Abstract
Context
Understanding the menopause association with body weight is important because excess weight increases risk for stroke, incident cardiovascular disease, cardiovascular mortality, and all-cause mortality among the middle-aged.
Objective
To examine chronological age and ovarian age and consider how these could influence body size and composition in mid-life women.
Design and Setting
The Study of Women’s Health Across the Nation is a longitudinal, community-based study. This report uses data from the Michigan SWAN site.
Participants
543 pre- or early perimenopausal African-American and Caucasian women aged 42–52 years at baseline examination.
Main Outcome Measures
Waist circumference, fat mass and skeletal muscle mass, from bioelectrical impedance, was assessed in 7 annual serial measures. Annual follicle-stimulating hormone (FSH) values were assayed by ELISA. The final menstrual period (FMP) was defined retrospectively following 12 months of amenorrhea.
Results
There was an absolute cumulative six-year increase in fat mass of 3.4 kg and a six-year decrease in skeletal muscle mass of ~0.23 kg. There was an absolute cumulative six-year increase of ~5.7 cm in waist circumference. The logFSH change was positively correlated with log(fat mass) change. Waist circumference increased over the time period, but one year following FMP, the rate of increase slowed. Fat mass continued to increase with no change in rate.
Conclusions
Both time (chronological aging) and ovarian aging contributed to substantial changes in body composition (fat and skeletal muscle mass) and waist circumference. These changes have important ramifications for establishing a metabolic environment that can be healthy or unhealthy.
Keywords: fat mass, lean mass, body composition, menopause, aging
INTRODUCTION
Obesity has been labeled as the epidemic of the 21st century, but until recently, the role of body composition compartments, especially fat mass, was not considered central to understanding the health-related implications of the obesity epidemic. Now, fat mass is no longer considered just a passive storage unit for fat, but an active contributor to the metabolic profiles affecting health so a more precise estimate is increasingly valuable as compared to the proxy body mass index (BMI; kg/m2) measure (1). The relative changes in body composition, particularly fat mass and skeletal muscle mass, during the menopause, are less well-documented than weight changes with age. Cross-sectional studies suggest an age-related increase in the prevalence of overweight and obesity, as measured by BMI (2,3), more often occurring in women than men, across multiple populations (2,4). Longitudinal studies of body weight change document a rapid increase in weight among young adults, followed by a lesser increase among middle-aged adults, and a decrease in weight in elderly adults (4,5).
Some, but not all, cross-sectional studies (6) suggest greater fat mass and less lean mass in postmenopausal as compared to premenopausal women, but no longitudinal studies have evaluated body composition changes across the menopause transition. Studies of fat distribution or topology suggest that there may be increased likelihood of a menopause effect (7). In longitudinal studies, measures of abdominal adiposity, such as waist circumference or abdominal skinfold thickness, steadily increase with aging (5,8). This would suggest a general increase in fat mass, but lack of change or decrease in fat-free mass, with aging (9,10).
Understanding the menopause contribution to weight and its distribution is important because of the potential impact on health status in women. Excess weight increases risk for stroke, incident cardiovascular disease, cardiovascular mortality, and all-cause mortality among the middle-aged, therefore identifying a potential contribution of menopause to this risk is important (11,12). Further, these risks take on new relevance as current research increasingly identifies that adipose tissue functions as an endocrine organ, secreting a variety of cytokines including leptin, adiponectin, resistin, plasminogen activator inhibitor-1 (PAI-1), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 (13) with immunological, vascular, and metabolic actions (14). The purpose of this study was to determine, in women at the mid-life, if changes in body size and body composition were related to chronological aging and/or to ovarian aging. In this longitudinal study, we used time in study (from 7 annual serial examinations) to describe the effect of chronological aging. Ovarian aging was characterized using annual measures of follicle-stimulating hormone (FSH) or menopause status defined by the interval lengths between self-reported menses and time since final menstrual period (FMP).
METHODS
Sample
While the Study of Women’s Health Across the Nation (SWAN) is a multi-site, multi-race/ethnic longitudinal study of women at mid-life as they approach and traverse menopause, this report is limited to the Michigan SWAN data, the location of a body composition site-specific study begun at the SWAN baseline.
