Abstract
Background: Prophylactic systemic antibiotics significantly lower the risk of postoperative infection, and injection of antibiotics directly into the wound cavity has been found to be even more effective. In this study, we investigated the efficacy of direct injection of antibiotics into a wound cavity after wound closure, both alone and in combination with systemic administration of antibiotics. We hypothesized that a combination of preoperative systemic administration and postoperative local injection would be the most effective treatment.
Methods: Rats were divided into six treatment groups: no treatment, local gentamicin, systemic cefazolin, local cefazolin, systemic cefazolin plus local gentamicin, and systemic cefazolin plus local cefazolin. A wound cavity was opened along the femur, an implant was placed, and the wound was inoculated with 2.5 × 108 colony forming units of Staphylococcus aureus. Systemic antibiotics were injected subcutaneously thirty minutes before the initial incision. Local antibiotics were injected percutaneously into the wound cavity after closure. The rats were killed at forty-eight hours postoperatively, and quantitative cultures were performed.
Results: All groups that received antibiotics showed significantly lower bacterial counts than the no-treatment control group (p < 0.0003). Local gentamicin treatment decreased the number of colony-forming-unit isolates by approximately two orders of magnitude as compared with the number in the group treated with systemic cefazolin (p = 0.00005) and five orders of magnitude as compared with the number in the control group (p = 0.00003). The combination of systemic cefazolin and local gentamicin decreased the bacterial count by approximately seven orders of magnitude as compared with the count in the no-treatment control group and significantly decreased the count as compared with that in the group treated with local gentamicin alone (p = 0.00006).
Conclusions: As we hypothesized, the combination of systemic cefazolin and local gentamicin proved to be the most effective regimen. Local injection of gentamicin proved more effective than systemic administration of cefazolin but was not as effective as the combination of both antibiotics. The initially high concentrations of locally applied antibiotic and the utilization of two different classes of antibiotics may have contributed to the observed efficacy.
Clinical Relevance: If our findings are supported by those in clinical trials, the combination of local gentamicin and systemic cefazolin could prove valuable as a regimen for prophylaxis against surgical wound infection.
Postoperative infections have been shown to significantly increase morbidity, extend the patient's hospital stay, drastically increase the cost to the medical system, and cause severe physical limitations that diminish quality of life1. Postoperative infection has been estimated to occur following 1% to 2% of all total hip arthroplasties and 2% to 4% of all total knee arthroplasties in the United States2,3. In 2002, more than 193,000 total hip arthroplasties and 381,000 total knee arthroplasties were performed4. Thus, even with relatively low infection rates, a substantial number of infections will still occur. With the serious implications of postoperative infection, it is imperative that measures, including the use of prophylactic antibiotics, be taken to prevent infection.
Sterile surgical technique and intravenous administration of antibiotics such as cefazolin, cefuroxime, vancomycin, or clindamycin prior to incision are the current standard of care in orthopaedic surgery. However, the prolonged and indiscriminate use of antimicrobials has been associated with the emergence of resistant strains of bacteria5-7. Staphylococcus species are the most important group of bacteria responsible for postoperative infection, with Staphylococcus aureus and Staphylococcus epidermidis accounting for 50% to 60% of all infections contracted during total hip arthroplasties since 19802. An increasing proportion of these infections has been caused by antimicrobial-resistant strains such as methicillin-resistant Staphylococcus aureus. Therefore, the development of a more effective prophylactic regimen to reduce the rate of emergence of resistant strains would be beneficial.
Various antibiotic delivery methods, such as implantable pumps, antibiotic-containing plaster of Paris, antibiotic-containing bone cement, topical agents, and local administration, have been developed for the treatment of established infection8-13. A recent study from our laboratory showed an injection of gentamicin into the surgical wound cavity after closure of the incision to be two orders of magnitude more effective than preoperative systemic administration of antibiotics for reducing in-wound bacterial counts two days postoperatively11. Thus, the local administration of gentamicin for prophylaxis against surgical wound infection is promising, but to our knowledge it has not been compared with the administration of other antibiotics locally or with combinations of local and systemic antibiotics14,15.
