand is due to the presence of two conserved asymmetrical bases (T and G) that bulge out from the folded DNA sequence (Fig. 1C; Johansson et al. 2004). The crystal structure of the VchIntIA-VCRbs synapse (class 4 integron integrase monomers bound to two antiparallel VCRbs duplexes) showed that integrase binds its attC substrate as a dimer (MacDonald et al. 2006). The extrahelical base T is bound by cis-interaction with one integrase subunit, and is important for DNA site recognition, whereas the extrahelical base G is bound by trans-interaction with a second integrase subunit bound to the other VCRbs. The trans-interactions are important features to hold the synaptic complex together during recombination.
FIGURE 1.
Integron structure and attC recombination sites. (A) General schematic representation of an integron. intI, integrase gene; Pi, integrase promoter; Pc, cassette promoter; attI, integrase recombination site (integrase specific); ORF, cassette open reading frame; attC, integrase recombination site (cassette specific). (B) Sequence of the attCaadA1 site. 2L-2R and 1L-1R boxes are inverted repeats; arrows, integrase recombination site and intron insertion site; Vienna output (dots and parentheses between the top-strand [ts] and bottom-strand [bs] sequences), base pairings from a potential single-stranded secondary structure based on MFOLD prediction. (C) Predicted stem–loop structures of the attC top- and bottom-strand sequences based on MFOLD predictions. IBS1 and IBS3, intron binding sites 1 and 3; asterisk (*), extrahelical bases T38 and G45 upon folding of the attC bottom strand.
To address how bacterial class IIC-attC introns insert specifically into the bottom strand of attC sites, we used the S.ma.I2 intron in a two-plasmid mobility assay. S.ma.I2 was found in a class 1 integron, from the multiresistant clinical isolate Serratia marcescens SCH909, adjacent to the aadB (aminoglycoside resistance) gene cassette (Centron and Roy 2002). We generated several mutants of the attCaadA1 site from the aadA1-qacE cassette junction in Tn21 (Fig. 1B) in order to find stem–loop features that influence intron homing. We used this site because it is widespread among plasmid-borne cassettes and is almost a perfect palindromic sequence (Fig. 1C), but most importantly because of previous observations in vitro demonstrating specific binding of integrase to the bottom strand of attCaadA1 (Francia et al. 1999; Johansson et al. 2004; Bouvier et al. 2005). The quantitative results showed that mobility into the wild-type (WT) site occurs at a frequency (the fraction of homing site plasmids containing introns) of 18% and is strongly biased by the orientation of the homing site relative to the direction of DNA replication. S.ma.I2 mobility results into mutant attCaadA1 sites are consistent with recognition of stem–loop motifs in unwound DNA, as in the Bacillus halodurans class IIC intron (Robart et al. 2007). The homing frequency results showed that, while the entire attC sequence is not necessary for recognition of the insertion site, short deletions of the attC stem–loop motif inhibited the intron mobility. Moreover, our data show that S.ma.I2 requires a bulged base in the folded attC stem for high homing frequency. We demonstrate that the IBS1/IBS3 motifs and two bulge bases conserved among attC sites are determinants for S.ma.I2 homing specificity for the attC bottom strand.
RESULTS
In vivo assay of mobility into the wild-type attCaadA1 site
To address how bacterial class IIC-attC introns insert specifically into the bottom strand of attC sites, we used an Escherichia coli two-plasmid mobility assay in which S.ma.I2 inserted into WT or mutant attCaadA1 sites (Figs. 2, 3). For each target, oligonucleotides of both complementary DNA strands were annealed, cloned, and tested as homing sites (Supplemental Table 1). Intron mobility was screened using a qualitative PCR method in combination with a quantitative colony patch hybridization method to measure and compare intron mobility frequencies into WT and mutant attCaadA1 sites (Table 1; see Materials and Methods). Three targets were cloned in both orientations such that reverse transcription of the inserted intron RNA could potentially use either a nascent lagging (LAG) or leading (LEAD) DNA strand as a primer at the replication fork. These clones were tested in order to detect a probable strand bias for homing in our experiment. It was previously reported that different group II introns with IEPs lacking the endonuclease domain prefer either a nascent lagging strand (Martinez-Abarca et al. 2004) or leading strand (Zhong and Lambowitz 2003) as a primer. Figure 4 shows the orientation of intron insertion using a highly sensitive PCR-based approach, whereas Figure 5 shows degrees of intron insertion (semiquantitatively) without information on the orientation. Mobility assays with the WT site show that S.ma.I2 homes specifically into the attC bottom strand (Fig. 4). Sequencing of the PCR amplicon confirmed that S.ma.I2 used the expected 5′-TTGT/T sequence (IBS1/IBS3, respectively) as its intron binding sites (data not shown) (Quiroga et al. 2008). However, comparison of intron mobility levels into the WT (LAG) and WT (LEAD) sites is consistent with a replication orientation bias (Fig. 5). Indeed, quantitative results suggest that reverse transcription of the inserted intron RNA preferred a nascent lagging (LAG) strand DNA as a primer over a leading (LEAD) strand DNA (18.0 ± 2.5% and <1.0% homing frequencies, respectively) (Table 1). Figure 4 shows that despite the low homing frequencies observed using the LEAD construct, mobility events into the WT (LEAD) site were amplified by PCR. The identities of the amplicons were confirmed by sequencing (data not shown). In order to find the structural determinants for recognition of the attCaadA1 site, we designed and cloned 26 attCaadA1 mutants and tested them as potential target sites for S.ma.I2, taking into account the effect of homing site orientation in pACYC184 (Fig. 3).