The SWAN study population was recruited using a two-stage design with different criteria for inclusion at each stage, and this has been previously described (15). In the first stage, conducted in 1995, potential Michigan SWAN enrollees were identified from a census of 24,283 households located in Census tracts associated with two suburban communities near Detroit, Michigan. We successfully interviewed 2,621 (65%) eligible women, aged 40–55 years, residing in the pre-selected Census tracts, telephone (25% of contacts) or in-person contact (75%). The list of successfully-interviewed women in the first stage served as the sampling frame for identification of women eligible for enrollment in the longitudinal cohort (2nd stage). Women eligible for the longitudinal cohort were aged 42–52 years, either African American or Caucasian by self-definition, reported menstrual bleeding within the previous three months of enrollment and no use of hormone replacement therapy within three months of enrollment. A longitudinal cohort of 543 (72%) women (325 African-American; 218 Caucasian) was enrolled. This number was reduced in the first follow-up year to reduce study costs but in the 5th follow-up, 62 enrollees with baseline data were re-recruited. Since then, participation has been more than 80% of the baseline sample. There have been 18 deaths among members of the cohort. Table 1 describes the baseline sample numbers and characteristics of those women whose data contributed to this report.
TABLE 1.
Baseline characteristics of women age 42–52 years at baseline, Michigan SWAN
| African American | Caucasian | |||||
|---|---|---|---|---|---|---|
| Overall (n=543) | ||||||
| (n=325) | (n=218) | |||||
| Mean | SD* | Mean | SD | Mean | SD | |
| Age (years) | 45.6 | 2.7 | 45.5 | 2.8 | 45.7 | 2.6 |
| Body Size Measures | ||||||
| Height (cm) | 163.5 | 6.2 | 163.8 | 6.1 | 162.9 | 6.2 |
| Weight (kg) | 85.8 | 21.8 | 86.7 | 22.0 | 84.3 | 21.5 |
| Body Mass Index (kg/m2) | 32.1 | 8.1 | 32.3 | 8.1 | 31.8 | 8.0 |
| Waist circumference (cm) | 94.1 | 17.2 | 94.8 | 16.9 | 93.2 | 17.6 |
| Hip circumference (cm) | 113.9 | 16.2 | 113.8 | 16.3 | 113.9 | 16.1 |
| Waist:Hip Ratio | 0.83 | 0.1 | 0.83 | 0.1 | 0.82 | 0.1 |
| Fat mass (kg) | 36.4 | 16.4 | 37.2 | 16.9 | 35.0 | 15.5 |
| Lean mass (kg) | 49.4 | 8.7 | 49.3 | 8.7 | 49.6 | 8.6 |
| Skeletal muscle mass (kg) | 21.8 | 3.4 | 21.7 | 3.4 | 21.8 | 3.3 |
| SMI (kg/m2)† | 8.1 | 1.2 | 8.1 | 1.2 | 8.2 | 1.1 |
| LogFSH (mIU/ml)‡ | 2.9 | 0.7 | 2.9 | 0.8 | 2.8 | 0.6 |
| FSH (mIU/ml)§ | 17.8 | 13.1 | 18.9 | 13.9 | 16.3 | 12.0 |
SD is the standard deviation
SMI is the Skeletal Muscle Index
Log transformed values are presented due to the skewed distribution of FSH
FSH values presented have been transformed back to their original scale
Measures
Michigan SWAN enrollees participated in annual examinations that were scheduled to coincide with the anniversary of their initial assessment and within a window to allow phlebotomy to be indexed to the early follicular phase of the menstrual cycle, if women were still menstruating. Written informed consent was obtained annually from all participants, and data were collected via protocols reviewed and endorsed by the University of Michigan Institutional Review Board.
Body composition measures
The SWAN Core protocol included annual measures of weight and height, measured with calibrated electronic or balance beam scales and stadiometer, and those data were used to calculate BMI. Other annual measures included waist circumference, measured at the narrowest part of the torso, and hip circumference, measured at the iliac crest. The former was used to approximate central adiposity in this report. To supplement this information, in 1996/97, a site-specific substudy of body composition and physical functioning was implemented in the Michigan SWAN longitudinal population. The Michigan Sub-study included the use of bioelectrical impedance analysis (BIA) to assess body composition since study inception; other sites added this measure at the 6th annual follow-up.
BIA is based on measurement of the transmission speed of a ¼ volt electrical pulse between electrodes attached at the feet and electrodes attached across the knuckles of the hand. Because fat-free mass is comprised of water, proteins and electrolytes, conductivity is greater in fat-free mass than in fat mass (16). Resistance and reactance are used to estimate total body water, and by extension, fat mass and lean mass, with the latter including bone (17). Skeletal muscle mass (SMM) was calculated by the method of Janssen et al. (18) who subsequently indexed SMM to height for a skeletal muscle index (SMI) and developed cutpoints relating to the risk of disability associated with SMI (19). These variables were treated as time-varying covariates.