One benefit of local administration is that the antibiotic is delivered directly to the wound cavity rather than diffusing into the cavity from the bloodstream as occurs with systemic administration. Another advantage is the ability to obtain a high antibiotic concentration within the wound cavity while maintaining safe systemic levels. In many cases, at this high concentration, bacteria that are normally considered resistant to an antibiotic fall within its spectrum of effective activity16. However, care must be taken to avoid excessively high local concentrations as some antibiotics have demonstrated cytotoxicity at very high concentrations, which may inhibit new bone growth and fracture union17-19.
We hypothesized that a combined treatment consisting of preoperative systemic and postoperative local antibiotics would prove more effective than administering antibiotics by either route alone for prophylaxis against wound infection. We also hypothesized that using two different classes of antibiotics for local and systemic administration would prove more effective than administering only a single class. We selected gentamicin because it has been proven to be effective in previous studies and it demonstrates good activity against Staphylococcus aureus11. We selected cefazolin because it is routinely used for prophylaxis in humans and it has also shown good activity against Staphylococcus aureus. The synergistic action of the aminoglycoside and cephalosporin classes of antibiotics has been well documented for many years, and many experiments have demonstrated improvement in the efficacy of gentamicin when it is combined with cephalosporin antibiotics20. We did not include a group treated with systemically administered gentamicin because gentamicin is not routinely given systemically for prophylaxis before orthopaedic procedures as it is associated with severe side effects, including ototoxicity and renal toxicity in up to a third of patients21. Also, we previously performed a study in which local gentamicin proved to be more effective than systemic gentamicin11.
Materials and Methods
Bacterial Preparation
Staphylococcus aureus (American Type Culture Collection [ATCC] number 25923) was grown in trypticase soy broth (BD Diagnostics, Sparks, Maryland) at 37°C for seventy-two hours. This strain is susceptible to both gentamicin and cefazolin. The broth was then separated into 1-mL aliquots and stored in a freezer at a temperature of −80°C. A series of 1:10 serial dilutions was then performed on four thawed samples; the solutions were plated on trypticase soy media and demonstrated an average viable concentration of 5.0 × 108 colony forming units (CFUs)/mL.
Study Design
After approval of the protocol by our institutional animal care and use committee, female Sprague-Dawley retired breeder rats weighing between 250 and 400 g were obtained from a commercial breeder. Animal selection was randomized, and antibiotic administration was blinded. The surgical procedure that was used in the animals was demonstrated to be effective in producing substantial and consistent wound infection in a previous study11, and therefore no pilot study was undertaken. Our study consisted of six different treatment groups with ten or more rats each (described below).
Surgical Procedure
Cefazolin and gentamicin were obtained from the distributors and diluted to the appropriate concentrations with use of 0.9% sterile saline-solution irrigant. The cefazolin was diluted to a concentration of 7.0 mg/mL for local administration and 50 mg/mL for systemic administration. The gentamicin was diluted to 2.0 mg/mL for local administration. The systemic administration of cefazolin consisted of a subcutaneous injection of 1 mL of 50 mg/mL cefazolin above the right thigh thirty minutes prior to the incision. Isoflurane anesthesia (2% to 3% to effect) was then administered until the toe pinch reflex was absent. Electric clippers were used to depilate the lateral portion of the left thigh. The wound site was prepared with an iodine-povidone swab, an alcohol swab, and a final iodine-povidone swab. A 12-mm incision was made along the length of the femur with scissors. A number-10 scalpel blade was then used to puncture the fascia. Blunt dissection through the quadriceps was used to expose the femur, and then a 1.5 × 1.5 × 1.5-cm wound cavity was created by spreading anteriorly and posteriorly along the length of the femur, with a final spread perpendicular to the femur. A 30-gauge stainless-steel suture wire was placed as a cerclage around the femur at approximately the midpart of the shaft to simulate an orthopaedic implant. A 0.5-mL aliquot of 5.0 × 108 CFUs/mL gentamicin-sensitive Staphylococcus aureus was then pipetted into the wound cavity and allowed to remain there for 2.5 minutes. The wound was then irrigated with 0.5 mL of sterile saline solution, and the irrigant was allowed to run out of the wound. The wound was closed superficially only with stainless-steel surgical skin clips. After wound closure, the rats that were to receive local antibiotics had a needle inserted percutaneously down to the femur to be sure that it was within the wound cavity and were given an injection of 1 mL of either 2 mg/mL gentamicin or 7 mg/mL cefazolin depending on the treatment group. Postoperatively, rats were given an acetaminophen elixir (6 mg/mL of their drinking water) for pain control until they were killed.