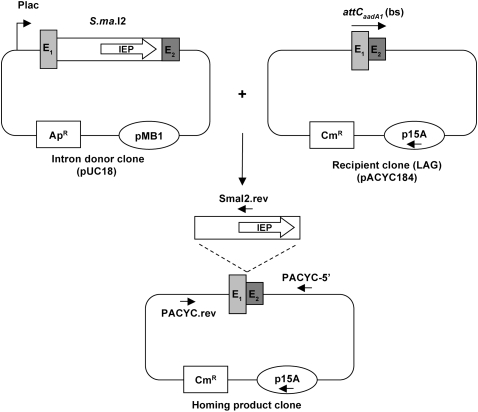
FIGURE 2.
Schematic of the two-plasmid mobility assay in E. coli (see the Materials and Methods section for a more detailed protocol). E1 and E2, exon sequences; IEP, S.ma.I2 intron encoded protein; Plac, lac promoter; ApR and CmR, ampicillin and chloramphenicol resistance genes; pMB1 and p15A, plasmid replication origins.
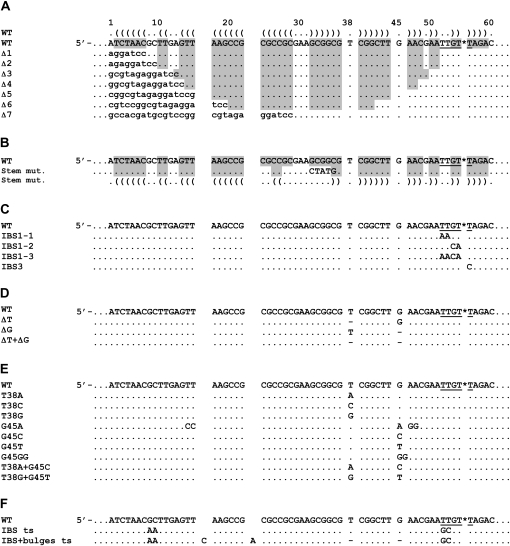
FIGURE 3.
Sequence alignments of the attCaadA1 mutants tested as homing sites in this study. (A) Mutation of the stem lengths; (B) mutation of the stem pairings; (C) mutation of IBS1 or IBS3; (D) deletion of the extrahelical bases; (E) mutation of the extrahelical bases; and (F) inversion of the intron binding sites with or without the extrahelical bases. The figure shows only the bottom-strand sequences of the wild-type (WT) and mutant attCaadA1 sites. Capital letters, attC sequences; lower case letters, pACYC184 sequences; gray shading and Vienna outputs, nucleotide pairings from a potential single strand stem–loop structure determined using MFOLD. Asterisk (*), intron homing site; underlined bases, IBS1 (TTGT) and IBS3 (T); dot, sequence identity to WT; dash, base deletion.
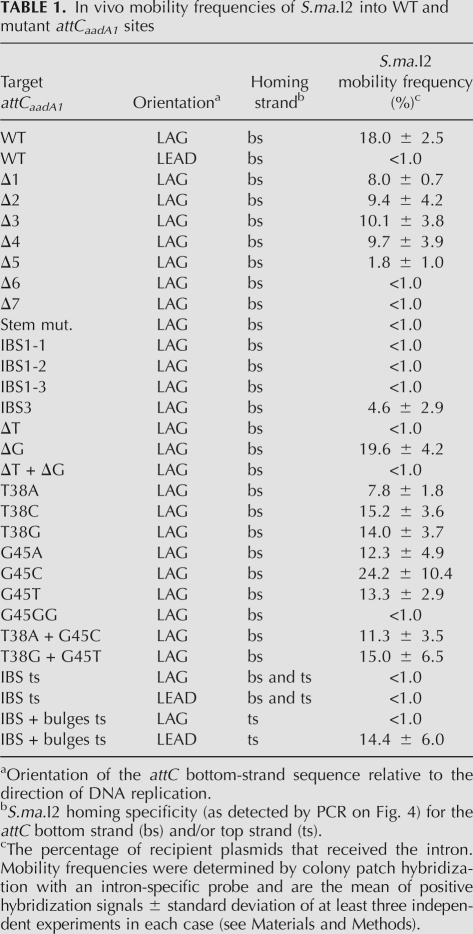
TABLE 1.
In vivo mobility frequencies of S.ma.I2 into WT and mutant attCaadA1 sites
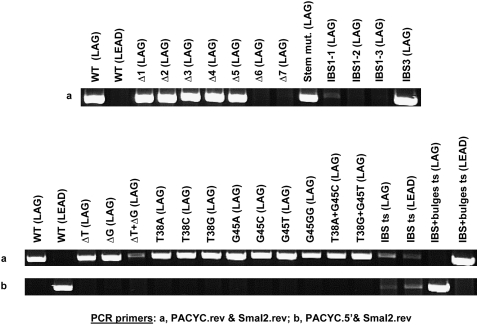
FIGURE 4.
S.ma.I2 in vivo mobility results into attCaadA1 mutants. Agarose gels of the PCR products using primer pair “a” or “b” in order to detect the orientation of intron insertion. The figure shows the 5′ intron–exon integration junction (see Fig. 2 for primer position). “WT,” “IBS ts,” and “IBS + bulges ts” attCaadA1 were cloned in both orientations (LAG or LEAD) relative to the direction of DNA replication in pACYC184 (see Materials and Methods).