FSH assay
The phlebotomy protocol specified a blood draw following 12-hour fast and in the 2–5 day window of the early follicular phase of the menstrual cycle. If a blood sample could not be obtained in the day 2–5 window in the 60 days following the anniversary visit date (usually because of irregular menstrual cycles), blood was obtained in the subsequent 30 days without respect to menstrual bleeding. Serum was analyzed for FSH. Serum FSH concentrations were measured with a two-site chemiluminometric immunoassay using constant amounts of two monoclonal antibodies. Each antibody is directed to different regions on the beta subunit (one coupled to paramagnetic particles and the other labeled with a dimethylacridinium ester (DMAE)) with specificity for intact FSH. Inter- and intra-assay coefficients of variation (CV) were 12.0% and 6.0%, respectively, at an FSH level of 15 mIU/mL.
Menopausal status measures
Menopausal status assignment was based on annual reports about menstrual bleeding and its regularity. Premenopause was identified as no decreased regularity in menstrual bleeding during the previous year. Other classifications were early perimenopause (decreased menses regularity in the three months before the interview), late perimenopause (no menses for 3–11 months) and postmenopause (no menses for 12 or more months). Surgical menopause was defined by report of either hysterectomy or oophorectomy.
The number of women classified as premenopausal declined progressively from 50.4% at the baseline examination to 8% at the 6th follow-up examination. Conversely, the number of women who became postmenopausal (without hormone therapy use) rose progressively from 1% at the first follow-up examination to 15% at the 6th follow-up examination. Hormone therapy (HT) use and medication use was identified from interviews and, when possible, confirmed by observing the medication packaging. There was no use of HT at the baseline examination as a condition of study eligibility, though by the 6th follow-up visit, cumulatively, 20.4% of women had reported HT use. Data from women reporting HT use were classified into a separate menopause group; these data were treated as time-varying covariates. FMP is defined retrospectively as the last menstrual period prior to 12 months amenorrhea.
Other measures
Self-administered questionnaires were used to assess current smoking status (yes/no) (20). Physical activity, assessed at baseline and at the 6th follow-up exam, was a summary score reflecting activities of living, home, recreation, plus work, if relevant (21,22). A variable of 6-year physical activity change was created for analysis purposes.
Analysis approaches
Analyses were based on a total of 3426 observations from 543 women, with women contributing 1–7 data points, depending upon the frequency of their participation in follow-up visits. There were 830 observations from 130 women with an FMP during the study period. Univariate distributions of eight continuous measures of body composition were examined for normality. To meet the assumptions of normality and to reduce skewness, natural log transformations were applied to FSH concentrations and seven of the eight body composition measures (excluding height). The frequencies of categorical covariates were examined overall and by year of visit.
The variability of the eight body composition outcomes was evaluated and expressed as a CV for each visit and the output plotted. The lowest CV (not surprisingly) were in height—cm (3.7%) followed by waist—cm (3.8%), lean mass—kg (4.4%), skeletal muscle—kg (5%), weight—kg (5.5%), BMI (7%), and fat mass—kg (12%). The variation was consistent over the 7-year data collection period being examined (no drift upward with time or increasing body size) and similar in both African-American and Caucasian women.
The nature of influential individuals and/or data points was examined using likelihood distance (LD). Influential values were retained as real biological fluctuations (e.g., some body measures/compositions may exhibit cycling patterns within certain time frame).
Participant characteristics at baseline and each of the 6 follow-up visits were described with means and standard deviations calculated in the transformed scale and back-transformed to original scale. Differences in these characteristics according to race/ethnicity were tested for statistical significance at α = 0.05.
Repeated measures mixed modeling (SAS, Proc Mixed) for continuous variables was used to evaluate the relationship between body composition and menopause status (approximated by levels of FSH and intervals of bleeding frequency). See the Appendix for model development. This approach combined the cross-sectional (between women) and longitudinal (within women) estimates of association while correcting those estimates for multiple and correlated measures within women. Random intercepts, random slopes and/or quadratic terms were included as appropriate. These mixed models also allowed the specification of the variance and covariance of measures so that standard errors used in testing significance of associations were robust. Sample variograms and goodness-of-fit statistics were used to evaluate the covariance structures and we selected the auto-regressive (AR) option. The appropriateness of model fitting was assessed both graphically and using residual analyses. Within the general frame of linear mixed-effect model, a piecewise linear mixed-effect model was developed to evaluate the change of slope at or near FMP for body composition measures.