There were six experimental groups: (1) no treatment (negative control), in which eleven rats were given neither systemic nor local antibiotics; (2) local gentamicin (positive control), in which thirteen rats were given a local injection of gentamicin (1 mL of a 2.0 mg/mL concentration) into the wound cavity after closure of the incision; (3) systemic cefazolin, in which ten rats were given a subcutaneous injection of cefazolin (1 mL of a 50 mg/mL concentration) thirty minutes preoperatively; (4) local cefazolin, in which thirteen rats were given a local injection of cefazolin (1 mL of a 7.0 mg/mL concentration) into the wound cavity after closure of the incision; (5) systemic cefazolin and local gentamicin, in which fourteen rats were given a subcutaneous injection of cefazolin (1 mL of a 50 mg/mL concentration) thirty minutes preoperatively and a local injection of gentamicin (1 mL of a 2.0 mg/mL concentration) into the wound cavity after closure of the incision; and (6) systemic cefazolin and local cefazolin, in which twelve rats were given a subcutaneous injection of cefazolin (1 mL of a 50 mg/mL concentration) thirty minutes preoperatively and a local injection of cefazolin (1 mL of a 7.0 mg/mL concentration) into the wound cavity after closure of the incision.
Outcome Measurement
Two days postoperatively, the rats were killed with a CO2 overdose. The incision site was then prepared with an alcohol swab to prevent contamination from the skin flora. The wound clips were removed, and the incision was reopened. Sterile hemostats were used to spread open the wound cavity, and a cotton-tipped swab was then passed throughout the cavity for five seconds. An attempt was made to establish uniform contact with all surfaces within the surgical pocket. The swab was then placed in a 10-mL centrifuge tube containing 1 mL of trypticase soy broth and vortexed on a setting of 10 for five seconds. A series of 1:10 dilutions was performed, and the diluents were plated on trypticase soy media and then spread with use of a bent glass Pasteur pipette. The plates were incubated at 37°C for twenty-four hours, after which time the number of CFUs was counted. Only plates containing between ten and 200 colonies were considered valid. If more than one plate in each series had a countable number of colonies, the average was calculated. If either no difference or a difference of greater than two orders of magnitude was observed between one serial dilution and the next, the data were discarded, as such findings were interpreted as representing methodological error.
Statistical Analysis
The data and therefore the variances were logarithmic as a result of the nature of microbiological studies. Thus, the Mann-Whitney U test, a nonparametric method of analysis that does not assume homoscedasticity, was chosen for statistical analysis.
Source of Funding
The funding for this project was provided by the University of North Carolina orthopaedic department and a summer student research stipend ($2000) was received from the National Institutes of Health.
Results
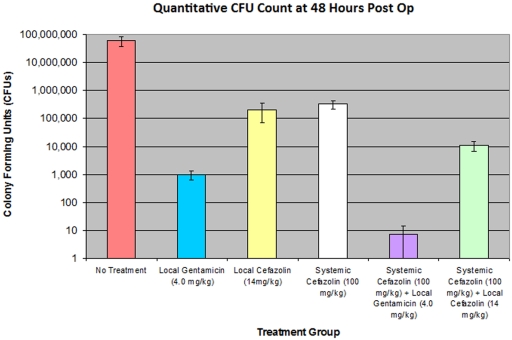
Although the control rats demonstrated marked lethargy and a decreased capacity for movement, all rats survived the two-day postoperative period and were killed at forty-eight hours after the initial surgery. Figure 1 displays, on a logarithmic scale, the mean number of CFUs cultured from each of the six treatment groups. All groups treated with antibiotics showed a significant decrease (p < 0.0003) in the mean bacterial count when compared with the control group (5.80 × 107 CFUs). No significant difference (p = 0.16) was demonstrated between the group treated with systemic cefazolin (3.30 × 105 CFUs) and the group treated with local cefazolin (2.06 × 105 CFUs). There was a significant difference (p = 0.00005) between the group treated with systemic cefazolin and that treated with local gentamicin (9.81 × 102 CFUs). There also was a significant difference (p = 0.00006) between the group treated with local gentamicin and that treated with systemic cefazolin plus local gentamicin (7.41 CFUs). Only a single wound showed growth (100 CFUs) in the group treated with systemic cefazolin plus local gentamicin; the rest of the wounds were sterile.