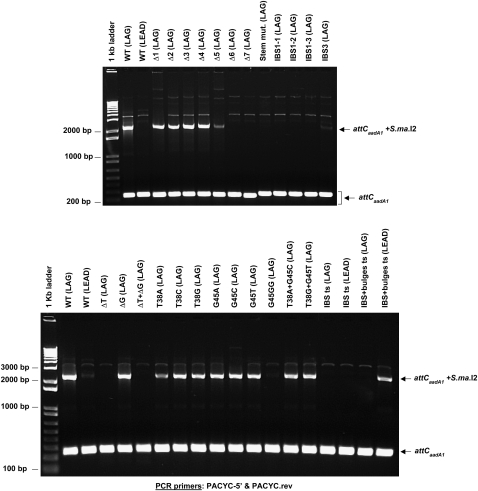
FIGURE 5.
S.ma.I2 in vivo mobility results into attCaadA1 mutants. Agarose gels of the PCR products using PACYC-5′ and PACYC.rev primers in order to detect variations in the degree of intron insertion for each mutant compared with the WT site (see Fig. 2 for primer position). The Figure shows attCs that were and were not targeted by S.ma.I2.
Mutation of the 5′-exon stem lengths
The attCaadA1 bottom strand is almost a perfect palindromic sequence except for the “spacer” bases (between the 1L-1R and 2L-2R boxes), the two conserved asymmetrical bases (T38 and G45) and the central loop (GAA) (Fig. 1C). According to the predicted secondary structure, we designed seven stem–loop mutants (Δ1–Δ7), which are 5′-end truncations of the WT attCaadA1 bottom strand sequence (homing strand) (Fig. 3A). The homing results showed that the minimal target was a 44-bp attCaadA1 (mutant Δ5) that includes 39 bp of 5′ exon (Fig. 4). The 5′ exon sequence can fold into a stem (12 bp) and loop (3 nucleotides [nt]) motif, which is separated from the IBS1 sequence (5′-TTGT) by a 7-nt spacer (Fig. 3A). For this target, residual S.ma.I2 mobility was 1.8 ± 1.0%, whereas 18.0 ± 2.5% mobility was observed for the WT attCaadA1 (Table 1). Further deletion resulted in the absence of detectable mobility into the homing site (Fig. 4). The homing frequencies showed that, while the entire attCaadA1 sequence is not necessary for intron homing, even short deletions, like the 7-bp Δ1 mutant, inhibited the intron mobility (8.0 ± 0.7%). Interestingly, base deletions of the attC ranging from the 1R to the beginning of the 2R inverted repeats (Δ1–Δ4) resulted in a ∼50% (mean) diminution of the homing reaction in vivo. Additional deletions, including half (Δ5), or all, of the 2R site (Δ6 and Δ7), resulted in a ∼90% diminution or a complete inhibition of the in vivo mobility, respectively.
Mutation of the stem pairings
For this mobility assay, we substituted five nucleotides in attCaadA1 in order to disrupt the stem before the central loop (Fig. 3B). It was shown that an identical stem mutation on attCaadA7 inhibits the recombination frequency by integrase (Bouvier et al. 2005). Here, intron mobility into the attC bottom strand was detected by PCR (Fig. 4) but quantitative results showed an important loss of efficiency (<1.0%) (Table 1). This result was confirmed by the absence of PCR amplicon using two pACYC184-specific primers that flank the attC site (Fig. 5).
Mutation of IBS1 or IBS3
Retrohoming of group II introns is normally highly specific because of base pairings between the intron RNA and the DNA exons. Bacterial class IIC intron base pairings are limited to the short IBS1-EBS1 and IBS3-EBS3 interactions (Robart et al. 2007). Here, we designed three IBS1 motif mutants (IBS1-1 to IBS1-3) and one IBS3 mutant (IBS3) (Fig. 3C). PCR results confirmed the importance of a specific IBS1 sequence (5′-TTGT) and, to a lesser proportion, a specific IBS3 (T) base. Indeed, a weak PCR amplicon was obtained only for the first IBS1 mutant (IBS1-1) and a strong PCR amplicon for the unique IBS3 mutant (IBS3) (Fig. 4). Hybridization results confirmed the PCR results obtained with the IBS1-1 and IBS3 mutants, respectively, as the corresponding homing frequencies were <1.0% and 4.6 ± 2.9% (Table 1).
Deletion of the extrahelical bases T38 and G45
Quantitative results of mobility of S.ma.I2 into attCaadA1 with mutated 5′-exon stems (above) showed that short disruptions of the stem pairings strongly decrease the mobility frequency. To assess whether inhibition of mobility into stem mutants is a consequence of the absence of the extrahelical bases (T and G), we cloned three attC mutants deleting either the T38 or G45 bulge bases or both (Figs. 1C, 3D). Deletion of the thymine at position 38 (T38) resulted in the inhibition of the homing reaction in attCaadA1 in vivo (Fig. 5). The mobility frequency of S.ma.I2 into the ΔT mutant was >18-fold lower than into the WT (LAG) site (Table 1). Nevertheless, Figure 4 shows that we were able to amplify homing events into the ΔT mutant bottom strand, showing that the T38 bulge base is not essential for attC site recognition but enhances the mobility frequency. Sequencing of the PCR amplicon from the 5′ exon–intron integration junction within the ΔT mutant (Fig. 4) confirmed the specificity of the intron for the homing site 5′-TTGT/T (data not shown). Deletion of the guanine at position 45 (G45) had no effect on target site recognition by the intron. The ΔG mutant had mobility frequencies similar to those of the WT (LAG) site. Deletion of both T38 and G45 bases (ΔT + ΔG mutant) resulted in mobility frequencies comparable to ΔT alone, and a weak PCR amplicon corresponding to specific bottom-strand insertion was observed (Fig. 4).