The “chronological aging” or time effect was decomposed into a cross-sectional effect (approximated by baseline age) and a longitudinal “time” effect (baseline and 6 annual follow-up visits). Models included a quadratic "time" term (centered at visit 3). The effect of “ovarian age” was modeled by incorporating terms for menopause status based on timing of menstrual bleeding and by incorporating values of FSH.
FSH concentrations (continuous variable) or menopausal status (categorical variable) were treated as time-varying covariates in the models while baseline age and baseline body composition variables were treated as single-time variables. Interaction terms were incorporated to assess the relative effects of ovarian age on body composition with greater chronological age. Covariates were retained in models if their inclusion changed the beta coefficients for either FSH or menopause status (independent variables) by 10% or more. Models of associations between ovarian or chronological aging with measures of body composition are adjusted for race and not stratified by race because there were no differences in associations between races.
The population-averaged annual change and accumulated change (6th follow-up visit vs. baseline) for each body composition measure were reported in absolute and/or relative (percent) values. The mean and 95% confidence interval from bootstrapping were used for Figure 3. The marginal mean from mixed models at each year related to FMP were constructed and graphed. SAS 9.1 and Macro facilities (SAS Institute, Cary, NC) were used to perform the statistical analyses and plot the findings. The experimental features in SAS 9.1 were used to perform the influence analysis.
Figure 3.
RESULTS
At baseline in 1996/7, the 543 participants, of whom approximately 60% were African American, had a mean age of 45 years and mean BMI and waist circumference of 32.1 kg/m2 and 94.1 cm, respectively (see Table 1). Mean baseline FSH was 18.9 (SD=13.9) and 16.3 (SD=12.0) mIU/ml for African-American and Caucasian women, respectively, with the value for African-American women being statistically significantly higher than for Caucasian women.
Body composition and race
The average amount of fat mass in African-American women was 8.1% (95% confidence interval (CI): 0.5%, 16.3%) higher than the average fat mass in Caucasian women, as estimated using linear mixed models. There were no other statistically significant differences in other body composition measures between African-American and Caucasian women.
Body composition changes with time
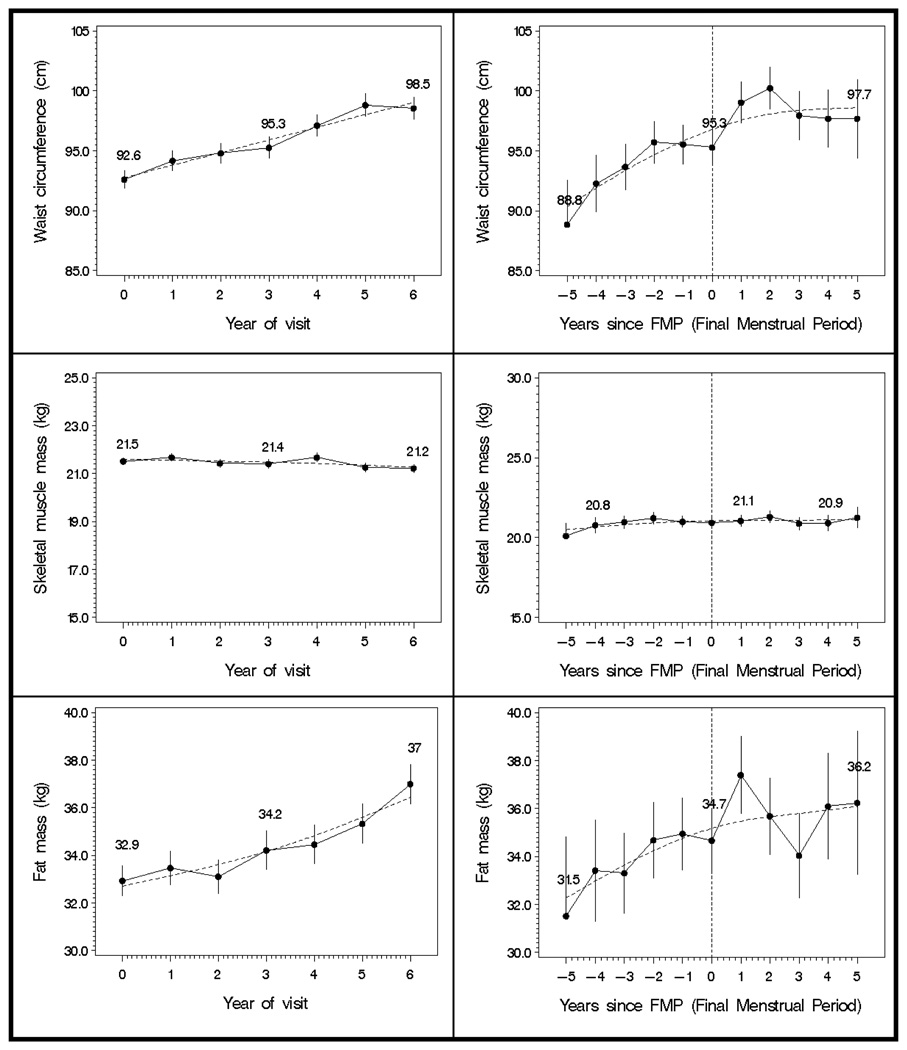
Body composition measures changed over time with increasing age at each annual visit (see Figure 1, left side). The changes in weight, BMI, and waist circumference were linear whereas the changes in height, fat mass, lean mass and skeletal muscle mass had a more curvilinear pattern over time.