Fig. 1.
The mean CFU counts for each treatment group displayed on a logarithmic scale with standard error bars. All groups treated with antibiotics showed a significant decrease relative to the control group (p < 0.0003). The number of bacteria in the local gentamicin group was significantly lower than that in the systemic cefazolin group (p = 0.00005), and the number in the group treated with systemic cefazolin plus local gentamicin was significantly less than that in the local gentamicin group (p = 0.00006).
Discussion
The combination of systemic cefazolin and local gentamicin decreased the bacterial count by approximately seven orders of magnitude compared with the count in the no-treatment control group (p = 0.000005). As we hypothesized, the combination of systemic cefazolin and local gentamicin was the most effective treatment, with a decrease in the bacterial count within the wound by approximately two orders of magnitude as compared with the count associated with the next most effective treatment (local gentamicin alone) (p = 0.00006). Local gentamicin treatment decreased CFU isolates by approximately two orders of magnitude as compared with that following treatment with systemic cefazolin (p = 0.00005) and five orders of magnitude as compared with the control value (p = 0.00003). A significant difference was not demonstrated between the systemic cefazolin and local cefazolin groups (p = 0.16), but it should be noted that the dosage of systemic cefazolin was 100 mg/kg whereas the dosage of local cefazolin was 14 mg/kg.
In a previous study conducted in our laboratory, similar methodology was used to investigate local gentamicin antibiotic prophylaxis after inoculation of the wound cavity with 8.0 × 105 CFUs of Staphylococcus aureus11. In the current study, the surgical wound cavity was inoculated with a much greater bacterial concentration (2.5 × 108 CFUs), and the no-treatment control group demonstrated a much higher bacterial count (5.80 × 107 CFUs in this study compared with approximately 3.0 × 105 CFUs in the previous study). In the previous study, we found that a local gentamicin injection after wound closure decreased the in-wound bacterial number by nearly five orders of magnitude compared with the value in the no-treatment control group, a finding that is similar to the improvement in the present study.
The particular strain of Staphylococcus aureus that we used in this study has been used by other investigators as an example of a methicillin-sensitive Staphylococcus aureus, and different results would be likely if a methicillin-resistant strain were employed. The methicillin-sensitive Staphylococcus aureus that we used is a virulent organism as indicated in our previous study, in which many of the untreated control animals died prior to the forty-eight-hour experimental end point11. None of the control rats died in this study; however, they all appeared moribund before they were killed.
The current standard of care for prophylaxis for human patients being treated with an orthopaedic surgical procedure is a preoperative 1-g dose of a cephalosporin antibiotic, typically cefazolin. Such systemically administered antibiotics penetrate perfused capillary beds well and diffuse from them into the wound cavity in low concentrations. Thus, the hematoma that forms in the wound contains some antibiotic. The dosage of systemic cefazolin (approximately 100 mg/kg) used in this study is based on the finding, in studies of rats, that such a dose simulates the human serum pharmacokinetics associated with the 1-g intravenous bolus typically given to humans as preoperative prophylaxis22. Some surgeons use antibiotic irrigants during the procedure, but they are typically evacuated by surgical suction prior to wound closure and the duration of bacterial exposure to these antibiotics is short. The findings in the present study support the administration of a local antibiotic directly into the wound cavity after closure of the wound, thus ensuring that the antibiotic reaches the site of potential infection in a high concentration and is not removed except by diffusion. Furthermore, with these high antibiotic concentrations, organisms that are normally considered to be resistant to the antibiotic could fall within the spectrum of activity16. It has been shown that local administration results in high antibiotic concentrations within the wound cavity while still maintaining safe systemic concentrations. Studies have demonstrated that locally applied vancomycin can reach levels twenty times toxic serum levels while maintaining a safe systemic concentration23. However, there is concern that the extremely high concentrations that can be reached during local application of antibiotics can result in local cytotoxic effects on human cells. The inhibitory effects of antibiotics on osteoblast or osteoblast-like cell lines have been investigated in various studies. Isefuku et al. found that gentamicin decreases osteoblast metabolism at concentrations above 100 μg/mL and inhibits cell replication at levels above 700 μg/mL24. The estimated volume of the wound cavity in our study was approximately 3.38 cm3, and 2.0 mg of gentamicin was injected. This would result in an approximate concentration of 600 μg/mL in the wound cavity, a level that is below the replication threshold established by Isefuku et al. Edin et al. determined that osteoblast replication drops off at a cefazolin concentration of 1000 μg/mL and death occurs at concentrations higher than 10,000 μg/mL17. We selected a locally applied dose that yields an estimated wound concentration of approximately 2100 μg/mL.