Mutation of the extrahelical bases
Compilation of several integron cassette attC sites and folding of the bottom-strand sequence into the most probable stem–loop motifs showed that the two asymmetrical bases in the stem are a conserved thymine and guanine (Stokes et al. 1997; MacDonald et al. 2006). In order to determine if S.ma.I2 homing involves a specific bulge base, nine different mutants of the extrahelical bases T38 and G45 were examined (Fig. 3E). We observed that substitution of T38 with an adenine (T38A mutant) resulted in a more than twofold decrease of the intron mobility frequency compared with WT (LAG) (Fig. 5; Table 1). However, such differences were not observed with the T38C and T38G mutants. Substitution of G45 with any of the alternative bases (G45A, G45C, and G45T mutants) did not alter intron homing (Fig. 5). In the G45A mutant, additional mutations where made in order to prevent bulging of one of the two adjacent adenines (A46 or A47) instead of the G45A in the folded structure. In the G45GG mutant, another guanine was inserted next to the G45. This unusual mutant was tested as a homing site because it was previously shown that a GG bulge mutant of the attCaadA1 resulted in a complete loss of IntI1 integrase binding in vitro (Johansson et al. 2004). Strikingly, this mutant displayed <1.0% homing efficiency, whereas the ΔG mutant showed homing levels comparable to those of WT (LAG). Figure 4 shows that we successfully amplified homing events into the GG mutant bottom strand. Sequencing confirmed the specificity of the intron for the homing site 5′-TTGT/T (data not shown). Finally, intron mobility was tested using attCaadA1 mutants for which extrahelical bases were either changed for the complementary base (T38A + G45C) or exchanged between themselves (T38G + G45T). The T38A + G45C mutant showed a weak (∼1.5-fold) decrease of homing frequency compared with WT (LAG) (most probably due to the T38A mutation), whereas no inhibition of the mobility was observed with the T38G + G45T mutant (Table 1).
Inversion of the intron binding sites with or without the extrahelical bases
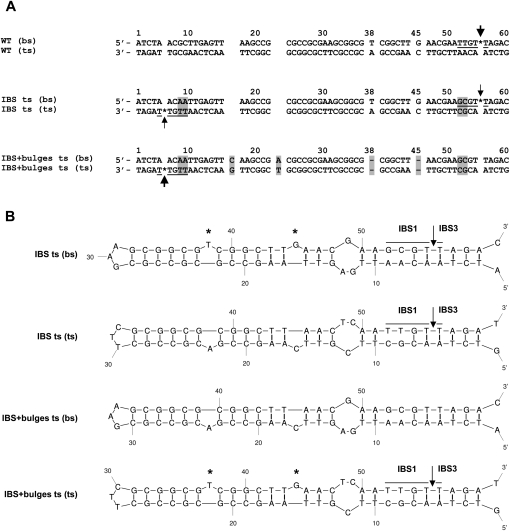
Class IIC–attC introns identified so far are specifically inserted into attC bottom strands. Quantitative results show the unexpected importance of the T extrahelical base for a high level of mobility into attCaadA1 (above). A parallel can be drawn with integron integrase, for which the T base dictates the position of the integrase monomer along attC bottom strands (MacDonald et al. 2006). It was shown that minor alterations of the attCaadA1, involving nested inversions of both the bulge bases (T and G) and central loop, resulted in strong integrase binding to the top strand with the reciprocal loss of binding to the bottom strand (Johansson et al. 2004). Here, in order to change the S.ma.I2 bottom strand specificity, two minimal attCaadA1 inversions were examined (Fig. 3F). First, intron-binding sequences, IBS1 and IBS3 (5′-TTGT/T), were symmetrically moved from the attC bottom strand to the top strand in the “IBS ts” (LAG) mutant. This inversion leads to an >18-fold decrease of mobility compared with WT (LAG) (Table 1). Interestingly, Figure 4 shows two weak PCR amplicons corresponding to mobility events that occurred in either strand. Similar homing frequencies (<1.0%) and PCR results were obtained when the orientation of the target site was inverted relative to the direction of plasmid replication (“IBS ts” LEAD). Sequencing of the two PCR amplicons obtained from either LAG or LEAD mutant clones confirmed intron mobility into the new top strand IBS motifs, 5′-TTGT/T (IBS1/IBS3), but also into the bottom strand sequence, 5′-GCGT/T (IBS1/IBS3) (Fig. 6) corresponding to the mutated wild-type IBS motifs (5′-TTGT/T → 5′-GCGT/T). Second, intron-binding sequences and bulge bases (T38 and G45) were symmetrically moved from the attC bottom strand to the top strand within the “IBS+bulges ts” (LAG) mutant (Fig. 3F). These mutations led to an important inhibition of mobility into the attC site (<1.0% homing efficiency), but strong PCR amplicons corresponding to specific top strand mobility events were observed (Fig. 4). Cloning of the same attC mutant into the opposite orientation (“IBS + bulges ts” LEAD), increased the intron mobility frequency to 14.4 ± 6.0%, comparable to WT (LAG) (Table 1). This increase of mobility is related to the same replication orientation bias observed with the WT attC site. Similarly, PCR amplification of the target site shows an increase of mobility events relative to the “IBS + bulges ts” (LAG) mutant (Fig. 5). Figure 4 shows a switch in the intron specificity for the attC top strand and the absence of mobility events into the bottom strand. Sequencing of the PCR amplicons confirmed the top-strand homing and specificity of the insertion site into the new IBS motifs (Fig. 6). Therefore, moving the IBS1-IBS3 sites to the opposite strand of the attC site retains high-level mobility only when accompanied by the bulge bases and the correct orientation with regard to DNA replication.