Figure 1.
There was a 0.6% (~0.5 kg) annual increase in weight [β: 0.0056 (95% CI: 0.004, 0.007)] which represents a relative and absolute cumulative six-year increase of 3.4% and ~2.9 kg, respectively (Table 2). There was a decrease in height of 0.064 cm per year (95% CI: −0.073, −0.055) and a cumulative six-year decrease of 0.38 cm. With the decrease in height and increase in weight, there was a significant annual increase of 0.7% in BMI (~0.2 kg/m2) [β: 0.0065 (95% CI: 0.005, 0.008)] which represents a relative and absolute cumulative six-year increase of 4% and ~1.2 kg/m2, respectively.
TABLE 2.
Estimates of body composition changes with time from statistical models with body composition measures as the dependent variables and time as the linear or curvilinear independent variable(s)
| Body Size/Composition Measures |
Annual Change | Relative 6-Year Change |
Absolute Cumulative 6-Year Change |
|---|---|---|---|
| Weight (kg) | 0.6% (~0.5 kg) | 3.4% | 2.9 kg |
| Height (cm) | −0.064 cm* | 0.24% | −0.38 cm |
| BMI (kg/m2) | 0.7% (~0.2 kg/m2) | 4% | 1.2 kg/m2 |
| Waist (cm) | 1% (~0.9 cm) | 6.2% | 5.7 cm |
| Fat mass (kg) | 1.6% (~0.57 kg) | 10.1% | 3.4 kg |
| Skeletal muscle mass (kg) | −0.18% (~0.04 kg) | −1.06% | −0.23 kg |
Percent of annual change depends on time of visit
There was a 1% (~0.9 cm) annual increase in waist circumference [β: 0.0099 (95% CI: 0.009, 0.011)] which represents a relative and absolute cumulative six-year increase of 6.2% and ~5.7 cm, respectively.
A 1.6% (~0.57 kg) increase in fat mass [β: 0.016 (95% CI: 0.013, 0.02)] was observed, which corresponds to a relative and absolute cumulative six-year increase of 10.1% and 3.4 kg. There was an annual 0.18% decrease in skeletal muscle mass [(~0.04 kg) β: −0.0018 (95% CI: −0.003, −0.0007)] reflected in relative and absolute cumulative, six-year decreases in skeletal muscle mass of 1.06% and ~0.23 kg, respectively. Annual measurements of lean mass did not change across the six-year time period.
Body composition and menopause stages
Three approaches were used to evaluate a potential ovarian aging contribution to body composition change including the amount explained by menopause stages, by FSH and its change, and the change before and after the FMP. There was no discernible change in body composition parameters associated with menopause stages defined by frequency of menstrual bleeding, independent of the effect of time.