In the in vitro studies discussed above, the antibiotic concentrations on tissue-cultured osteoblasts were maintained for days. We expect that a single local injection would result in an initially high local concentration but this concentration would drop rapidly as the antibiotic is absorbed into the systemic circulation. This rapid drop in local concentration was demonstrated by Humphrey et al., who implanted a bovine collagen sponge containing 3 mg/kg of gentamicin (12 mg in a 4-kg rabbit) into a 2 × 2-cm wound in rabbits23. The local gentamicin level was measured to be 600 μg/mL at four hours and <7 μg/mL at twenty-four hours. Thus, any toxic effects of locally applied antibiotics may be transient, especially without a sustained-release local delivery vehicle. Antibiotic-containing bone cement is frequently used for prophylaxis for patients undergoing total knee or total hip arthroplasty. Since such “depot” administration would result in sustained high levels of antibiotic, it would be better to seek levels well below the locally toxic concentrations with such methodology. In our previous study of local antibiotic prophylaxis, we evaluated the use of powdered plaster of Paris as a material that could be implanted in surgical wounds, would elute local antibiotics over a few days, and (unlike acrylic) would not need to be removed from a patient treated with non-joint-replacement surgery11. Although the elution from plaster of Paris was effective, it was not as effective as the simple injection of aqueous gentamicin; thus, we are no longer pursuing that avenue.
It must be noted that local administration of antibiotics does not have the same predictability of distribution as a systemic dose, as some antibiotic may leak from the surgical field when the closure is not watertight. However, it was quite effective in this study even if some antibiotic may have been lost.
Limitations of this study include the low volume of surgical irrigation that we used. We chose to irrigate the wound with only 0.5 mL of 0.9% sterile saline solution because this volume was shown to allow consistent establishment of infection in our previous study11. We recognize, however, that adequate irrigation with larger volumes than we used should remain an important infection-control measure and plays a crucial role in surgical prophylaxis against wound infection. Furthermore, we investigated only a single species of bacteria and only two types of antibiotics. These antibiotics were quite successful in this model but might not be as effective with a different type of bacterial contamination or in humans. Also, we administered the cefazolin through a subcutaneous route for ease of treatment, but there are little data demonstrating the kinetics of cefazolin distribution after various routes of administration in rats. Additionally, we did not include an experimental group treated with systemic gentamicin, so we could not compare locally administered gentamicin with systemic gentamicin. The inclusion of a group treated with systemic gentamicin could potentially yield information on the mechanism of bacterial killing, but we did not include such a group in the present experiment as systemic gentamicin is not routinely used for orthopaedic prophylaxis because of toxicity. We demonstrated in a previous study that locally applied gentamicin performed better than systemically applied gentamicin, with a decrease in bacterial counts in wound cultures by more than two orders of magnitude11.