FIGURE 6.
(A) Alignment of the sequenced PCR products from the S.ma.I2 mobility assays into the WT, “IBS ts,” and “IBS + bulges ts” attCaadA1 sites. Arrows indicate the intron homing site determined by PCR (Fig. 4). The thickness of the arrows corresponds to the mobility frequencies observed in Table 1. Asterisk (*), intron homing site; underlined bases, intron binding site; highlighted bases, difference from the WT sequence; dash, base deletion. (B) Predicted stem–loop structures of the attCaadA1 mutant top- and bottom-strand sequences based on MFOLD predictions. IBS1 and IBS3, intron binding sites 1 and 3; asterisk (*), extrahelical bases T38 and G45 upon folding of the attC bottom strand.
DISCUSSION
In this study, we searched for stem–loop features of attCaadA1 that could explain the site specificity of class IIC-attC introns. Our results, based on qualitative PCR and quantitative hybridization methods, indicate that target specificity of S.ma.I2 occurs by recognition of short intron-binding sites (5′-TTGT/T as IBS1/IBS3, respectively) adjacent to a bulged hairpin structure within the attCaadA1 bottom strand. This hairpin structure implies that the inverted-repeat motifs (1L-1R and 2L-2R), conserved among attC sites, could form a robust double-strand DNA-like structure in vivo, which can be bound by nonspecific phosphate–backbone interaction or specific base contacts by the intron RNP amino acids. It was shown for several group II introns, that in the absence of second-strand cleavage, the intron RNA reverse splices at the DNA replication fork into either single-stranded or double-stranded DNA and then uses a nascent strand as primer for reverse transcription (Ichiyanagi et al. 2002; Zhong and Lambowitz 2003; Martinez-Abarca et al. 2004). We found that homing of S.ma.I2 displays a strong replication orientation bias and predominantly occurs using a nascent lagging DNA strand as a primer for reverse transcription of the inserted intron RNA. This bias reflects the absence of second-strand cleavage, because of the missing endonuclease domain of the IEP (Centron and Roy 2002), and suggests that S.ma.I2 reverse splices into single-stranded DNA targets at the DNA replication fork (Ichiyanagi et al. 2002; Zhong and Lambowitz 2003; Coros et al. 2005). Together, these results support the hypothesis that the DNA replication fork (rather than transcription bubbles) is the most probable source of single-strand DNA, allowing the attC bottom strand to fold into a stem–loop motif used by class IIC-attC introns as a structural determinant for target site recognition. In addition to group II introns, other mobile elements, including the IS605 group of insertion sequences and transposon Tn7, which use different mobility pathways, demonstrate lagging-strand preference during transposition (Peters and Craig 2001; Barabas et al. 2008).
The crystal structure of the VchIntIA-VCRbs complex confirms that the integrase binds the attC bottom strand as a DNA stem–loop with extrahelical bases (MacDonald et al. 2006). Our results suggest that S.ma.I2 uses the conserved attC structure in order to compensate for the shortened sequence-specific IBS1-EBS1 interaction and the missing EBS2-IBS2 motifs, which are normally involved in 5′ exon recognition. Therefore, there are two major determinants for attCaadA1 recognition by S.ma.I2 in vivo. First, a four-base IBS1 (TTGT) and a one-base IBS3 (T) base pair to complementary intron RNA exon-binding sites (as predicted by its secondary structure), and are conserved among several targeted attC sites (Quiroga et al. 2008). We showed that mutations of the IBS1 and (to a lesser degree) IBS3 motifs strongly inhibited the homing reaction into attCs in vivo. Second, there is an imperfect stem–loop motif adjacent to the intron binding sites. Compilation of insertion sites of several subgroup IIC introns shows that a stem–loop structure is located a few bases (≤8) before the intron binding sites (Robart et al. 2007). Assays of homing into mutated attCaadA1 sites showed mobility into a minimal 5′-exon sequence folded into a 12-base stem and a three-base loop motif. The IBS motifs and the minimal 5′-exon sequence requirement identified in this study is consistent with the consensus structure for several class C targets (Robart et al. 2007). We showed that deletion of the 1R region of attC (Δ1) resulted in a ∼50% diminution of the homing reaction in vivo (Table 1), while deletion of the region between 1R and 2R (Δ2–Δ4) gave no further loss of mobility. Additional deletions including half (Δ5) or the entire 2R site (Δ6 and Δ7) resulted in a ≥90% inhibition of mobility. Further studies showed that mutations disrupting the stem before the central loop led to an important inhibition of intron mobility (Table 1). Interestingly, an identical stem mutation on attCaadA7, which differs by two nucleotides from attCaadA1 (Johansson et al. 2004), leads to a 10-fold decrease of the class 1 integrase recombination frequency (Bouvier et al. 2005). Together, these results show that correct folding of the attC bottom strand is essential for substrate recognition, and suggest that S.ma.I2 makes DNA–protein contacts with the same attC site determinants (1L-1R and 2L-2R subsites) as integron integrase (MacDonald et al. 2006; Johansson et al. 2009).