Body composition, FSH and FMP
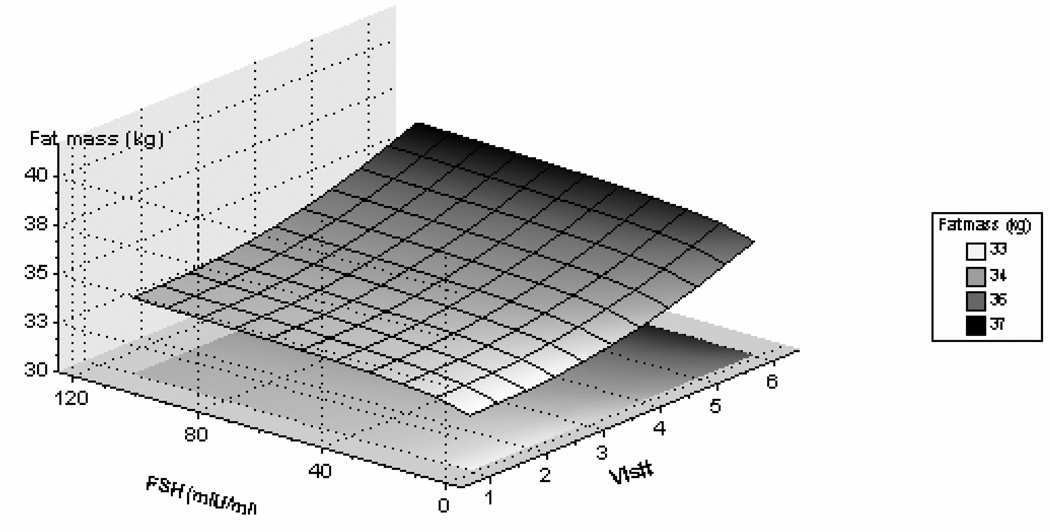
Increasing levels of logFSH were associated with increasing fat mass and waist circumference, before and after adjusting for baseline age and baseline fat mass or baseline waist circumference, respectively. As shown in Figure 2, there was a positive relationship between change in logFSH and change in log(fat mass) [correlation coefficient = 0.12 (95%CI: 0.09, 0.16) ]. Increasing FSH was associated with decreasing lean mass and skeletal muscle mass. These associations were not related to either baseline age or to the interaction of baseline age with FSH concentration.
Figure 2.
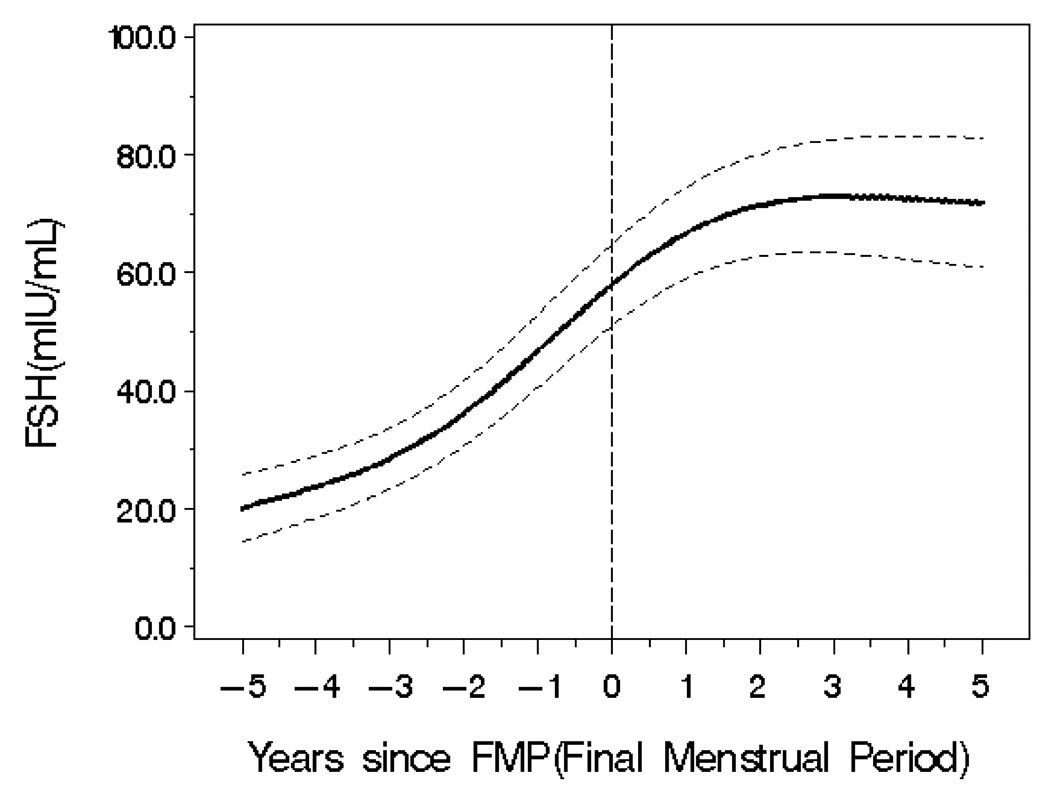
Of the 543 enrollees, 130 (24%) experienced the FMP during the study time interval. When FSH values were considered in relation to time and FMP, the increasing average FSH was cubic, that is, FSH increased between −5 to −2 years from the FMP, then increased markedly from −2 years to 1 year postmenopause and then the fitted line tended to flatten, as shown in Figure 3.