The results of this investigation will need to be confirmed in human studies. The strengths of this study include the control of a number of variables and the evaluation of the ability of these antibiotics to prevent infection despite very heavy contamination of the wound with bacteria, features not possible in a human study. 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants of less than $10,000 from the National Institutes of Health. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at the Department of Orthopaedics, University of North Carolina, Chapel Hill, North Carolina
References
- 1.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23:183-9. [DOI] [PubMed] [Google Scholar]
- 2.An YH, Friedman RJ. Prevention of sepsis in total joint arthroplasty. J Hosp Infect. 1996;33:93-108. [DOI] [PubMed] [Google Scholar]
- 3.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247-54. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487-97. [DOI] [PubMed] [Google Scholar]
- 5.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101:2916-21. [DOI] [PubMed] [Google Scholar]
- 6.Eggimann P, Pittet D. Infection control in the ICU. Chest. 2001;120:2059-93. [DOI] [PubMed] [Google Scholar]
- 7.Hecker MT, Aron DC, Patel NP, Lehmann MK, Donskey CJ. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972-8. [DOI] [PubMed] [Google Scholar]
- 8.Klemm K. Antibiotic bead chains. Clin Orthop Relat Res. 1993;295:63-76. [PubMed] [Google Scholar]
- 9.Perry CR, Rice S, Ritterbusch JK, Burdge RE. Local administration of antibiotics with an implantable osmotic pump. Clin Orthop Relat Res. 1985;192:284-90. [PubMed] [Google Scholar]
- 10.Picknell B, Mizen L, Sutherland R. Antibacterial activity of antibiotics in acrylic bone cement. J Bone Joint Surg Br. 1977;59:302-7. [DOI] [PubMed] [Google Scholar]
- 11.Yarboro SR, Baum EJ, Dahners LE. Locally administered antibiotics for prophylaxis against surgical wound infection. An in vivo study. J Bone Joint Surg Am. 2007;89:929-33. Erratum in: J Bone Joint Surg Am. 2008;90:384. [DOI] [PubMed] [Google Scholar]
- 12.Dahners LE, Funderburk CH. Gentamycin-loaded plaster of Paris as a treatment of experimental osteomyelitis in rabbits. Clin Orthop Relat Res. 1987;219:278-82. [PubMed] [Google Scholar]
- 13.Gerhart TN, Roux RD, Horowitz G, Miller RL, Hanff P, Hayes WC. Antibiotic release from an experimental biodegradable bone cement. J Orthop Res. 1988;6:585-92. [DOI] [PubMed] [Google Scholar]
- 14.Clawson DK, Davis FJ, Hansen ST Jr. Treatment of chronic osteomyelitis with emphasis on closed suction-irrigation technique. Clin Orthop Relat Res. 1973;96:88-97. [PubMed] [Google Scholar]
- 15.Christian EP, Bosse MJ, Robb G. Reconstruction of large diaphyseal defects, without free fibular transfer, in grade-IIIB tibial fractures. J Bone Joint Surg Am. 1989;71:994-1004. [PubMed] [Google Scholar]
- 16.Burdon DW. Principles of antimicrobial prophylaxis. World J Surg. 1982;6:262-7. [DOI] [PubMed] [Google Scholar]
- 17.Edin ML, Miclau T, Lester GE, Lindsey RW, Dahners LE. Effect of cefazolin and vancomycin on osteoblasts in vitro. Clin Orthop Relat Res. 1996;333:245-51. [PubMed] [Google Scholar]
- 18.Chang Y, Goldberg VM, Caplan AI. Toxic effects of gentamicin on marrow-derived human mesenchymal stem cells. Clin Orthop Relat Res. 2006;452:242-9. [DOI] [PubMed] [Google Scholar]
- 19.Holtom PD, Pavkovic SA, Bravos PD, Patzakis MJ, Shepard LE, Frenkel B. Inhibitory effects of the quinolone antibiotics trovafloxacin, ciprofloxacin, and levofloxacin on osteoblastic cells in vitro. J Orthop Res. 2000;18:721-7. [DOI] [PubMed] [Google Scholar]
- 20.Giamarellou H. Aminoglycosides plus beta-lactams against gram-negative organisms. Evaluation of in vitro synergy and chemical interactions. Am J Med. 1986;80:126-37. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Huang W-G, Zha D-J, Qiu JH, Wang JL, Sha SH, Schacht J. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 2007;226:178-82. [DOI] [PubMed] [Google Scholar]
- 22.Woodnutt G, Berry V, Mizen L. Simulation of human serum pharmacokinetics of cefazolin, piperacillin, and BRL 42715 in rats and efficacy against experimental intraperitoneal infections. Antimicrob Agents Chemother. 1992;36:1427-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphrey JS, Mehta S, Seaber AV, Vail TP. Pharmacokinetics of a degradable drug delivery system in bone. Clin Orthop Relat Res. 1998;349:218-24. [DOI] [PubMed] [Google Scholar]
- 24.Isefuku S, Joyner CJ, Simpson AH. Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma. 2003;17:212-6. [DOI] [PubMed] [Google Scholar]