Sequence variation between the 2L and 2R repeats of several attC sites reveals a lack of absolute sequence requirement in the stem except for two conserved extrahelical bases (T and G) that are usually separated by a six-base spacer (Stokes et al. 1997). Mutation or deletion of the extrahelical base T results in inhibition of the homing reaction in vivo (Table 1). Interestingly, the crystal structure of the VchIntIA-VCRbs synapse showed that the extrahelical base T is bound by cis-interaction with one integrase subunit and is important for DNA site recognition (MacDonald et al. 2006). Deletion of the T or G extrahelical bases in VCRbs resulted in inhibition of excision frequency by VchIntIA integrase (MacDonald et al. 2006). Furthermore, it was demonstrated that a GG bulge mutant of the attCaadA1 resulted in a complete loss of IntI1 integrase binding in vitro (Johansson et al. 2004). This mutation also inhibits S.ma.I2 homing into attCaadA1, whereas deletion (or mutation for another base) of the G45 bulge has no effect on intron mobility. These results show that the G45 bulge is not recognized by S.ma.I2, and suggest that the G45GG mutation resulted in a change of the attC structure, which potentially inhibited an important contact with the T38 bulge. Consequently, these results suggest that S.ma.I2 makes an unexpected additional DNA–protein contact with the T bulge, in order to position the intron RNP along the DNA, facilitate the IBS–EBS pairings, and enhance the homing frequency.
Similarly, recognition of extrahelical bases from stem–loop motifs is also used by a particular class of DNA transposases coded by the IS605 group of insertion sequences. The transposases specifically bind to conserved 22–23 base imperfect inverted repeats located at the transposon ends. The crystal structure of the ISHp608 transposase from Helicobacter pylori with stem–loop DNA, from the folded inverted repeats, shows how DNA/protein interactions direct the transposase toward its cleavage site (Ronning et al. 2005). The transposase interacts with two unpaired thymine bases (separated by a six-base spacer) in these stem–loops, forming the basis for discrimination between the top and bottom strand stem–loops. Interactions between the ISHp608 transposase and the extrahelical bases are both protein side-chain and backbone interactions. Here, we demonstrated that full homing of the intron to the attC top strand was possible when both bulge bases and intron binding sites were symmetrically moved to the top strand (Fig. 6). Therefore, the conserved extrahelical bases (most importantly the T bulge) from the folded attC sites and the IBS1/IBS3 (5′-TTGT/T) motifs are the determinants for directing the intron to the attC bottom strand. Finally, these results suggest that class IIC–attC introns and integron integrases have convergently evolved to recognize attC sites and to tolerate attC variation by recognition of a bulged hairpin DNA motif rather than a specific sequence.
MATERIALS AND METHODS
Plasmids and media
Plasmids are described in Supplemental Table 1. E. coli DH5-α (supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1; Invitrogen) was grown in Luria-Bertani broth at 30°C or 37°C as indicated below. Antibiotics were used at the following concentrations: ampicillin (Ap), 100 μg/mL; chloramphenicol (Cm), 68 μg/mL. Isopropyl-β-D-thiogalactopyranoside (IPTG) was added to 1-mM final concentration.
Polymerase chain reaction procedures
Polymerase chain reaction (PCR) for plasmid assembly and other PCR reactions used the Phusion DNA polymerase (Finnzymes) according to the manufacturer's instructions. PCR primers (Supplemental Table 2) were synthesized using the DNA Synthesizer ABI-3900 from Applied Biosystems, Inc.
Nucleic acid folding
Target site DNA single-stranded secondary structures were determined using the MFOLD 3.2 program (Zuker 2003).
In vivo mobility assay
Intron mobility was determined by PCR amplification of the targeted sites using an E. coli two-plasmid assay in which S.ma.I2 cloned into pUC18 inserts into mutant attCaadA1 sites cloned into pACYC184 (Supplemental Table 1). pACYC184-based clones were obtained by cloning full-length complementary oligonucleotides (Supplemental Table 2) of each target site. Targets were cloned such that reverse transcription of the inserted intron RNA could potentially use either a nascent lagging (LAG) or leading (LEAD) DNA strand as a primer at the replication fork.
For each mobility assay, the intron donor (ApR) and recipient (CmR) plasmids were cotransformed into E. coli DH5-α competent cells (recA1−). One colony of each double transformant was grown in 5 mL of LB media in the presence of Ap and Cm at 30°C overnight. The preculture (100 μL) was pelleted and inoculated in 5 mL of LB medium with both antibiotics at 37°C until O.D600 nm = 0.5. Expression of the intron was then induced by addition of 1 mM IPTG, followed by incubation at 37°C for 3 h. Plasmid DNAs were extracted using a QIAprep kit (Qiagen). Mobility was screened by PCR using different combinations of primers (Fig. 2; Supplemental Table 2) and insertion sites were confirmed by sequencing.
Homing frequency was determined by colony patch hybridization. Purified plasmids (50–100 ng) from S.ma.I2 mobility assays, were digested with five units of PvuI. This enzyme cuts only once in the intron donor plasmid, outside of the intron sequence. E. coli DH5-α cells were transformed with the digested products and plated on LB/Cm plates to select for cells with recipient plasmids containing or lacking the intron. To calculate the intron mobility efficiency, measured by the percentage of recipient plasmids that received the intron, 100 isolated CmR colonies were patched onto LB/Cm plates. The patches were lifted onto nylon membranes and hybridized with 5′-end-labeled probe (SmaI2.for) using [γ-33P]ATP (3000 Ci/mmol; GE Healthcare). For each target site tested, positive colonies, randomly chosen, were cultured and the homing site-specificity confirmed by PCR amplification of the target site (using the PACYC-5′ and PACYC.rev primers) and sequencing.