Over the study time, waist circumference continued to increase but approximately one year after FMP, the rate of increase slowed (See Figure 1, right side). Women continued to gain fat mass, and no point was identified at which the rate of change increased or decreased. There was a modest loss of skeletal muscle mass until the year following the FMP after which there was little evidence of change.
DISCUSSION
Using longitudinal data with seven annual serial measures, we identified that the 6-year increase in weight of women at the mid-life was associated with both a substantially increased fat mass (an increase of 10%) and a smaller, nonetheless real, loss of skeletal muscle mass (a decrease of 1%). As expected, there was also an average 6% increase in waist circumference over the same time period. These body composition changes were explained, not only with the increasing age associated with the passage of time, but also with ovarian aging including progressively higher FSH concentrations and occurrence of the last menstrual period. Further, these changes in body composition over this 6-year period occurred similarly in both African-American and Caucasian women.
These data suggest that increase is waist circumference is more sensitive to ovarian aging than the increase in fat mass. The increase in waist circumference becomes less pronounced after the FMP coincident with a flattening of the FSH levels in the year following the FMP, whereas fat mass continues to increase with no evidence of a node when the rate of change is altered. Further investigation will have to explain why this alteration in waist should be observed in a year following the FMP. One might question whether this represents the response to a consolidation of a more androgenic profile; however, such a consolidation would appear to be more appropriate for an increase in the waist circumference rather than a diminished rate of change.
It is valuable to consider not only the change in weight with the menopause, but also additional measures of body composition. Adipose tissue became an acknowledged endocrine organ rather than just a passive reservoir for energy storage with the identification that adipocytes were sites of sex steroid metabolism and leptin production (23,24). For example, leptin affects not only energy homeostasis but also regulates neuroendocrine function including the activation and suppression of the hypothalamic-pituitary axis (13). These are associated with metabolic attributes of cardiovascular disease including vascular injury and inflammation, disordered cholesterol metabolism, and hypertension (25). Thus, the increases in fat mass and its distribution to the waist around the menopause represent compelling risk factors for heart disease.
The changes in body composition also included a small but detectable loss of skeletal muscle mass. Loss of skeletal muscle mass also has implications for health in women following the menopause. A smaller skeletal muscle mass bed could be accompanied by diminished opportunity for synthesis of the Glut4 transport protein. In some (26,27) but not all studies (28–30), levels of Glut4 mRNA and protein in adipose cells have been shown to be low in insulin-deficient diabetics, in those with impaired glucose tolerance, and in Type 2 diabetes. Kahn concluded that insulin resistance was due, in part, to defective translocation or activation of glucose transporters in the plasma membrane of skeletal muscle rather than changes in the levels of Glut4 in intracellular pools (27).
A number of factors are important in considering this study. These study results reflect information from women whose transition to postmenopause from a baseline pre- or early perimenopause designation was relatively rapid. Additional longitudinal observations are needed to establish whether the greater changes in FSH associated with greater changes in fat mass are sustained in the postmenopause and how long this effect will be relevant. Additional observations will be required to assure that a similar pattern is replicated in those women with a longer transition time.
We used bioelectrical impedance analysis to estimate body composition. This technique, while sensitive to hydration in the physically active, is quite useful in studies of middle-aged women. Size of women is not restricted in using bioelectrical impedance whereas other technologies, such as dual energy x-ray densitometry, are restricted to those whose weight is within the examination table specifications, a requirement that would have excluded more than 7% of the women being evaluated. Indeed, BIA was the measurement methodology used in the National Health and Nutrition Examination Survey (NHANES) III (31). Additionally, there was little evidence of drift in this measure over time and there was substantial precision in the measures as shown with low coefficients of variation.
In summary, we have shown that there were marked changes in body composition and body topology reflected in 7 annual serial measures of women transitioning the menopause. Furthermore, both chronological age and ovarian age contributed to the increase in fat mass, decrease in skeletal muscle mass and increase in waist circumference. These changes have important ramifications for health in that adipose tissue, adipose tissue distribution, and skeletal muscle mass all make substantial contributions to the health-related environment.
Supplementary Material
Acknowledgments
GRANT SUPPORT
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women’s Health (Grants NR004061, AG017104, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495).
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 – 1999; Joel Finkelstein, PI 1999-present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark–Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Footnotes
This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
Disclosure summary:
M-F.S. consults for and received lecture fees from Wyeth. H.Z., K.T., C.K-G., M.J., X.L, and M.Y. have nothing to declare. J.S. was previously employed by and has equity interests in Pfizer.