SUPPLEMENTAL MATERIAL
Supplemental material can be found at http://www.rnajournal.org.
ACKNOWLEDGMENTS
This work was supported by Canadian Institutes of Health Research (CIHR) Grant MT-13564 to P.H.R.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1649309.
REFERENCES
- Barabas O, Ronning DR, Guynet C, Hickman AB, Ton-Hoang B, Chandler M, Dyda F. Mechanism of IS200/IS605 family DNA transposases: Activation and transposon-directed target site selection. Cell. 2008;132:208–220. doi: 10.1016/j.cell.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L, Vogel J. The ins and outs of group II introns. Trends Genet. 2001;17:322–331. doi: 10.1016/s0168-9525(01)02324-1. [DOI] [PubMed] [Google Scholar]
- Bouvier M, Demarre G, Mazel D. Integron cassette insertion: A recombination process involving a folded single strand substrate. EMBO J. 2005;24:4356–4367. doi: 10.1038/sj.emboj.7600898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centron D, Roy PH. Presence of a group II intron in a multiresistant Serratia marcescens strain that harbors three integrons and a novel gene fusion. Antimicrob Agents Chemother. 2002;46:1402–1409. doi: 10.1128/AAC.46.5.1402-1409.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coros CJ, Landthaler M, Piazza CL, Beauregard A, Esposito D, Perutka J, Lambowitz AM, Belfort M. Retrotransposition strategies of the Lactococcus lactis Ll.LtrB group II intron are dictated by host identity and cellular environment. Mol Microbiol. 2005;56:509–524. doi: 10.1111/j.1365-2958.2005.04554.x. [DOI] [PubMed] [Google Scholar]
- Cousineau B, Smith D, Lawrence-Cavanagh S, Mueller JE, Yang J, Mills D, Manias D, Dunny G, Lambowitz AM, Belfort M. Retrohoming of a bacterial group II intron: Mobility via complete reverse splicing, independent of homologous DNA recombination. Cell. 1998;94:451–462. doi: 10.1016/s0092-8674(00)81586-x. [DOI] [PubMed] [Google Scholar]
- Ferat JL, Michel F. Group II self-splicing introns in bacteria. Nature. 1993;364:358–361. doi: 10.1038/364358a0. [DOI] [PubMed] [Google Scholar]
- Francia MV, Avila P, de la Cruz F, Garcia Lobo JM. A hot spot in plasmid F for site-specific recombination mediated by Tn21 integron integrase. J Bacteriol. 1997;179:4419–4425. doi: 10.1128/jb.179.13.4419-4425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia MV, Zabala JC, de la Cruz F, Garcia Lobo JM. The IntI1 integron integrase preferentially binds single-stranded DNA of the attC site. J Bacteriol. 1999;181:6844–6849. doi: 10.1128/jb.181.21.6844-6849.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granlund M, Michel F, Norgren M. Mutually exclusive distribution of IS1548 and GBSi1, an active group II intron identified in human isolates of group B streptococci. J Bacteriol. 2001;183:2560–2569. doi: 10.1128/JB.183.8.2560-2569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Zimmerly S, Perlman PS, Lambowitz AM. Group II intron endonucleases use both RNA and protein subunits for recognition of specific sequences in double-stranded DNA. EMBO J. 1997;16:6835–6848. doi: 10.1093/emboj/16.22.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RM, Brookes DE, Stokes HW. Site-specific insertion of genes into integrons: Role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991;5:1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Ichiyanagi K, Beauregard A, Lawrence S, Smith D, Cousineau B, Belfort M. Retrotransposition of the Ll.LtrB group II intron proceeds predominantly via reverse splicing into DNA targets. Mol Microbiol. 2002;46:1259–1272. doi: 10.1046/j.1365-2958.2002.03226.x. [DOI] [PubMed] [Google Scholar]
- Jacquier A, Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987;50:17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Jimenez-Zurdo JI, Garcia-Rodriguez FM, Barrientos-Duran A, Toro N. DNA target site requirements for homing in vivo of a bacterial group II intron encoding a protein lacking the DNA endonuclease domain. J Mol Biol. 2003;326:413–423. doi: 10.1016/s0022-2836(02)01380-3. [DOI] [PubMed] [Google Scholar]
- Johansson C, Kamali-Moghaddam M, Sundstrom L. Integron integrase binds to bulged hairpin DNA. Nucleic Acids Res. 2004;32:4033–4043. doi: 10.1093/nar/gkh730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Boukharta L, Eriksson J, Aqvist J, Sundstrom L. Mutagenesis and homology modeling of the Tn21 integron integrase IntI1. Biochemistry. 2009;48:1743–1753. doi: 10.1021/bi8020235. [DOI] [PubMed] [Google Scholar]
- Lambowitz AM, Zimmerly S. Mobile group II introns. Annu Rev Genet. 2004;38:1–35. doi: 10.1146/annurev.genet.38.072902.091600. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Demarre G, Bouvier M, Mazel D, Gopaul DN. Structural basis for broad DNA-specificity in integron recombination. Nature. 2006;440:1157–1162. doi: 10.1038/nature04643. [DOI] [PubMed] [Google Scholar]
- Martinez-Abarca F, Toro N. Group II introns in the bacterial world. Mol Microbiol. 2000;38:917–926. doi: 10.1046/j.1365-2958.2000.02197.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Abarca F, Garcia-Rodriguez FM, Toro N. Homing of a bacterial group II intron with an intron-encoded protein lacking a recognizable endonuclease domain. Mol Microbiol. 2000;35:1405–1412. doi: 10.1046/j.1365-2958.2000.01804.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Abarca F, Barrientos-Duran A, Fernandez-Lopez M, Toro N. The RmInt1 group II intron has two different retrohoming pathways for mobility using predominantly the nascent lagging strand at DNA replication forks for priming. Nucleic Acids Res. 2004;32:2880–2888. doi: 10.1093/nar/gkh616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura M, Saldanha R, Ma H, Wank H, Yang J, Mohr G, Cavanagh S, Dunny GM, Belfort M, Lambowitz AM. A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: Biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes & Dev. 1997;11:2910–2924. doi: 10.1101/gad.11.21.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D. Integrons: Agents of bacterial evolution. Nat Rev Microbiol. 2006;4:608–620. doi: 10.1038/nrmicro1462. [DOI] [PubMed] [Google Scholar]
- Michel F, Ferat JL. Structure and activities of group II introns. Annu Rev Biochem. 1995;64:435–461. doi: 10.1146/annurev.bi.64.070195.002251. [DOI] [PubMed] [Google Scholar]
- Munoz-Adelantado E, San Filippo J, Martinez-Abarca F, Garcia-Rodriguez FM, Lambowitz AM, Toro N. Mobility of the Sinorhizobium meliloti group II intron RmInt1 occurs by reverse splicing into DNA, but requires an unknown reverse transcriptase priming mechanism. J Mol Biol. 2003;327:931–943. doi: 10.1016/s0022-2836(03)00208-0. [DOI] [PubMed] [Google Scholar]
- Peters JE, Craig NL. Tn7 recognizes transposition target structures associated with DNA replication using the DNA-binding protein TnsE. Genes & Dev. 2001;15:737–747. doi: 10.1101/gad.870201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar M, Chu VT, Pyle AM, Perlman PS. Group II intron splicing in vivo by first-step hydrolysis. Nature. 1998;391:915–918. doi: 10.1038/36142. [DOI] [PubMed] [Google Scholar]
- Pyle AM, Lambowitz AM. Group II introns: Ribozymes that splice RNA and invade DNA. In: Gesteland RF, et al., editors. The RNA World. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2006. pp. 469–505. [Google Scholar]
- Quiroga C, Roy PH, Centron D. The S.ma.I2 class C group II intron inserts at integron attC sites. Microbiology. 2008;154:1341–1353. doi: 10.1099/mic.0.2007/016360-0. [DOI] [PubMed] [Google Scholar]
- Robart AR, Seo W, Zimmerly S. Insertion of group II intron retroelements after intrinsic transcriptional terminators. Proc Natl Acad Sci. 2007;104:6620–6625. doi: 10.1073/pnas.0700561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronning DR, Guynet C, Ton-Hoang B, Perez ZN, Ghirlando R, Chandler M, Dyda F. Active site sharing and subterminal hairpin recognition in a new class of DNA transposases. Mol Cell. 2005;20:143–154. doi: 10.1016/j.molcel.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Simon DM, Clarke NA, McNeil BA, Johnson I, Pantuso D, Dai L, Chai D, Zimmerly S. Group II introns in eubacteria and archaea: ORF-less introns and new varieties. RNA. 2008;14:1704–1713. doi: 10.1261/rna.1056108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NN, Lambowitz AM. Interaction of a group II intron ribonucleoprotein endonuclease with its DNA target site investigated by DNA footprinting and modification interference. J Mol Biol. 2001;309:361–386. doi: 10.1006/jmbi.2001.4658. [DOI] [PubMed] [Google Scholar]
- Stokes HW, Hall RM. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: Integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Stokes HW, O'Gorman DB, Recchia GD, Parsekhian M, Hall RM. Structure and function of 59-base element recombination sites associated with mobile gene cassettes. Mol Microbiol. 1997;26:731–745. doi: 10.1046/j.1365-2958.1997.6091980.x. [DOI] [PubMed] [Google Scholar]
- Toor N, Hausner G, Zimmerly S. Coevolution of group II intron RNA structures with their intron-encoded reverse transcriptases. RNA. 2001;7:1142–1152. doi: 10.1017/s1355838201010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor N, Robart AR, Christianson J, Zimmerly S. Self-splicing of a group IIC intron: 5′ exon recognition and alternative 5′ splicing events implicate the stem–loop motif of a transcriptional terminator. Nucleic Acids Res. 2006;34:6461–6471. doi: 10.1093/nar/gkl820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor N, Keating KS, Taylor SD, Pyle AM. Crystal structure of a self-spliced group II intron. Science. 2008;320:77–82. doi: 10.1126/science.1153803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro N. Bacteria and Archaea Group II introns: Additional mobile genetic elements in the environment. Environ Microbiol. 2003;5:143–151. doi: 10.1046/j.1462-2920.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- Zhong J, Lambowitz AM. Group II intron mobility using nascent strands at DNA replication forks to prime reverse transcription. EMBO J. 2003;22:4555–4565. doi: 10.1093/emboj/cdg433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerly S, Guo H, Eskes R, Yang J, Perlman PS, Lambowitz AM. A group II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell. 1995;83:529–538. doi: 10.1016/0092-8674(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Zimmerly S, Hausner G, Wu X. Phylogenetic relationships among group II intron ORFs. Nucleic Acids Res. 2001;29:1238–1250. doi: 10.1093/nar/29.5.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]