REFERENCES
- 1.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 3.Iwao S, Iwao N, Muller DC, Elahi D, Shimokata H, Andres R. Effect of aging on the relationship between multiple risk factors and waist circumference. J Am Geriatr Soc. 2000;48:788–794. doi: 10.1111/j.1532-5415.2000.tb04754.x. [DOI] [PubMed] [Google Scholar]
- 4.Williamson DF. Descriptive epidemiology of body weight and weight change in U.S. adults. Ann Intern Med. 1993;119:646–649. doi: 10.7326/0003-4819-119-7_part_2-199310011-00004. [DOI] [PubMed] [Google Scholar]
- 5.Noppa H, Andersson M, Bengtsson C, Bruce A, Isaksson B. Longitudinal studies of anthropometric data and body composition. The population study of women in Gotenberg, Sweden. Am J Clin Nutr. 1980;33:155–162. doi: 10.1093/ajcn/33.1.155. [DOI] [PubMed] [Google Scholar]
- 6.Svendsen OL, Hassager C, Christiansen C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy Xray absorptiometry. Metabolism. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 7.Tremollieres FA, Pouilles JM, Ribot CA. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol. 1996;175:1594–1600. doi: 10.1016/s0002-9378(96)70111-4. [DOI] [PubMed] [Google Scholar]
- 8.Shimokata H, Andres R, Coon PJ, Elahi D, Muller DC, Tobin JD. Studies in the distribution of body fat. II. Longitudinal effects of change in weight. Int J Obes. 1989;13:455–464. [PubMed] [Google Scholar]
- 9.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfield SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 10.Nassis P, Geladas D. Age-related pattern in body composition changes for 18–69 year old women. J Sports Med Phys Fitness. 2003;43:327–333. [PubMed] [Google Scholar]
- 11.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 12.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 13.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 14.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- 15.Sowers MF, Crawford S, Sternfeld B, Morgenstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. Design, survey sampling and recruitment methods of SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 16.Lukaski HC, Bolonchuk WW. Estimation of body fluid volumes using tetrapolar bioelectrical impedance measurements. Aviat Space Environ Med. 1988;59:1163–1169. [PubMed] [Google Scholar]
- 17.Boulier A, Fricker J, Thomasset AL, Apfelbaum M. Fat-free mass estimation by the two-electrode impedance method. Am J Clin Nutr. 1990;52:581–585. doi: 10.1093/ajcn/52.4.581. [DOI] [PubMed] [Google Scholar]
- 18.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–471. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 20.Ferris B. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 21.Baecke J, Burema J, Fritjers J. A short questionnaire of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Sternfeld B, Ainsworth B, Quesenberry CJ. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 23.Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr. 1987;45:277–282. doi: 10.1093/ajcn/45.1.277. [DOI] [PubMed] [Google Scholar]
- 24.Havel PJ. Peripheral signals conveying metabolic information to the brain: short-term and long-term regulation of food intake and energy homeostasis. Exp Biol Med. 2001;226:963–977. doi: 10.1177/153537020122601102. [DOI] [PubMed] [Google Scholar]
- 25.Fortuno A, Rodriguez A, Gomez-Ambrosi J, Fruhbeck G, Diez J. Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003;59:51–60. doi: 10.1007/BF03179868. [DOI] [PubMed] [Google Scholar]
- 26.Pantanetti P, Garrapa GG, Mantero F, Boscaro M, Faloia E, Venarucci D. Adipose tissue as an endocrine organ? A review of recent data related to cardiovascular complications of endocrine dysfunctions. Clin Exp Hypertens. 2004;26:387–398. doi: 10.1081/ceh-120034142. [DOI] [PubMed] [Google Scholar]
- 27.Kahn BB. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J Clin Invest. 1992;86:1367–1374. doi: 10.1172/JCI115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman JE, Caro JF, Pories WJ, Azevedo JL, Dohm GL. Glucose metabolism in incubated human muscle: effect of obesity and non-insulin-dependent diabetes mellitus. Metabolism. 1994;43:1047–1054. doi: 10.1016/0026-0495(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen O, Bak JF, Andersen PH, Lund S, Moller DE, Flier JS, Kahn BB. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 1990;39:865–870. doi: 10.2337/diab.39.7.865. [DOI] [PubMed] [Google Scholar]
- 30.Handberg A, Vaag A, Damsbo P, Beck-Nielsen H, Vinten J. Expression of insulin regulatable glucose transporters in skeletal muscle from Type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1990;33:625–627. doi: 10.1007/BF00400207. [DOI] [PubMed] [Google Scholar]
- 31.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from NHANES III bioelectrical impedance dataBody composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.